Abstract
Tuberous sclerosis complex (TSC) is an autosomal dominant genetic disorder caused by inactivating mutations in either the TSC1 or TSC2 genes. It is characterized by the development of multiple, benign tumors in several organs throughout the body. Lesions occur in the brain, kidneys, heart, liver, lungs, and skin and result in seizures and epilepsy, mental retardation, autism, and renal and pulmonary organ system dysfunction, as well as other complications. Elucidation of the molecular pathways and etiological factors responsible for causing TSC has led to a paradigm shift in the management and treatment of the disease. TSC1 or TSC2 mutations lead to constitutive upregulation of the mammalian target of rapamycin pathway, which affects many cellular processes involved in tumor growth. By targeting mammalian target of rapamycin with everolimus, an orally active rapamycin derivative, clinically meaningful and statistically significant reductions in tumor burden have been achieved for the main brain (subependymal giant cell astrocytoma) and renal manifestations (angiomyolipoma) associated with TSC. This review provides an overview of TSC, everolimus, and the clinical trials that led to its approval for the treatment of TSC-associated subependymal giant cell astrocytoma and renal angiomyolipoma.
Epidemiology and etiology of subependymal giant cell astrocytomas, angiomyolipomas, skin lesions, and lymphangioleiomyomatosis associated with tuberous sclerosis complex
Tuberous sclerosis complex (TSC) is an autosomal dominant genetic disorder with an approximate birth incidence of 1:6,000 newborns.Citation1–Citation3 Up to 1 million people worldwide are affected.Citation4 Although TSC can be inherited, approximately two-thirds of all cases are sporadic.Citation5 Patients with TSC have a severely debilitating disorder that is associated with multiple benign tumors (hamartomas), most commonly in the brain, kidney, lungs, and skin.Citation4,Citation6 Common symptoms include seizures (90%); renal tumors, also known as angiomyolipomas (up to 80%); skin lesions, ie, ash leaf macules and cutaneous fibromas (∼70%); pulmonary involvement, specifically lymphangioleiomyomatosis (LAM) (≤40%); autism and autism spectrum disorders (30%–40%); and neurocognitive impairments (50%–60%), which often occur in individuals with a normal intelligence quotient.Citation5,Citation7,Citation8
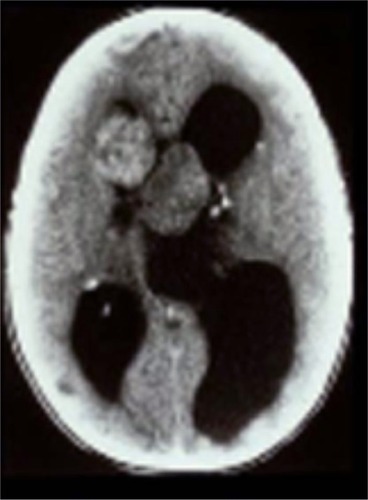
In the brain, up to 20% of TSC patients develop subependymal giant cell astrocytomas (SEGAs)—slow-growing glioneuronal tumors that typically arise near the foramen of Monro.Citation2,Citation8,Citation9 SEGAs may grow and obstruct cerebrospinal fluid and increase cerebral pressure, causing headaches, blurred vision, or sudden death from acute hydrocephalus ().Citation8–Citation10 The extent of elevated intracranial pressure and hydrocephalus is proportional to tumor diameter.
Figure 1 Computed tomography scan demonstrating acute presentation of bilateral subependymal giant cell astrocytomas with marked obstructive hydrocephalus. After emergency surgical resection, the patient with tuberous sclerosis complex was left blind and mentally retarded.
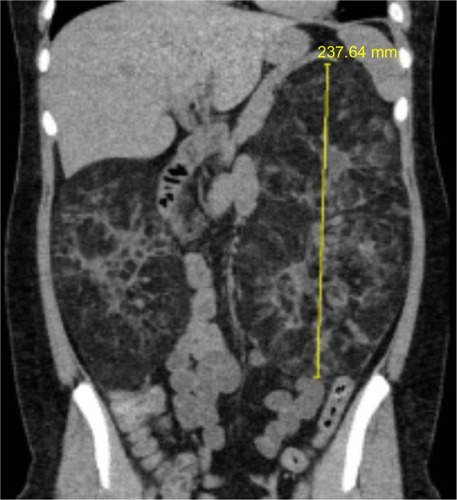
In the kidney, angiomyolipoma is one of the leading causes of death in TSC patients.Citation11 Angiomyolipomas occur in up to 80% of patients and typically are multiple and located bilaterally in the kidneys. They tend to increase in size over time, with some becoming >25–30 cm in their longest dimension (). Patients are at risk of serious complications such as life-threatening hemorrhage (25%–50%) and often present in the emergency room with shock (~20%–30%).Citation4,Citation7,Citation12 As angiomyolipomas enlarge, they often develop micro- and macroaneurysms. Angiomyolipomas ≥4 cm in diameter are at increased risk for spontaneous bleeding as a result of ruptured vessels. The presence of aneurysms >5 mm is a strong predictor of spontaneous hemorrhage.Citation13 Renal failure resulting in end-stage renal disease is largely due to encroachment of angiomyolipomas on normal renal parenchyma. Many patients require dialytic replacement therapy.Citation7
Figure 2 Renal computed tomography scan demonstrating significant angiomyolipomata burden in both kidneys of a patient with tuberous sclerosis complex.
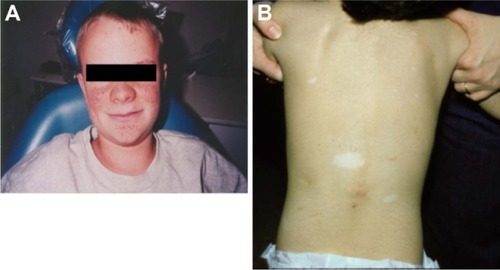
Skin lesions are detected in >70% of patients with TSC and include hypomelanotic macules (ash leaf spots), shagreen patches, confetti-like lesions, forehead fibrous plaque, facial angiofibromas, and periungual and ungual fibromas ( and ).Citation5,Citation14,Citation15 Facial angiofibromas can result in decreased quality of life for patients because they affect appearance, may cause disfigurement, and are prone to bleeding, which increases the possibility of infection.Citation14,Citation16
Figure 3 Examples of tuberous sclerosis complex-associated skin lesions: (A) angiofibromas and (B) shagreen patches and ash leaf macules.
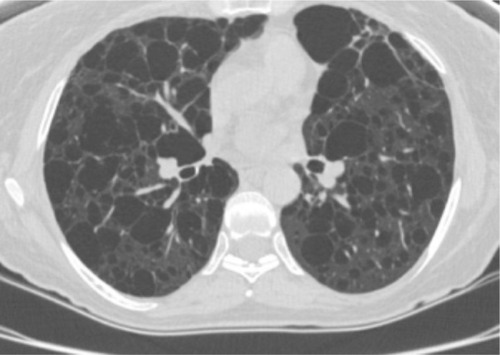
Pulmonary LAM occurs in up to 40% of TSC patients and predominantly affects women.Citation1,Citation7,Citation17 LAM occurs more rarely in patients without TSC (sporadic LAM). It is characterized by cystic destruction of the lung caused by infiltration of smooth muscle cells ().Citation7
Figure 4 Chest computed tomography scan showing parenchymal destruction associated with lymphangioleiomyomatosis in a patient with tuberous sclerosis complex.
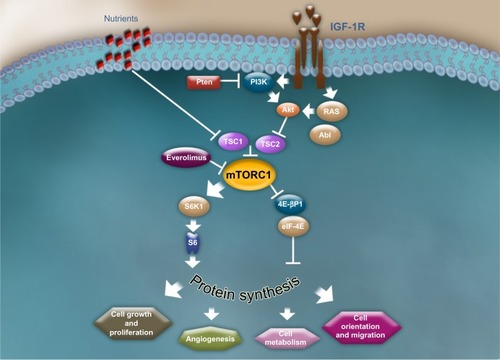
The molecular pathogenesis of TSC is thought to be caused by mutations in either the TSC1 (encoding hamartin) or TSC2 (encoding tuberin) genes, both of which are integral to the normal functioning of the mammalian target of rapamycin (mTOR) pathway.Citation18–Citation20 The mTOR pathway integrates numerous cellular inputs to affect a multitude of downstream signaling cascades that are involved in cellular processes such as cellular metabolism, growth, proliferation, angiogenesis, and survival.Citation1,Citation6,Citation8,Citation21 Hamartin and tuberin interact to form a dimer that activates a GTPase, preventing phosphorylation of the GAP protein—Ras homolog enriched in brain (Rheb)—and inhibiting activation of mTOR complex 1 (mTORC1), a serine threonine kinase.Citation22,Citation23 In patients with TSC, the hamartin/tuberin dimer is unable to form because of mutations in TSC1 and TSC2. As a result, mTORC1 is constitutively activated, which leads to hyperactive mTOR signaling of the downstream kinase signaling cascade and consequent abnormalities in many cellular processes, including cell cycle progression, transcription, translation, and metabolic control ().Citation22,Citation24
Figure 5 The mammalian target of rapamycin signaling pathway.
Abbreviations: 4E-BP1, 4E binding protein 1; Abl, Abelson kinase; Akt, protein kinase B; eIF-4E, eukaryotic initiation factor 4E; IGF-1R, insulin-like growth factor-1 receptor; PI3K, phosphoinositide 3-kinase; Pten, phosphatase and tensin homolog; RAS, rat sarcoma; S6K1, 40 S ribosomal S6 kinase; TSC1, tuberous sclerosis complex 1; TSC2, tuberous sclerosis complex 2.
TSC is thought to develop from complete loss of functional TSC1 or TSC2 and follows Knudson’s two-hit hypothesis whereby the first “hit,” causing a mutation in one allele of the TSC1 or TSC2 gene, is followed by a second “hit,” somatic mutation, in the corresponding TSC wildtype allele.Citation8 Disease severity can be variable, even within families, and may reflect differential expression of normal and mutant TSC alleles.Citation25
TSC2 mutations, which occur in 70%–80% of cases, are more common than TSC1 mutations, which occur in 20%–30% of cases.Citation26 Studies of TSC patients have found that the clinical manifestations of TSC are more frequent and often more severe in patients with TSC2 mutations.Citation18,Citation19 Data from genotype/phenotype studies suggest that different types and locations of TSC germline mutations may be associated with distinct neurocognitive phenotypes.Citation19,Citation27,Citation28
Management and current therapeutic strategies
Recently, a subcommittee at the Tuberous Sclerosis Complex Clinical Consensus Conference (sponsored by the Tuberous Sclerosis Alliance) reviewed and updated the recommendations for surveillance and management of patients with TSC.Citation29 Specific guidance on screening and follow-up evaluations, as well as clearly defined time intervals for clinical evaluations, is expected in the TSC guidelines and will help to standardize and improve the clinical care of TSC patients. These guidelines will soon be published and available to clinicians. Historically, treatment of TSC-associated hamartomas has focused on a combination of active and watchful waiting, palliative treatments with drug therapy, and surgical procedures to reduce tumor burden ().
Table 1 Common treatment modalities for SEGA, angiomyolipoma, skin lesions, and pulmonary LAM
SEGA
Active surveillance with serial neuroimaging is recommended even in asymptomatic patients, because SEGAs have the potential to grow over time.Citation30,Citation31 Surgical resection has been the standard treatment option for symptomatic SEGAs; however, scientific evidence for the effectiveness of SEGA surgery in reducing SEGA-related conditions is limited.Citation30,Citation32–Citation35 Postoperative morbidity is variable and may occur in ~50% of patients.Citation36 Complications include increased prevalence of seizures, hydrocephalus, vision disorders, headaches, stroke, hemiparesis, and autism.Citation32,Citation33,Citation35,Citation37
Tumor recurrence invariably is seen in the absence of gross total resection. This may occur with very large, locally invasive, or bilateral lesions.Citation32,Citation33 The timing of surgical intervention for SEGA is controversial, with some surgeons advocating early surgery, and others waiting for symptomatic lesions or development of hydrocephalus, which can facilitate a transcortical approach to the lesion.Citation30,Citation32,Citation38,Citation39 If surgery is chosen, gradual formation of an operative corridor using balloon dilation is an example of a minimally invasive technique.Citation40 This approach has resulted in gross total resection with resolution of ventricular dilation and minimal cortical disruption.Citation40 Clinical recommendations made by a panel of European experts who met in Rome, Italy, in March 2012, endorse surgery in symptomatic patients and asymptomatic patients with documented tumor growth.Citation41 The panel also recommends everolimus (Afinitor®, Novartis, East Hanover, NJ, USA) for the treatment of adults and children ≥3 years of age with SEGA associated with TSC who require therapeutic intervention but are not amenable to surgery.Citation41 Contraindications to surgery include an inability to tolerate anesthesia and cases in which the risks of surgery outweigh the benefits (total resection is unachievable or poses significant risk to the patient).Citation41 Endoscopic procedures are associated with lower morbidity, but they are limited to lesions with a diameter ≤2 cm.Citation32 Gamma Knife stereotactic radiosurgery has been used, but its role in treating SEGA has not been clearly defined because efficacy and safety data are sufficient to support its use.Citation31,Citation37 Standard chemotherapy is not advised owing to the fact that therapeutic efficacy data are lacking and risk of remote, second malignancies is increased.Citation42
Angiomyolipomas
Appropriate management of angiomyolipomas is determined by lesion size and symptoms. An association between angiomyolipoma size (>4 cm), aneurysm size (≥5 mm), and risk of hemorrhage has been noted.Citation13 Embolization is preferred in the setting of recent or active hemorrhage, or when large or multiple aneurysms are present. Embolization may require multiple procedures over time and is associated with modest reductions in tumor bulk.Citation43 Complications of embolization include a condition called postembolization syndrome, which is characterized by pain, fever, and malaise resulting from the presence of necrotic tissue in the retroperitoneum.Citation41 This can be prevented by the use of prophylactic steroids.Citation44
Skin lesions
Currently, treatments for skin lesions such as facial angiofibromas include dermabrasion, cryosurgery, curettage, chemical peeling, electrodesiccation, excision, and laser therapy; however, repeated treatment is often needed because lesions may recur.Citation5,Citation14
Pulmonary LAM
Bronchodilators are used to help control symptoms in patients with reversible air flow obstruction. Other treatment approaches include oxygen support and treatment with estrogen antagonists; however, effective treatment options for this condition are lacking.Citation1,Citation45
Pharmacology and mode of action of everolimus
Pharmacodynamics
The pivotal role of the mTOR pathway in the etiology of TSC and its associated conditions provides a strong rationale for the use of mTOR inhibition as a targeted therapy. Everolimus is a rapamycin derivative that inhibits the mTOR pathway by acting on mTORC1 ().Citation46,Citation47 It binds to FKBP-12, forming an inhibitory complex with mTORC1, thereby inhibiting mTOR kinase activity and downstream pathways.Citation46,Citation48,Citation49 Everolimus reduces the phosphorylation of downstream effectors of mTOR, such as the translational repressor eukaryotic elongation factor 4E binding protein and the S6 ribosomal protein kinase 1, which are involved in protein translation.Citation46,Citation50,Citation51
Pharmacokinetics
In healthy volunteers, everolimus was rapidly absorbed within 30 minutes and reached a maximum blood concentration of 44.2 ± 13.3 μg/L after a single 4 mg oral dose (time to reach maximal concentration [Tmx] = 0.5 hour [range 0.5–1 hour]) ().Citation52 In adult patients with solid tumors, once-daily dosing with 5 mg or 10 mg of everolimus resulted in rapid absorption within 1 hour.Citation53 The mean (standard deviation) maximum steady-state blood concentration was 32 ng/L (9 ng/L) and 61 ng/L (17 ng/L) after the 5 mg and 10 mg dose, respectively. The median (range) time to maximum serum concentration at the 5 mg and 10 mg dose was 1 hour (1 hour) and 1 hour (1–6 hours), respectivelyCitation53 For these same doses, the mean (standard deviation) area under the curve at steady state was 238 ng·h/mL (77 ng·h/mL) and 514 ng·h/mL (231 ng·h/mL), respectivelyCitation53 In healthy human subjects, a high-fat meal delayed the median time to maximum concentration by 1.25 hours (median) and reduced the maximum serum concentration by 60% and the area under the concentration–time curve by 16%.Citation54 Therefore, everolimus should be consistently administered with or without food to avoid fluctuations in therapeutic drug.
Table 2 Pharmacokinetic properties of everolimus in various patient populations
Everolimus is a substrate of CYP3A4 and P-glycoprotein. Therefore, administration of CYP3A4 inhibitors or inducers affects its metabolism.Citation46 A comparison of the relative amount of parent compound in blood after 1.5 mg/kg oral and 2-hour 1.0 mg/kg intravenous administration of everolimus suggests that intestinal metabolism (first-pass effect) accounts for a large percentage of metabolism.Citation55
Everolimus is eliminated mainly in bile and, to a lesser extent, in urine.Citation52 The mean elimination half-life is approximately 30 hours. In healthy subjects, the elimination half-life after a 4 mg dose of everolimus was 32.2 ± 6 hours.Citation46,Citation52 Hepatic impairment increases exposure of everolimus.Citation46,Citation52 The extent of exposure depends on whether hepatic impairment is mild, moderate, or severe. Dose reductions should be implemented in TSC patients with SEGA and/or renal angiomyolipoma and mild to moderate hepatic impairment.Citation46 If the benefit outweighs the risk, reductions in doses of everolimus are warranted in TSC patients with severe hepatic impairment and renal angiomyolipoma; however, in TSC patients with SEGA and severe hepatic impairment, everolimus should be avoided.Citation46
Clinical efficacy
The efficacy of everolimus in treating TSC patients was first demonstrated in an open-label Phase I/II clinical trial in 28 patients with SEGA associated with TSC (NCT00411619).Citation56 The core treatment phase lasted 6 months, after which patients could transition into an extension phase. This trial led to US Food and Drug Administration approval of everolimus for the treatment of SEGA in TSC patients; it was the first trial to show marked reductions in SEGA volume within the first 3 months of treatment that were sustained as long as therapy continued.Citation56 Patients (median age 11 years [range 3–34 years]) received everolimus initiated at a dose of 3 mg/m2 and subsequently adjusted to whole blood trough concentrations of 5–15 ng/mL.Citation56 The median duration of treatment was 21.5 months (range 4.7–34.4 months).Citation56 Clinically meaningful reductions in the volume of primary SEGA, as assessed by independent central review, were achieved, and the median reduction was −0.80 cm3 (95% confidence interval [CI]: 0.4–1.2; P < 0.001).Citation56 No new lesions, evidence of increased intracranial pressure, worsened hydrocephalus, or need for surgical resection or any other therapy was reported. Everolimus was associated with a significant reduction in the overall frequency of clinical and subclinical seizures (median change −1 seizure; P = 0.02).Citation56 Quality of Life in Childhood Epilepsy scores improved over time (58.74 ± 14 at baseline; 63.4 ± 12.4 at 3 months; 62.1 ± 14.2 at 6 months).Citation53 This trial continues in the extension phase, and results for patients treated up to 3 years were recently reported by Krueger et al.Citation57 Of the original 28 patients enrolled 25 were continuing treatment at the data cutoff for the 3-year extension studyCitation57 After a median exposure of 34.2 months, the primary SEGA volume was reduced from 1.74 cm3 at baseline to 0.97 cm3 at 36 months.Citation57 Reductions ≥30% from baseline at 24 months, 30 months, and 36 months were seen in 79.2%, 64.7%, and 77.8% of patients, respectively, and reductions ≥50% were seen in 50.0%, 41.2%, and 55.6% of patients, respectivelyCitation57 At 24 months, improvements in facial angiofibromas compared with the previous visit were observed in eight of nine patients (88.9%).Citation57 A subgroup analysis of patients enrolled in this trial reported a significant change in white matter diffusion in patients treated with everolimus, suggesting that treatment alters the genetic defects in normal-appearing white matter in the brain of TSC patients.Citation58 Significant changes in fractional anisotropy and radial diffusivity were observed in the corpus callosum, internal capsule, and geniculocalcarine regions of patients treated with everolimus, and no changes were observed in the age- and gender-matched control group of patients with TSC.Citation58 Mean (95% CI) fractional anisotropy significantly increased by 0.04 (0.019–0.062) in all three brain regions after 12–18 months of everolimus treatment (P < 0.05), and this was driven by a decrease in radial diffusivity in the corpus callosum and the geniculocalcarine tract (P < 0.01 for each).Citation58
A Phase III, randomized double-blind placebo-controlled trial, A Randomised Double-blind Placebo-controlled Study of Everolimus in the Treatment of Patients With Subependymal Gaint Cell Astrocytomas (SEGA) Associated With Tuberous Sclerosis Complex (TSC) (EXIST-1)(NCT 00789828Citation59), investigated the efficacy of everolimus versus placebo in 117 TSC patients with SEGA ().Citation36 Patients were randomized to everolimus 4.5 mg/m2 per day (n = 78) or placebo (n = 39), and baseline demographics and disease characteristics were generally well balanced with the exception of a higher proportion of males and the presence of hydrocephalus in the everolimus group.Citation36 The median age of patients was 9.5 years (range 0.8–26.6 years), and 51% and 64% of patients in the everolimus and placebo arms, respectively, had one target SEGA lesion; 44% and 36% of patients in the everolimus and placebo arms, respectively, had two SEGA lesions.Citation36 One or more skin lesions were present in 110 patients (92% [n = 72] of patients in the everolimus arm and 97% of patients in the placebo arm [n = 38]), and 38% (n = 30) of patients in the everolimus arm and 36% (n = 14) of patients in the placebo arm had one or more angiomyolipomas.Citation36 After a median follow-up of 9.7 months, the SEGA response rate—the proportion of patients with a reduction in sum of volumes of target SEGA lesions ≥50% relative to baseline in the absence of nontarget lesion worsening, new lesions ≥1 cm in diameter, or new/worsening hydrocephalus—was 35% (95% CI: 24%–46%) in the everolimus group and 0% (95% CI: 0%–9%) in the placebo group (difference in response rate 35%, 95% CI: 15%–52%; P < 0.0001).Citation36
Table 3 Efficacy and safety analyses from EXIST-1 and EXIST-2
The median time to SEGA progression was not reached in either treatment arm, and estimated progression-free rates at 6 months were 100% for everolimus and 86% for placebo.Citation36 Analysis of change in seizure frequency was inconclusive—a fact that was attributed to the method of assessment, a single 24-hour video electroencephalography, which likely limited the number of evaluable patients. The median change from baseline to week 24 in seizure frequency was 0 in both treatment arms (P = 0.2004), yielding an inconclusive analysis, as most patients had no seizures at baseline or follow-up.Citation36 Thirty patients (42%) in the everolimus arm and four patients (11%) in the placebo arm had a skin lesion response (P = 0.0004), and 16 patients (53%) had an angiomyolipoma response, defined as reduction in the sum of volumes of all target lesions ≥50% relative to baseline; no patient in the placebo arm had a response.Citation36
In another large, Phase III, randomized, double-blind, placebo-controlled—A Randomized, Double-blind, Placebo-controlled Study of RAD0001 in the Treatment of Angiomyolipoma in Patients With Either Inberous Sclerusis Complex (TSC) or Sporadic Lymphangioleiomysmatosis (LAM) (EXIST 2)—the efficacy of everolimus in treating angiomyolipoma associated with TSC or sporadic LAM was assessed in 118 patients.Citation60 Patients (median age 31 years [range 18.0–61.0 years]) were randomized to receive everolimus 10 mg per day (n = 79) or placebo (n = 39).Citation60 The percentages of patients with angiomyolipoma lesions with longest diameter ≥4 cm but not larger than 8 cm in the everolimus and placebo arms were 57% and 49%, respectively.Citation60 Of note, lesions ≥4 cm are at risk for hemorrhagic rupture. SEGAs were noted as a major feature in a higher percentage of everolimus patients (54%) compared with placebo patients (36%) at baseline, but patient demographics were well balanced between the treatment arms and were generally representative of disease epidemiology.Citation60 Skin lesion response was determined for the 114 patients with one or more skin lesions at baseline.
The angiomyolipoma response rate, defined as the proportion of patients with confirmed ≥50% reductions in the sum of volumes of all target angiomyolipomas relative to baseline, was 42% (95% CI: 31%–53%) for everolimus compared with 0% (95% CI: 0%–9%) for placebo, translating to a significant difference of 42% (95% CI: 23.5%–58.4%; P < 0.0001).Citation60 A higher proportion of patients in the everolimus arm had a ≥50% reduction from baseline in the sum of volumes of target angiomyolipoma lesions at week 24 (55% versus 0%).Citation60 Similarly, a higher proportion of patients in the everolimus arm had a reduction in the sum of volumes of target angiomyolipoma lesions ≥30% at this same time point (80% versus 3%).Citation60 The median time to angiomyolipoma response with everolimus was 2.9 months.Citation60
The everolimus arm had a significantly higher skin lesion response rate: response rates were 26% (95% CI: 17%–37%) in the everolimus arm and 0% (95% CI: 0%–10%) in the placebo arm (P = 0.0002).Citation60 Time to angiomyolipoma progression was significantly longer with everolimus versus placebo (hazard ratio: 0.08 [95% CI: 0.02–0.37]), and progression-free rates for everolimus versus placebo, respectively, were 98% (95% CI: 89%–100%) versus 83% (95% CI: 65%–93%) at 6 months, and 92% (95% CI: 65%–98%) and 25% (95% CI: 1%–64%) at 12 months.Citation60 The median time to angiomyolipoma progression was 11.4 months for placebo and was not reached for everolimus.Citation60
Safety and tolerability
In all three clinical trials, everolimus yielded a comparable safety profile: adverse events (AEs) were generally mild and grade 1 or 2 in severity, as assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events.Citation36,Citation57,Citation60,Citation61
Stomatitis (79%) and upper respiratory tract infection (79%) accounted for the majority of AEs in the Phase I/II trial; grade 3 AEs were reported in ten patients, and one grade 4 event (convulsion) occurred in one patient.Citation61 No consistent laboratory abnormalities were noted, with the exception of increases in the concentrations of total cholesterol, low-density lipoprotein cholesterol, and triglycerides.Citation61 The safety profile in the extension phase continues to be consistent with that reported in the original analysis, and no patient discontinued treatment because of an AE.Citation57 No patients discontinued because of AEs in EXIST-1, and few patients discontinued because of AEs in EXIST-2.Citation36,Citation60 Reasons for discontinuation in the everolimus arm included one case of grade 2 blood phosphorus decrease; one patient with concurrent grade 3 hypersensitivity, grade 3 angioedema, and grade 3 bronchospasm; and one patient with convulsion due to intractable seizures deemed not related to study drug.Citation60 In the EXIST-1 study, the most common AEs in the everolimus versus placebo groups, respectively, were mouth ulceration (32% versus 5%), stomatitis (31% versus 21%), convulsion (23% versus 26%), and pyrexia (22% versus 15%).Citation36 The most common grade 3 AEs in the everolimus versus placebo group were stomatitis (8% versus 3%), pyrexia (6% versus 0%), and convulsion (5% versus 5%).Citation36 In the EXIST-2 study, the most common AEs in the everolimus versus placebo group, respectively, were stomatitis (48% versus 8%), nasopharyngitis (24% versus 31%), acne-like skin lesions (22% versus 5%), and headache (22% versus 18%).Citation60 The most common grade 3 AEs in the everolimus versus placebo group were aphthous stomatitis (3% versus 0%) and mouth ulceration (3% versus 0%).Citation60 Grade 4 events were rare in both studies. Secondary amenorrhea was observed in EXIST-1 and EXIST-2. In EXIST-1, three of eight everolimus-treated and no placebo-treated female patients experienced amenorrhea.Citation36 In EXIST-2, seven of 52 everolimus-treated females and one of 26 placebo-treated females experienced amenorrhea.Citation60 As a result, amenorrhea is being further investigated as a potential AE associated with everolimus.
Biomarkers
Loss of the TSC1 or TSC2 gene has been associated with increased production of vascular endothelial growth factor (VEGF), and inhibitors of mTOR have been shown to have an inhibitory effect on production of VEGF, tumor growth, and angiogenesis, both in vitro and in vivo.Citation62,Citation63
The effect of everolimus on several angiogenic biomarkers is being assessed in the EXIST-1 and EXIST-2 trials. Compared with placebo, a sustained ~30% and ~60% increase in VEGF-A was observed in the everolimus arm of EXIST-1 and EXIST-2, respectively.Citation64 A concomitant decrease in collagen type IV (~25% EXIST-1; ~45% EXIST-2) and soluble VEGF receptor (sVEGFR2) (~25% both trials) was observed in the everolimus arm.Citation64 A sustained decrease (~60%) in VEGF-D was observed in the everolimus arm of EXIST-2, but not in EXIST-1.Citation64 In both studies, no change was observed in placental growth factor, sVEGFR1, or c-Kit plasma concentrations in the everolimus arm or in any biomarkers evaluated in the placebo arm.Citation64 Baseline sVEGFR2 and VEGF-D were ~40% and ~four-fold higher, respectively, while VEGF-A was ~50% lower in EXIST-2 compared with in EXIST-1.Citation64 A similar baseline plasma concentration for the other biomarkers was noted in both studies.Citation64 The inclusion of biomarkers in future clinical trials may provide new predictive or prognostic biomarkers when evaluating disease burden and everolimus efficacy and safety.
Conclusion
Everolimus is approved for the treatment of adults with renal angiomyolipoma and TSC not requiring immediate surgery, and of adults and children ≥3 years of age with SEGA associated with TSC who require therapeutic intervention but are not candidates for curative surgical resection. The therapeutic efficacy and safety of everolimus have been demonstrated in multicenter, international, randomized, double-blind, placebo-controlled trials. These studies continue in their extension phases with the goal of establishing the long-term safety and efficacy profile of everolimus in patients with TSC. Everolimus, an orally administered mTOR inhibitor, is the first pharmacological therapy approved for the treatment of TSC. Targeted systemic therapies such as everolimus are particularly useful in that they treat the multiple manifestations of TSC and offer patients both a means of symptom relief and a treatment alternative to invasive surgical procedures.
Acknowledgments
Dr Franz acknowledges ApotheCom for medical editorial assistance, and Novartis for supporting this trial and for funding medical editorial assistance.
Disclosure
Dr Franz has received honoraria and travel support from Novartis and Lundbeck. His institution, Cincinnati Children’s Hospital, has received research support and consulting fees from Novartis.
References
- FranzDNBisslerJJMcCormackFXTuberous sclerosis complex: neurological, renal and pulmonary manifestationsNeuropediatrics201041519920821210335
- TurnerSGPetersKBVredenburghJJDesjardinsAFriedmanHSReardonDAEverolimus tablets for patients with subependymal giant cell astrocytomaExpert Opin Pharmacother201112142265226921806479
- HallettLFosterTLiuZBliedenMValentimJBurden of disease and unmet needs in tuberous sclerosis complex with neurological manifestations: systematic reviewCurr Med Res Opin20112781571158321692602
- BuddeKGaedekeJTuberous sclerosis complex-associated angiomyolipomas: focus on mTOR inhibitionAm J Kidney Dis201259227628322130643
- SchwartzRAFernandezGKotulskaKJozwiakSTuberous sclerosis complex: advances in diagnosis, genetics, and managementJ Am Acad Dermatol200757218920217637444
- CuratoloPBombardieriRJozwiakSTuberous sclerosisLancet2008372963965766818722871
- BisslerJJKingswoodJCRenal angiomyolipomataKidney Int200466392493415327383
- CrinoPBNathansonKLHenskeEPThe tuberous sclerosis complexN Engl J Med2006355131345135617005952
- AdriaensenMESchaefer-ProkopCMStijnenTDuyndamDAZonnenbergBAProkopMPrevalence of subependymal giant cell tumors in patients with tuberous sclerosis and a review of the literatureEur J Neurol200916669169619236458
- GohSButlerWThieleEASubependymal giant cell tumors in tuberous sclerosis complexNeurology20046381457146115505165
- ShepherdCWGomezMRLieJTCrowsonCSCauses of death in patients with tuberous sclerosisMayo Clin Proc19916687927961861550
- DixonBPHulbertJCBisslerJJTuberous sclerosis complex renal diseaseNephron Exp Nephrol20111181e15e2021071977
- YamakadoKTanakaNNakagawaTKobayashiSYanagawaMTakedaKRenal angiomyolipoma: relationships between tumor size, aneurysm formation, and ruptureRadiology20022251788212354988
- HofbauerGFMarcollo-PiniACorsencaAThe mTOR inhibitor rapamycin significantly improves facial angiofibroma lesions in a patient with tuberous sclerosisBr J Dermatol2008159247347518547304
- SeibertDHongCHTakeuchiFRecognition of tuberous sclerosis in adult women: delayed presentation with life-threatening consequencesAnn Intern Med20111541280681321690595
- YatesJRTuberous sclerosisEur J Hum Genet200614101065107316868562
- CostelloLCHartmanTERyuJHHigh frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complexMayo Clin Proc200075659159410852420
- DaboraSLJozwiakSFranzDNMutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organsAm J Hum Genet2001681648011112665
- SancakONellistMGoedbloedMMutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: genotype:phenotype correlations and comparison of diagnostic DNA techniques in tuberous sclerosis complexEur J Hum Genet200513673174115798777
- AuKSWilliamsATRoachESGenotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United StatesGenet Med2007928810017304050
- HuangJManningBDThe TSC1-TSC2 complex: a molecular switchboard controlling cell growthBiochem J2008412217919018466115
- FranzDNAgricolaKDTudorCAKruegerDAEverolimus for tumor recurrence after surgical resection for subependymal giant cell astrocytoma associated with tuberous sclerosis complexJ Child Neurol201328560260722805244
- LiYInokiKGuanKLBiochemical and functional characterizations of small GTPase Rheb and TSC2 GAP activityMol Cell Biol200424187965797515340059
- DowlingRJTopisirovicIFonsecaBDSonenbergNDissecting the role of mTOR: lessons from mTOR inhibitorsBiochim Biophys Acta20101804343343920005306
- JentarraGMRiceSGOlfersSSaffenDNarayananVEvidence for population variation in TSC1 and TSC2 gene expressionBMC Med Genet2011122921345208
- FranzDNEverolimus: an mTOR inhibitor for the treatment of tuberous sclerosisExp Rev Anticancer Ther201111811811192
- van EeghenAMBlackMEPulsiferMBKwiatkowskiDJThieleEAGenotype and cognitive phenotype of patients with tuberous sclerosis complexEur J Hum Genet201220551051522189265
- JansenACSancakOD’AgostinoMDUnusually mild tuberous sclerosis phenotype is associated with TSC2 R905Q mutationAnn Neurol200660552853917120248
- KruegerDNorthrupH2012TSC Clinical Consensus Conference: summary of updates diagnosis, treatment and surveillance guidelinesSilver Spring, MDTuberous Sclerosis Alliance2012
- BerhoumaMManagement of subependymal giant cell tumors in tuberous sclerosis complex: the neurosurgeon’s perspectiveWorld J Pediatr20106210311020490765
- RoachESDiMarioFJKandtRSNorthrupHTuberous Sclerosis Consensus Conference: recommendations for diagnostic evaluation. National Tuberous Sclerosis AssociationJ Child Neurol199914640140710385849
- BeaumontTLLimbrickDDSmythMDAdvances in the management of subependymal giant cell astrocytomaChilds Nerv Syst201228796396822562196
- SunPKohrmanMLiuJGuoARogerioJKruegerDOutcomes of resecting subependymal giant cell astrocytoma (SEGA) among patients with SEGA-related tuberous sclerosis complex: a national claims database analysisCurr Med Res Opin201228465766322375958
- CucciaVZuccaroGSosaFMongesJLubieniekyFTaratutoALSubependymal giant cell astrocytoma in children with tuberous sclerosisChilds Nerv Syst200319423224312715190
- SunPKruegerDLiuJGuoARogerioJKohrmanMSurgical resection of subependymal giant cell astrocytomas (SEGAs) and changes in SEGA-related conditions: a US national claims database studyCurr Med Res Opin201228465165622375957
- FranzDNBelousovaESparaganaSEfficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trialLancet2013381986112513223158522
- CampenCJPorterBESubependymal giant cell astrocytoma (SEGA) treatment updateCurr Treat Options Neurol201113438038521465222
- de RibaupierreSDorfmüllerGBulteauCSubependymal giant-cell astrocytomas in pediatric tuberous sclerosis disease: when should we operate?Neurosurgery2007601838917228255
- AminSCarterMEdwardsRJThe outcome of surgical management of subependymal giant cell astrocytoma in tuberous sclerosis complexEur J Paediatr Neurol2013171364423183057
- LevineNBCollinsJFranzDNCroneKRGradual formation of an operative corridor by balloon dilation for resection of subependymal giant cell astrocytomas in children with tuberous sclerosis: specialized minimal access technique of balloon dilationMinim Invasive Neurosurg200649531732017163349
- JozwiakSNabboutRCuratoloPManagement of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC): clinical recommendationsEur J Paediatr Neurol201317434835223391693
- MatsumuraHTakimotoHShimadaNHirataMOhnishiTHayakawaTGlioblastoma following radiotherapy in a patient with tuberous sclerosisNeurol Med Chir (Tokyo)19983852872919640965
- SooriakumaranPGibbsPCoughlinGAngiomyolipomata: challenges, solutions, and future prospects based on over 100 cases treatedBJU Int2010105110110619493268
- BisslerJJRacadioJDonnellyLFJohnsonNDReduction of postembolization syndrome after ablation of renal angiomyolipomaAm J Kidney Dis200239596697111979340
- KohrmanMHEmerging treatments in the management of tuberous sclerosis complexPediatr Neurol201246526727522520346
- Afinitor (everolimus) tablets for oral administration [summary of product characteristics]Stein, SwitzerlandNovartis Pharma AG2012
- O’ReillyTMcSheehyPMBiomarker development for the clinical activity of the mTOR inhibitor everolimus (RAD001): processes, limitations, and further proposalsTransl Oncol201032657920360931
- LebwohlDThomasGLaneHAResearch and innovation in the development of everolimus for oncologyExpert Opin Drug Discov20116332333822647206
- HoughtonPJEverolimusClin Cancer Res20101651368137220179227
- LaneHAWoodJMMcSheehyPMmTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitorClin Cancer Res20091551612162219223496
- TanakaCO’ReillyTKovarikJMIdentifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic dataJ Clin Oncol200826101596160218332467
- KirchnerGIMeier-WiedenbachIMannsMPClinical pharmacokinetics of everolimusClin Pharmacokinet2004432839514748618
- O’DonnellAFaivreSBurrisHA3rdPhase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumorsJ Clin Oncol200826101588159518332470
- KovarikJMHartmannSFigueiredoJEffect of food on everolimus absorption: quantification in healthy subjects and a confirmatory screening in patients with renal transplantsPharmacotherapy200222215415911837553
- CroweABruelisauerADuerrLGuntzPLemaireMAbsorption and intestinal metabolism of SDZ-RAD and rapamycin in ratsDrug Metab Dispos199927562763210220493
- KruegerDACareMMHollandKEverolimus for subependymal giant-cell astrocytomas in tuberous sclerosisN Engl J Med2010363191801181121047224
- KruegerDACareMMAgricolaKTudorCMaysMFranzDNEverolimus long-term safety and efficacy in subependymal giant-cell astrocytomaNeurology201380657458023325902
- TillemaJMLeachJLKruegerDAFranzDNEverolimus alters white matter diffusion in tuberous sclerosis complexNeurology201278852653122262746
- Novartis PharmaceuticalsEfficacy and Safety of Everolimus (RAD001) in Patients of All Ages With Subependymal Giant Cell Astrocytoma Associated With Tuberous Sclerosis Complex (TSC)(EXIST-1) Available from http://www.clinicaltrials.gov/ct2/show/NCT00789828. NLM identifier: NCT00789828Accessed September 11, 2013
- BisslerJJKingswoodJCRadzikowskaEEverolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trialLancet2013381986981782423312829
- WanXHarkavyBShenNGroharPHelmanLJRapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanismOncogene200726131932194017001314
- El-HashemiteNWalkerVZhangHKwiatkowskiDJLoss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycinCancer Res200363175173517714500340
- DaboraSLFranzDNAshwalSMulticenter phase 2 trial of sirolimus for tuberous sclerosis: kidney angiomyolipomas and other tumors regress andPLoS One201169e2337921915260
- FranzDNKingswoodCJozwiakSEffect of everolimus on angiogenic biomarkers in patients with tuberous sclerosis complex (TSC): results From EXIST-1 and EXIST-2Poster presented at: American Society of Clinical OncologyJune 1–5, 2012Chicago, IL
- EkiciMAKumandasSPerHSurgical timing of the subependymal giant cell astrocytoma (SEGA) with the patients of tuberous sclerosis complexTurk Neurosurg201121331532421845566
- KovarikJMHartmannSFigueiredoJRouillyMPortARordorfCEffect of rifampin on apparent clearance of everolimusAnn Pharmacother200236698198512022896
- FouladiMLaninghamFWuJPhase I study of everolimus in pediatric patients with refractory solid tumorsJ Clin Oncol2007253018061812
