Abstract
Purpose
Chemotherapy with anthracyclines, taxanes, or alkylating agents often causes cutaneous side effects. Nonspecific inhibition of the proliferative activity of keratinocytes has antidifferentiation effects that lead to defects in the barrier function and, thus, to dry, itchy, and irritable skin. These cutaneous symptoms reduce the quality of life of the patients considerably. Conditioning with topical application of niacinamide uses the cytoprotective and barrier stabilizing effect of vitamin B3.
Patients and methods
A multicenter randomized crossover study investigated the influence of the test preparation on the quality of life compared to standard care for 73 patients with breast cancer undergoing adjuvant or neoadjuvant cytostatic therapy. Primary target parameter was the Dermatology Life Quality Index with its respective subscales after 6 weeks of a twice-daily application of the respective preparations. Additionally, specific symptoms such as pruritus, dryness, and irritability have been assessed using visual analog scales.
Results
Regarding the total score of the Dermatology Life Quality Index, no relevant differences could be observed. However, the results for the “symptoms and feelings” subscale show a significant advantage in favor of the test preparation. Significant superiority of the test preparation could also be observed in the secondary target parameters, the visual analog scales (P<0.05).
Conclusion
The results show for the first time a significant superiority of prophylactic application of niacinamide for maintaining quality of life while undergoing cytostatic treatment.
Introduction
The prevalence of cancers in industrial nations is steadily increasing.Citation1 With approximately 75,000 new cases each year, breast cancer is a common tumor in Germany and by far the most frequent malignant tumor in women.Citation2 Based on guidelines, therapeutic regimens are chosen depending on clinical grading, hormone receptor state, lymph node involving, and the patient’s age. For intermediate and high-risk conditions, this almost always involves adjuvant systemic chemotherapy.Citation3 Usually, combinations of cytostatics are used, sometimes together with targeted therapies. Depending on dosage schedule, treatment period, and the type of combination, therapies show considerable unwanted side effects. Additionally, the severe psychological stress for patients with cancer as well as its social impact has to be taken into account.Citation4 Therefore, more and more supportive measures are validated in oncology.Citation5–Citation7
Unwanted side effects affecting the skin organ are mostly non-life-threatening, but can seriously reduce patients’ quality of life and, thus, endanger therapeutic success by reducing compliance.Citation8 From a dermatological point of view, various clinical patterns can be determined; these occur quite frequently and, therefore, have a high practical relevance.Citation9,Citation10 Recommendations for the therapeutical management for some of these patterns exist, the evidence of which is worked on progressively.Citation11,Citation12 Recommendations for prophylactic measures, on the other hand, have not gone beyond general notes on care so far.Citation13–Citation15
However, the success of specific prophylactic strategies depends very much on the pathomechanism of individual reaction patterns, and these are not necessarily specific to substances or their combinations.Citation16 Also, it is important to distinguish between toxic and nontoxic reactions. Unspecific cytotoxic effects on the epithelium or skin appendages caused by the cytostatics themselves or by their metabolites are the most common events and can be observed in up to 30% of all cancer patients, regardless of the type of the primary tumor, but dependent on the regimen and the combination of chemotherapy. Furthermore, individual pathologic factors such as pharmacogenetics, comorbidities, concomitant medication, sweating, cutaneous vulnerability, and genetic disposition seem to be important. On the whole rather rare, but quite frequent in connection with targeted therapies, specific antiproliferative effects can be observed. The intrinsic tyrosine kinase activity of the epidermal growth factor and its receptors on the cell surface (EGFR) play a major role here.Citation17 The specific blockade of EGFR by therapeutic antibodies as well as the unspecific inhibition by multikinase inhibitors can cause alterations associated primarily with skin appendages (acne-like rash, paronychia).Citation9,Citation18–Citation22 Elimination of cytostatics and their metabolites by eccrine sweat can cause both direct toxic effects due to accumulation in the stratum corneum, especially in palmar and plantar skin, and inflammatory phenomena due to depletion of the antioxidative capacity of the skin (chemotherapy-induced acral erythema, also known as palmar-plantar erythrodysesthesia or hand–foot syndrome).Citation11
Most frequently, however, barrier disorders caused by complex dysfunction of proliferation and differentiation of interfollicular keratinocytes and epidermal stem cells can be observed. Clinical symptoms are dryness and scaling of the skin, which after latency can lead to inflammatory irritations along with pruritus and, therefore, scratching.Citation23 Up to now, only individual recommendations for supportive skin care, aiming at barrier substitution, can be found in literature.Citation16 These recommendations are based on concepts that have been established for chronic inflammatory skin diseases with endogenous barrier deficiency (eg, atopic dermatitis) and have been empirically applied to oncological situations. Here, the different pathomechanism of barrier dysfunction under therapy with cytostatics is ignored, and proactive use is propagated without scientific evidence.Citation16 The clinical use of a niacinamide-containing preparation implements the concept of using the well-known effects of the natural vitamin proactively to prevent clinically relevant chemotherapy-induced barrier dysfunction.Citation24–Citation28 The advantages of niacinamide are its anti-inflammatory effects due to inhibition of proinflammatory factors, as well as its ability to increase the expression of serine palmitoyltransferase as the key enzyme for ceramide synthesis.Citation29–Citation33 Even after repeated epicutaneous application of preparations with 4% niacinamide, no systemic toxic effects or interactions with other systemically applied active substances are to be expected.Citation34
A study complying with the principles of Good Clinical Practice investigated the clinical benefits of the proactive use of a semisolid niacinamide-containing preparation in patients with breast cancer undergoing cytostatic therapy.
Patients and methods
Study objective and study design
The objective of this multicenter prospective randomized reference-controlled crossover study was to validate the clinical relevance of the preventive use of a niacinamide-containing barrier-protective preparation under real life conditions in patients undergoing cytostatic therapy. Female patients aged 18 years or older and diagnosed with breast cancer were enrolled in the study on condition that they had an indication for adjuvant or neoadjuvant chemotherapy with anthracyclines or taxanes; combination with trastuzumab was allowed. Not enrolled were patients who: had clinical signs of a barrier dysfunction before study start; had indications for an atopic or psoriatic disposition in their medical history; or were using pharmaceutical or over-the-counter products that have vasoactive, anti-inflammatory, or diuretic effects, or those that affect the lipid metabolism. Study start was the first day patients received chemotherapy. One study group first used the test preparation (TP) for 6 weeks and, subsequently, standard care (SC) for 6 weeks. The other study group first used SC for 6 weeks and, subsequently, TP for 6 weeks. The Dermatology Life Quality Index (DLQI) as primary target parameter was recorded over the time of 12 weeks.Citation35–Citation38 In addition to the total DLQI score, the six DLQI subscales were included in the analysis (Figure S1).Citation39,Citation40 As second target parameters, the symptoms pruritus, dryness, and irritability were quantified and recorded via visual analog scales.Citation41
Test preparation
TP was a lipophilic cream containing 4% niacinamide, shea butter as lipophilic, and thermal spring water from La Roche-Posay as hydrophilic phase (Lipikar® Baume AP, La Roche-Posay Laboratoire Pharmaceutique, La Roche-Posay, France). TP was applied twice daily on the whole body. Standard care (SC), which was defined as the patients usual body care in their individual quantity and frequency, was chosen as control arm.
Statistics
The confirmative evaluation of the principal target parameter was done by analysis of variance. All other parameters were evaluated descriptively. The number of the values, missing values, mean value, standard deviation, median, quartile, minimum, and maximum were specified for all continuous values. For all other values, frequency tables were generated. The statistical evaluation was carried out using the SAS package version 9.1 (SAS Institute Inc., Cary NC, USA).
Ethics
The study was approved by the Ethics Committee of the Faculty of Medicine Charité, Humboldt University Berlin; the respective ethics committees of the study sites agreed with this approval. All patients gave their written informed consent to their participation in this study. The study was conducted according to the guidelines of Good Clinical Practice.
Results
Patient characteristics
The study was conducted between February 2012 and April 2013 in six breast cancer centers in Germany. A total of 95 patients aged between 25 and 77 years were enrolled (). Via block randomization, 46 patients were randomized in group TP/SC and 48 in group SC/TP. Twenty-one patients dropped out before the end of the study and one patient had to be excluded for protocol deviation. A total of 73 patients were included in the analysis.
Treatment details
The most frequent chemotherapies received by patients were: epirubicin plus cyclophosphamide plus docetaxel or paclitaxel; cyclophosphamide plus epirubicin plus 5-fluorouracil; and doxorubicin plus cyclophosphamide plus docetaxel, sometimes combined with trastuzumab. The respective regimens and their frequencies are shown in . The study had no influence on the therapeutic decision.
Table 1 Characteristics of study population
DLQI results
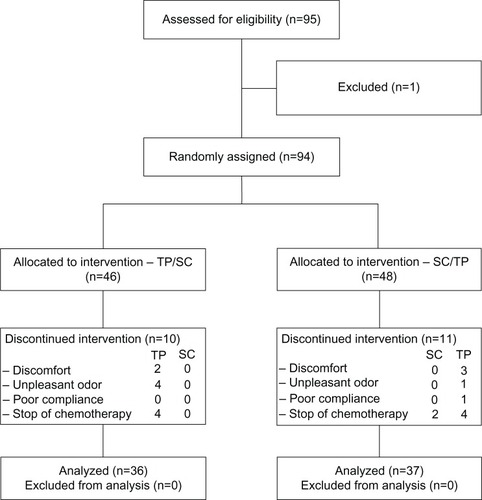
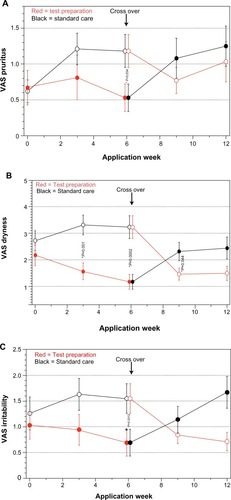
Comparing the total DLQI score between TP and SC after 6 weeks did not show any significant differences (). However, the analysis of the six subscales showed significant superiority of TP for the “symptoms and feelings” aspect after week 4. After crossover, superiority of TP can be observed after week 8 (). For the other subscales, no significant differences could be found. The visual analog scale values for the secondary target parameters of pruritus, dryness, and irritability show significant superiority for TP after 6 weeks ().
Figure 2 DLQI: comparison of both groups over application time.
Abbreviations: DLQI, Dermatology Life Quality Index;Citation35 SF, subscale Symptoms and Feelings.
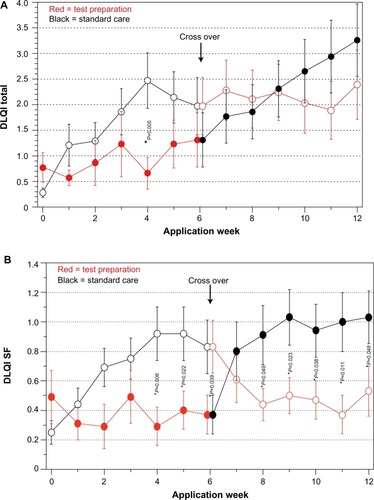
Figure 3 VAS: comparison of both groups over application time.
Abbreviation: VAS, visual analog scale.
Discussion
The need for supportive therapy in oncology is obvious and widely acknowledged.Citation5 Supportive measures depend on the underlying condition, the stage of the disease, the treatment regimen, as well as on individual pathologic and social factors. If possible, evidence based recommendations should be aspired to. From a dermatological point of view, a prophylactic or proactive approach must be distinguished from therapeutic or reactive strategies. Both strategies interact in the management of unwanted cutaneous reactions and have high practical relevance. Referring to safety data of published studies, the probability of pathogenetic cutaneous symptoms depending on dosage and combination of cytostatics can be estimated. The most common symptom, especially when using anthracyclines, taxanes, or alkylating agents, is dry and itchy skin as expression of complex dysfunctional differentiation. This becomes phenotypically relevant usually after 7–14 days. Substitution, specifically targeted to condition the barrier function, starting at the same time as cytostatic therapy can prevent, slow down, or reduce clinical symptoms. For this conditioning effect, niacinamide provides favorable preconditions. It has cytoprotective effects due to stimulation of DNA repair mechanisms.Citation42,Citation43 In addition, there are indications that niacinamide supports the release of cytostatics from cells, depending on concentration, and therefore reduces their antiproliferative effects.Citation44 These effects of the topically applied and, therefore, only cutaneously bioavailable active substance are the basis for the hypothesis of a conditioning proactive use during cytostatic therapy. The above-mentioned additional effects concerning ceramide synthesis and anti-inflammation are cumulative.Citation29,Citation45,Citation46
To recruit enough patients that met the inclusion and exclusion criteria in a timely manner, and to avoid seasonal influences, six breast centers were selected for competitive recruitment. The high cumulative dropout rate of 23% in this study is mainly due to discontinuation of chemotherapy for medical reasons. Additionally, some cases of early termination can be attributed to chemotherapy-induced alterations of sweat gland functions (hyperhidrosis) and the olfactory sense (dysosmia). The present study data and published data raise no safety concerns for clinical use.Citation47–Citation49 Furthermore, niacinamide has been used systemically as a perfusion enhancer for palliative care in patients with breast cancer due to its relaxant effects on vascular smooth muscle cells.Citation50,Citation51
The results of this study suggest that cytostatic cutaneous symptoms among other health problems have a negative impact on quality of life for the patients. That supports the usefulness of a proactive prophylactic skin care, which should be applied with the start of cytostatic therapy at the latest. Despite the psychological stress that accompanies the start of a cytostatic therapy, the patients experienced the offer of supportive skin care as motivating rather than as an additional burden. The superiority of TP over SC, regarding the mentioned aspect of quality of life and regarding pruritus, dryness, and irritability, proves the efficacy of TP for prophylactic use within the study setting. Statements regarding the efficacy of the preparation for other pathogenetic patterns of unwanted drug reactions, eg, palmar-plantar erythrodysesthesia, acne-like rash, or toxic exanthema, cannot be made.Citation9,Citation20,Citation52–Citation54 Also, no therapeutic effects can be propagated regarding established, inflammatory skin conditions.
Conclusion
The results of this study favor the niacinamide-containing TP for proactive treatment accompanying cytostatic therapies with classic antiproliferative substances. Certainly, further investigations are necessary in order to strengthen the evidence for the supportive use of topical niacinamide in oncology.
Disclosure
The study was fully sponsored by La Roche-Posay Laboratoire Dermatologique L’Oréal Deutschland GmbH Georg-Glock-Straße 18 40474 Düsseldorf, Germany. J Wohlrab and D Lüftner received lecture fees, were co-operation partners in scientific projects and investigators in clinical studies sponsored by L’Oréal Deutschland GmbH. R Richter and S Seite are employed by the sponsor. The other authors were investigators in this clinical study sponsored by L’Oréal Deutschland GmbH and declare no conflict of interest.
Acknowledgments
The authors wish to thank Ms Andrea Stennett for excellent assistance in study management and Dr Gerd Franke for biometrical evaluation.
References
- AssiHAKhouryKEDboukHKhalilLEMouhieddineTHEl SaghirNSEpidemiology and prognosis of breast cancer in young womenJ Thorac Dis20135S2S823819024
- ThomssenCMarschnerNUntchMABC1 Consensus Conference – a German Perspective: First International Consensus Conference on Advanced Breast Cancer (ABC1), Lisbon, November 5, 2011Breast Care (Basel)20127525922553474
- TruongPTOlivottoIAWhelanTJClinical practice guidelines for the care and treatment of breast cancer: 16. Locoregional post-mastectomy radiotherapyCMAJ20041701263127315078851
- KroenkeCHQuesenberryCKwanMLSweeneyCCastilloACaanBJSocial networks, social support, and burden in relationships, and mortality after breast cancer diagnosis in the Life After Breast Cancer Epidemiology (LACE) studyBreast Cancer Res Treat201313726127123143212
- PetrieWLoganJDeGrasseCResearch review of the supportive care needs of spouses of women with breast cancerOncol Nurs Forum2001281601160711759307
- García-EstévezLTusquetsIAlvarezISupportive care for patients with early breast cancerClin Transl Oncol201012324220080469
- FreedmanOAmirEZimmermannCClemonsMFilling in the gaps: reporting of concurrent supportive care therapies in breast cancer chemotherapy trialsSupport Care Cancer20111931532221203780
- VialePHChemotherapy and cutaneous toxicities: implications for oncology nursesSemin Oncol Nurs20062214415116893743
- GutzmerRWollenbergAUgurelSHomeyBGanserAKappACutaneous side effects of new antitumor drugs: clinical features and managementDtsch Arztebl Int201210913314022419954
- HartmannJTHaapMKoppHGLippHPTyrosine kinase inhibitors – a review on pharmacology, metabolism and side effectsCurr Drug Metab20091047048119689244
- Webster-GandyJDHowCHarroldKPalmar-plantar erythrodysesthesia (PPE): a literature review with commentary on experience in a cancer centreEur J Oncol Nurs20071123824617350337
- KumarSJuresicEBartonMShafiqJManagement of skin toxicity during radiation therapy: a review of the evidenceJ Med Imaging Radiat Oncol20105426427920598015
- HaleyACCalahanCGandhiMWestDPRademakerALacoutureMESkin care management in cancer patients: an evaluation of quality of life and tolerabilitySupport Care Cancer20111954555420336328
- MASCCCaring for your skin, hait and nails whenon “targeted therapies”2011 Available from: (http://www.mascc.org/assets/documents/skin_toxicity_egfri_patientbrochure.pdf)Accessed April 10, 2014
- American Cancer SocietyTargeted therapy2013 Available from:(http://www.cancer.org/acs/groups/cid/documents/webcontent/003024-pdf.pdf)Accessed April 10, 2014
- DrenoBBensadounRJHumbertPAlgorithm for dermocosmetic use in the management of cutaneous side-effects associated with targeted therapy in oncologyJ Eur Acad Dermatol Venereol2013271071108023368717
- PotthoffKHofheinzRHasselJCInterdisciplinary management of EGFR-inhibitor-induced skin reactions: a German expert opinionAnn Oncol20112252453520709812
- PatriziAVenturiMDikaEMaibachHTacchettiPBrandiGCutaneous adverse reactions linked to targeted anticancer therapies bortezomib and lenalidomide for multiple myeloma: new drugs, old side effectsCutan Ocul Toxicol2014331623638756
- MateusCRobertCNew drugs in oncology and skin toxicityRev Med Interne200930401410 French19299041
- LacoutureMEMeloskyBLCutaneous reactions to anticancer agents targeting the epidermal growth factor receptor: a dermatology-oncology perspectiveSkin Therapy Lett2007121517762902
- Galimont-CollenAFVosLELavrijsenAPOuwerkerkJGelderblomHClassification and management of skin, hair, nail and mucosal side-effects of epidermal growth factor receptor (EGFR) inhibitorsEur J Cancer20074384585117289377
- LacoutureMEAnadkatMJBensadounRJClinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicitiesSupport Care Cancer2011191079109521630130
- AghaRKinahanKBennettCLLacoutureMEDermatologic challenges in cancer patients and survivorsOncology (Williston Park)20072114621472 discussion 1473, 1476, 1481 passim18077993
- ZhangZRiviereJEMonteiro-RiviereNAEvaluation of protective effects of sodium thiosulfate, cysteine, niacinamide and indomethacin on sulfur mustard-treated isolated perfused porcine skinChem Biol Interact1995962492627750164
- NamaziMRNicotinamide in dermatology: a capsule summaryInt J Dermatol2007461229123118173513
- BissettDTopical niacinamide and barrier enhancementCutis200270812 discussion 21–2312498532
- GehringWNicotinic acid/niacinamide and the skinJ Cosmet Dermatol20043889317147561
- OtteNBorelliCKortingHCNicotinamide – biologic actions of an emerging cosmetic ingredientInt J Cosmet Sci20052725526118492206
- TannoOOtaYKitamuraNKatsubeTInoueSNicotinamide increases biosynthesis of ceramides as well as other stratum corneum lipids to improve the epidermal permeability barrierBr J Dermatol200014352453110971324
- DharajiyaNChoudhuryBKBacsiABoldoghIAlamRSurSInhibiting pollen reduced nicotinamide adenine dinucleotide phosphate oxidase-induced signal by intrapulmonary administration of antioxidants blocks allergic airway inflammationJ Allergy Clin Immunol200711964665317336614
- WhiteSDRosychukRAReinkeSIParadisMUse of tetracycline and niacinamide for treatment of autoimmune skin disease in 31 dogsJ Am Vet Med Assoc1992200149715001535346
- FabbrociniGCantelliMMonfrecolaGTopical nicotinamide for seborrheic dermatitis: an open randomized studyJ Dermatolog Treat20142524124523763270
- NamaziMRNicotinamide as a potential addition to the anti-atopic dermatitis armamentariumInt Immunopharmacol20044670971215135312
- Cosmetic Ingredient review expert panelFinal report of safety assessment of niacinamide and niacinInt J Toxicol200524Suppl 5131
- FinlayAYKhanGKDermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical useClin Exp Dermatol1994192102168033378
- LewisVFinlayAY10 years experience of the Dermatology Life Quality Index (DLQI)J Investig Dermatol Symp Proc20049169180
- NijstenTMeadsDMMcKennaSPDimensionality of the dermatology life quality index (DLQI): a commentaryActa Derm Venereol200686284285 author reply 285–28616710607
- FinlayAYBasraMKPiguetVSalekMSDermatology life quality index (DLQI): a paradigm shift to patient-centered outcomesJ Invest Dermatol20121322464246522592153
- HongboYThomasCLHarrisonMASalekMSFinlayAYTranslating the science of quality of life into practice: What do dermatology life quality index scores mean?J Invest Dermatol200512565966416185263
- BasraMKFenechRGattRMSalekMSFinlayAYThe Dermatology Life Quality Index 1994–2007: a comprehensive review of validation data and clinical resultsBr J Dermatol2008159997103518795920
- CraigBMBusschbachJJSalomonJAModeling ranking, time trade-off, and visual analog scale values for EQ-5D health states: a review and comparison of methodsMed Care20094763464119433996
- NamaziMRNicotinamide-containing sunscreens for use in Australasian countries and cancer-provoking conditionsMed Hypotheses20036054454512615518
- SurjanaDHallidayGMDamianDLNicotinamide enhances repair of ultraviolet radiation-induced DNA damage in human keratinocytes and ex vivo skinCarcinogenesis2013341144114923349012
- GuptaNSaleemAKotzBKötzBCarbogen and nicotinamide increase blood flow and 5-fluorouracil delivery but not 5-fluorouracil retention in colorectal cancer metastases in patientsClin Cancer Res2006123115312316707610
- GrangePARaingeaudJCalvezVDupinNNicotinamide inhibits Propionibacterium acnes-induced IL-8 production in keratinocytes through the NF-kappaB and MAPK pathwaysJ Dermatol Sci20095610611219726162
- MonfrecolaGGaudielloFCirilloTFabbrociniGBalatoALemboSNicotinamide downregulates gene expression of interleukin-6, interleukin-10, monocyte chemoattractant protein-1, and tumour necrosis factor-alpha gene expression in HaCaT keratinocytes after ultraviolet B irradiationClin Exp Dermatol20133818518823397947
- Cosmetic Ingredient Review Expert PanelFinal report of the safety assessment of niacinamide and niacinInt J Toxicol200524Suppl 5131
- KnipMDouekIFMooreWPSafety of high-dose nicotinamide: a reviewDiabetologia2000431337134511126400
- VasanthaJSoundararajanPVanitharaniNSafety and efficacy of nicotinamide in the management of hyperphosphatemia in patients on hemodialysisIndian J Nephrol20112124524922022084
- PigottKDischeSSaundersMIShort communication: the addition of carbogen and nicotinamide to a palliative fractionation schedule for locally advanced breast cancerBr J Radiol1995682152187537598
- RuddockMWHirstDGNicotinamide relaxes vascular smooth muscle by inhibiting myosin light chain kinase-dependent signaling pathways: implications for anticancer efficacyOncol Res20041448348915559762
- HoeslyFJBakerSGGunawardaneNDCotliarJACapecitabine-induced hand-foot syndrome complicated by pseudomonal superinfection resulting in bacterial sepsis and death: case report and review of the literatureArch Dermatol20111471418142322184763
- KhandanpourCDührsenURöthAHand-Foot syndrome: Common presentation in an uncommon situationEur J Haematol20139147223859621
- YokomichiNNagasawaTColer-ReillyAPathogenesis of Hand-Foot Syndrome induced by PEG-modified liposomal DoxorubicinHum Cell20132681823386177