Abstract
The rapid development of new drugs, therapies, and devices has created a dramatic increase in the number of clinical research studies that highlights the need for greater participation in research by physicians as well as patients. Furthermore, the potential of clinical research is unlikely to be reached without greater participation of physicians in research. Physicians face a variety of barriers with regard to participation in clinical research. These barriers are system-or organization-related as well as research-and physician-related. To encourage physician participation, appropriate organizational and operational infrastructures are needed in health care institutes to support research planning and management. All physicians should receive education and training in the fundamentals of research design and methodology, which need to be incorporated into undergraduate medical education and postgraduate training curricula and then reinforced through continuing medical education. Medical schools need to analyze current practices of teaching–learning and research, and reflect upon possible changes needed to develop a ‘student-focused teaching–learning and research culture’. This article examines the barriers to and benefits of physician participation in clinical research as well as interventions needed to increase their participation, including the specific role of undergraduate medical education. The main challenge is the unwillingness of many physicians and patients to participate in clinical trials. Barriers to participation include lack of time, lack of resources, trial-specific issues, communication difficulties, conflicts between the role of clinician and scientist, inadequate research experience and training for physicians, lack of rewards and recognition for physicians, and sometimes a scientifically uninteresting research question, among others. Strategies to encourage physician participation in clinical research include financial and nonfinancial incentives, adequate training, research questions that are in line with physician interests and have clear potential to improve patient care, and regular feedback. Finally, encouraging research culture and fostering the development of inquiry and research-based learning among medical students is now a high priority in order to develop more and better clinician-researchers.
Introduction
… the mind is not a vessel to be filled, but a fire to be kindled.
(Plutarch, ca 46–127 AD)
In recent years, a paradigm shift has been noticed from experience-based to evidence-based practice in medicine and education.Citation1–Citation3 Research is the cornerstone of evidence-based medical practice, which translates new knowledge and technological capability into powerful tools for prevention and treatment of disease.Citation4 Published research studies, especially landmark trials, have resulted in major changes in medical education and practice.Citation1 Clinical trials offer ‘a way to pool controlled observations in an objective and scientific way, allowing clinicians to decide with the best available data what therapy will work best for each patient’.Citation1 The rapid development of new drugs, therapies, and devices has created a dramatic increase in the number of clinical research projects, and one of the key challenges to conducting a research project is recruiting the target sample size within a stipulated timescale. To meet these challenges, there is a need for greater participation in research by the physicians, including clinicians, as well as patients.
There is a growing concern that many countries, especially the developing world, have not been exploiting the enormous research potential offered by health care services. It has been observed that most clinical research fails to meet its recruitment targets. For example, multicenter trials funded by Health Technology Assessment (HTA) and Medical Research Council (MRC) in the United Kingdom showed that 45% failed to reach 80% of the target; less than half of participating clinicians succeeded in recruiting any patients.Citation5,Citation6 Commercial trials also reported similar problems: 30% of sites failing to recruit a single patient and 70% failing to meet agreed recruitment targets.Citation7 A study demonstrated that 50%–80% of eligible patients are not recruited in clinical trials because of their doctors’ decision not to offer the trial to that patient.Citation8 An analysis of 333 randomized controlled trials (RCTs) conducted in the United Kingdom between 1971 and 2000 revealed that just over one-half failed to recruit the desired sample size, one-fifth recruited at least 75% of the target sample, while a further fifth recruited <25% of the planned number of patients.Citation9 It is also worrying that the number of physicians pursuing a career in research has also declinedCitation10,Citation11 and ‘physician-scientist’ has now become an ‘endangered species’.Citation12 There is clearly a need to examine why both physicians and patients are reluctant to take part in clinical research. Problems in patient recruitment to a trial may limit the statistical power of the trial to detect a treatment effect,Citation13,Citation14 and the reduction in statistical power is considered one of the main reasons for abandoning trials early.Citation15,Citation16 In addition, a less representative sample size also reduces the external validity of the trial.Citation13 All these problems may thus delay the potential introduction of new treatments and more detailed evaluation of existing ones.Citation13
Physician-researchers can act as ‘double agents’ who can enrich the quality of both services and research studies. Although clinical research is considered the key to the advancement of medical knowledge, physicians face a variety of barriers with regard to participation in clinical research and trials.Citation4 All physicians should receive education and training in the fundamentals of research design and methodology, which need to be incorporated into undergraduate medical education and postgraduate training curricula and reinforced through continuing medical education.Citation17 This article examines the barriers to and benefits of physician participation in clinical research and interventions needed to increase physician participation, including the specific role of undergraduate medical education.
Barriers to participation in clinical research
Many factors related to physician, patient, and trial characteristics may influence participation of physicians in clinical research.Citation13,Citation15,Citation18–Citation21 The barriers that physicians usually encounter in conducting clinical research are highlighted in many studies.Citation13,Citation16,Citation21,Citation22 Fayter et alCitation13,Citation18 identified these barriers as system-, organization-, research-, and physician-related. The details of the barriers are highlighted in .
Table 1 Barriers to physician participation in clinical research and trialsCitation4,Citation7,Citation8,Citation17,Citation19–Citation23
Ross et alCitation15 conducted the most comprehensive systematic review related to barriers to participation in RCTs for cancer and other illnesses and identified lack of time as a major barrier. Ellis et alCitation20 examined the barriers to participation in clinical trials for early breast cancer among Australian cancer specialists and identified lack of resources and issues related to specific trials as the major barriers. Another surveyCitation23 conducted among 357 clinicians to examine their attitudes to clinical trials of cancer therapy identified constraints imposed by the health care system which impede trial participation, including lack of time, communication difficulties, and conflicts between the role of clinician and scientist. Dev et alCitation4 examined the factors influencing the participation of gastroenterologists and hepatologists in clinical research and identified the greatest barrier to participation in clinical research as lack of adequate resources.
Clinical practice and management duties deter physicians from participating in research.Citation21,Citation24–Citation26 Time demands of recruitment, the consent process, and follow-up in trials, and additional management and administrative duties may also be considered as barriers.Citation26–Citation29 Physicians’ inadequate research experience and trainingCitation26,Citation30–Citation33 and lack of support staff Citation24,Citation25,Citation34,Citation35 are also blamed for poor participation.
There is a concern that research may alter the doctor–patient relationship,Citation15,Citation36 and physicians’ rapport with patients may be damaged by participation in research/trials.Citation27,Citation30,Citation36 The main issues highlighted were the difficulty for clinicians of admitting that they do not know which treatment is bestCitation29,Citation30 and the perceived conflict between the clinician role and the research (or scientific) role.Citation37–Citation39 Sometimes concerns regarding patients may affect physicians’ decision to take part in a research trial, which include treatment toxicity or side effects,Citation24,Citation40 patients’ travel time and cost,Citation8,Citation24,Citation27 recruitment of more severely ill patients,Citation41 and the effectiveness of treatment patients receive.Citation33 Other barriers include loss of clinical autonomy, including loss of decision-making power and independence, being accountable to a third party, and restriction of the ability to individualize patient care.Citation15,Citation21,Citation29–Citation32,Citation37,Citation38 Some studies mentioned that lack of rewards and recognition is a deterrent to physician participation.Citation15,Citation24,Citation31–Citation33 Scientifically uninteresting trials and research questions may sometimes fail to attract physicians to research.Citation15,Citation24,Citation33
Strategies to encourage physician participation in clinical research
In the absence of physician scientists, the bridge between bench and bedside will weaken, perhaps even collapse.
Dr Leon Rosenberg
To encourage physician participation in clinical research, organizational and operational infrastructures need to be strengthened by establishing effective relationships among structure, process, and outcome of research planning and management process.Citation42 Effective operational and organizational structures are needed to encourage physician participation in research and these are summarized in .
Table 2 Operational and organizational structures needed to encourage physician participation in research
Resources
Financial incentives have been shown to be among the most important factors motivating physician involvement in research.Citation43–Citation45 Clinical research and industry-sponsored trials in particular, which often carry greater reimbursement, are now viewed as essential sources of income for the maintenance of research programs and staff.Citation4,Citation46 Research also indicates that academic–industry relationships in medicine have substantial benefits for industry sponsors and that the rate of industry support for clinical research is likely to increase in future.Citation4,Citation47
Training clinician researchers
Physicians need adequate training in research methodology and biostatistics in order to build research skills on ‘core’ clinical knowledge.Citation22 Need-based training will help role integration of care providers and scientists and will develop physicians to become patient-oriented clinician-researchers. Research fellowships and mentoring programs, research bursaries, and workshops/seminars intended for physicians will help to develop appropriate understanding of research and will provide opportunities to work with research groups and role models to discuss the practical issues of conducting clinical research. Appropriate provisions should be adopted to integrate research methodology in undergraduate education, postdoctoral training, career awards, and intensive training conferences. Physicians can be encouraged to undertake appointments in out-of-program research, leading to a higher degree, usually a PhD.Citation48 The dual-degree programs MD–PhD or MD–MPHCitation49 in the United States, and NIHR Academic Clinical Fellowships (50% of time undertaking research or educationalist training) and NIHR Clinical Lectureships (50% of time) in the United KingdomCitation50 can be widely used in other countries to produce physician-researchers to handle a growing number of clinical research studies. In Singapore, a program has been launched to encourage doctors under specialty training to pursue a higher degree in research (either a 3- to 4-year PhD or a 1-year MSc) in order to equip them with research knowledge and skills that would allow them to develop translational research parallel to their clinical careers in the long term.Citation51 Programs can be specifically designed to encourage the practicing physician (including private or academic clinical practice) to engage in clinical research while maintaining an active role in clinical practice, for example, the Clinical Research/Reproductive Scientist Training Program supported by the National Institute of Child Health and Human Development, Duke University, and the American Society for Reproductive Medicine.Citation12 All these will help physicians to relate clinical experience to research and research knowledge to clinical work.
Creating research environment
The contention, ‘doctors simply don’t want to take part in clinical trials’,Citation52 is not true. Studies showed that physicians are eager to participate in clinical research if an adequate trial infrastructure and environment is present.Citation19–Citation22 To ensure physician participation in clinical research, an organizational culture needs to be developed that values research and nourishes evidence- based medicine and practice. A ‘centralized support services’ organization outside the physician group should facilitate the business of research by undertaking the clerical and other administrative tasks, including human subject approvals, institutional agreements, progress reports to funding agencies, and communications among the research team.Citation4,Citation19,Citation21,Citation22 This will create a research environment that will ensure patient safety, increase economic and medical efficiency, and promote a more standardized and regulatory-compliant process for conducting clinical research.Citation4 Physician-focused structures and forums, research groups, and networks should be created within the academic and health care organizations and appropriate collaboration with industry should be established to secure research funds.
Motivation for research participation
To facilitate physician participation, the research topic/area should be in-line with physician interest, relevant and important to their field, linked to the real world of clinical practice, and above all, should have clear potential to improve patient care.Citation12,Citation19,Citation45,Citation51,Citation53 Research activities integrated into the usual patterns of patient care which do not interfere with the flow of patient care increase the likelihood of physician participation. Citation19 As mentioned earlier, there should be a responsible party to handle the logistics and deal with research-related problems and issues.Citation4,Citation19,Citation21,Citation22 This will ensure minimal impact on clinical practice. Regular feedback and support along with financial compensation will motivate physicians to participate in clinical research.Citation19,Citation53,Citation54 Feedback regarding the progress and the degree of achievement of goals will help busy clinicians to assess their contributions and to maintain enthusiasm for the research.Citation19
Policies and guidelines
There is a need for developing appropriate policies for managing time for the physicians to minimize interference with clinical commitments and for payments and recognition for physician involvement in research. To manage time, appropriate provision for research administrative support should be established with efficient use of a study coordinator and other staff.Citation4,Citation19,Citation22 Reward and recognition should include financial and nonfinancial incentives;Citation19,Citation55 financial recognition usually includes pay increases, promotion, grants for attending conferences, etc, and nonfinancial incentives include formal institutional recognition/awards, news in the institute’s newsletters, news in the local media, coauthorship, etc. Financial incentives have ‘symbolic’ and ‘material’ significance which highlight the importance of the research.Citation19
Benefits of clinical research
Clinical research provides benefits to physicians, patients, health care organizations, and the country as a whole.
Benefits to physicians
A clinician-researcher is considered to be an important figure in health research and emphasis has been given to involving more clinicians in patient-oriented research.Citation53 Clinical research contributes to the expanding knowledge base of medicine and provides physicians an opportunity to offer patients latest cutting-edge therapies.Citation1,Citation56 Participation opens their eyes to medical innovation, and they are benefited by satisfying intellectual curiosity, increasing research provisions, and assisting career advancement.Citation56 Participation in clinical research may add prestige to physicians’ practice or institution.Citation1 A study conducted in the United States showed that a substantial number of physicians engaged in pharmaceutical industry-sponsored clinical trials and/or lectures in an effort to supplement their incomes as well as enhance their prestige, knowledge, and professional reputation.Citation45
Benefits to patients
Clinical research offers patients access to cutting-edge therapy, which could be lifesaving in addition to providing them with state-of-the-art quality care.Citation56 It plays an important role in improving the diagnosis and treatment of diseases and quality of life of the patients and people. Research has resulted in hundreds of innovations that offer earlier diagnosis of illness, result in better outcomes, and minimize side effects, including less demanding administration regimes. Clinical research may also provide direct benefits for those patients involved in clinical trials due to patients receiving closer medical attention and better follow-up and continuity of care. There is substantial evidence that participation in clinical research, irrespective of whether enrolment is in the placebo or in the treatment arm, improves health outcomes.Citation57
Operational and financial benefits to health care organizations
Research helps hospitalsCitation58 and educational institutesCitation59 with additional funding for capacity building in core academic, clinical, and research activities. Hospitals usually receive reimbursements for participating in clinical trials, either in cash, equipments, or additional staff. In India, for example, Pfizer has donated a US $100,000 bone density testing machine to each of six hospitals to investigate its osteoporosis drug.Citation57 Pharmaceutical companies support high-profile one-off investments for setting-up clinical laboratory and other large- scale research facilities, for example, GlaxoSmithKline’s £72 million investment in Imperial College London’s Clinical Imaging Centre.Citation58 In addition, industry collaborations sometimes contribute part of the overall hospital budget that provides infrastructure, part funding of personnel, day-to-day activities, and other subsidies. For example, 2% of the overall budget of Royal Marsden, specialist cancer hospital in the United Kingdom, comes from industry collaborations.Citation58
Scientific benefits to health care organizations
The clinician-researcher is able to make an important contribution to the quality of clinical services by facilitating an interactive flow of ideas between the clinical and research fields and disseminating evidence-based treatment approaches. Citation53 Physicians have the opportunities to attract funds and resources through research grants, and this ultimately helps health care organizations to retain their talents, knowledge, and skills in a competitive global economy.
Overall economic and strategic benefits
The economic opportunities created by clinical research draw more talented people into the medical profession in a country. The scope for clinical trials is increasing in developing countriesCitation60 and opens the door for wider employment opportunities. Clinical research creates employment for site personnel, study monitors, and ancillary services, with an economic impact on the whole community. In 2003, the UK pharmaceutical industry had a trade surplus of £3.6 billion and has been shown to have an employment multiplier effect of 6.7 and an economic contribution multiplier of 3.9.Citation58 It is estimated that the Indian clinical research industry will attract US$1.5 billion of revenue from international sponsors by 2010 which will create job demands for ~10,000 investigators and 50,000 clinical research professionals.Citation61
Research in medical curricula: implications for future physician-scientist
… undergraduate research should … be at the center of the undergraduate experience.
Hodge (2007)
The ‘teaching–research nexus’ should be central to medical education.Citation62 Research training should be considered an essential component in an innovative undergraduate medical curriculum.Citation54,Citation59,Citation63–Citation66 This issue is crucial as medical research is not given high priority by the medical and scientific community.Citation67–Citation71 The research activities should be boosted by incorporating research methodology in medical curricula, appointing researchers in clinical and academic departments, and allocating more funds to conduct research. A study reported that medical students are largely unaware of the research activities in their host institutionCitation59 and it is emphasized that adequate training should be provided on research methodology and biostatistics.Citation72 There has been much discussion regarding the decline in medical graduates choosing clinician-scientist careers and decrease of physician-scientists in medical practice.Citation67,Citation73–Citation78 Encouraging research culture and fostering the development of inquiry and research-based learning among medical students are now a high priority.Citation79–Citation81 A recent review conducted by Bierer and ChenCitation82 has shown that engaging in research projects can influence students’ choice of clinical specialty or interest in research. Various authorities strongly emphasized the development of research-specific skills among undergraduate medical students along with other transferable skills.Citation63,Citation83 To address this issue effectively, a research-informed approach to pedagogic development should be undertaken in medical schools to establish a sustainable link between teaching and research. In recent years, an increasing number of medical schools have implemented or are considering implementing structured research activities.Citation8,Citation59,Citation77,Citation84–Citation89 Research involvement should be an obligatory part of medical schools’ curricula,Citation67,Citation71 for example, involving medical students in designing and implementing research studies (Aga Khan University, PakistanCitation68), awarding medical degrees to medical students only after they have authored a research project (GermanyCitation89), the Medical Student Research Fellowship (MSRF) Program (United States),Citation77 Duke Clinical Research Fellowship (CRF) program (United States),Citation84 Norwegian Medical Student Research Programme,Citation85 student selected components (SSC) program (United Kingdom),Citation88 and introducing dual-degree programs (MD–PhD, MD–MPH, United States).Citation49 The Mount Sinai School of Medicine (United States) established a multifaceted research program to encourage students to involve basic or patient-oriented research, provide information about available research opportunities, help students in obtaining financial support from existing sources and developing new sources, implement strategies to reward student participation in research, and create new and innovative programs.Citation90 It is also important to examine ways to increase faculty involvement in student research. This can be encouraged through formal training in student supervision and protection of faculty time for student project work.Citation59
Medical schools should analyze current practices of teaching–learning and research while reflecting upon possible changes to develop a research culture using the model proposed by Healey and JerkinsCitation91 (). The model suggested four main ways of engaging undergraduates with research and inquiry:Citation92
Figure 1 Curriculum design and the research–teaching nexus.Citation91
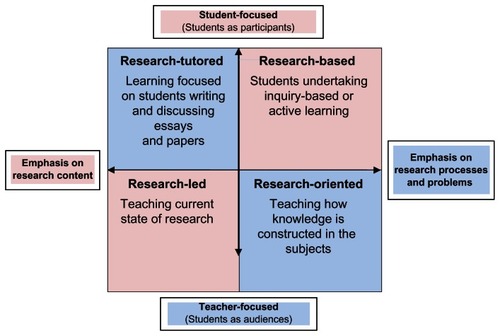
Research-led: learning about current research in the discipline.
Research-oriented: developing research skills and techniques.
Research-based: undertaking research and inquiry.
Research-tutored: engaging in research discussions.
This model has two axes: one classifies the ways students may be engaged in research and inquiry according to the extent to which students are treated primarily as the audience or as participants, while the second axis classifies the approach as emphasizing research content or research processes and problems. All four ways of engaging students with research and inquiry are valid and valuable, and it is advocated that curricula should contain elements of all of them.Citation92 In medical schools, relatively too much teaching and learning is in the bottom half of the model, and most students would benefit from spending more time in the top half. However, students should not spend nearly all their time in the top half, as tends to happen in some problem-based learning curricula.Citation92
Conclusion
Physician-researchers can serve as effective ‘bridges’ between the research and practice communities and can facilitate both development of clinically relevant research and dissemination of evidence-based treatments into routine clinical services. The organization should have adequate structure to support process and achieve outcomes. A well- planned implementation of these structures is likely to encourage clinicians to participate in clinical research. In addition, medical schools should develop effective institutional strategies and policies to highlight student awareness and experience of undergraduate research and inquiry using the following strategies:
Embed research in the mission and vision of the medical school.
Link undergraduate research and inquiry to institutional policies.
Develop supportive institutional curricula frameworks and structures.
Develop student-focused teaching, learning, and research policies.
Embed undergraduate research and inquiry from the first day students enter medical schools.
Raise student awareness of research and create a research environment.
Provide opportunities for all students to undertake undergraduate research and inquiry within and outside the curriculum.
Acknowledgement
The authors would like to thank the Open University Press for copyright permission to use .
Disclosure
The authors report no conflicts of interest in this work.
References
- LaderEWCannonCPOhmanEMAmerican College of Cardiology FoundationThe clinician as investigator: participating in clinical trials in the practice settingCirculation2004109212672267915173050
- HardenRMLilleyPMEditorial: best evidence medical education: the simple truthMed Teach2000222117119
- HartIRHardenRMBest evidence medical education (BEME): a plan for actionMed Teach2000222131135
- DevATKaufTLZekryAFactors influencing the participation of gastroenterologists and hepatologists in clinical researchBMC Health Serv Res2008820818842135
- JackWJChettyURodgerARecruitment to a prospective breast conservation trial: why are so few patients randomised?BMJ1990301674383852390587
- PetoVCoulterABondAFactors affecting general practitioners’ recruitment of patients into a prospective studyFam Pract19931022072118359613
- Department of HealthClinical Research Report: Pharmaceutical Industry Competitive Task Force (PICTF)2002 Available from: http://www.advisorybodies.doh.gov.uk/pictf/clinicalresearch.htmAccessed November 18, 2010.
- BensonAB3rdPreglerJPBeanJARademakerAWEshlerBAndersonKOncologists’ reluctance to accrue patients onto clinical trials: an Illinois Cancer Center studyJ Clin Oncol1991911206720751941065
- ValeCStewartLTierneyJUK Coordinating Committee for Cancer Research National Register of CancerTrends in UK cancer trials: results from the UK Coordinating Committee for Cancer Research National Register of Cancer TrialsBr J Cancer200592581181415756251
- DeMariaANClinical investigation … an impending crisis?J Am Coll Cardiol200341112100210112798588
- EastmanPNumber of US physicians participating in clinical research continuing to drop (Association of Clinical Research Organizations Survey)Oncology Times201032142123
- ArmstrongAYDecherneyALeppertPRebarRMaddoxYTKeeping clinicians in clinical research: the Clinical Research/Reproductive Scientist Training ProgramFertil Steril200991366466619144332
- FayterDMcDaidCEastwoodAA systematic review highlights threats to validity in studies of barriers to cancer trial participationJ Clin Epidemiol20076010990100117884592
- BaumMThe ATAC (Arimidex, Tamoxifen, Alone or in Combination) adjuvant breast cancer trial in postmenopausal patients: factors influencing the success of patient recruitmentEur J Cancer200238151984198612376201
- RossSGrantACounsellCGillespieWRussellIPrescottRBarriers to participation in randomised controlled trials: a systematic reviewJ Clin Epidemiol199952121143115610580777
- HoldenGRosenbergGBarkerKTuhrimSBrennerBThe recruitment of research participants: a reviewSoc Work Health Care19931921448153843
- ChenDTMillerFGRosensteinDLClinical research and the physician- patient relationshipAnn Intern Med2003138866967212693890
- FayterDMcDaidCRitchieGStirkLEastwoodASystematic Review of Barriers, Modifiers and Benefits Involved in Participation in Cancer Clinical TrialsYork (UK)Centre for Reviews and Dissemination2006 CRD Report 31
- AlbersLLSedlerKDClinician perspectives on participation in researchJ Midwifery Womens Health2004491475014710140
- EllisPMButowPNSimesRJTattersallMHDunnSMBarriers to participation in randomized clinical trials for early breast cancer among Australian cancer specialistsAust N Z J Surg199969748649110442918
- YanagawaHKishukuMAkaikeMAzumaHIraharaMView of physicians on and barriers to patient enrollment in a multicenter clinical trial: experience in a Japanese rural areaInt Arch Med20103720525325
- SumiEMurayamaTYokodeMA survey of attitudes toward clinical research among physicians at Kyoto University HospitalBMC Med Educ200997520025782
- FallowfieldLRatcliffeDSouhamiRClinicians’ attitudes to clinical trials of cancer therapyEur J Cancer19973313222122299470810
- FoleyJFMoertelCGImproving accrual into cancer clinical trialsJ Cancer Educ1991631651731931596
- SmythJFMossmanJHallRConducting clinical research in the new NHS: the model of cancer. United Kingdom Coordinating Committee on Cancer ResearchBMJ199430969524574617920132
- DickinsonCJClinical research in the NHS todayJ R Coll Physicians Lond19942854604637807437
- FisherWBCohenSJHammondMKTurnerSLoehrerPJClinical trials in cancer therapy: efforts to improve patient enrollment by community oncologistsMed Pediatr Oncol19911931651682023564
- SchaefferMHKrantzDSWichmanAMasurHReedEVinickyJKThe impact of disease severity on the informed consent process in clinical researchAm J Med199610032612688629670
- TaylorKMMargoleseRGSoskolneCLPhysicians’ reasons for not entering eligible patients in a randomized clinical trial of surgery for breast cancerN Engl J Med198431021136313676717508
- TaylorKMKelnerMInterpreting physician participation in randomized clinical trials: the Physician Orientation ProfileJ Health Soc Behav19872843894003429808
- TaylorKMPhysician participation in a randomized clinical trial for ocular melanomaAnn Ophthalmol19922493373441471822
- TaylorKMFeldsteinMLSkeelRTPandyaKJNgPCarbonePPFundamental dilemmas of the randomized clinical trial process: results of a survey of the 1,737 Eastern Cooperative Oncology Group investigatorsJ Clin Oncol1994129179618058083703
- TognoniGAlliCAvanziniFRandomised clinical trials in general practice: lessons from a failureBMJ199130368089699711954424
- SheaSBiggerJTJrCampionJEnrollment in clinical trials: institutional factors affecting enrollment in the cardiac arrhythmia suppression trial (CAST)Control Clin Trials19921364664861334819
- MorseEVSimonPMBeschCLWalkerJIssues of recruitment, retention, and compliance in community-based clinical trials with traditionally underserved populationsAppl Nurs Res1995818147695360
- Maslin-ProtheroSEFactors Affecting Recruitment to Breast Cancer Clinical TrialsAn Examination of the British Association of Surgical Oncology II Trial and the International Breast Cancer Intervention StudyNottingham (UK)Nottingham University122000
- TaylorKMShapiroMSoskolneCLMargoleseRGPhysician response to informed consent regulations for randomized clinical trialsCancer1987606141514223621123
- TaylorKMIntegrating conflicting professional roles: physician participation in randomized clinical trialsSoc Sci Med19923522172241509310
- SiminoffLAFettingJHAbeloffMDDoctor–patient communication about breast cancer adjuvant therapyJ Clin Oncol198979119212002671280
- WinnRJMiranskyJKernerJFKennellyLMichaelsonRASturgeonSRAn evaluation of physician determinants in the referral of patients for cancer clinical trials in the community settingProg Clin Biol Res198415663736473453
- AntmanKAmatoDWoodWSelection bias in clinical trialsJ Clin Oncol198538114211474020412
- GawlinskiAThe power of clinical nursing research: engage clinicians, improve patients’ lives, and forge a professional legacyAm J Crit Care200817431532618593830
- PrescottRJCounsellCEGillespieWJFactors that limit the quality, number and progress of randomised controlled trialsHealth Technol Assess1999320114310683591
- KeinonenTKeränenTKlaukkaTSaanoVYlitaloPEnlundHInvestigator barriers and preferences to conduct clinical drug trials in Finland: a qualitative studyPharm World Sci200325625125914689812
- AsharBHMillerRGGetzKJPoweNRPrevalence and determinants of physician participation in conducting pharmaceutical-sponsored clinical trials and lecturesJ Gen Intern Med200419111140114515566444
- BlumenthalDAcademic-industrial relationships in the life sciencesN Engl J Med2003349252452245914681513
- BlumenthalDCampbellEGCausinoNLouisKSParticipation of life-science faculty in research relationships with industryN Engl J Med199633523173417398929266
- NHS Education for ScotlandScottish Clinical Research Excellence Development Scheme (SCREDS): A Guide to the Scheme2009 Available from: http://www.nes.scot.nhs.uk/media/4911/scredsfinalrevisedguidetoscheme16thjanuary09.pdfAccessed December 18, 2010.
- Association of American Medical Colleges Available from: http://www.aamc.org/students/considering/exploring_medical/research/mdphd/Accessed December 18, 2010.
- National Institute for Health ResearchNIHR Integrated Academic Training Programme Available from: http://www.nihrtcc.nhs.uk/intetacatrain/Accessed December 18, 2010.
- Salto-TellezMOhVMLeeEHHow do we encourage clinician scientists in Singapore?Ann Acad Med Singapore2007361187988018071592
- McCurryJJapan unveils 5-year plan to boost clinical researchLancet200736995701333133617455378
- YanosPTZiedonisDMThe patient-oriented clinician-researcher: advantages and challenges of being a double agentPsychiatr Serv200657224925316452704
- LloydTPhillipsBRAberRCFactors that influence doctors’ participation in clinical researchMed Educ200438884885115271045
- ThomasPADiener-WestMCantoMIMartinDRPostWSStreiffMBResults of an academic promotion and career path survey of faculty at the Johns Hopkins University School of MedicineAcad Med200479325826414985201
- JamesonSThe benefits and challenges of conducting clinical trialsCommun Oncol200633163167
- MaitiRRaghavendraMClinical trials in IndiaPharmacol Res200756111017391981
- UK Clinical Research Collaboration (UKCRC)Clinical Research in the UK: Towards a Single System that Reliably Delivers Distinctive Quality and Rapid Access at Reasonable Cost (The McKinsey Report)2005 Available from: http://www.ukcrc.org/publications/reports.aspxAccessed December 18, 2010.
- BurgoyneLNO’FlynnSBoylanGBUndergraduate medical research: the student perspectiveMed Educ Online2010155212
- RashidAGlobal clinical trials in Bangladesh: a call for actionBAPA J2006152631
- White Paper on Global Clinical Trials in India: Prospects and ChallengesBioBusiness Summit 20052005 Nov 14–15Federation House, FICCI, New Delhi, India
- JenkinsAHealeyMZetterRLinking Teaching and Research in Disciplines and DepartmentsYork (UK)Higher Education Academy2007
- General Medical CouncilTomorrow’s Doctors Education: Outcomes and Standards for Undergraduate Medical EducationUKGeneral Medical Council2009
- SimpsonJGFurnaceJCrosbyJThe Scottish Deans’ Medical Curriculum GroupThe Scottish doctor – learning outcomes for the medical undergraduate in Scotland: a foundation for competent and reflective practitionersMed Teach200224213614312098432
- IllingJThinking About Research: Frameworks, Ethics and ScholarshipEdinburgh (UK)ASME2007
- World Federation for Medical EducationInternational standards in medical education: assessment and accreditation of medical schools’ – educational programmes. A WFME position paper. The Executive Council, The World Federation for Medical EducationMed Educ199832554955810211301
- MajumderMAAIssues and priorities of medical education research in AsiaAnn Acad Med Singapore200433225726315098645
- KhanHKhawajaMRWaheedARaufMAFatmiZKnowledge and attitudes about health research amongst a group of Pakistani medical studentsBMC Med Educ200665417081286
- KhanHKhanSIqbalAKnowledge, attitudes and practices around health research: the perspective of physicians-in-training in PakistanBMC Med Educ200994619615071
- PaiSACareer preferences of medical students who joined Grant Medical College, Bombay in 1957 and 1982Natl Med J India200922312112319764686
- AslamFShakirMQayyumMAWhy medical students are crucial to the future of research in South AsiaPLoS Med2005211e32216288553
- Murdoch-EatonDDrewerySEltonSWhat do medical students understand by research and research skills? Identifying research opportunities within undergraduate projectsMed Teach2010323e152e16020218832
- WyngaardenJBThe clinical investigator as an endangered speciesN Engl J Med19793012312541259503128
- GillNGThe end of the physician scientist?Am Sch198453353368
- RosenbergLEThe physician-scientist: an essential – and fragile – link in the medical research chainJ Clin Invest1999103121621162610377167
- LeyTJRosenbergLEThe physician-scientist career pipeline in 2005: build it, and they will comeJAMA2005294111343135116174692
- SolomonSSTomSCPichertJWassermanDPowersACImpact of medical student research in the development of physician-scientistsJ Investig Med2003513149156
- SiemensDRPunnenSWongJKanjiNA survey on the attitudes towards research in medical schoolBMC Med Educ201010420096112
- DetskyMEDetskyASEncouraging medical students to do research and write papersCMAJ2007176121719172117548386
- BoningerMTroenPGreenEImplementation of a longitudinal mentored scholarly project: an approach at two medical schoolsAcad Med201085342943720182115
- ParsonnetJGruppusoPAKanterSLBoningerMRequired vs elective research and in-depth scholarship programs in the medical student curriculumAcad Med201085340540820182112
- BiererSBChenHCHow to measure success: the impact of scholarly concentrations on students – a literature reviewAcad Med201085343845220182116
- National Alliance for Physician CompetenceGood Medical Practice – USA: The National Alliance. Version 1.02007 Available from: http://www.gmpusa.orgAccessed December 18, 2010.
- GallinEKLe BlancqSMClinical Research Fellowship Program LeadersLaunching a new fellowship for medical students: the first years of the Doris Duke Clinical Research Fellowship ProgramJ Investig Med20055327381
- HunskaarSBreivikJSiebkeMTømmeråsKFigenschauKHansenJBEvaluation of the medical student research programme in Norwegian medical schools. A survey of students and supervisorsBMC Med Educ200994319602226
- Van EykHJHooiveldMHWvan LeeuwenTNvan der WurffBLJDe CraenAJMDekkerFWScientific output of Dutch medical studentsMed Teach201032323123520218838
- GreenEPBorkanJMProssSHEncouraging scholarship: medical school programs to promote student inquiry beyond the traditional medical curriculumAcad Med201085340941820182113
- RobinsonLDrewerySEllershawJSmithJWhittleSMurdoch- EatonDResearch governance: impeding both research and teaching? A survey of impact on undergraduate research opportunitiesMed Educ200741872973617661880
- DiezCArkenauCMeyer-WentrupFThe German medical dissertation–time to change?Acad Med200075886186310965870
- ZierKStagnaro-GreenAA multifaceted program to encourage medical students’ researchAcad Med200176774374711448834
- HealeyMLinking research and teaching to benefit student learningJ Geogr High Educ2005292183201
- HealeyMJenkinsADeveloping Undergraduate Research and InquiryYork (UK)Higher Education Academy2009
