Abstract
Pertussis has recently re-emerged in well-vaccinated populations most likely due to a combination of pathogen adaptation and waning of vaccine-induced pertussis immunity. Changes in genomic content of the etiologic agent, Bordetella pertussis, observed in the postvaccination era can have a bearing on the efficacy of vaccines currently in use. Moreover, protective immune responses in vaccinees wane gradually depending on their originally induced size and breadth, and memory responses may not be as regularly boosted by circulating strains as was the case in the prevaccination era. This pertussis scenario asks for new, improved vaccines with at least a longer duration of protection. Pertussis vaccine research, development and postmarketing surveillance require re-evaluation and innovation of the currently available pertussis animal models, with emphasis on the use of circulating B. pertussis strains.
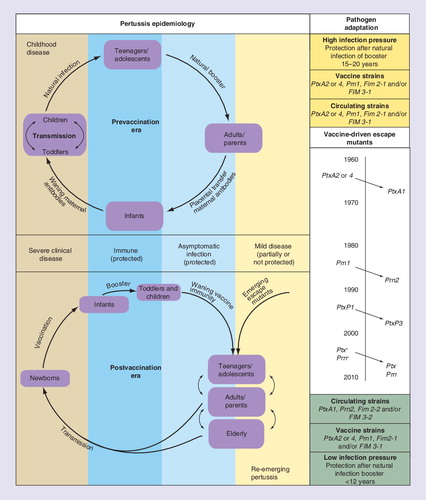
Pertussis changed from a classical childhood disease in the prevaccination era to a disease that affects all ages except recently vaccinated groups. B. pertussis resurged in well-vaccinated populations largely due to waning of immunity and pathogen adaptation (left panel). Genomic and antigenic shifts in vaccine antigens Ptx, Prn and Fim occurred in time, causing an increased antigenic mismatch between vaccines in use and circulating strains (right panel).
Whooping cough, or pertussis, has attracted renewed interest because of its recent resurgence in highly vaccinated populations. This respiratory disease is still one of the leading causes of vaccine preventable deaths in infants under 1 year of age. The WHO estimated that in 2008 approximately 16 million cases of pertussis occurred worldwide, 95% of which were in developing countries, and that approximately 195,000 children died from the disease Citation[1]. Pertussis is a highly contagious disease caused by the Gram-negative rod Bordetella pertussis, which binds to the ciliated epithelial cells in the human nasopharynx of the upper respiratory tract. The disease is characterized by three stages, a catarrhal, paroxysmal and convalescence stage, and derives its name from the ‘whoop’ sound made from the inspiration of air after a cough. Disease presentation varies with age and history of previous infection or vaccination. Young infants can present apnea and cyanosis, with or without other disease symptoms. Adults and adolescents can exhibit only mild symptoms or have the typical prolonged paroxysmal cough. In all persons, the cough can continue for months Citation[2]. A small proportion of pertussis is caused by the closely related Bordetella parapertussis and mild respiratory disease sporadically by Bordetella bronchiseptica. Recently, it has been reported that infection with B. holmesii may induce symptoms similar to pertussis Citation[3].
B. pertussis and B. parapertussis are independent derivatives of B. bronchiseptica-like ancestors, and evolved as human-specific pathogens. These three species, known as classical Bordetellae, express a wide array of virulence antigens to colonize the respiratory tract of their natural hosts, to impair the function of immune mechanisms and/or to invade host tissues Citation[4]. The expression levels of the majority of these virulence antigens are controlled by one master two-component regulatory system, BvgS/BvgA. BvgS is a transmembrane sensor protein that can phosphorylate itself and BvgA. The system controls the adherence of Bordetella (bvg+), the forming of biofilms (bvgi) and survival outside the host (bvg−) in response to environmental stimuli Citation[5]. The repression of the regulated genes is dependent upon the third gene, bvgR Citation[6]. Progressive loss and inactivation of B. pertussis genes has been seen over the past 60 years and may have altered pathogen virulence due to loss of regulatory or control functions Citation[7]. Furthermore, although the human Bordetellae are considered as genetically monomorphic, there is increasing evidence for accumulation of genetic variation in B. pertussis, such as through expansion of insertion sequence elements and SNPs Citation[4,8,9]. This process may underlie selection of B. pertussis strains that are more adapted to survive in dense and immune human populations.
Pertussis vaccines & quality control
To date, there are two different types of licensed pertussis vaccines, the first generation being whole cell vaccines (WCVs), which are gradually being replaced by the second generation of acellular vaccines (ACVs). Both types of vaccines differ considerably in composition, side effects and steering of immune responses, but also greatly with respect to their release requirements after production. WCVs contain killed bacteria and cause more adverse reactions than ACVs, which contain one to five purified virulence factors, being: pertactin (Prn), filamentous hemagglutinin (FHA), two fimbriae (Fim) serotypes (Fim 2 and 3) and chemically detoxified pertussis toxin (Ptx). These ACV products, whichare available from diverse manufacturers, differ not only in the number of components but also in their concentrations, detoxification methods, their degree of adsorption to different adjuvants and consequently in their immunogenicity.
WCVs and ACVs induce different types of humoral, as well as cellular, immune responses in human and animals Citation[10,11]. WCV induce a T-helper cell response of a predominant type 1 (Th1) cytokine profile, and a broad antibody response against a whole range of surface antigens yet moderate or low levels against the major protective antigens, such as Ptx and Prn Citation[12]. ACV induces a more Th2-cytokine dominated cellular immune response and high antibody responses against the vaccine antigens involved Citation[13–15]. The short-term efficacy of both vaccines is regarded similarly high, but neither type of vaccine has a satisfactory long-term duration of protection. Protection after vaccination with WCV is estimated at 4–12 years while protection by pediatric ACV may last 5–7 years, depending on the quality of the vaccine and immunization schedule in use Citation[16,17]. For release of vaccines, national and international authorities require the performance of extensive quality control testing, including a potency test before each vaccine batch is released for human use.
Currently, the potency of WCV is assessed by an intracerebral mouse protection test (ic-MPT) based on the Kendrick test Citation[18] but this test is not applicable for ACV batch release. In Japan, China and Korea, a modified ic-MPT (suckling mice test) is used to assess the potency of ACV with varying pertussis antigen compositions Citation[19]. In Europe and North America, consistency in production of licensed ACV is demonstrated by immunogenicity assays instead of by assessing potency Citation[20,21]. Recently, recommendations to assure the quality, safety and efficacy of ACV were published by the WHO Citation[201]. In this document, details are provided for the modified ic-MPT for potency testing of ACV. Simultaneously, the WHO recommends use of the intranasal mouse challenge model (in-MCM) in nonclinical testing for registration of new vaccine candidates. The in-MCM is known as a valid research model in vaccine research and development Citation[22–24]. The WHO does not recommend this model for routine potency testing Citation[201].
Resurgence of pertussis
Mass vaccination programs against pertussis with WCV in the 1950s led to a dramatic decrease in mortality and morbidity among children. Pertussis virtually disappeared in the industrialized world during the 1970s and 1980s, and herd immunity was initially observed Citation[25]. Clearly, pertussis vaccination prevents severe illness and reduces infection- and transmission rates but not to the same extent as seen for other vaccine preventable childhood diseases Citation[26,27]. In addition to reducing its incidence, 50–60 years of vaccination either with WCV and/or ACV profoundly changed the epidemiology of pertussis. Infants too young to be fully immunized have become the most vulnerable group at highest risk for severe clinical disease, whereas adolescents and adults, previously protected, have become populations susceptible to mild disease Citation[28,29]. Since the 1990s, a steady increase in the number of pertussis cases was observed in various countries with successful and long-lasting vaccination programs, such as Argentina, Finland, Norway, Spain, Switzerland, Israel, Canada, Australia, the USA and The Netherlands Citation[30–34]. In these countries, different types of pertussis vaccines were being used with proven efficacy in clinical trials. Improved diagnostics and/or increased awareness have been implicated in the observed increased notifications and, to some extent, differences in efficacy of the various vaccines and/or national vaccination schemes in use could also play a role. However, the two key factors held responsible for the pertussis resurgence in vaccinated populations are, on the one hand, the appearance of new B. pertussis strain variants, and on the other hand, the gradual loss of vaccine-induced protective immunity in vaccinees, in an era of less circulation and natural boosting . The relative impact of these factors in the resurgence of pertussis is under investigation and may differ between subpopulations and countries Citation[35–38].
Pathogen adaptation in immune populations
Coinciding with the observed increase of pertussis in various vaccinating countries, antigenic mutations were noted in circulating B. pertussis strains all over the world Citation[32,39,40]. In The Netherlands, a country with an overall high vaccination coverage of more than 95%, the rise in pertussis notifications in the 1990s concurred highly with the switch in the Ptx promoter (ptxP) region from the ptxP1 allele to the ptxP3 allele Citation[41]. Earlier antigenic drift from the Ptx S1 subunit PtxA2 to PtxA1, and from Prn1 to Prn2, had no significant effects on the pertussis incidence, but could have contributed to the reduced long-term effectiveness of the WCV in use Citation[41,42]. To date, the most prominent circulating strains are typed PtxA1/Prn2/Fim2-2 or -/Fim3-2, as opposed to those in the prevaccination era that were predominantly PtxA2/Prn1/Fim2-1 or -/Fim3-1 and are still the base for our first- and second-generation vaccines Citation[31,32,40,43,44]. In addition to the above allelic shifts, multiple other SNPs were revealed by comparative genomics of strains isolated before or after the introduction of vaccination, that might be linked to successful transmission in vaccinated populations Citation[8]. Hence, comparative genomics data suggest that B. pertussis, despite its high population homogeneity, is evolving in response to vaccination pressure, resulting in expansion of clones carrying new variants of genes encoding immunogenicity and pathogenicity-associated antigens. The fact that new variant B. pertussis strains are more often isolated from vaccinated than from nonvaccinated patients further supports this notion Citation[45,46]. Immune pressure on B. pertussis antigens can eventually even result in functional inactivation or entire deletion of the genes coding for them, as has been described for ptx and prn Citation[45]. Prn deficiency does not seem to be such a rare phenomenon. In France, Bouchez et al. noted that a significant percentage (5.6%) of the strains from studied hospitalized newborns below 6 months of age did not produce Prn Citation[45]. Recently, Otsuka et al reported that Prn-deficient strains have significantly increased in B. pertussis populations since the early 2000s in Japan Citation[47]. Both countries have molecular surveillance systems in place and report stable pertussis vaccination coverage for primary series Citation[47,202,203].
Antigenic mismatches between pertussis vaccine strains and circulating vaccine-evasive B. pertussis strains may alter the effectiveness of vaccine-induced immune responses through escape from immune recognition, as will be discussed below. However, adaptation may also affect strain virulence properties or the biological impact of antigens, as is under investigation by several research groups. In preliminary studies, Prn-deficient strains were found to have competing growth advantage in vitro Citation[47], but reduced multiplication in adult mice Citation[45]. By producing 1.6-times more of the virulence factor and immune-modulating agent, Ptx, the emerging ptxP3 strains may alter the course or severity of an infection before the immune response can become effective Citation[41]. Allelic shifts therefore may indeed have provided a selection bias on pathogen colonization, survival and offspring. Taken together, the genetic and antigenic drift of B. pertussis in well-vaccinated populations is an acknowledged fact, and is one of the factors jeopardizing the efficacy of current pertussis vaccines. In this, overall gain of advantageous biological properties and loss of immunologic recognition of circulating strains occur side by side.
Induction & maintenance of pertussis immunity
The general view of human pertussis-specific immune mechanisms is that they protect against disease rather than against infection, and are not long-lived. In the search for a correlate of protection, pertussis-specific serological responses have mostly been studied. Antipertussis antibodies can minimize the infection and its symptoms by preventing bacterial adherence to the respiratory epithelium by neutralizing bacterial toxins and by removing bacteria through opsonization and complement-mediated killing Citation[11,23,48]. Human pertussis vaccination induces initial high levels of specific IgG antibodies Citation[49–52] and pertussis infection also induces specific IgA Citation[53]. Although the presence of certain specificities of antipertussis antibodies has been associated with protective immunity in a household exposure setting and higher levels are generally regarded as more protective, no accepted threshold of specific antibodies to a single pertussis antigen, such as for tetanus or diphtheria toxins, is established as a correlate of protection. In a mouse model, protection against infection was shown in the absence of detectable antibodies Citation[54,55], suggesting some kind of protection by recall of immunologic memory. In such case, pertussis specific memory B cells, which can be enumerated in immunized humans and mice Citation[56–59], are triggered to provide a rapid rise in the number of plasma cells and in levels of antibodies with improved affinity.
Because T cells highly regulate antibody responses and are responsible for the production of antimicrobial cytokines, a wide interest also exists for human and murine T-cell responses specific for B. pertussis antigens after vaccination or infection. These responses have been associated with a variety of functional cytokines Citation[11,13,60,61]. Recovery from whooping cough in older infants is associated with induction of T lymphocytes producing Th1-type cytokines IFN-γ and IL-2 Citation[61]. Studies in very young children (2 months of age) show that natural infection with B. pertussis or a first administration of WCV can promote maturation of Th1-type responses Citation[61,62]. By contrast, the widely used ACV vaccines also induce Th2-type cytokine (IL-4 and IL-5) responses to concomitant vaccine antigens in infants Citation[60,62], becoming more pronounced when given closer to birth Citation[63,64]. Although suggested earlier, current infant immunization with ACV does not seem to be a risk factor for the development of atopic disease in children Citation[65,66], despite similarities in Th2 polarization of vaccine-induced and allergic T-cell responses. Recently, in a systems-level approach, it was demonstrated that the Th2 component of ACV-induced responses was counterbalanced by parallel Th2-antagonistic and antimicrobial immunity, which was either not present or actively downregulated within the Th2-polarized responses associated with allergic inflammation Citation[67]. However, strong Th2 polarization of ACV-induced T-cell immunity might be an unfavorable effect, as it has been implied as a potential cause of local reactions to booster vaccinations Citation[68,69]. In addition to Th1 cytokines Citation[23], IL-17 has recently been identified as another protective cytokine in preclinical models of pertussis vaccination and challenge Citation[70–72]. IL-17 has also been implied in in vivo and in vitro programming of human T-cell responses by B. pertussis Citation[73,74]. However, IL-17 and any other proinflammatory cytokines could mediate immunopathology in the lung. Probably to avoid this, pertussis T-cell responses are strongly controlled by regulatory T cells Citation[11].
Clearly, both humoral and cell-mediated immune responses readily react to B. pertussis antigens, but so far no single specificity, type or level of effector mechanism has been implied as a preclinical or clinical correlate of protection. Likely, protection involves a threshold of mucosal and systemic immunity to prevent colonization of B. pertussis in the nasopharynx and early production of bacterial toxins. The duration of the protected status then depends on the initially induced level of immunity and its subsequent natural contraction and maintenance in time. While for some classical examples of viruses maintenance of protective immunity may last for decades with or without boosting Citation[75–77], this clearly seems not to be the case for pertussis.
Mechanisms underlying progressive loss of protective immunity against pertussis
Although initially induced at a sufficiently protective level, acquired pertussis-specific immunity is progressively lost with time, as indicated by epidemiological data. Vaccinees may become at risk of natural infection with currently circulating B. pertussis strains as early as 2 years after vaccination, as suggested for children after preschool booster by De Greeff et al. Citation[78]. Hence the duration of protection against pertussis compared with other vaccine-preventable diseases is relatively short, implying that pertussis-specific immunological memory mechanisms are not effective in the long term. Ready induction of specific serum antibody levels after vaccination or infection, as well as their rapid fall, are well documented in humans and mice Citation[13,52,56–59,79–83]. Recently, Hendrikx et al. reported that despite waning of antibody titers several years after primary or preschool booster vaccination, children may have detectable circulating pertussis-specific memory B cells Citation[57]. Moreover, levels of these Ptx, FHA and Prn specific memory B cell populations can be shown to rise shortly after a booster vaccination, yet their waning is also observed within 1 year Citation[81,84]. This exemplifies that natural expansion and contraction of human pertussis-specific B-cell mechanisms occur after vaccination. Little knowledge is available on the long-term behavior of human pertussis-specific T-cell responses. Based on 3H-thymidine incorporation, pertussis-specific cellular responses were found to wane 3–4 years after their induction by primary pertussis vaccination of infants, which in part seemed masked by ongoing natural boosting through subclinical infections Citation[13]. With the benefits of a pathogen-specified environment and accessibility of various tissue samples, long-term persistence of immune mechanisms to pertussis can also be studied in mice. Such analysis revealed a lack of prolonged maintenance of mouse splenic memory B-cell populations specific for Ptx, FHA and Prn Citation[58], as opposed to third-party vaccine antigen Citation[85], suggesting a possible weakness in the self-renewal capacity of the pertussis antigen-induced memory B cells. On the other hand, follow-up of pertussis vaccine-induced murine T-cell responses suggested their prolonged persistence, but more importantly emphasized the sustained Th1 versus Th2 cytokine imprinting of cell-mediated immune responses induced by WCV and ACV, respectively [van Els C, unpublished data] Citation[55]. More long-term clinical and preclinical studies on the characteristics of specific memory T and B-cell populations following pertussis vaccination or infection are needed to shed more light on their typical rise and contraction, but more so on the mechanisms responsible for the progressive loss of protective immunity to pertussis Citation[86].
Basically, defects in durable immunological protection to circulating B. pertussis strains may arise at two different encounters between the host immune system and pertussis antigens. First, during the primary immune response to WCV or ACV, the initial clonal burst size, the differentiation of various lymphocyte specificities and their maintenance into a memory phase may be suboptimal. ACV antigens have been studied for immune-modulating properties in primary immune responses and were implied to have an effect on immunogenicity of coadministered vaccine components Citation[87]. McGuirk et al. studied the in vivo immunomodulatory effect of detoxified Ptx when formulated with other antigens and did not find any effect Citation[88]. On the other hand, FHA was found to suppress in vitro IL-12 production and to enhance IL-6 and IL-10 production by macrophages Citation[89]. In mice, maintenance of memory B-cell populations to ACV antigens was found to be compromised compared with other vaccine antigens Citation[58,90]. Hence, ACV antigens may have intrinsic properties affecting the primary immune response. This might not become critical in the first few years after vaccination but only after some natural waning.
Second, when encountering live B. pertussis the secondary immune response may lack efficiency. At this level, various mechanisms of evasion of protective immunity can be envisaged. The B. pertussis genus has acquired various evolutionary properties able to evade the innate phagocyte system as an important effector arm of adaptive immune responses. Induction of innate inflammatory chemokines, cytokines and of other immune mediators is needed for phagocytosis and bacterial killing. Ptx delays neutrophil recruitment and bacterial killing by inhibiting the production of early chemokines (RANTES, MCP-1, MIP-1 and IL-8) Citation[91–93]. In addition, FHA, adenylate cyclase hemolysin (AC-Hly) and tracheal cytotoxin, as well as antibodies to lipopolysaccharide and surface-localized adherence factors interfere with recruitment, bacterial uptake, bactericidal activity and cell survival of monocytes and neutrophils Citation[90]. Furthermore, lipopolysaccharide, CyaA, tracheal cytotoxin, type III secretion system, Ptx and (indirectly) FHA can subvert innate Toll-like receptor signaling pathways, resulting in inefficient antigen presentation and production of proinflammatory cytokines by DCs, important for the recall of memory T cells Citation[71,72,94]. Ongoing evolutionary adaptation of B. pertussis in immune populations could lead to selection of variants even more efficient in escaping recall immunity. Whether allelic variants of PtxS1 or Prn have differential capacity to undermine secondary immune responses is, to our knowledge, unknown, but increased levels of Ptx expression, such as relating to the ptxP3 mutation Citation[41], will likely have an impact.
On the other hand, antigenic mismatches between vaccine-induced immune responses and the antigenic profile of circulating strains, accumulating through ongoing strain adaptation, will compromise long-term effectiveness of protective immune mechanisms in the vaccinated population. Antibody or T-cell cross reactivity between allelic variants of Ptx S1, Prn and fimbriae may still ensure protection, depending on the absolute level of the memory response, but loss of entire expression of important vaccine antigens such as Ptx and/or Prn in circulating strains will unambiguously narrow the effective breadth of the recall response Citation[45].
Hence, suboptimal long-term maintenance of protective immunity against whooping cough relates to complex interplay between B. pertussis components and the host immune system at various time points of their interaction. More fundamental understanding of the essential levels and mechanisms of immunity correlating with protection against disease, as well as of the major obstruction(s) for their long-term persistence, is needed to help design improved vaccines with a longer duration of protection. Animal research is instrumental in developing such knowledge, as well as in investigating novel vaccine candidates.
Expert commentary
Various animal models are available for pertussis research Citation[95,96]; however, these should be reviewed and optimized in the context of the emergence of novel B. pertussis strain variants in the vaccinated host, and the need for innovation of vaccines or schedules to improve the duration of protection. Ideally, new vaccination strategies for pertussis should be studied in a reasonably accessible animal model that can sense differences in clinical manifestations (more or less resembling human pathophysiology) after infection with B. pertussis strain variants, and can assess protection and duration of immune mechanisms relevant to human, preferably against acceptable costs. However, it is unlikely that one animal species or one model will answer all research questions. Also, all experimental animal species will have lifespan restrictions when testing durability of protection. Moreover, no model, not even the human, has disclosed a defined correlate or surrogate of protection for pertussis Citation[12,18,22,96–113]. Although some animal models (ic-MPT and in-MCM) showed an indirect relationship between vaccine-induced short-term protection in humans and animals Citation[18,19,24], this correlation may fade in time due to the continuous genetic and antigenic evolution of B. pertussis. It is our view that the ongoing pathogen adaptation of B. pertussis asks for innovation of presently available animal models throughout the chain of vaccine research, development and postmarketing surveillance, introducing the use of circulating B. pertussis strains. Notably, excluded from this innovation are mandatory animal tests for the quality control of vaccine batches – that is, so-called toxicity and potency tests – since these are used to demonstrate safety and consistency in production and do not relate to the efficacy of vaccines.
Introducing currently circulating pertussis strains in various animal models will have consequences for their design, as the models are generally optimized for the use of laboratory-attenuated infectious strains Citation[114–116]. Pertussis animal models imply various animal species, each allowing the assessment of one or more experimental parameters, such as strain pathogenicity, host response to infection, or immunogenicity and effectiveness of candidate or registered vaccines, but also having their limitations Citation[95,96]. For instance, nonhuman primates are the most suitable experimental animals for pathogenicity research since these animals can display classical whooping cough or persistent coughing Citation[97,106,109–111]. When compared with rodents their immune responses are likely more similar to humans, and these animals live longer, allowing duration of protection to be studied over time. However, these models are expensive and ethically not acceptable for wide-scale use. A more accessible and the most commonly used animal model in pertussis vaccine research is the in-MCM Citation[22,95]. This model was shown to predict efficacy of ACV, as well as WCV, in a relatively reproducible manner Citation[22,23], but in fact measures prevention of B. pertussis infections by host defense mechanisms of the upper rather than the lower respiratory tract Citation[99]. Using this model, Mills and coworkers demonstrated similarities to human immune parameters such as the requirement of specific T and B cells for protection Citation[23], and, in accordance with clinical studies, no clear correlation between specific antibody responses and protection Citation[11,24]. However, the initially found similar ranking of the ACV under test in terms of estimated efficacy in children and potency in the in-MCM could not formally be confirmed in an international collaborative study Citation[117]. As an exception, the coughing rat model displays symptoms reminiscent of human disease and could therefore serve as a more relevant infection model in a small experimental animal. This model has only been used to a limited extent due to the intrabronchial infection procedure, involving agarose-embedded bacteria Citation[101–104,108]. However, recently we demonstrated the feasibility of the coughing rat model to study the pathogenesis of different circulating strains and that the route of infection, intrabronchial instead of intranasal, determines the level of disease and immunity against B. pertussis Citation[118]. Until now however, reasons of convenience, such as availability of species-specific immunochemical reagents and in-house animal husbandry experience, often guide the choice of the pertussis animal species in pertussis models.
Depending on the phase in pertussis vaccine research and development, and for each topic to be studied an experimental animal species should be chosen (e.g., mouse, rat, monkey and others). Furthermore, the animal model of choice should be optimized with respect to: covariates for infection (e.g., clinical isolate, laboratory strain, recombinant strain, inoculum and route); covariates for vaccination (e.g., vaccine or immunogen, dose, route and schedule); and end points of disease, protection and/or immunity (e.g., disease features, reduction of disease features, clearance, magnitude, quality and duration of immunological features), as is summarized in . In the following section, we discuss improvements of basic infection, vaccination and challenge models and their use to substantiate the protectiveness of current pertussis vaccines and novel vaccine candidates against newly circulating B. pertussis strain variants.
Infection models
Infection models are needed in basic pertussis research and also form the basis for challenge models to test effectiveness of vaccine candidates or regimens in the preclinical phase. The choice of covariates and end points are highly decisive for whether differences in pathogenicity between B. pertussis isolates can be demonstrated or not, and should be made carefully . The most commonly used B. pertussis strains in animal models of infection, 18323, Wellcome 28 and Tohama I, are laboratory subcultured and attenuated strains, and hence genetically different from clinical isolates Citation[37,114,119]. Since differences in genome sequences may affect the pathogenicity of B. pertussis in animals and humans, these laboratory strains are not representative for current B. pertussis strains. In fact, the same is true for clinical isolates and vaccine strains descending from the prevaccination era, from which currently circulating B. pertussis strains also differ genetically through loss, rearrangement and adaptation of genes during 50 years of mass vaccination Citation[120,121]. Infection models should therefore be optimized for the use of relevant clinical and recombinant B. pertussis strains in addition to laboratory strains. Recently data from Van Gent et al. suggested that an allelic shift from prn1 to prn2 had a trade-off in the ability of ptxP1 clinical isolates to colonize naive hosts in a rodent animal model, prn2 strains being less capable of colonizing the mouse lung Citation[122]. Earlier, using isogenic mutants of the B. pertussis strain Tohama I expressing the alleles ptxA1 or ptxA2 and prn1 or prn2, Komatsu et al. found that the emerged nonvaccine-type mutant ptxA1/prn2 displayed a prolonged survival in naive mice compared with the other mutants investigated Citation[107]. These results illustrate the feasibility of carefully designed experimental animal infection to unravel the role of allele shifts in B. pertussis.
The severity and/or course of infection are generally demonstrated by measuring the reduction in colonization of B. pertussis in the lungs in the animal model of infection. The clearance of B. pertussis in the nasopharynx and lungs, however, depends highly on the infectious dose, route of infection and the age of the animal of choice. The incubation period is often shorter and natural clearance of the bacteria is faster in animals compared with humans Citation[96]. In particular, mice and rats are highly effective in eliminating bacteria in an aspecific manner from the nasopharynx, by contrast to, for example, monkeys having a more human-like clearance. High infectious doses and artificial routes of infection are therefore inevitable in mice and rats. However, these high pathogen loads will trigger inflammatory responses due to high levels of endotoxins and the bacteria may already have been cleared aspecifically from the respiratory tract before a genuine infection can develop. As an alternative, immobilization and inoculation of the bacterium deep in the lower airways allows the bacteria to colonize in a rat as well as a piglet model Citation[98,101]. Therefore, animals infected with B. pertussis embedded in agarose to prevent aspecific clearance of B. pertussis can develop pertussis-specific clinical symptoms. Consequently, multiple disease features, such as lung pathology, lung clearance and leukocytosis, and even coughing (in rats), can be monitored and these clinical animal models may therefore underpin similarity or differences in the course or severity of infection between B. pertussis strains [van der Ark AAJ. Manuscript in preparation]. To a limited scale, results could be refined and confirmed in nonhuman primate studies Citation[97,106,109–111]. Recently, Warfel et al. developed a novel pertussis infection model in Olive baboons (Papio anubis) Citation[123]. They demonstrated that all pertussis-infected baboons developed clinical symptoms of pertussis as opposed to only 25% of rhesus macaques (Macaca mulatta). Although the exact mechanism remains unclear, it was postulated that the growth of virulent B. pertussis is temperature-dependently modulated. The lower normal body temperature of baboons (37–39°C) allows B. pertussis to grow in a virulent phase while in rhesus macaques, having a higher normal body temperature (38.7–39.8°C), the virulence of B. pertussis is reduced due to inactivation of CyaA at temperatures higher than 39°C.
Vaccination & challenge models
Vaccination models are used in the discovery phase of vaccine development to assess the immunogenicity of vaccine leads and in the preclinical phase of vaccine development to characterize the quality, quantity and duration of innate and/or adaptive immune responses induced by promising vaccine candidates. Challenge models, combining vaccination and infection, are generally used to assess the quality and level of vaccine-induced protection against a particular B. pertussis strain and often use high vaccine doses, up to one-quarter human dose (HD), to immunize the animals. This vaccine dose should be carefully reconsidered. Although high doses of vaccine induce higher immune responses that are easier to measure, they bear little or no relevant information about the effectiveness of the underlying immune effector mechanisms. When immunized with one-fourth HD, mice receive 12.5 HD per kilogram of bodyweight, while infants and adults are immunized with approximately 0.3 and <0.02 HD per kilogram of bodyweight, respectively.It is like that immune responses induced in mice by one-quarter HD do not wane rapidly, and are still maximal at the time of challenge Citation[58]. This makes challenge models in their current form unsuitable to study the phenomenon of waning immunity, especially, if the sensitivity of the animal to the B. pertussis challenge inoculum may alter with increasing age. All of this complicates long-term follow-up studies (>1 year) in rodents after vaccination. Hence, the most‑used pertussis research model, the in-MCM, in its current form may not be an appropriate model to test vaccine potency at prolonged term. It should be optimized per vaccine candidate with respect to vaccine dose and strain, and dose and time point of the bacterial challenge inoculum. Another animal species with promising perspectives for long-term protection studies is the baboon Citation[123]. This experimental animal shows features of waning pertussis specific immune responses, has a longer lifespan than rodents, and seems to remain susceptible to pertussis infection with increasing age.
Finally, optimized challenge models should be introduced in the so-called ‘postmarketing surveillance phase’. Despite being a suboptimal animal species for B. pertussis infection, mice are often used to assess vaccine-induced protection against B. pertussis after administration of an infectious challenge inoculum to vaccinated animals Citation[95]. In view of the increasing antigenic mismatch between vaccine strains and clinical isolates and other evaluating features observed, we recommend that the potency of the current pertussis vaccines be periodically tested against emerging B. pertussis mutants in an optimized pertussis challenge model. This could reveal the risk of vaccine failure, especially in the face of new strains with functionally altered or inactivated genes, and serve as a feedback loop into the discovery phase of the pertussis vaccine research and development chain.
Scientific substantiation of mechanisms of protection
As stated earlier, direct correlates of protection are still not established for whooping cough, probably due to the fact that multiple target antigens or mechanisms together play a role. Correlates of protection are badly missed in pertussis animal models throughout the various phases of vaccine development . The results of animal experiments are often decisive for the continuation of a vaccine candidate and for the release of vaccines. Also valuable would be the identification of early biomarkers predicting the outcome of adaptive immune mechanisms, such as their magnitude, multispecificity, type and long-term maintenance. An integrated strategy to study the mechanisms that form and sustain a highly protective immune response against pertussis could be ‘systems biology’. This new field targets complex interactions in biological systems, such as the entire immune system, and uses, visualizes and interprets extensive data sets obtained from various technology platforms, such as genomics, transcriptomics and (immuno)proteomics. Currently, a few examples are described in the literature illustrating the advances of systems biology in vaccine research and development Citation[124–126]. After vaccination or infection, the immune system reacts with the up- or down-regulation of genes associated with host defense. The corresponding proteins are involved in the elimination of the pathogen or the vaccine antigens – that is, associated with cytokine expression, cell differentiation, isotype switching or complement activation. Protection against pertussis is not based on one single feature, such as a minimal concentration of neutralizing antibodies, but on a detailed network of many, yet still hardly known, factors. Information obtained from initial clinical data sets using the technology platforms could be integrated, and predictive gene signatures could be further validated and optimized in animal models of protective immune responses against pertussis. Pulendran et al. suggested the development of a vaccination chip consisting of a minimal set of host genes which can elucidate the protective activity induced by a specific vaccine Citation[127]. We suggest that such a vaccination chip should be developed for the most-used and informative animal species in pertussis vaccine research, the mouse, the rat and, eventually, monkeys. The systems biology approach would then allow an in depth evaluation of early gene signatures of vaccine leads, adjuvants and others, and the effect on the quality and duration of a protective immune response. Moreover, the systems biology approach in animal models should be linked to early human signatures related to durable specific immune responses in Phase I and II clinical studies of improved vaccines against pertussis.
Five-year view
The continuous need for animal models in pertussis vaccine research, development and release is unambiguous. However, a re-evaluation of the current pertussis animal models is necessary to find a solution for the resurgence of pertussis in well-vaccinated populations by innovating pertussis vaccines and vaccination. Depending on the phase of vaccine research and development, we suggest a focus on particular points for optimization or renewal of these models for the next 5 years . The focus in the discovery phase is especially on improving infection models and studying immunogenicity of new vaccine leads. In preclinical development, an optimized challenge model with a relevant dose of vaccine candidate and choice of challenge strain is most important to predict efficacy. In this phase, we recommend to study not only the short-term but also the long-term features of immunity, protection and level of cross protection to various isolates. For some candidates, this can eventually also be performed in more sophisticated animal models in which more extended evaluation of protective immunity is feasible. Mandatory release and consistency testing of clinical batches of vaccines can still be performed in the available standard testing procedures including animal tests. Finally, in the postmarketing surveillance phase, alongside clinical ‘Phase IV’ studies, the use of animal models should be introduced to predict and measure loss of protection against currently circulating strains. Antigenic drift of B. pertussis vaccine antigens has so far been observed in Ptx, Prn and fimbriae, but more virulence factors of B. pertussis are evolving. These may not be direct targets of vaccine-induced immunity, but could rather influence the effectiveness of the memory host response or affect colonization or survival Citation[90]. Hence, it is important in this phase to continuously collect clinical isolates in the vaccinated population, not only for assessment of virulence and immunomodulation properties in basic animal models, but also for periodical testing of effectiveness of licensed and used ACV or WCV in an optimized animal challenge model using the newest strains. In our view, a suitable animal model for such ‘surveillance of protection’, should sense changing biological properties of genomically drifting B. pertussis species and would preferably display clinical symptoms reminiscent of human disease.
Given the limited duration of protection of current WCVs as well as ACVs, new vaccination strategies should be developed avoiding a too narrow basis of protective antigens, as well as early exhaustion of host immune mechanisms. Insight into the induction of long-term protection could lead to a novel generation of optimized pertussis vaccines and vaccine components (antigens, adjuvants), immunization routes and vaccination schedules, possibly with the use of the systems biology approach linking early gene signatures to protective immune responses. Candidates include new vaccine concepts such as the live attenuated vaccine strain BPZE1 Citation[100,128–131] and outer membrane vesicle vaccines Citation[105,132–134], which all can be administered intranasally and may induce a wider, more effective and tissue-targeted long-term protective response. Alternatively, new adjuvants could be introduced that direct the vaccine-induced immune responses more effectively towards the preferred Th1 responses without evoking side effects. However, each new vaccination strategy will lead to further ongoing pathogen adaptation and it is therefore of great importance that animal testing and in vitro immunoassays are optimized and re-evaluated for strain usage on a regular basis.
Table 1. Brief summary of features of pertussis animal models compared with human infection and immunity.
Table 2. Re-evaluation of pertussis animal models in various phases of vaccine research and development.
Key issues
• Pertussis resurged in well-vaccinated populations worldwide, largely due to rapid loss of protective immunity and pathogen adaptation. Bordetella pertussis remained endemic because available pertussis vaccines mainly prevent disease and to a lesser extent infection and transmission.
• Adaptation of currently circulating B. pertussis strains to the immune population may cause immune evasion through altered features either of pathogenicity or of the interaction with the host immune system. These altered features may allow the bacterium to (more) successfully circumvent vaccine-induced immunity, compromising the efficacy of current pertussis vaccines, at the short and long term.
• Re-evaluation and innovation of current pertussis animal models, to imply the use of newly circulating B. pertussis strains, is highly necessary in pertussis vaccine research and development. Models should be optimized with regard to choice of animal, infection and immunization procedures (e.g., route, doses, schedule and strains), and end points of infection, immunity and protection, to be able to monitor alterations in pathogenicity, and evasion of immunity and vaccine effectiveness as B. pertussis evolves.
• Direct correlates of protection for pertussis are still not established but are urgently needed throughout the various phases of vaccine research and development. Therefore, the identification of early biomarkers predicting the magnitude, multispecificity, type and long-term maintenance of adaptive immune mechanisms against the continuously evolving B. pertussis is of great importance.
• Postmarketing ‘surveillance of protection’ of registered vaccines against emerging vaccine-evasive strains of B. pertussis in improved animal challenge models, should be an extended activity of continuous disease surveillance programs. For this, collecting isolates in vaccinated populations is essential.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- WHO. Pertussis vaccines: WHO position paper. Wkly Epidemiol. Rec. 85(40), 385–400 (2010).
- Halperin S, De Serres, G. Pertussis. In: Bacterial Infections of Humans. Brachman PS, Elias A (Eds). Springer, NY, USA, 577–595 (2009).
- Yih WK, Silva EA, Ida J, Harrington N, Lett SM, George H. Bordetella holmesii-like organisms isolated from Massachusetts patients with pertussis-like symptoms. Emerging Infect. Dis. 5(3), 441–443 (1999).
- Diavatopoulos DA, Cummings CA, Schouls LM, Brinig MM, Relman DA, Mooi FR. Bordetella pertussis, the causative agent of whooping cough, evolved from a distinct, human-associated lineage of B. bronchiseptica. PLoS Pathog. 1(4), e45 (2005).
- Locht C. Molecular aspects of Bordetella pertussis pathogenesis. Int. Microbiol. 2(3), 137–144 (1999).
- Merkel TJ, Boucher PE, Stibitz S, Grippe VK. Analysis of bvgR expression in Bordetella pertussis. J. Bacteriol. 185(23), 6902–6912 (2003).
- King AJ, van Gorkom T, van der Heide HG, Advani A, van der Lee S. Changes in the genomic content of circulating Bordetella pertussis strains isolated from The Netherlands, Sweden, Japan and Australia: adaptive evolution or drift? BMC Genomics 11, 64 (2010).
- Bart MJ, van Gent M, van der Heide HG et al. Comparative genomics of prevaccination and modern Bordetella pertussis strains. BMC Genomics 11, 627 (2010).
- Mooi FR. Bordetella pertussis and vaccination: the persistence of a genetically monomorphic pathogen. Infect. Genet. Evol. 10(1), 36–49 (2010).
- Canthaboo C, Xing D, Douglas A, Corbel M. Investigation of an aerosol challenge model as alternative to the intracerebral mouse protection test for potency assay of whole cell pertussis vaccines. Biologicals 28(4), 241–246 (2000).
- Mills KH. Immunity to Bordetella pertussis. Microbes Infect. 3(8), 655–677 (2001).
- van der Ark A, van Straaten-van de Kappelle I, Hendriksen C, van de Donk H. Pertussis serological potency test as an alternatively to the intracerebral mouse protection test. Dev. Biol. Stand. 86, 271–281 (1996).
- Ausiello CM, Lande R, Urbani F et al. Cell-mediated immune responses in four-year-old children after primary immunization with acellular pertussis vaccines. Infect. Immun. 67(8), 4064–4071 (1999).
- Hendrikx LH, Schure RM, Oztürk K et al. Different IgG-subclass distributions after whole-cell and acellular pertussis infant primary vaccinations in healthy and pertussis infected children. Vaccine 29(40), 6874–6880 (2011).
- Mascart F, Hainaut M, Peltier A, Verscheure V, Levy J, Locht C. Modulation of the infant immune responses by the first pertussis vaccine administrations. Vaccine 25(2), 391–398 (2007).
- Lugauer S, Heininger U, Cherry JD, Stehr K. Long-term clinical effectiveness of an acellular pertussis component vaccine and a whole cell pertussis component vaccine. Eur. J. Pediatr. 161(3), 142–146 (2002).
- Wendelboe AM, Van Rie A, Salmaso S, Englund JA. Duration of immunity against pertussis after natural infection or vaccination. Pediatr. Infect. Dis. J. 24(Suppl. 5), S58–S61 (2005).
- Kendrick PL, Eldering G. Mouse protection tests in the study of pertussis vaccine; a comparative series using the intracerebral route for challenge. Am. J. Public Health Nations. Health 37(7), 803–810 (1947).
- Gaines-Das R, Horiuchi Y, Zhang SM et al. Modified intra-cerebral challenge assay for acellular pertussis vaccines: comparisons among whole cell and acellular vaccines. Vaccine 27(49), 6824–6832 (2009).
- André M, Poirier B, Bornstein N, Marmonier D, El Zaouk A, Fuchs F. Key points for the development of mouse immunogenicity test as potency assay for acellular pertussis vaccines. Biologicals 28(4), 217–225 (2000).
- Mastrantonio P, Spigaglia P, van Oirschot H et al. Antigenic variants in Bordetella pertussis strains isolated from vaccinated and unvaccinated children. Microbiology (Reading, Engl.) 145(Pt 8), 2069–2075 (1999).
- Guiso N, Capiau C, Carletti G, Poolman J, Hauser P. Intranasal murine model of Bordetella pertussis infection. I. Prediction of protection in human infants by acellular vaccines. Vaccine 17(19), 2366–2376 (1999).
- Mills KH, Brady M, Ryan E, Mahon BP. A respiratory challenge model for infection with Bordetella pertussis: application in the assessment of pertussis vaccine potency and in defining the mechanism of protective immunity. Dev. Biol. Stand. 95, 31–41 (1998).
- Mills KH, Ryan M, Ryan E, Mahon BP. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect. Immun. 66(2), 594–602 (1998).
- Taranger J, Trollfors B, Bergfors E et al. Mass vaccination of children with pertussis toxoid – decreased incidence in both vaccinated and nonvaccinated persons. Clin. Infect. Dis. 33(7), 1004–1010 (2001).
- Kim TH, Johnstone J, Loeb M. Vaccine herd effect. Scand. J. Infect. Dis. 43(9), 683–689 (2011).
- Rashid H, Khandaker G, Booy R. Vaccination and herd immunity: what more do we know? Curr. Opin. Infect. Dis. 25(3), 243–249 (2012).
- Hewlett EL, Edwards KM. Clinical practice. Pertussis – not just for kids. N. Engl. J. Med. 352(12), 1215–1222 (2005).
- Tan T, Trindade E, Skowronski D. Epidemiology of pertussis. Pediatr. Infect. Dis. J. 24(Suppl. 5), S10–S18 (2005).
- Hallander HO, Advani A, Donnelly D, Gustafsson L, Carlsson RM. Shifts of Bordetella pertussis variants in Sweden from 1970 to 2003, during three periods marked by different vaccination programs. J. Clin. Microbiol. 43(6), 2856–2865 (2005).
- Hozbor D, Mooi F, Flores D et al. Pertussis epidemiology in Argentina: trends over 2004–2007. J. Infect. 59(4), 225–231 (2009).
- van Amersfoorth SC, Schouls LM, van der Heide HG et al. Analysis of Bordetella pertussis populations in European countries with different vaccination policies. J. Clin. Microbiol. 43(6), 2837–2843 (2005).
- Octavia S, Sintchenko V, Gilbert GL et al. Newly emerging clones of Bordetella pertussis carrying prn2 and ptxP3 alleles implicated in Australian pertussis epidemic in 2008–2010. J. Infect. Dis. 205(8), 1220–1224 (2012).
- Peppler MS, Kuny S, Nevesinjac A et al. Strain variation among Bordetella pertussis isolates from Québec and Alberta provinces of Canada from 1985 to 1994. J. Clin. Microbiol. 41(7), 3344–3347 (2003).
- Aguas R, Gonçalves G, Gomes MG. Pertussis: increasing disease as a consequence of reducing transmission. Lancet Infect. Dis. 6(2), 112–117 (2006).
- Bamberger ES, Srugo I. What is new in pertussis? Eur. J. Pediatr. 167(2), 133–139 (2008).
- Packard ER, Parton R, Coote JG, Fry NK. Sequence variation and conservation in virulence-related genes of Bordetella pertussis isolates from the UK. J. Med. Microbiol. 53(Pt 5), 355–365 (2004).
- van Boven M, Mooi FR, Schellekens JF, de Melker HE, Kretzschmar M. Pathogen adaptation under imperfect vaccination: implications for pertussis. Proc. Biol. Sci. 272(1572), 1617–1624 (2005).
- Hallander H, Advani A, Riffelmann M et al. Bordetella pertussis strains circulating in Europe in 1999 to 2004 as determined by pulsed-field gel electrophoresis. J. Clin. Microbiol. 45(10), 3257–3262 (2007).
- He Q, Mertsola J. Factors contributing to pertussis resurgence. Future Microbiol. 3(3), 329–339 (2008).
- Mooi FR, van Loo IH, van Gent M et al. Bordetella pertussis strains with increased toxin production associated with pertussis resurgence. Emerging Infect. Dis. 15(8), 1206–1213 (2009).
- Van Loo IH, Mooi FR. Changes in the Dutch Bordetella pertussis population in the first 20 years after the introduction of whole-cell vaccines. Microbiology (Reading, Engl.) 148(Pt 7), 2011–2018 (2002).
- Godfroid F, Denoël P, Poolman J. Are vaccination programs and isolate polymorphism linked to pertussis re-emergence? Expert Rev. Vaccines 4(5), 757–778 (2005).
- Kallonen T, He Q. Bordetella pertussis strain variation and evolution postvaccination. Expert Rev. Vaccines 8(7), 863–875 (2009).
- Bouchez V, Brun D, Cantinelli T, Dore G, Njamkepo E, Guiso N. First report and detailed characterization of B. pertussis isolates not expressing pertussis toxin or pertactin. Vaccine 27(43), 6034–6041 (2009).
- Njamkepo E, Cantinelli T, Guigon G, Guiso N. Genomic analysis and comparison of Bordetella pertussis isolates circulating in low and high vaccine coverage areas. Microbes Infect. 10(14–15), 1582–1586 (2008).
- Otsuka N, Han HJ, Toyoizumi-Ajisaka H et al. Prevalence and genetic characterization of pertactin-deficient Bordetella pertussis in Japan. PLoS ONE 7(2), e31985 (2012).
- Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin. Infect. Dis. 47(3), 401–409 (2008).
- Gustafsson L, Hessel L, Storsaeter J, Olin P. Long-term follow-up of Swedish children vaccinated with acellular pertussis vaccines at 3, 5, and 12 months of age indicates the need for a booster dose at 5 to 7 years of age. Pediatrics 118(3), 978–984 (2006).
- Olin P, Hallander HO, Gustafsson L, Reizenstein E, Storsaeter J. How to make sense of pertussis immunogenicity data. Clin. Infect. Dis. 33(Suppl. 4), S288–S291 (2001).
- Pebody RG, Gay NJ, Giammanco A et al. The seroepidemiology of Bordetella pertussis infection in Western Europe. Epidemiol. Infect. 133(1), 159–171 (2005).
- Storsaeter J, Hallander HO, Gustafsson L, Olin P. Levels of antipertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16(20), 1907–1916 (1998).
- Hendrikx LH, Öztürk K, de Rond LG et al. Serum IgA responses against pertussis proteins in infected and Dutch wP or aP vaccinated children: an additional role in pertussis diagnostics. PLoS ONE 6(11), e27681 (2011).
- Leef M, Elkins KL, Barbic J, Shahin RD. Protective immunity to Bordetella pertussis requires both B cells and CD4(+) T cells for key functions other than specific antibody production. J. Exp. Med. 191(11), 1841–1852 (2000).
- Mahon BP, Brady MT, Mills KH. Protection against Bordetella pertussis in mice in the absence of detectable circulating antibody: implications for long-term immunity in children. J. Infect. Dis. 181(6), 2087–2091 (2000).
- Buisman AM, de Rond CG, Oztürk K, Ten Hulscher HI, van Binnendijk RS. Long-term presence of memory B-cells specific for different vaccine components. Vaccine 28(1), 179–186 (2009).
- Hendrikx LH, Oztürk K, de Rond LG et al. Identifying long-term memory B-cells in vaccinated children despite waning antibody levels specific for Bordetella pertussis proteins. Vaccine 29(7), 1431–1437 (2011).
- Stenger RM, Smits M, Kuipers B et al. Impaired long-term maintenance and function of Bordetella pertussis specific B cell memory. Vaccine 28(40), 6637–6646 (2010).
- Stenger RM, Smits M, Kuipers B, Kessen SF, Boog CJ, van Els CA. Fast, antigen-saving multiplex immunoassay to determine levels and avidity of mouse serum antibodies to pertussis, diphtheria, and tetanus antigens. Clin. Vaccine Immunol. 18(4), 595–603 (2011).
- Ausiello CM, Urbani F, la Sala A, Lande R, Cassone A. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect. Immun. 65(6), 2168–2174 (1997).
- Mascart F, Verscheure V, Malfroot A et al. Bordetella pertussis infection in 2-month-old infants promotes type 1 T cell responses. J. Immunol. 170(3), 1504–1509 (2003).
- Ryan M, Murphy G, Ryan E et al. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology 93(1), 1–10 (1998).
- Vermeulen F, Verscheure V, Damis E et al. Cellular immune responses of preterm infants after vaccination with whole-cell or acellular pertussis vaccines. Clin. Vaccine Immunol. 17(2), 258–262 (2010).
- White OJ, Rowe J, Richmond P et al. Th2-polarisation of cellular immune memory to neonatal pertussis vaccination. Vaccine 28(14), 2648–2652 (2010).
- Nilsson L, Kjellman NI, Björkstén B. A randomized controlled trial of the effect of pertussis vaccines on atopic disease. Arch. Pediatr. Adolesc. Med. 152(8), 734–738 (1998).
- Ryan EJ, Nilsson L, Kjellman N, Gothefors L, Mills KH. Booster immunization of children with an acellular pertussis vaccine enhances Th2 cytokine production and serum IgE responses against pertussis toxin but not against common allergens. Clin. Exp. Immunol. 121(2), 193–200 (2000).
- White OJ, McKenna KL, Bosco A, H J van den Biggelaar A, Richmond P, Holt PG. A genomics-based approach to assessment of vaccine safety and immunogenicity in children. Vaccine 30(10), 1865–1874 (2012).
- Rowe J, Yerkovich ST, Richmond P et al. Th2-associated local reactions to the acellular diphtheria-tetanus-pertussis vaccine in 4- to 6-year-old children. Infect. Immun. 73(12), 8130–8135 (2005).
- Scheifele DW, Ochnio JJ, Halperin SA. Cellular immunity as a potential cause of local reactions to booster vaccination with diphtheria and tetanus toxoids and acellular pertussis antigens. Pediatr. Infect. Dis. J. 28(11), 985–989 (2009).
- Andreasen C, Carbonetti NH. Role of neutrophils in response to Bordetella pertussis infection in mice. Infect. Immun. 77(3), 1182–1188 (2009).
- Banus S, Stenger RM, Gremmer ER et al. The role of Toll-like receptor-4 in pertussis vaccine-induced immunity. BMC Immunol. 9, 21 (2008).
- Higgins SC, Jarnicki AG, Lavelle EC, Mills KH. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J. Immunol. 177(11), 7980–7989 (2006).
- Fedele G, Bianco M, Debrie AS, Locht C, Ausiello CM. Attenuated Bordetella pertussis vaccine candidate BPZE1 promotes human dendritic cell CCL21-induced migration and drives a Th1/Th17 response. J. Immunol. 186(9), 5388–5396 (2011).
- Stenger RM, Poelen MC, Moret EE et al. Immunodominance in mouse and human CD4+ T-cell responses specific for the Bordetella pertussis virulence factor P.69 pertactin. Infect. Immun. 77(2), 896–903 (2009).
- Panum Pl. Boebachtungen uber das maserncontagium. Vichows Archives 1847(1), 492–503.
- Paul JR, Riordan JT, Melnick JL. Antibodies to three different antigenic types of poliomyelitis virus in sera from North Alaskan Eskimos. Am. J. Hyg. 54(2), 275–285 (1951).
- Sawyer WA. Persistence of yellow fever immunity. J. Prevent. Med. 5, 413–428 (1930).
- De Greeff SC, de Melker HE, van Gageldonk PG et al. Seroprevalence of pertussis in The Netherlands: evidence for increased circulation of Bordetella pertussis. PLoS ONE 5(12), e14183 (2010).
- Greco D, Salmaso S, Mastrantonio P et al. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. Progetto Pertosse Working Group. N. Engl. J. Med. 334(6), 341–348 (1996).
- Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N. Engl. J. Med. 334(6), 349–355 (1996).
- Hendrikx LH, de Rond LG, Oztürk K et al. Impact of infant and preschool pertussis vaccinations on memory B-cell responses in children at 4 years of age. Vaccine 29(34), 5725–5730 (2011).
- Simondon F, Yam A, Gagnepain JY, Wassilak S, Danve B, Cadoz M. Comparative safety and immunogenicity of an acellular versus whole-cell pertussis component of diphtheria-tetanus-pertussis vaccines in Senegalese infants. Eur. J. Clin. Microbiol. Infect. Dis. 15(12), 927–932 (1996).
- Storsaeter J, Hallander HO, Gustafsson L, Olin P. Low levels of antipertussis antibodies plus lack of history of pertussis correlate with susceptibility after household exposure to Bordetella pertussis. Vaccine 21(25–26), 3542–3549 (2003).
- Hendrikx LH, Felderhof MK, Oztürk K et al. Enhanced memory B-cell immune responses after a second acellular pertussis booster vaccination in children 9 years of age. Vaccine 30(1), 51–58 (2011).
- Luijkx TA, van Gaans-van den Brink JA, van Dijken HH, van den Dobbelsteen GP, van Els CA. Hyperproliferation of B cells specific for a weakly immunogenic PorA in a meningococcal vaccine model. Clin. Vaccine Immunol. 15(10), 1598–1605 (2008).
- Smits K, Smet J, Van Twillert I et al. Evaluation of Bordetella pertussis-specific immune responses after infection or after different vaccination programs in the framework of the FP7 Child-INNOVAC program. Presented at: 9th International Bordetella Symposium, Baltimore, MD, USA, 30 September–3 October 2010.
- Eskola J. Analysis of Haemophilus influenzae type B conjugate and diphtheria-tetanus-pertussis combination vaccines. J. Infect. Dis. 174(Suppl. 3), S302–S305 (1996).
- McGuirk P, Mills KH. Direct anti-inflammatory effect of a bacterial virulence factor: IL-10-dependent suppression of IL-12 production by filamentous hemagglutinin from Bordetella pertussis. Eur. J. Immunol. 30(2), 415–422 (2000).
- McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J. Exp. Med. 195(2), 221–231 (2002).
- de Gouw D, Diavatopoulos DA, Bootsma HJ, Hermans PW, Mooi FR. Pertussis: a matter of immune modulation. FEMS Microbiol. Rev. 35(3), 441–474 (2011).
- Carbonetti NH. Immunomodulation in the pathogenesis of Bordetella pertussis infection and disease. Curr. Opin. Pharmacol. 7(3), 272–278 (2007).
- Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 18(2), 326–382 (2005).
- Pichichero ME. Booster vaccinations: can immunologic memory outpace disease pathogenesis? Pediatrics 124(6), 1633–1641 (2009).
- Hickey FB, Brereton CF, Mills KH. Adenylate cycalse toxin of Bordetella pertussis inhibits TLR-induced IRF-1 and IRF-8 activation and IL-12 production and enhances IL-10 through MAPK activation in dendritic cells. J. Leukoc. Biol. 84(1), 234–243 (2008).
- Corbel MJ, Xing DK. Toxicity and potency evaluation of pertussis vaccines. Expert Rev. Vaccines 3(1), 89–101 (2004).
- Elahi S, Holmstrom J, Gerdts V. The benefits of using diverse animal models for studying pertussis. Trends Microbiol. 15(10), 462–468 (2007).
- Culotta CS, Harvey DF, Gordon EF. Whooping-cough: II. Experimental study. J.Pediatr. 6, 743–752 (1935).
- Elahi S, Brownlie R, Korzeniowski J et al. Infection of newborn piglets with Bordetella pertussis: a new model for pertussis. Infect. Immun. 73(6), 3636–3645 (2005).
- Elahi S, Buchanan RM, Attah-Poku S, Townsend HG, Babiuk LA, Gerdts V. The host defense peptide β-defensin 1 confers protection against Bordetella pertussis in newborn piglets. Infect. Immun. 74(4), 2338–2352 (2006).
- Feunou PF, Kammoun H, Debrie AS, Mielcarek N, Locht C. Long-term immunity against pertussis induced by a single nasal administration of live attenuated B. pertussis BPZE1. Vaccine 28(43), 7047–7053 (2010).
- Hall E, Parton R, Wardlaw AC. Cough production, leucocytosis and serology of rats infected intrabronchially with Bordetella pertussis. J. Med. Microbiol. 40(3), 205–213 (1994).
- Hall E, Parton R, Wardlaw AC. Differences in coughing and other responses to intrabronchial infection with Bordetella pertussis among strains of rats. Infect. Immun. 65(11), 4711–4717 (1997).
- Hall E, Parton R, Wardlaw AC. Responses to acellular pertussis vaccines and component antigens in a coughing-rat model of pertussis. Vaccine 16(17), 1595–1603 (1998).
- Hall E, Parton R, Wardlaw AC. Time-course of infection and responses in a coughing rat model of pertussis. J. Med. Microbiol. 48(1), 95–98 (1999).
- Hamstra HJ, Kuipers B, Schijf-Evers D, Loggen HG, Poolman JT. The purification and protective capacity of Bordetella pertussis outer membrane proteins. Vaccine 13(8), 747–752 (1995).
- Huang CC, Chen PM, Kuo JK et al. Experimental whooping cough. N. Engl. J. Med. 266, 105–111 (1962).
- Komatsu E, Yamaguchi F, Abe A, Weiss AA, Watanabe M. Synergic effect of genotype changes in pertussis toxin and pertactin on adaptation to an acellular pertussis vaccine in the murine intranasal challenge model. Clin. Vaccine Immunol. 17(5), 807–812 (2010).
- Parton R, Hall E, Wardlaw AC. Responses to Bordetella pertussis mutant strains and to vaccination in the coughing rat model of pertussis. J. Med. Microbiol. 40(5), 307–312 (1994).
- Rich AR, Long PH, Brown JH, Bliss EA, Holt LE Jr. Experiments upon the cause of whooping cough. Science 76(1971), 330–331 (1932).
- Shibley GS, Hoelscher H. Studies on whooping cough: i. type-specific (s) and dissociation (R) forms of hemophilus pertussis. J. Exp. Med. 60(4), 403–418 (1934).
- Stanbridge TN, Preston NW. Experimental pertussis infection in the vaccinated and unvaccinated marmoset: similarities to natural infection in the child. J. Clin. Pathol. 25(6), 551 (1972).
- van der Ark A, van Straaten-van de Kappelle I, Akkermans A, Hendriksen C, van de Donk H. Development of pertussis serological potency test. Serological assessment of antibody response induced by whole cell vaccine as an alternative to mouse protection in an intracerebral challenge model. Biologicals 22(3), 233–242 (1994).
- van der Ark A, van Straaten-van de Kappelle I, Olander RM et al. The Pertussis serological potency test. Collaborative study to evaluate replacement of the mouse protection test. Biologicals 28(2), 105–118 (2000).
- Caro V, Bouchez V, Guiso N. Is the Sequenced Bordetella pertussis strain Tohama I representative of the species? J. Clin. Microbiol. 46(6), 2125–2128 (2008).
- Fry NK, Duncan J, Vaghji L, George RC, Harrison TG. Antimicrobial susceptibility testing of historical and recent clinical isolates of Bordetella pertussis in the United Kingdom using the Etest method. Eur. J. Clin. Microbiol. Infect. Dis. 29(9), 1183–1185 (2010).
- Gaillard ME, Bottero D, Castuma CE, Basile LA, Hozbor D. Laboratory adaptation of Bordetella pertussis is associated with the loss of type three secretion system functionality. Infect. Immun. 79(9), 3677–3682 (2011).
- Xing DK, Corbel MJ, Dobbelaer R, Knezevic I. WHO working group on standardisation and control of acellular pertussis vaccines–report of a meeting held on 16–17 March 2006, St. Albans, United Kingdom. Vaccine 25(15), 2749–2757 (2007).
- Van Der Ark A, Kuipers B, Van Amerongen G et al. Infection procedure determines disease and immunity induced by B. pertussis strain variants in the coughing rat model. (2010). Presented at: 9th International Bordetella Symposium, Baltimore, MD, USA, 30 September – 3 October 2010.
- Fennelly NK, Sisti F, Higgins SC et al. Bordetella pertussis expresses a functional type III secretion system that subverts protective innate and adaptive immune responses. Infect. Immun. 76(3), 1257–1266 (2008).
- Heikkinen E, Xing DK, Olander RM et al. Bordetella pertussis isolates in Finland: serotype and fimbrial expression. BMC Microbiol. 8, 162 (2008).
- King AJ, van Gorkom T, Pennings JL et al. Comparative genomic profiling of Dutch clinical Bordetella pertussis isolates using DNA microarrays: identification of genes absent from epidemic strains. BMC Genomics 9, 311 (2008).
- van Gent M, Bart MJ, van der Heide HG et al. SNP-based typing: a useful tool to study Bordetella pertussis populations. PLoS ONE 6(5), e20340 (2011).
- Warfel JM, Beren J, Kelly VK, Lee G, Merkel TJ. Nonhuman primate model of pertussis. Infect. Immun. 80(4), 1530–1536 (2012).
- Berry MP, Graham CM, McNab FW et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466(7309), 973–977 (2010).
- Nakaya HI, Wrammert J, Lee EK et al. Systems biology of vaccination for seasonal influenza in humans. Nat. Immunol. 12(8), 786–795 (2011).
- Querec TD, Akondy RS, Lee EK et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 10(1), 116–125 (2009).
- Pulendran B. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat. Rev. Immunol. 9(10), 741–747 (2009).
- Feunou PF, Bertout J, Locht C. T- and B-cell-mediated protection induced by novel, live attenuated pertussis vaccine in mice. Cross protection against parapertussis. PLoS ONE 5(4), e10178 (2010).
- Mielcarek N, Debrie AS, Mahieux S, Locht C. Dose response of attenuated Bordetella pertussis BPZE1-induced protection in mice. Clin. Vaccine Immunol. 17(3), 317–324 (2010).
- Mielcarek N, Debrie AS, Raze D et al. Live attenuated B. pertussis as a single-dose nasal vaccine against whooping cough. PLoS Pathog. 2(7), e65 (2006).
- Skerry CM, Mahon BP. A live, attenuated Bordetella pertussis vaccine provides long-term protection against virulent challenge in a murine model. Clin. Vaccine Immunol. 18(2), 187–193 (2011).
- Asensio CJ, Gaillard ME, Moreno G et al. Outer membrane vesicles obtained from Bordetella pertussis Tohama expressing the lipid A deacylase PagL as a novel acellular vaccine candidate. Vaccine 29(8), 1649–1656 (2011).
- Hozbor D, Rodriguez ME, Fernández J, Lagares A, Guiso N, Yantorno O. Release of outer membrane vesicles from Bordetella pertussis. Curr. Microbiol. 38(5), 273–278 (1999).
- Roberts R, Moreno G, Bottero D et al. Outer membrane vesicles as acellular vaccine against pertussis. Vaccine 26(36), 4639–4646 (2008).
Websites
- WHO Expert Committee on Biologigal Standardization. WHO/BS/2011.2158. www.who.int/biologicals/vaccines/BS2158_Recommendations_aP_with_line_numbers_JULY.pdf
- Pertussis incidence 2008. Institut De Veille Sanitaire, France. www.invs.sante.fr/beh/2008/51_52/beh_51_52_2008.pdf
- WHO program on immunization, surveillance, assesment and monitoring. www.who.int/immunization_monitoring/data/data_subject/en/index.html
