Abstract
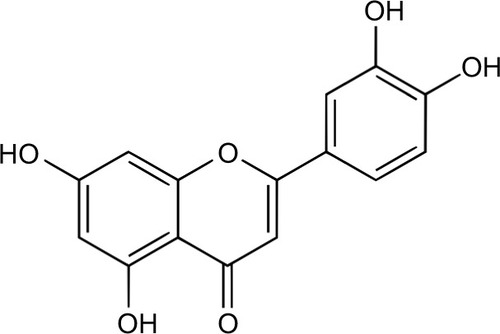
Metastatic breast cancer is typically an extremely aggressive cancer with poor prognosis. Metastasis requires the orchestration of homeostatic factors and cellular programs, many of which are potential therapeutic targets. Luteolin (2-[3,4-dihydroxyphenyl]-5,7-dihydroxy-4-chromenone), is a naturally occurring flavonoid found in fruits and vegetables that exhibits many anticancer properties. Luteolin obstructs metastasis through both direct and indirect mechanisms. For instance, luteolin may suppress breast cancer invasion by acting as an antiangiogenic therapeutic inhibiting VEGF production and its receptor’s activity. Furthermore, luteolin decreases epithelial–mesenchymal transition markers and metastatic proclivity. Luteolin also acts as an antiproliferative by suppressing receptor tyrosine-kinase activity and apoptosis, both of which could prevent incipient colonization of breast cancer. Many of these antimetastatic characteristics accredited to luteolin are likely functionally related. For instance, the PI3K/Akt pathway, which is impeded by luteolin, has several downstream programs involved in increased proliferation, survival, and metastatic potential in breast cancer. In this review, luteolin’s ability to ameliorate breast cancer is summarized. The paper also offers insight into the molecular mechanisms by which luteolin may suppress breast cancer metastasis.
Introduction
Breast cancer afflicts millions of women worldwide and is the second-leading cause of cancer related death in US women.Citation1 Early detection, awareness, and treatment options have significantly improved quality of life in breast cancer survivors. Over a century ago, Paget postulated that metastatic cancer is not determined by chance, but instead depends upon coordinated crosstalk between circulating tumor cells (seeds) and organ-specific microenvironments (soil).Citation2 In agreement with Paget’s hypothesis, metastatic breast cancer tends preferentially to metastasize to the bone, brain, liver, and lungs. Only 5%–10% of all new cancers are diagnosed as metastatic,Citation1 and the lack of therapeutic options likely explains why 90% of cancer-related deaths are attributed to metastasis and not primary tumor encumbrance.Citation1,Citation3,Citation4 Preventing tumor metastasis is arguably the paramount challenge to curing cancer, as well-defined localized tumors are manageable with current options.
All potential secondary tumors must first invade, disseminate, and subsequently colonize a hostile environment.Citation3,Citation5 These biological events are orchestrated through intrinsic and extrinsic homeostatic factors and molecular pathways, of which many may provide clinical opportunities to mitigate the metastatic propensity of breast cancer. Currently, most chemotherapeutic approaches revolve around obstructing established and incipient tumors through a combination of surgical resection and adjuvant therapy. These therapies have exceptional outcomes for breast cancer patients with confined primary tumors; however, metastatic cancer, being systemic in nature and resistant to chemotherapeutics, tends to have poorer prognosis.Citation6 This article addresses the aptitude of luteolin (2-[3,4-fihydroxyphenyl]-5,7-dihydroxy-4-chromenone), a natural occurring flavonoid found in fruit and vegetablesCitation7 (), to combat metastatic breast cancer by focusing on research that exemplifies the capacity of luteolin to abrogate metastatic steps involved in invasion, migration, and colonization ().
Table 1 Effect of luteolin on breast cancer at a glance
Angiogenesis
The invasion–metastasis cascade begins with neovascularization, the transition from epithelial to mesenchymal subtype, and the dissolution of the proteinaceous extracellular matrix and basement membrane.Citation8 The “angiogenic switch” in carcinogenesis increases potent angiogenic factors, such as platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF), increasing tumorigenicity.Citation9–Citation11 VEGF plays an instrumental role in tumor-cell survival, proliferation, and migration.Citation9–Citation13 As such, luteolin significantly reduces VEGFA production in MDA-MB-231 triple-negative breast cancer cells, which lack ER, PR, and HER2 (aka HER2/neu).Citation14,Citation15 An antibody blockade against VEGFR-2, a receptor tyrosine kinase (RTK), has produced results similar to that of luteolin;Citation14 however, a causative link was not established. An in vivo study using progestin-driven T47D breast cancer cells showed that luteolin significantly reduced progestin-stimulated blood vessel formation.Citation16 A similar reduction in blood vessel formation by luteolin is seen in prostate cancer xenografts.Citation17 Luteolin has also been reported in two unrelated studies to reduce MDA-MB-231 lung metastasis in nude mice,Citation14,Citation18 which in part may be attributed to reduced blood vessel formation.
One plausible explanation for the reduction in blood-vessel formation is that luteolin suppresses endothelial cell proliferation and migration, a necessity for angiogenesis and nascent tumor growth.Citation12 Interestingly, MDA-MB-231-conditioned media from cells treated with luteolin reduced tube formation and cell migration in human umbilical vein endothelial cells (HUVECs).Citation15 A gastric cancer study reported luteolin reduced VEGF production in both gastric cancer cells and HUVECs, as well as reduced endothelial cell migration and proliferation.Citation19 Furthermore, luteolin prevented cancer-promoting effects when cocultured with HUVECs and gastric cancer cells.Citation19 Prospectively, this reduced migratory potential may be attributed to luteolin’s efficacy to inhibit VEGF-induced PI3K activity by blocking Akt phosphorylation.Citation14,Citation20 In separate studies, luteolin suppressed VEGF and angiotensin II-induced endothelial cell migration through the downregulation of VEGFR-2 phosphorylationCitation21 and the PI3K/Akt pathway.Citation22 Another article regarding endothelial cells showed that luteolin decreased VEGF-induced VEGFR-2 phosphorylation and downstream effectors, such as Akt ().Citation17 Pratheeshkumar et al showed that luteolin reduced kinase activity and proinflammatory cytokines in prostate cancer in vitro.Citation17 Taken together, these reports provide a potential relationship between the ability of luteolin to obstruct VEGF-induced angiogenesis and the prospective colonization of metastatic sites. Speculatively, this relationship may be due to luteolin impeding paracrine signaling between tumors and their microenvironment. However, the mechanism of action needs further research to determine the involvement of VEGF and luteolin in suppression of metastatic breast cancer cells.
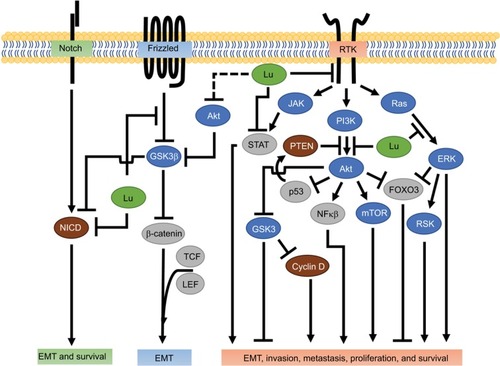
Figure 2 Luteolin inhibition of receptor-mediated tumor progression.
Abbreviations: EMT, epithelial–mesenchymal transition; JAK, Janus kinase; Lu, luteolin; NICD, notch intracellular domain; RSK, ribosomal S6 kinase; RTK, receptor tyrosine kinase; TCF, transcription factor.
Membrane degradation
A key step in neovascularization and tumor invasion is the degradation of the basement membrane, which is facilitated by matrix metalloproteinases (MMPs).Citation23,Citation24 In breast cancer, deregulation of MMP expression promotes invasion and metastasis.Citation25 In particular, several studies implicate MMP-2 and MMP-9 in enabling progression through the metastatic cascade, and MMP-9 being a possible prognostic factor for invasive breast cancer.Citation25 An early kinetic analysis characterized luteolin as a potent non-competitive inhibitor of MMP-9.Citation26 Recent evidence reported in MCF7 and MDA-MB-231 cells confirms the ability of luteolin to decrease both MMP-2 and MMP-9 mRNA and protein expression significantly.Citation15 Likewise, treatment of 12-O-tetradeconylphorbol-13-acetate-treated MCF7 cells with a luteolin derivative mitigated MMP-9 expression and subsequently reduced the invasive potential of these breast cancer cells.Citation27 Park et al attributed the reduced invasive potential to the downregulation of the MAPK pathway resulting in suppression of the proliferative transcription factors AP-1 and NF-κB.Citation27 MMPs promote cancer progression by cleaving an array of growth-factor precursors, which increases cell proliferation, invasion, and metastasis.Citation28 The link between luteolin-driven inhibition of MMPs and decreased metastatic potential is also seen in various other models,Citation29–Citation32 and thus warrants further investigation into the mechanism of action for luteolin-induced MMP inhibition.
MMP-mediated degradation of the extracellular matrix releases numerous growth factors and signaling molecules that enable tumor expansion.Citation33 One such factor normally sequestered in the matrix is the ubiquitous hyaluronic acid (HA). HA is degraded by hyaluronidase into various molecular weights, which then potentiates tumor progression and angiogenesis through one of two receptors: CD44 and RHAMM.Citation34 In breast cancer, the cell-surface proteins’ CD44+/CD24− profile is indicative of cancer stem-cell-like propertiesCitation35 and increased metastatic potential.Citation36,Citation37 Luteolin has been shown to be a potent inhibitor of hyaluronidase at relatively high concentrationsCitation38 and to prevent progestin-induced increases in CD44+ expression and ALDH activity at low doses.Citation16 In a combination treatment, luteolin prevented paclitaxel-induced CD44+ enrichment.Citation39 Along the same lines, luteolin has been reported to reduce CD44+ expression in prostate cancer,Citation40 as well as reduce CD44+ and ALDH activity in oral cancer cells.Citation41 These observations provide evidence suggesting that luteolin may prevent breast cancer cells from transitioning to a more tumorigenic stem-cell-like phenotype, though the exact mechanism is unknown.
Tumor microenvironment
The tumor microenvironment is involved in numerous aspects of tumorigenicity.Citation42,Citation43 Cancer cells interact with surrounding fibroblast, stromal, immune, and endothelial cells through paracrine factors that promote angiogenesis, membrane degradation, epithelial–mesenchymal transition (EMT), colonization, and cancer stem-cell upkeep.Citation8,Citation43 Luteolin, a known anti-inflammatory and antioxidant,Citation44 potentially modifies the tumor microenvironment. As described, luteolin decreases VEGF production, MMPs, and endothelial migration, all of which promote tumor progression. In breast cancer, cytokines coordinate communication between cancer cells and the microenvironment.Citation43 Recent evidence showed a luteolin derivative suppressed 12-O-tetradeconylphorbol-13-acetate-induced IL-8 mRNA expression.Citation27 The authors attributed the reduction in IL-8, a chemokine associated with advanced-stage breast cancer,Citation45 to MAPK inhibition.Citation27 In pancreatic cancer, luteolin has been shown to reverse IL-6-stimulated EMT by inhibiting STAT3 signaling.Citation32 Similarly, luteolin prevents IL-6/STAT3 signaling in oral cancer.Citation41 In a murine model, luteolin suppressed tumor-associated macrophage (TAM) secretion of promigratory chemokine CCL2 (aka MCP1) and attenuate CCL2-induced Lewis lung carcinoma-cell migration.Citation46 In general, TAMs and other tumor-associated immune cells promote metastasis through release of growth factors and cytokines, the latter of which may recruit more immune cells, resulting in signal amplification.Citation43 In light of this research, luteolin’s ability to reduce cytokine secretion from TAMs may contribute to the perturbation of metastasis. However, the nature by which luteolin arbitrates communication between breast cancer cells and the “soil” remains unclear.
Wnt/β-catenin signaling
The Wnt/β-catenin pathway plays a crucial role in morphogenesis and EMT.Citation47,Citation48 EMT is strongly associated with increased stem-cell-like properties like self-renewal, and is essential for development of breast cancer metastasis.Citation47 EMT progression requires concomitant activity of various signal-transduction pathways, such as the Wnt/β-catenin pathway.Citation49 In the canonical Wnt pathway, an unbound receptor allows for intracellular GSK3β to phosphorylate β-catenin and initiate proteolytic degradation, thereby reducing β-catenin levels.Citation50 In contrast, when Wnt conjugates with its receptor, GSK3β activity decreases, enabling β-catenin accumulation and its nuclear localization, resulting in increased β-catenin-orchestrated transcriptional activity and gene expression.Citation50,Citation51 Recent evidence has shown that luteolin effectively reduces β-catenin mRNA and protein levels in the triple-negative breast cancer cell lines MDA-MB-231 and BT-549.Citation18 Lin et al further showed that luteolin was associated with downregulation of the EMT markers Slug, N-cadherin, vimentin, and Snail and upregulation of the epithelial marker E-cadherin, reinforcing luteolin’s antimetastatic potential.Citation18 Wnt/β-catenin signaling can result in high nuclear Snail expression driving EMT; furthermore, the inverse relationship between Snail and E-cadherin is indicative of poor breast cancer prognosis.Citation52 As such, Lin et al suggested that the anti-EMT capacity of luteolin was mediated through β-catenin.Citation18 They supported this hypothesis in vitro and in vivo with the observation that overexpression of β-catenin rescued the migratory capacity of MDA-MB-231 cells in the presence of luteolin.Citation18 Of similar note, in colon cancer cells luteolin reduced the expression of the transcriptionally active forms of β-catenin and the phosphorylation of GSK3β ().Citation53 These data suggest that luteolin may act through the Wnt/β-catenin signaling pathway to suppress EMT, a pinnacle step in invasion and metastasis.
Notch signaling
In breast cancer, Notch signaling potentiates EMT through a variety of pathways, including Wnt/β-catenin, TGFβ, and the RTKs.Citation48,Citation54 Ligand-receptor-bound Notch initiates the cleavage of Notch intracellular domain (NICD) which then relocates to the nucleus, where it complexes with transcriptional activators that regulate Myc, p21, Hey1, and Hes1.Citation48 Luteolin suppresses Notch1 signaling and its downstream effectors Hes1 and Hey1 in MDA-MB-231 and MCF7 breast cancer cells.Citation15 These findings are of particular interest, because Hes1 inhibits the tumor-suppressor PTEN and Hey1 promotes EMT genes,Citation55 so suppressing Hes1 and Hey1 would likely translate to decreased metastatic propensity.
Reipas et al found that in SUM-149 cells enriched for stem-cell-like properties (CD44+/CD24−), luteolin inhibited Notch 4, with associated loss in cell viability and mammosphere formation.Citation39 In that study, luteolin acted as a novel p90 ribosomal S6 kinase (RSK) inhibitor in breast cancer cells.Citation39 The RSK family of proteins are highly conserved downstream effectors of the ERK/MAPK signaling pathway, and regulate cell survival, proliferation, and migration.Citation56 Also in Reipas et al, in MDA-MB-231 cells, RSK inhibition was accompanied by attenuation of the downstream-transcription factor YB-1 and an associated reduction in Notch4 mRNA signaling. siRNA knockdown of YB-1 resulted in a marked decrease in Notch4 and an associated loss in the NICD, implicating possible mediation between YB-1 and Notch4. Interestingly, the authors noted that siRNA knockdown of YB-1 also increased Notch1 mRNA transcripts; however, they did not attribute this effect to luteolin treatment.Citation39 Taken together, these studies implicate both Notch1 and Notch4 as possible targets by which luteolin could regulate the metastatic potential of breast cancer, which is encouraging, because the inhibition of Notch4 signaling has been shown to abolish breast cancer tumor formation in vivo completely.Citation57
The Notch and Wnt/β-catenin pathways crosstalk, specifically through GSK3β.Citation55 GSK3β-dependent phosphorylation of the NICD effectively inhibits transcriptional activation of Notch target genes in vitro and in vivo.Citation58 Luteolin attenuates GSK3β inactivation in the Wnt/β-catenin pathway,Citation53 and because Akt is known to inactivate GSK3β,Citation59 it is plausible to suspect that luteolin-induced NICD decrease may be the result of Akt inhibition and increased GSK3β activity. The ability of luteolin to suppress NICDs may be through direct interaction, indirectly through RSK/YB-1, or by preventing inactivation of GSK3β (). Luteolin’s capacity to mitigate NICD signaling and by extension metastatic potential could correlate with improved quality of life in breast cancer patients.
Antiproliferation capability of luteolin
In the later steps of the metastatic cascade, metastatic “seeds” must extravasate and colonize new tissue. During colonization, cells transition from a quiescent state to a proliferative state under the control of the cell cycle. The cell cycle controls mitotic division and is regulated through cyclin-dependent kinases (CDKs), cyclins, and their inhibitors p21 (p21Cip1) and p27 (p27Kip1).Citation60 Deregulation of cell-cycle proteins, such as in upregulation of CDKs in normal mammary tissue, can cause breast cancer.Citation60
In light of this, luteolin has been shown to cause cell cycle arrest in many different cancers. In MDA-MB-231 cells, luteolin prevented 3H-thymidine DNA incorporation and arrested cells in the cell cycle G2/M phase.Citation61 Further analysis of cell cycle-dependent regulatory proteins showed that luteolin induced an increase in p21 expression, as well as an associated suppression of S-phase cell-cycle modulators cyclin A and CDK2 and M-phase protein cyclin B1.Citation61 Intriguingly, luteolin shows a biphasic response, causing an increase in p21 at low dosesCitation61,Citation62 and a decrease in p21 at higher doses.Citation63 P21, a major target of the tumor suppressor p53, inhibits CDK2 preventing cell cycle progression and promotes cell survival, though the latter is dependent upon Akt inhibition of p21 nuclear translocation.Citation61,Citation64 Cotreatment of luteolin with p21 siRNA mitigates p21-induced survival advantage.Citation63 The observed increase in p21 is likely due to luteolin-induced Akt inhibition ();Citation61 however, other possibilities include the ability of luteolin indirectly to modify p53 activity by suppressing DNA topoisomerases I and II (essential DNA-repair proteins)Citation65–Citation67 and stabilizing p53,Citation68 or by a loss of negative feedback from CDK2.Citation69 Either way, luteolin-mediated regulation of p21, CDKs, and cyclins potentiates cell cycle arrest in breast cancer cells.Citation53,Citation65,Citation70,Citation71 However, more studies are necessary to elucidate the biphasic response of luteolin on p21 and whether or not luteolin induces changes in p21 localization.
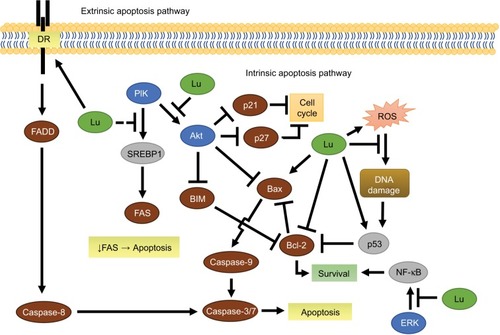
Figure 3 Luteolin-mediated extrinsic and intrinsic apoptosis in breast cancer.
Abbreviations: DR, death receptor; Lu, luteolin; ROS, reactive oxygen species; TCFs, transcription factors.
Human epidermal growth-factor receptor 2
The HER family consists of four membrane-bound trans-membrane receptors, and is associated with cell survival, proliferation, and metastasis in breast cancer.Citation72 Once a ligand binds to a HER receptor other than an orphan receptor, it preferentially dimerizes with HER2 and subsequently activates the downstream effectors PI3K/Akt and mTOR.Citation73 HER2 overexpression is diagnosed in approximately 20%–25% of all new breast cancer cases, and even though targeted therapy exists, HER2 breast cancers are associated with poorer prognosis.Citation73 In MDA-MB-453 breast cancer cells, luteolin at low concentrations has increased HER2 protein degradation in two separate reports.Citation63,Citation74 Chiang et al reported that luteolin inhibited cell proliferation, upregulated p27, and downregulated p21 at high doses.Citation63 P27 regulates cell-cycle progression, and low levels of p27 are typically associated with poorer prognosis in breast cancer patients.Citation75 The beneficial properties of increased cytosolic p27 may depend upon compartmentalization, as Akt inhibits p27 through Akt-mediated nuclear exclusion, preventing p27-induced cell cycle inhibition (). Investigation into MDA-MB-453 cells showed that low/high doses of luteolin, respectively, induced transient and complete suppression of Akt and its effector mTOR.Citation63 Akt resurgence is prevented when low dose luteolin is coincubated with rapamycin, an mTOR inhibitor, suggesting Akt/mTOR involvement. Additional studies have shown that moderate–high doses of luteolin resulted in decreased cyclin D1 (a principal regulator of G1/S cell cycle transition)Citation62 and increased p27 accumulation.Citation63,Citation76
In general, HER2-driven tumors are considered “oncogene-addicted”, meaning that without the oncogene, they cannot survive.Citation77 Unfortunately, targeting these addicted cancers with TK inhibitors (TKIs) has proven only moderately successful, due to the HER2-driven cancers developing resistance.Citation77 Recent evidence has shown that the transient effect of TKIs may be due to Akt-mediated negative feedback resulting in HER3 recruitment and thus PI3K/Akt signal restoration.Citation77 A report using trastuzumab, a HER2 antibody, blockade resulted in suppressed Akt and cyclin D1 activity, with a corresponding increase in p27 in BT-474 and SKBR3 cells, but not in MDA-MB-361 or MDA-MB-453 breast cancer cells.Citation78 This suggests that the efficacy of TKIs is contingent on whether HER2 inhibition is followed by PI3K/Akt attenuation, and whether or not the TKI can sustain Akt inhibition.Citation78 In combination therapy, luteolin synergistically enhances lapatinib, a HER2 and epidermal growth-factor receptor (EGFR) inhibitor, and inhibits Akt- and ERK-signaling pathways, resulting in decreased cell proliferation.Citation79 Interestingly, unlike luteolin, the PI3K inhibitor LY294002 causes complete Akt suppression over 24 hours, but only marginally decreases cyclin D1 in MDA-MB-453 cells.Citation63 These studies suggest that luteolin may arrest cells by suppressing HER2 signaling through the PI3K/Akt and ERK/MAPK pathways, which may prove advantageous in preventing TKI resistance in breast cancer. The ability of luteolin to degrade HER2, reduce Akt activity, and arrest cells will reduce metastatic potential by preventing incipient colonization.
EGFR
EGFR (aka HER1) is an HER-family transmembrane RTK that relays signals through the JAK/STAT, PI3K/Akt, and MAPK-signaling pathways that ultimately affect cell proliferation, apoptosis inhibition, angiogenesis, migration, adhesion, and invasion ().Citation80,Citation81 While EGFR and its ligands are essential for normal mammary development in the presence or absence of the sex hormones estrogen and progesterone,Citation80,Citation81 dysregulation of EGFR leads to breast cancer and metastasis. EGFR is frequently expressed in basal-like breast cancer and is associated with brain metastasis, and the inhibition of EGFR and its ligands reduces breast cancer metastatic potential.Citation82,Citation83 Low doses of luteolin inhibit EGFR signaling in a dose-dependent manner by downregulating EGF-induced EGFR phosphorylation and subsequently decreasing the activation of the downstream kinases Akt, ERK, and p38 in MDA-MB-231 breast cancer cells.Citation61 Similar findings have been reported in MCF7 cells in which moderate doses of luteolin abolished EGF-induced increased pEGFR, Akt, and ERK kinase activity, as well as the transcription factor STAT3.Citation84 Increasing RTK activity, such as EGFR and HER2, leads to STAT3-induced tumor progression and metastasis.Citation85,Citation86 In combination therapy, luteolin and paclitaxel (a cytoskeletal drug) work together to increase expression of the death receptor Fas and reduce STAT3 activation, which results in increased apoptosis in MDA-MB-231 cells ().Citation87 These reports provide a multifaceted approach in which luteolin may reduce breast cancer invasion, metastasis, and colonization by itself or in combination with current chemotherapeutics through modulation of EGFR, PI3k/Akt, and MAPK activity, as well as JAK/STAT signaling.
Insulin-like growth-factor 1 receptor
IGF1R is instrumental in normal mammary development. However, in breast cancer IGF1R intrinsically confers survival properties in and of itself, as well as through other RTKs via crosstalk.Citation88,Citation89 Clinical studies utilizing mono-therapeutic antibodies for EGFR and HER2 breast cancers have resulted in cancers gaining therapeutic resistance, in part due to IGF1R crosstalk.Citation88,Citation89 For example, after gaining resistance to gefitinib (an EGFR inhibitor) in MCF7 breast cancer cells, IGF1R dimerizes with EGFR and rescues downstream kinase activity.Citation90 In the same cell line, albeit not a chemotherapeutically resistant version, luteolin abrogates IGF-induced phosphorylation of IGF1R, and the loss in IGFR activity is accompanied by a decrease in Akt but not ERK activity.Citation91 Considering the ability of luteolin to suppress IGF1R and EGFR pathways as described, luteolin may be able to combat TKI drug-resistant breast cancer.Citation91 Interestingly, Wang et al went on to implicate the transcription factor ERα as the mechanism of action.Citation91 They showed that silencing ERα ameliorated luteolin inhibition of IGF-induced responses.Citation91 Conventionally speaking, estrogen-induced proliferation is determined by the ERα:β ratio, with ERα acting in a proproliferative manner and ERβ being a functional antagonist.Citation92,Citation93 In regard to this, luteolin has been shown to bind irreversibly to estrogen elementsCitation94 and have antiestrogenic or weak estrogenic effects at a similar dosage to that used to inhibit the aforementioned IGF1R pathway.Citation95,Citation96 These reports suggest that luteolin may antagonize IGF-induced ERα-mediated proliferation by acting as an ERα-competitive inhibitor, resulting in Akt pathway attenuation.
Luteolin-mediated apoptosis
The canonical apoptotic pathways include an intrinsic and extrinsic route (). The intrinsic apoptotic pathway is mediated by Bcl-2 family members in response to myriad nonreceptor stimuli that ultimately induce changes in mitochondrial membrane integrity, resulting in apoptosis. Bcl-2 family members control apoptosis through varying amounts of pro- and antiapoptotic Bcl-2 proteins (Bad, Bax, BAK, Bcl-2, Bcl-xL),Citation97 essentially acting like a pan balance in determining cell fate. When cytoplasmic Bad increases, Bcl-2 and Bcl-xL dissociate from Bax and BAK and bind to Bad.Citation97 In doing so, Bax and BAK are free to integrate into the mitochondrial membrane, decreasing its integrity, and initiate mitochondrial-mediated apoptosis via caspase-9 ().Citation97 In the extrinsic pathway, activated death receptors (DRs) (Fas, TNFR, TRAIL) signal the formation of the death-inducing signaling complex, which subsequently initiates the caspase cascade through caspase-8 and caspase-10.Citation97
In MCF7 breast cancer cells, luteolin induces apoptosis through both extrinsic and intrinsic pathways. Luteolin increases DR expression,Citation76,Citation87 activates caspase-8, and subsequently induces caspase-3 activity in the extrinsic apoptotic pathway. In the intrinsic path, Park et al showed luteolin increases Bax expression and decreases Bcl-2 expression, which in turn decreases mitochondrial membrane integrity, contributing to increased caspase-3 activity via caspase-9 activation.Citation76 Additionally, in MDA-MB-231 cells, low doses of luteolin significantly increase the Bax:Bcl-xL ratio, favoring apoptosis.Citation61 Somewhat confounding are results concerning a luteolin–doxorubicin combination. Sato et al reported that, low-dose luteolin ameliorated doxorubicin (topoisomerase inhibitor) cytotoxicity in part by decreasing doxorubicin-induced reactive oxygen species (ROS) and increasing Bcl-2 in MCF7 cells.Citation98 Flavonoids, including luteolin, are well known for their antioxidative properties;Citation99 however, moderate concentrations of luteolin have been shown to induce ROS in cancer cells ().Citation100 In this case, luteolin is likely acting as an antioxidant, but this maybe a dose-dependent effect.
As previously mentioned, luteolin can modify p53 activity indirectlyCitation62,Citation65 or directly,Citation68 and p53 mediates the intrinsic apoptotic pathway by regulating Bcl-2 family proteins, specifically through inhibition of Bcl-2 and Bax integration into the mitochondrial membrane.Citation97 In MCF7 cells, luteolin increased p53 protein levels, which results in the release of the intrinsic apoptosis mediator cytochrome C and PARP activation.Citation101 Additional reports confirm the ability of luteolin to increase p53 and decrease Bcl-2 mRNA in MDA-MB-231 cells.Citation102 This presents a novel avenue for luteolin to reduce metastatic colony formation, especially when considering a significant amount of breast cancer contains wild-type P53.Citation103,Citation104 However, the mechanism by which luteolin stabilizes the tumor-suppressor protein p53 and subsequently causes apoptosis in breast cancer is ambiguous.
FOXO-dependent apoptosis
In MCF7 breast cancer cells, luteolin shows a marked suppression in active Akt protein, cell migration, and subsequently an increase in apoptosis.Citation101,Citation105 Observations suggest that luteolin decreases cytosolic FOXO3a levels, while it increases nuclear FOXO3a levels over 48 hours.Citation101 Lin et al hypothesized that the nuclear accumulation of FOXO3a was due to the observed loss in Akt ().Citation101 In support, recent evidence suggests that Akt inhibition can promote FOXO3a-dependent apoptosis.Citation106 Unphosphorylated nuclear FOXO3a acts as a transcription factor to orchestrate p21 and p27 transcription, in addition to BIM-dependent apoptosis.Citation107 PI3K/Akt-pathway inhibition would prevent phosphorylation of the tumor suppressor FOXO3a, which causes its nuclear exportation and proteolytic degradation.
FAS-induced apoptosis
Repressing fatty-acid synthesis in breast cancer has been shown to induce programmed cell death ().Citation108 In MDA-MB-231 cells, luteolin reduces FAS (an instrumental lipogenic enzyme) activity, achieving a nearly 50% reduction in cell viability at relatively low doses.Citation109 In liver cancer cells, luteolin decreases both SREB-1 and FAS mRNA.Citation110 In agreement with the luteolin studies, inhibition of MAPK or PI3K signals in breast cancer cells results in decreased SREB-1 transcription and a corresponding decrease in FAS mRNA and protein.Citation111 In vivo targeted inhibition of either FAS or SREB-1 has also been shown to reduce tumor burden substantially.Citation112 Taken together, these studies suggest that luteolin suppresses FAS through Akt inhibition.
Conclusion
Prevention and treatment of metastatic breast cancer will ultimately lead to increased and sustained quality of life for breast cancer patients. Metastasis requires breast cancer cells to undergo morphological changes, migrate, and then colonize at distant sites. Playing a central role is Akt, and its hyperactivity is involved in increased cancer metastasis.Citation113 RTKs regulate Akt activity; however, constitutively active Akt can occur in the absence of RTK signaling by deactivating the tumor suppressor PTEN, a possible TKI-escape mechanism.Citation114 The ability of luteolin preferentially to disrupt RTKs and/or the downstream effectors Akt, ERK, and STAT3 activity is seen extensively in breast cancer () and others.Citation32,Citation115–Citation117 These studies provide compelling evidence that luteolin can palliate RTK-induced invasion, metastasis, and colonization.Citation32,Citation116,Citation118 Furthermore, luteolin not only inhibits RTK/Akt activity but it may also reestablish therapeutic potency in breast cancers.Citation62,Citation119,Citation120 Of note, most of the mechanistic experiments performed utilizing luteolin have been in the metastatic breast cancer cell lines MDA-MB-231 and MCF7 (). Further studies utilizing the T47D and SKBR3 cell lines may add to the mechanisms of action by which luteolin suppresses metastatic breast cancer in vivo.
Disclosure
The author reports no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2017CA Cancer J Clin201767173028055103
- PagetSThe distribution of secondary growths in cancer of the breastLancet18891333421571573
- GuptaGPMassaguéJCancer metastasis: building a frameworkCell2006127467969517110329
- SteegPSTumor metastasis: mechanistic insights and clinical challengesNat Med200612889590416892035
- FidlerIJThe pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisitedNat Rev Cancer20033645345812778135
- WeigeltBPeterseJLVan’t VeerLJBreast cancer metastasis: markers and modelsNat Rev Cancer20055859116056258
- Lopez-LazaroMDistribution and biological activities of the flavonoid luteolinMini Rev Med Chem200991315919149659
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell2011144564667421376230
- PriceDJMiralemTJiangSSteinbergRAvrahamHRole of vascular endothelial growth factor in the stimulation of cellular invasion and signaling of breast cancer cellsCell Growth Differ200112312913511306513
- FerraraNGerberHPLeCouterJThe biology of VEGF and its receptorsNat Med20039666967612778165
- HanahanDFolkmanJPatterns and emerging mechanisms of the angiogenic switch during tumorigenesisCell19968633533648756718
- HicklinDJEllisLMRole of the vascular endothelial growth factor pathway in tumor growth and angiogenesisJ Clin Oncol20052351011102715585754
- ZhaoDPanCSunJVEGF drives cancer-initiating stem cells through VEGFR-2/Stat3 signaling to upregulate Myc and Sox2Oncogene201534243107311925151964
- CookMTLiangYBesch-WillifordCHyderSMLuteolin inhibits lung metastasis, cell migration, and viability of triple-negative breast cancer cellsBreast Cancer (Dove Med Press)2017991928096694
- SunDWZhangHDMaoLLuteolin inhibits breast cancer development and progression in vitro and in vivo by suppressing notch signaling and regulating miRNAsCell Physiol Biochem20153751693171126545287
- CookMTLiangYBesch-WillifordCGoyetteSMafuvadzeBHyderSMLuteolin inhibits progestin-dependent angiogenesis, stem cell-like characteristics, and growth of human breast cancer xenograftsSpringerPlus2015444426312209
- PratheeshkumarPSonYOBudhrajaALuteolin inhibits human prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesisPLoS One2013712e52279
- LinDKuangGWanJLuteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expressionOncol Rep201737289590227959422
- ZangMHuLZhangBLuteolin suppresses angiogenesis and vasculogenic mimicry formation through inhibiting Notch1-VEGF signaling in gastric cancerBiochem Biophys Res Commun2017490391391928655612
- BagliEStefaniotouMMorbidelliLLuteolin inhibits vascular endothelial growth factor-induced angiogenesis; inhibition of endothelial cell survival and proliferation by targeting phosphatidylinositol 3′-kinase activityCancer Res200464217936794615520200
- RavishankarDWatsonKABoatengSYGreenRJGrecoFOsbornHMExploring quercetin and luteolin derivatives as antiangiogenic agentsEur J Med Chem20159725927425984842
- ZhuMChenDLiDLuteolin inhibits angiotensin II-induced human umbilical vein endothelial cell proliferation and migration through downregulation of Src and Akt phosphorylationCirc J201377377277923171663
- HiraokaNAllenEApelIJGyetkoMRWeissSJMatrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysinsCell19989533653779814707
- DeryuginaEIQuigleyJPMatrix metalloproteinases and tumor metastasisCancer Metastasis Rev200625193416680569
- MerdadAKarimSSchultenHJExpression of matrix metalloproteinases (MMPs) in primary human breast cancer: MMP-9 as a potential biomarker for cancer invasion and metastasisAnticancer Res20143431355136624596383
- EndeCGebhardtRInhibition of matrix metalloproteinase-2 and-9 activities by selected flavonoidsPlanta Med200470101006100815490332
- ParkSHKimJHLeeDHLuteolin 8-C-β-fucopyranoside inhibits invasion and suppresses TPA-induced MMP-9 and IL-8 via ERK/AP-1 and ERK/NF-κB signaling in MCF-7 breast cancer cellsBiochimie201395112082209023933110
- VandoorenJvan den SteenPEOpdenakkerGBiochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decadeCrit Rev Biochem Mol Biol201348322227223547785
- JiangYXieKHuoHWangLZouWXieMInhibitory effect of luteolin on the angiogenesis of chick chorioallantoic membrane and invasion of breast cancer cells via downregulation of AEG-1 and MMP-2Sheng Li Xue Bao2013655513518 Chinese24129732
- LuXLiYXiaoXLiXInhibitory effects of luteolin on human gastric carcinoma xenografts in nude mice and its mechanismZhonghua Yi Xue Za Zhi2013932142146 Chinese23648354
- PanduranganADharmalingamPSadagopanSGanapasamSLuteolin inhibits matrix metalloproteinase 9 and 2 in azoxymethane-induced colon carcinogenesisHum Exp Toxicol201433111176118524532706
- HuangXDaiSDaiJLuteolin decreases invasiveness, deactivates STAT3 signaling, and reverses interleukin-6 induced epithelial–mesenchymal transition and matrix metalloproteinase secretion of pancreatic cancer cellsOnco Targets Ther20158298926527884
- KöhrmannAKammererUKappMDietlJAnackerJExpression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literatureBMC Cancer2009918819531263
- TurleyEANoblePWBourguignonLYSignaling properties of hyaluronan receptorsJ Biol Chem200227774589459211717317
- SheridanCKishimotoHFuchsRKCD44+/CD24-breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasisBreast Cancer Res200685R5917062128
- Charafe-JauffretEGinestierCIovinoFBreast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signatureCancer Res20096941302131319190339
- DraffinJEMcFarlaneSHillAJohnstonPGWaughDJCD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cellsCancer Res200464165702571115313910
- KuppusamyUKhooHDasNStructure-activity studies of flavonoids as inhibitors of hyaluronidaseBiochem Pharmacol19904023974012375774
- ReipasKMLawJHCoutoNLuteolin is a novel p90 ribosomal S6 kinase (RSK) inhibitor that suppresses Notch4 signaling by blocking the activation of Y-box binding protein-1 (YB-1)Oncotarget20134232934523593654
- TsaiPHChengCHLinCYDietary flavonoids luteolin and quercetin suppressed cancer stem cell properties and metastatic potential of isolated prostate cancer cellsAnticancer Res201636126367638027919958
- TuDGLinWTYuCCChemotherapeutic effects of luteolin on radio-sensitivity enhancement and interleukin-6/signal transducer and activator of transcription 3 signaling repression of oral cancer stem cellsJ Formos Med Assoc2016115121032103827742160
- HojillaCMohammedFKhokhaRMatrix metalloproteinases and their tissue inhibitors direct cell fate during cancer developmentBr J Cancer200389101817182114612884
- KorkayaHLiuSWichaMSBreast cancer stem cells, cytokine networks, and the tumor microenvironmentJ Clin Invest2011121103804380921965337
- SeelingerGMerfortISchemppCMAnti-oxidant, anti-inflammatory and anti-allergic activities of luteolinPlanta Med200874141667167718937165
- BenoyIHSalgadoRvan DamPIncreased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survivalClin Cancer Res200410217157716215534087
- ChoiHJChoiHJChungTWHaKTLuteolin inhibits recruitment of monocytes and migration of Lewis lung carcinoma cells by suppressing chemokine (C–C motif) ligand 2 expression in tumor-associated macrophageBiochem Biophys Res Commun2016470110110626766793
- ZhanTRindtorffNBoutrosMWnt signaling in cancerOncogene201736111461147327617575
- LamouilleSXuJDerynckRMolecular mechanisms of epithelial–mesenchymal transitionNat Rev Mol Cell Biol201415317819624556840
- TseJCKalluriRMechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironmentJ Cell Biochem2007101481682917243120
- NelsonWJNusseRConvergence of Wnt, β-catenin, and cadherin pathwaysScience200430356631483148715001769
- CleversHWnt/β-catenin signaling in development and diseaseCell2006127346948017081971
- BlancoMJMoreno-BuenoGSarrioDCorrelation of Snail expression with histological grade and lymph node status in breast carcinomasOncogene200221203241324612082640
- PanduranganAKDharmalingamPSadagopanSKARamarMMunusamyAGanapasamSLuteolin induces growth arrest in colon cancer cells through involvement of Wnt/β-catenin/GSK-3β signalingJ Environ Pathol Toxicol Oncol201332213113924099426
- ChenJImanakaNGriffinJHypoxia potentiates Notch signaling in breast cancer leading to decreased E-cadherin expression and increased cell migration and invasionBr J Cancer2010102235136020010940
- RanganathanPWeaverKLCapobiancoAJNotch signalling in solid tumours: a little bit of everything but not all the timeNat Rev Cancer201111533835121508972
- AnjumRBlenisJThe RSK family of kinases: emerging roles in cellular signallingNat Rev Mol Cell Biol200891074775818813292
- HarrisonHFarnieGHowellSJRegulation of breast cancer stem cell activity by signaling through the Notch4 receptorCancer Res201070270971820068161
- EspinosaLInglés-EsteveJAguileraCBigasAPhosphorylation by glycogen synthase kinase-3β down-regulates Notch activity, a link for Notch and Wnt pathwaysJ Biol Chem200327834322273223512794074
- FangXYuSXLuYBastRCWoodgettJRMillsGBPhosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase AProc Natl Acad Sci U S A20009722119601196511035810
- MalumbresMBarbacidMCell cycle, CDKs and cancer: a changing paradigmNat Rev Cancer20099315316619238148
- LeeEJOhSYSungMKLuteolin exerts anti-tumor activity through the suppression of epidermal growth factor receptor-mediated pathway in MDA-MB-231 ER-negative breast cancer cellsFood Chem Toxicol201250114136414322926442
- RaoPSSatelliAMoridaniMJenkinsMRaoUSLuteolin induces apoptosis in multidrug resistant cancer cells without affecting the drug transporter function: involvement of cell line-specific apoptotic mechanismsInt J Cancer2012130112703271421792893
- ChiangCTWayTDLinJKSensitizing HER2-overexpressing cancer cells to luteolin-induced apoptosis through suppressing p21WAF1/CIP1 expression with rapamycinMol Cancer Ther2007672127213817620442
- AbbasTDuttaAP21 in cancer: intricate networks and multiple activitiesNat Rev Cancer20099640041419440234
- CasagrandeFDarbonJMEffects of structurally related flavonoids on cell cycle progression of human melanoma cells: regulation of cyclin-dependent kinases CDK2 and CDK1Biochem Pharmacol200161101205121511322924
- ChowdhuryARSharmaSMandalSGoswamiAMukhopadhyaySMajumderHKLuteolin, an emerging anti-cancer flavonoid, poisons eukaryotic DNA topoisomerase IBiochem J2002366265366112027807
- YamashitaNKawanishiSDistinct mechanisms of DNA damage in apoptosis induced by quercetin and luteolinFree Radic Res200033562363311200093
- AminARWangDZhangHEnhanced anti-tumor activity by the combination of the natural compounds (−)-epigallocatechin-3-gallate and luteolin potential role of p53J Biol Chem201028545345573456520826787
- OvertonKWSpencerSLNodererWLMeyerTWangCLBasal p21 controls population heterogeneity in cycling and quiescent cell cycle statesProc Natl Acad Sci U S A201411141E4386E439325267623
- KobayashiTNakataTKuzumakiTEffect of flavonoids on cell cycle progression in prostate cancer cellsCancer Lett20021761172311790449
- CaiXYeTLiuCLuteolin induced G2 phase cell cycle arrest and apoptosis on non-small cell lung cancer cellsToxicol In vitro20112571385139121601631
- LiYMPanYWeiYUpregulation of CXCR4 is essential for HER2-mediated tumor metastasisCancer Cell20046545946915542430
- RossJSSlodkowskaEASymmansWFPusztaiLRavdinPMHortobagyiGNThe HER-2 receptor and breast cancer: ten years of targeted anti–HER-2 therapy and personalized medicineOncologist200914432036819346299
- WayTDKaoMCLinJKDegradation of HER2/neu by apigenin induces apoptosis through cytochrome c release and caspase-3 activation in HER2/neu-overexpressing breast cancer cellsFEBS Lett2005579114515215620704
- CatzavelosCBhattacharyaNUngYCDecreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancerNat Med1997322272309018244
- ParkSHHamSKwonTHLuteolin induces cell cycle arrest and apoptosis through extrinsic and intrinsic signaling pathways in MCF-7 breast cancer cellsJ Environ Pathol Toxicol Oncol201433321923125272060
- SerginaNVRauschMWangDEscape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3Nature2007445712643744117206155
- YakesFMChinratanalabWRitterCAKingWSeeligSArteagaCLHerceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor actionCancer Res200262144132414112124352
- ZhangLYangFHuangLLiuAZhangJLuteolin enhances the antitumor activity of lapatinib in human breast cancer cellsBiomed Res2017281149024907
- SebastianJRichardsRGWalkerMPActivation and function of the epidermal growth factor receptor and erbB-2 during mammary gland morphogenesisCell Growth Differ1998997777859751121
- KenneyNJBowmanAKorachKSBarrettJCSalomonDSEffect of exogenous epidermal-like growth factors on mammary gland development and differentiation in the estrogen receptor-alpha knockout (ERKO) mouseBreast Cancer Res Treat200379216117312825851
- BosPDZhangXHNadalCGenes that mediate breast cancer metastasis to the brainNature200945972491005100919421193
- HicksDGShortSMPrescottNLBreast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFRAm J Surg Pathol20063091097110416931954
- SuiJXieKXieMInhibitory effect of luteolin on the proliferation of human breast cancer cell lines induced by epidermal growth factorSheng Li Xue Bao2016681273426915319
- BrombergJStat proteins and oncogenesisJ Clin Invest200210991139114211994401
- DevarajanEHuangSSTAT3 as a central regulator of tumor metastasesCur Mol Med200995626633
- YangMYWangCJChenNFHoWHLuFJTsengTHLuteolin enhances paclitaxel-induced apoptosis in human breast cancer MDA-MB-231 cells by blocking STAT3Chem Biol Interact2014213606824525192
- NahtaRYuanLXZhangBKobayashiREstevaFJInsulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cellsCancer Res20056523111181112816322262
- OliveiraSSchiffelersRStormGHenegouwenPRooversRCrosstalk between epidermal growth factor receptor-and insulin-like growth factor-1 receptor signaling: implications for cancer therapyCurr Cancer Drug Targets20099674876019754359
- JonesHEGoddardLGeeJMInsulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cellsEndocr Relat Cancer200411479381415613453
- WangLMXieKPHuoHNShangFZouWXieMJLuteolin inhibits proliferation induced by IGF-1 pathway dependent ERα in human breast cancer MCF-7 cellsAsian Pac J Cancer Prev20121341431143722799344
- StrömAHartmanJFosterJSKietzSWimalasenaJGustafssonJAEstrogen receptor β inhibits 17β-estradiol-stimulated proliferation of the breast cancer cell line T47DProc Natl Acad Sci U S A200410161566157114745018
- CovaledaAMvan den BergHVervoortJInfluence of cellular ERα/ERβ ratio on the ERα-agonist induced proliferation of human T47D breast cancer cellsToxicol Sci2008105230331118644836
- MarkaverichBMRobertsRRAlejandroMAJohnsonGAMiddleditchBSClarkJHBioflavonoid interaction with rat uterine type II binding sites and cell growth inhibitionJ Steroid Biochem1988301–671783386279
- Le BailJCVarnatFNicolasJCHabriouxGEstrogenic and antiproliferative activities on MCF-7 human breast cancer cells by flavonoidsCancer Lett199813012092169751276
- MarkaverichBMShoularsKRodriguezMALuteolin regulation of estrogen signaling and cell cycle pathway genes in MCF-7 human breast cancer cellsInt J Biomed Sci20117210111121731475
- ElmoreSApoptosis: a review of programmed cell deathToxicol Pathol200735449551617562483
- SatoYSasakiNSaitoMEndoNKugawaFUenoALuteolin attenuates doxorubicin-induced cytotoxicity to mcf-7 human breast cancer cellsBiol Pharm Bul2015385703709
- Rice-EvansCAMillerNJPagangaGStructure-antioxidant activity relationships of flavonoids and phenolic acidsFree Radic Biol Med19962079339568743980
- JuWWangXShiHChenWBelinskySALinYA critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-κB pathway and sensitization of apoptosis in lung cancer cellsMol Pharmacol20077151381138817296806
- LinCHChangCYLeeKRLinHJChenTHWanLFlavones inhibit breast cancer proliferation through the Akt/FOXO3a signaling pathwayBMC Cancer20151595826675309
- Momtazi-BorojeniAABehbahaniMSadeghi-AliabadiHAntiproliferative activity and apoptosis induction of crude extract and fractions of Avicennia marinaIran J Basic Med Sci201316111203120824494074
- AllredDCClarkGMElledgeRAssociation of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancerJ Natl Cancer Inst19938532002068423624
- SjögrenSInganäsMNorbergTThe p53 gene in breast cancer: prognostic value of complementary DNA sequencing versus immunohistochemistryJ Natl Cancer Inst1996883–41731828632491
- JeonYWSuhYJSynergistic apoptotic effect of celecoxib and luteolin on breast cancer cellsOncol Rep201329281982523229294
- DasTSumanSAlatassiHAnkemMDamodaranCInhibition of AKT promotes FOXO3a-dependent apoptosis in prostate cancerCell Death Dis201672e211126913603
- SuntersAde MattosSFStahlMFoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell linesJ Biol Chem200327850497954980514527951
- PizerESJackischCWoodFDPasternackGRDavidsonNEKuhajdaFPInhibition of fatty acid synthesis induces programmed cell death in human breast cancer cellsCancer Res19965612274527478665507
- BrusselmansKVrolixRVerhoevenGSwinnenJVInduction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activityJ Biol Chem200528075636564515533929
- LiuJFMaYWangYDuZYShenJKPengHLReduction of lipid accumulation in HepG2 cells by luteolin is associated with activation of AMPK and mitigation of oxidative stressPhytother Res201125458859620925133
- YangYAHanWFMorinPJChrestFJPizerESActivation of Fatty acid synthesis during neoplastic transformation: role of mitogen-activated protein kinase and phosphatidylinositol 3-kinaseExp Cell Res20022791809012213216
- GuoDPrinsRMDangJEGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapySci Signal20092101ra8220009104
- XueGHemmingsBAPKB/Akt-dependent regulation of cell motilityJ Natl Cancer Inst2013105639340423355761
- SosMLKokerMWeirBAPTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFRCancer Res20096983256326119351834
- FangJZhouQShiXlJiangBHLuteolin inhibits insulin-like growth factor 1 receptor signaling in prostate cancer cellsCarcinogenesis200728371372317065200
- LeeLTHuangYTHwangJJTransinactivation of the epidermal growth factor receptor tyrosine kinase and focal adhesion kinase phosphorylation by dietary flavonoids: effect on invasive potential of human carcinoma cellsBiochem Pharmacol200467112103211415135307
- ShihYLLiuHCChenCSCombination treatment with luteolin and quercetin enhances antiproliferative effects in nicotine-treated MDA-MB-231 cells by down-regulating nicotinic acetylcholine receptorsJ Agric Food Chem2009581235241
- HuangYTHwangJJLeePPEffects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptorBr J Pharmacol19991285999101010556937
- TuSHHoCTLiuMFLuteolin sensitises drug-resistant human breast cancer cells to tamoxifen via the inhibition of cyclin E2 expressionFood Chem201314121553156123790951
- van ZandenJJGeraetsLWortelboerHMvan BladerenPJRietjensIMCnubbenNHStructural requirements for the flavonoid-mediated modulation of glutathione S-transferase P1-1 and GS-X pump activity in MCF7 breast cancer cellsBiochem Pharmacol20046781607161715041478
- ColemanDTBigelowRCardelliJAInhibition of fatty acid synthase by luteolin post-transcriptionally down-regulates c-Met expression independent of proteosomal/lysosomal degradationMol Cancer Ther20098121422419139131
- KimMJWooJSKwonCHKimJHKimYKKimKHLuteolin induces apoptotic cell death through AIF nuclear translocation mediated by activation of ERK and p38 in human breast cancer cell linesCell Biol Int201236433934422073986
- DuGJSongZHLinHHHanXFZhangSYangYMLuteolin as a glycolysis inhibitor offers superior efficacy and lesser toxicity of doxorubicin in breast cancer cellsBiochem Biophys Res Commun2008372349750218503759