Abstract
Background
Many rectovaginal fistulas(RVF), especially low RVF, do not involve/penetrate the RV-septum, but due to lack of proper nomenclature, such fistulas are also managed like RVF (undertaking repair of RV-septum) and inadvertently lead to the formation of a high RVF (involving RV-septum) in many cases. Therefore, REctovaginal Fistulas, Not Involving the Rectovaginal Septum, should be Treated like Anal fistulas(RENISTA) to prevent any risk of injury to the RV septum. This concept(RENISTA) was tested in this study.
Methods
RVFs not involving RV-septum were managed like anal fistulas, and the RV-septum was not cut/incised. MRI, objective incontinence scoring, and anal manometry were done preoperatively and postoperatively. High RVF (involving RV-septum) were excluded.
Results
Twenty-seven patients with low RVF (not involving RV-septum) were operated like anal fistula[age:35.2±9.2 years, median follow-up-15 months (3–36 months)]. 19/27 were low fistula[<1/3 external anal sphincter(EAS) involved] and fistulotomy was performed, whereas 8/27 were high fistula (>1/3 EAS involved) and underwent a sphincter-sparing procedure. Three patients were excluded. The fistula healed well in 22/24 (91.7%) patients and did not heal in 2/24 (8.3%). The healing was confirmed on MRI, and there was no significant change in mean incontinence scores and anal pressures on tonometry. RV-septum injury did not occur in any patient.
Conclusions
RVF not involving RV-septum were managed like anal fistulas with a high cure rate and no significant change in continence. RV-septum injury or formation of RVF with septum involvement did not occur in any patient. The RENISTA concept was validated in the present study. A new classification was developed to prevent any inadvertent injury to the RV-septum.
Introduction
Anal fistulas are challenging clinical conditions to manage, especially because of the high rate of recurrence and risk of fecal incontinence as a result of treatment.Citation1–3 The challenge becomes higher when the fistula is anterior in a female patient. This is so because the risk of incontinence is expected to be higher in these patients as the anal sphincter at the anterior position is thin and weak in females.Citation1 Moreover, the trauma to the anal sphincter mechanism during pregnancies further weakens the sphincters in females. Therefore, anterior fistulas in females are categorized as complex fistulas even in primary fistulas (no previous recurrence), and fistulotomy is not recommended in these fistulas.Citation1,Citation4,Citation5
The management of the anterior fistula becomes even more difficult when it is communicating with the vagina or is opening near the vicinity of the vagina. Such fistulas are labeled as rectovaginal (RVF) or anovaginal (AVF) fistulas.Citation1,Citation4,Citation5 As per the existing literature, there is no difference between these fistulas except that AVF is considered a type of low RVF. Apart from this, it is assumed that both fistulas may involve the rectovaginal septum, and the management may also be similar.Citation1,Citation4,Citation5
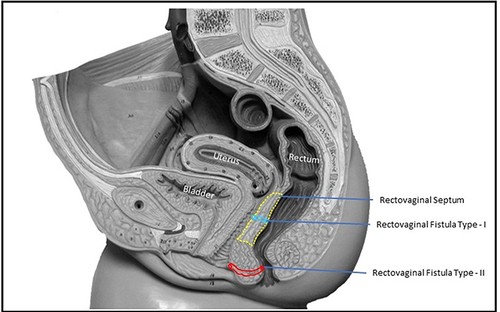
However, many low RVFs, including most AVFs and trans-perineal fistulas, do not involve or pass through the area of the rectovaginal septum but affect the anal sphincter system. If such fistulas are managed like conventional RVF with procedures that involve dissection of RV-septum [Endorectal advancement flap with or without sphincteroplasty, gracilis muscle or bulbocavernosus (Martius) flap, etc., with RV-septum repair],Citation1 then there is a risk of inadvertent injury to injury RV-septum. This is so because the RV-septum is very thin and fragile with precarious vascularity, and once an uninvolved RV-septum is operated upon, there is a high likelihood of its non-healing. In such cases, an RVF with a non-involved RV-septum gets converted to an RVF with an involved RV-septum. The latter would then be far more difficult to manage as the RV-septum is quite thin, making fistula management quite difficult. Therefore, we logically hypothesized that REctovaginal fistulas, Not Involving Septum, should be Treated like Anal fistulas (RENISTA). We, therefore, proposed a reclassification of RV fistulas, which could guide the surgeons to better and more safely manage the repair of these risky conditions () and help prevent any risk of injury to the RV-septum (). If the rectovaginal septum was involved, the fistula was categorized as RVF-I or High-RVF (RVF with RV-septum involved), and if the rectovaginal septum was not involved, the fistula was categorized as RVF-II or Low-RVF’ (RVF with RV-septum not involved)’. High or low RVF should not be confused with high or low anal fistula. Whereas the former (RVF) are classified based on RV-septum involvement, whereas the latter (anal fistulas) are classified based on the amount of involvement of the external anal sphincter.
Table 1 Classification of Rectovaginal Fistulas
Methods
The first Author of this manuscript (PG) was the single surgeon who performed all the operations reported in this paper between October 2020 and October 2023. This is an absolute strength of this paper. Moreover, preoperative and postoperative MRIs 3 months after surgery were obtained to objectively confirm healing. This is another strength of this paper. Objective fecal incontinence scoring and anal manometry were performed before surgery (baseline) and at three months after surgery (to detect any change in anal pressures).
The patients in whom the fistula was opening inside the vagina or at the vaginal introitus were short-listed for inclusion in the study. The patients suffering from Crohn’s disease or tuberculosis were excluded from the study. After careful perineal clinical examination and a detailed analysis of MRI by the joint team of the operating surgeon and the radiologist, it was determined whether the rectovaginal septum was involved or spared by the anal fistula tract. If the rectovaginal septum was involved, the fistula was categorized as RVF-1 or High-RVF (RVF with RV-septum involved). These were excluded from the study. If the rectovaginal septum was not involved, the fistula was categorized as RVF-II or Low-RVF’ (RVF with RV-septum not involved).’ These were included in the study.
Intervention
In included patients, RVF-II [Low-RVF (RVF with RV-septum not involved)], depending on the amount of anal sphincter involvement, the anal fistula could be low (<1/3 of external anal sphincter involved) or high (>1/3 of external anal sphincter involved). RVF-II with low anal fistulas were managed by fistulotomy, while RVF-II with high fistulas (involving more than one-third of the external anal sphincter) were treated by a sphincter-saving procedure. In the present study, the transanal opening of the intersphincteric space (TROPIS) procedure was performed as the sphincter-saving procedure in high fistulas.Citation6–10 RVF-1 or High-RVF (RVF with RV-septum involved) were not managed like anal fistulas but as conventionally the way RVF are managed. They were referred to a center managing a high turnover of RVFs.
TROPIS Procedure
The TROPIS procedure was performed through the transanal route, and an artery forceps was inserted into the internal opening to enter the fistula tract in the intersphincteric space.Citation6–8 Subsequently, the mucosa and the internal sphincter over the artery forceps were incised, and its edges were trimmed with electrocautery. Thus, the fistula tract in the intersphincteric space was laid open into the anal canal. This wound was left open to heal by secondary intention. The healing of the resultant wound closed the internal opening by granulation tissue (secondary intention).Citation6–8 So, the principle followed in TROPIS was that in the presence of sepsis, the healing was better and more secure with secondary intention rather than primary intention (attempting to close the internal opening by suture closure).Citation6–8The fistula tract lateral (external) to the EAS could be managed by any method convenient to the surgeon (excision or curettage with insertion of a drainage tube or laser ablation). In the present study, curettage of the external tracts with the insertion of a drainage tube was done.
The surgery was done under saddle block (spinal anesthesia). The patients were discharged from the hospital on the next day of surgery. Kegel exercises (pelvic floor exercises) were recommended to be done 50 times/day from the next day of surgery for one year. The purpose and benefits of doing KE were explained to the patients. They were counseled and motivated at every follow-up visit to perform KE regularly.
Primary Outcome Measure
Fistula healing: The fistula was diagnosed on clinical examination and confirmed on preoperative MRI. In the postoperative period (three months after surgery), the fistula was deemed healed clinically when all the external openings and tracts had closed with no pus discharge from any openings. In a fistula with multiple tracts, pus discharge from even one tract was taken as “non-healing” of the fistula. Postoperative MRI was done three months after surgery to confirm the “radiological healing” of the fistula. The fistula was taken as “healed” only when clinical healing was corroborated by ‘radiological healing.
Secondary Outcome Measure
Fecal incontinence: The baseline assessment of continence was done preoperatively by objective incontinence scores and anal manometry, and the postoperative assessment was done three months after surgery.
Injury to RV-Septum: The patients included in the study were “RVF with RV-septum not involved”. However, the injury to the RV-septum was monitored closed in the postoperative period.
The clinical objective incontinence scoring was done by Garg incontinence scores (GIS) ().Citation11,Citation12 GIS was utilized as they were scientifically better and improved the shortcomings in the previous scoring systems (Wexner’s and Vaizey’s), especially assigning equal weightage to all types of fecal incontinence.Citation11 Unlike previous scoring systems, in GIS, different weights were assigned to different types of fecal incontinence (derived by a robust statistical methodology).Citation11 The solid, liquid, flatus, mucous, stress, and urge fecal incontinence were assigned weights of 8, 8, 6, 6, 5, and 7, respectively, in GIS.Citation11 The scores for the three levels of frequency of each fecal incontinence were assigned as never = 0 (No episode of FI ever), occasional = 1 (≤1 episode of FI/ week), and common = 2 (>1 episode of FI/ week), and was termed as frequency score.Citation11 The score for each fecal incontinence would be derived by multiplying the frequency score and the weight for that fecal incontinence type. The maximum possible score was 80 (total incontinence), and the lowest score could be 0 (implied full continence) ().Citation11
Table 2 Garg Incontinence Scoring (GIS) system
Anal Manometry
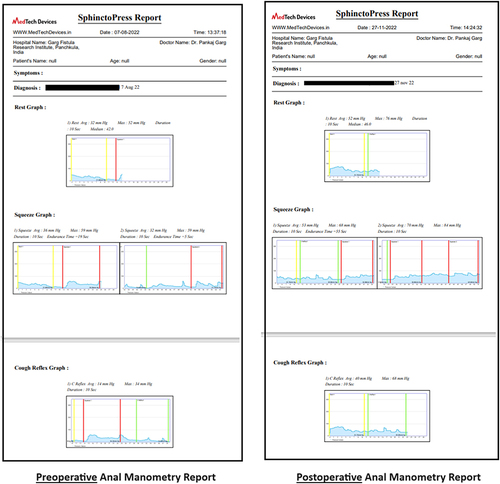
The anal manometry was performed by SphinctoPress (MedTech Devices, version 2.1.3, Baroda, Gujarat, India). The preoperative (baseline) and postoperative (after three months of surgery) measurements were taken in the outpatient clinic. The standard reference values in the study were 40–50 mm Hg (baseline anal pressure), >100 mm Hg (peak cough and squeeze pressures) ().
Figure 2 The graph of preoperative and postoperative anal manometry in a 25-year-old female patient who underwent fistulotomy for rectovaginal fistula with no rectovaginal septum involvement (RVF-II) fistula.
The study was approved by the Ethics Committee of Adesh Medical College and Hospital, Shahbad, India, via reference number AMCH/IEC/2022/02/03. The patients were informed about the purpose of the study, written informed consent was obtained, and the study was conducted in accordance with the Declaration of Helsinki.
Statistical Analysis
Categorical variables were compared by performing chi-squared or Fisher’s exact test. For normally distributed data, the continuous variables were tested using the Student’s t-test when there were two samples, and an ANOVA test was performed when there were more than two. For data that were not distributed normally, the Wilcoxon signed-rank test was performed for paired samples, and the Mann–Whitney U-test was applied for unpaired samples. The significant cut-off point was set at p<0.05.
Results
Eight hundred eighty patients presented with perianal fistula over a period of three years. Out of 880, 830 (94.4%) were anal fistula, 27 patients with RVF-II (low-RVF, not involving the rectovaginal septum but affecting the sphincter anal canal) were detected and operated like anal fistulas (), whereas during differential diagnosis, 23 RVF-I (high-RVF, fistulas involving the rectovaginal septum) were detected and were referred to other pelvic surgical departments for treatment. The etiology of rectovaginal fistulas could not be ascertained in most patients.
The mean age was 35.2 ± 9.2 years (range: 22–54 years). The median follow-up was 15 months (3–36 months). The demographics and main fistula characteristics of included patients as location, route, the participation of external anal sphincter, and surgical operations, were registered in all patients and are shown in . 19 RVF-II patients had low anal fistulas and were managed by fistulotomy, whereas 8 RVF-II patients had high anal fistulas and underwent a sphincter-sparing procedure. Three patients had short follow-ups and were excluded from the analysis. The fistula healed well in 22/24 (91.7%) patients ( and ) and did not heal in 2/24 (8.3%). The healing was confirmed on MRI in all the patients ( and ).
Table 3 Demography and Fistula Characteristics
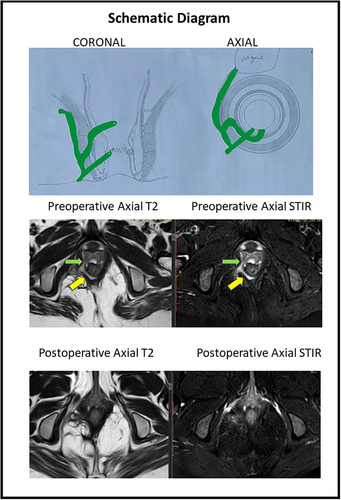
Figure 3 A 33-year old female patient with anterior horseshoe, anterior anal fistula and a RVF-II fistula. She underwent a fistulotomy. Upper panel: Schematic diagram of coronal (left side) and axial sections (right side). Middle panel: Preoperative magnetic resonance imaging - Axial sections: T2 weighted (left side) and Short Tau Inversion Recovery (STIR) (right side) (yellow arrows showing fistula tract). Lower panel: Postoperative magnetic resonance imaging- 3 months after surgery - Axial sections: T2 weighted (left side) and Short Tau Inversion Recovery (STIR) (right side showing the complete healing of the fistula.
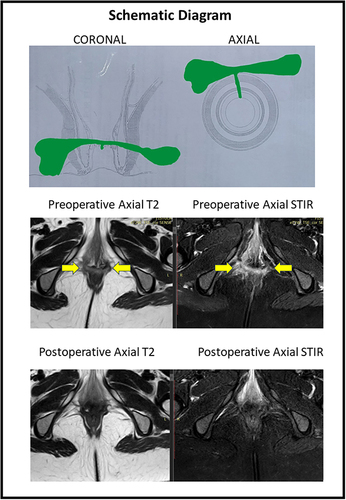
Figure 4 A 36-year old female patient with posterior horseshoe anal fistula and an RVF-II fistula opening in the left posterior vagina. She underwent the TROPIS procedure. Upper panel: Schematic diagram of coronal (left side) and axial sections (right side). Middle panel: Preoperative magnetic resonance imaging - Axial sections: T2 weighted (left side) and Short Tau Inversion Recovery (STIR) (right side) (yellow arrows showing fistula tract, green arrows showing fistula tract opening into vagina). Lower panel: Postoperative magnetic resonance imaging- 2 months after surgery - Axial sections: T2 weighted (left side) and Short Tau Inversion Recovery (STIR) (right side showing the complete healing of the fistula.
There was no significant change in objective mean incontinence scores (preoperative-1.05 ± 3.30, post-operative- 3.11 ± 4.55; p=0.06, not significant, Student’s t-test) (). The urge or flatus incontinence was present in three patients before surgery, and eight patients after surgery, but the change was not significant ((p=0.065, not-significant, Fisher’s exact test) (). The pre-operative and postoperative resting anal pressures were 25.6± 14.5 and 30.8 ± 17.2 mm Hg, respectively (p=0.53, not significant), peak squeeze anal pressures were 64.6± 32.9 and 65.3 ± 37.0 mm Hg respectively (p=0.11, not-significant), and peak cough anal pressures were 63.6± 38.1 and 63.0 ± 29.7 mm Hg respectively (p=0.95, not-significant, Student’s t-test) (). RV-septum injury or formation of RVF with septum involvement did not occur in any patient. This paper makes a meaningful contribution to the advancement of the field. It can help reduce the risks of low RVF correction and the invasiveness of the surgery. It introduces the novel concept of RENISTA, inviting the surgeon to consider and treat low RVFs like anal fistulas with good results.
Table 4 Change in Objective Incontinence Scores (Garg Incontinence Scores) After Surgery
Table 5 Change in Anal Manometry After Surgery
Discussion
Anovaginal fistula (AVF) and rectovaginal fistula (RVF) are conventionally defined as an abnormal connection between the anorectum and the vagina.Citation13 The main causative factors of RVF are obstetric trauma, Crohn’s disease, trauma, infection/ cryptoglandular infection, radiation injury, malignancy or postsurgical complications [hysterectomy, stapler misfire from low anterior resection (LAR), rectocele repair, and proctocolectomy with ileoanal pouch anastomosis].Citation1,Citation14,Citation15 The incidence of RVF varies as per etiology. RVF occurred in about 0.1% of patients who underwent episiotomy during delivery. RVF develops in 0.05% of patients who undergo a median episiotomy but in 1% of those patients who suffer from third- and fourth-degree perineal tears.Citation16,Citation17 The second most common cause of RVF is Crohn’s disease and is reported to occur between 8–10% of patients.Citation18,Citation19 Asymptomatic or low-symptomatic patients (a small minority in patients with RVF) may benefit from conservative treatment or a longer follow-up. Thus, the timing of surgery is strongly dependent on the surgeon’s experience.Citation20 The surgical treatment of RVF is challenging, and the success rate of surgical intervention across the globe is between 41–78%.Citation1,Citation21–23 In this study, most females with RVF were of middle age, in reproductive and sexually active age, all were symptomatic, and hence, there was a need for surgery. In the present study, none of the patients were in the older age group (>55 years), but RVF can also occur in older ages, especially in women with pelvic organ prolapse managed by surgery with pelvic mesh repair,Citation24 and even more in women with long-standing supporting intravaginal pessaries.Citation15
Not infrequently, the terms AVF and RVF are used interchangeably as the exact origin of the fistula from the anus or rectum cannot be differentiated distinctly in every patient. As per ASCRS’s latest guidelines, RVF and AVF are pathophysiologically same, except that AVF is a type of low RVF.Citation1 Therefore, the same management is recommended for both these fistulas, ie, AVF are managed like RVF.Citation13,Citation15
This is the first study in which the dogma of treating anovaginal fistulas like rectovaginal fistulas is challenged, intruding a new insight into this field and calling for a reclassification of RVFs because they are not all the same. When RVFs do not involve the RV septum (RVF-II), they should be treated differently, reducing the risks of the surgery. RVF-I may be managed like RVF, while RVF-II should be managed like anal fistulas. Due to this reason, in this study, it is recommended that these fistulas be classified separately as RVF-I (RVF involving RV-septum) and RVF-II (RVF not involving RV-septum). RVF-II would include most low-RVF, anovaginal, and transperineal fistulas.
The RENISTA [REctovaginal fistulas, Not Involving Septum (RVF-II), should be Treated like Anal fistulas] concept was vindicated by the results of the study in which 27 RVF-II patients were managed like anal fistula with a high success rate (91.7%) on a median follow-up of 15 months. The fistula healed in 91.7% of patients and did not heal in 8.3% (two) patients, but this recurrence rate is acceptable in RVF, which is notorious for high recurrence rate. But, importantly, RV-septum injury or the formation of RVF-I (RVF with septum involvement) did not occur in any patient (not even in two patients who had fistula recurrence), thereby vindicating the validity of the RENISTA concept.
In our study, there were 23 RVF-I patients. These cases were registered and safely differentiated from RVF-II, and they were referred to other pelvic surgery departments. It seems that RVF-II (that should be treated by anal surgeons, as the anal sphincter canal is affected) represents a significant proportion of RVF. In contrast, RVF-I presents difficult surgical management due to diversity in etiology. It seems that the most common reasons are pelvic radiation and rectal pelvic operations (more common than obstetric injuries)Citation25 and the most common operations are the diverting stoma followed by other complex pelvic plastic operations.Citation26 These operations present high failure rates, high morbidity, and a high index of reinterventions.
Against this background, the RENISTA concept assumes importance. This is so because by following RENISTA (managing RVF-II as an anal fistula), the management of this group (RVF-II) becomes simpler, and the final outcome and the prognosis can improve markedly, as demonstrated by the present study. RV-septum was not injured or involved subsequently in any of the patients in this study. This reiterates the fact that subjecting these patients to conventional RVF surgery would have led to unwarranted surgery on the RV-septum, increasing the risk of septum injury/ involvement in case the fistula recurred.
The vesicovaginal fistula is a similar challenging disease. It is difficult to manage as the vesicovaginal septum is also a very thin septum like the RV-septum. However, a good success rate has been described in managing vesicovaginal fistula by reducing the invasiveness of the surgical approach, particularly in centers of experience.Citation27
It is important that thorough assessment is pivotal before classifying RVFs as RVF-I or RVF-II. A meticulous clinical examination and MRI/ TRUS (Transrectal ultrasound) may be done to confirm the RV-septum involvement in case of any doubt. In the present study, a preoperative MRI was done on all the patients.
Our 27 patients with low RVF type II had a high success rate in surgery at 91.7%, showing the contribution of the correct anal surgery in the healing of these fistulas. After the classification of RVF in type II with the help of preoperative MRI (showing the not involvement of the RV-septum area and affection of the anal sphincter system by the fistulous track), the next step was the selection of patients for the most suitable surgical technique to achieve the highest healing rates. In this point, the correct estimation of the percentage of affected external anal sphincter determine the surgical procedure that should be performed; thus, if the length of the external anal sphincter affected by the fistulous track is <1/3 of the total length (calculation by the help of the pre-operative MRI and mainly by the anal surgeon during Examination Under Anesthesia), then fistulotomy could be performed safely. In well-selected patients, fistulotomy presents the highest healing rates (more than 95%). In our patients studied, the majority of patients (19/27-70%) with RVF-II were treated by fistulotomy. In patients with >1/3 of the external anal sphincter affected by the fistulous track (8/27-30%), a sphincter-saving procedure was performed. There are many sphincter-saving procedures in use. Our preferable choice was the TROPIS operation, which was shown to have the highest healing rates in a recent meta-analysis evaluating the effectiveness and safety of surgical operations for the treatment of anal fistulas.Citation9 There was an associated abscess in 30% (8/27) patients in the present study (), a strong and negative prognostic factor for the final healing. Thus, the high healing rates in our study reveal the effectiveness of surgical procedures performed: fistulotomy and TROPIS. The novelty and advantages in our strategy for the treatment of RVF-II fistulas are the following: a) both procedures could be easily performed, b) then no need to use other sphincter-saving procedures by the use of advanced technologic equipment, where the cost is added, c) the high healing rates more than 90%, d) the complete safety of the procedures, as there were no patients with post-operative incontinence, e) in patients with coexisting local abscesses ( and ), both procedures were suitable with excellent postoperative results, f) the only disadvantage of the TROPIS procedure was that it was a relatively new procedure in literature, not well spread worldwide.Citation6–8
Regarding the preoperative pelvic MRI, it was the most helpful examination to classify RVF as type II or I, to assess the participation of the fistulous tract in the anal sphincter system and to find any active local septic conditions (abscesses). Indeed, it was used postoperatively, at least three months after surgery, and complete healing was diagnosed in 22/24 (91.7%) of the patients. Therefore, confirming radiological healing of fistula (especially in complex fistulas) by postoperative MRI correlates quite well with long-term fistula healing rates.Citation28,Citation29
The evaluation of fecal incontinence by objective incontinence scoring and anal manometry added further strength to the study. In the present study, there was no significant deterioration in continence after the surgery. The main reason for this could be the right patient selection. The anterior anal sphincter is thin and weaker in the anterior position, especially in females. Due to this reason, the anterior fistulas (even primary fistulas) in females are categorized as “complex fistulas”, and fistulotomy is not recommended in these fistulas.Citation1,Citation4,Citation5 But in the present study, fistulotomy was performed in 19/27 patients with low fistulas and there was no deterioration in continence. This demonstrated that with appropriate patient selection, even fistulotomy can be safely done in anterior fistulas in a female patient.
Regarding the anatomy and function of the RV-septum, there are some controversies in the literature. It is known that the RV-septum divides the middle perineal compartment (uro-gynecologic) from the posterior perineal compartment (digestive). The existence of this septum may be a myth and probably reality. Many authors do not accept the existence of this septum. This anatomic structure is visible in MRI when vaginal agenesis exists in most women.Citation30 In anatomy, it is difficult to define the RV-septum; superior borders may be defined easily due to the existence of the Denonvillier’s fascia or perirectal fascia, the lower border is extended next to the perineal body, anteriorly there is the posterior vaginal wall, and posteriorly there is the perirectal fascia. In daily practice, the RV-septum is never described as an anatomic structure when a pelvic MRI is performed. At the moment, in the literature, there is no information regarding the function of the RV-septum, even though, in practice, many patients are undergoing reinforcement of the RV-septum area with synthetic meshes (posterior colporraphy). Anyway, whether it exists or does not exist in this septum, the area of this septum is shown in , which is helpful to classify RVF as type I or type II. The characteristics of these types of fistulas are clearly shown in the , with optimal surgical results in RVF type II when they are treated like anal fistula.
The study had limitations. All RVF-I found during the classification of the RVF were referred to other pelvic floor departments where RVF management was routinely done.
Conclusion
The important contribution of the present study could be that the involvement of RV-septum by RVF should be utilized to classify these fistulas as RVF-I (RVF involving RV-septum) and RVF-II (RVF not involving RV-septum). RVF-II would include most low-RVF, anovaginal, and transperineal fistulas. The RENISTA (REctovaginal fistulas Not Involving Septum are Treated like Anal fistulas) concept formed the basis of treatment. RVF-I should undergo conventional RVF treatment, while it’s the opinion of the authors that RVF-II can be safely and conveniently treated like anal fistulas and that this can prevent any accidental injury to the RV septum in type-II patients. Finally, fistulotomy can be safely performed also in anterior fistulas provided the patient selection is judiciously done. Preoperative pelvic MRI correctly classified RVF in types I and II and showed the participation of the sphincter anal canal in participation of the fistulous tract. The use of postoperative MRI diagnosed high postoperative healing rates. More data and longer follow-ups are needed in the future to reinforce and further confirm these findings.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Gaertner WB, Burgess PL, Davids JS, et al. The American society of colon and rectal surgeons clinical practice guidelines for the management of anorectal abscess, fistula-in-ano, and rectovaginal fistula. Dis Colon Rectum. 2022;65(8):964–985. doi:10.1097/DCR.0000000000002473
- Dekker L, Zimmerman DDE, Smeenk RM, Schouten R, Han-Geurts IJM. Management of cryptoglandular fistula-in-ano among gastrointestinal surgeons in the Netherlands. Tech Coloproctol. 2021;25:709–719. doi:10.1007/s10151-021-02446-3
- Halligan S, Tolan D, Amitai MM, et al. ESGAR consensus statement on the imaging of fistula-in-ano and other causes of anal sepsis. Eur Radiol. 2020;30(9):4734–4740. doi:10.1007/s00330-020-06826-5
- Ommer A, Herold A, Berg E, et al. German S3 guidelines: anal abscess and fistula (second revised version). Langenbecks Arch Surg. 2017;402(2):191–201. doi:10.1007/s00423-017-1563-z
- Vogel JD, Johnson EK, Morris AM, et al. Clinical practice guideline for the management of anorectal abscess, fistula-in-ano, and rectovaginal fistula. Dis Colon Rectum. 2016;59(12):1117–1133. doi:10.1097/DCR.0000000000000733
- Garg P, Kaur B, Menon GR. Transanal opening of the intersphincteric space: a novel sphincter-sparing procedure to treat 325 high complex anal fistulas with long-term follow-up. Colorectal Dis. 2021;23(5):1213–1224. doi:10.1111/codi.15555
- Li YB, Chen JH, Wang MD, et al. Transanal opening of intersphincteric space for fistula-in-ano. Am Surg. 2022;88(6):1131–1136. doi:10.1177/0003134821989048
- Huang B, Wang X, Zhou D, et al. Treating highly complex anal fistula with a new method of combined intraoperative endoanal ultrasonography (IOEAUS) and transanal opening of intersphincteric space (TROPIS). Wideochir Inne Tech Maloinwazyjne. 2021;16(4):697–703. doi:10.5114/wiitm.2021.104368
- Huang H, Ji L, Gu Y, Li Y, Xu S. Efficacy and safety of sphincter-preserving surgery in the treatment of complex anal fistula: a network meta-analysis. Front Surg. 2022;9:825166. doi:10.3389/fsurg.2022.825166
- Mishra S, Thakur DS, Somashekar U, Verma A, Sharma D. The management of complex fistula in ano by transanal opening of the intersphincteric space (TROPIS): short-term results. Ann Coloproctol. 2023. doi:10.3393/ac.2022.01018.0145
- Garg P, Sudol-Szopinska I, Kolodziejczak M, Bhattacharya K, Kaur G. New objective scoring system to clinically assess fecal incontinence. World J Gastroenterol. 2023;29(29):4593–4603. doi:10.3748/wjg.v29.i29.4593
- Clemente N, Singhal T, Yagnik VD. Incontinence scores (GIS): a paradigm shift in assessing fecal incontinence. Glob J Med Pharm Biomed Update. 2023;18:19–22. doi:10.25259/GJMPBU_70_2023
- Egal A, Etienney I, Atienza P. Endorectal advancement flap with muscular plication in anovaginal and anterior perineal fistulas. Ann Coloproctol. 2021;37(3):141–145. doi:10.3393/ac.2020.04.10.1
- Keighley MR, Williams NS, Keighley W. Surgery of the Anus, Rectum and Colon: Two-Volume Set. CRC Press; 2018.
- Das B, Snyder M. Rectovaginal fistulae. Clin Colon Rectal Surg. 2016;29(1):50–56. doi:10.1055/s-0035-1570393
- Homsi R, Daikoku NH, Littlejohn J, Wheeless CR Jr. Episiotomy: risks of dehiscence and rectovaginal fistula. Obstet Gynecol Surv. 1994;49(12):803–808. doi:10.1097/00006254-199412000-00002
- Wall LL, Karshima JA, Kirschner C, Arrowsmith SD. The obstetric vesicovaginal fistula: characteristics of 899 patients from Jos, Nigeria. Am J Obstet Gynecol. 2004;190(4):1011–1019. doi:10.1016/j.ajog.2004.02.007
- Schwartz DA, Loftus EV Jr, Tremaine WJ, et al. The natural history of fistulizing Crohn’s disease in Olmsted county, Minnesota. Gastroenterology. 2002;122(4):875–880. doi:10.1053/gast.2002.32362
- Radcliffe AG, Ritchie JK, Hawley PR, Lennard-Jones JE, Northover JMA. Anovaginal and rectovaginal fistulas in Crohn’s disease. Dis Colon Rectum. 1988;31(2):94–99. doi:10.1007/BF02562636
- Maeda K, Wada N, Shida A. Treatment of Rectovaginal Fistula. J Anus Rectum Colon. 2023;7(2):52–62. doi:10.23922/jarc.2023-007
- Corte H, Maggiori L, Treton X, et al. Rectovaginal fistula: what is the optimal strategy?: An analysis of 79 patients undergoing 286 procedures. Ann Surg. 2015;262(5):855–860. doi:10.1097/SLA.0000000000001461
- Halverson AL, Hull TL, Fazio VW, et al. Repair of recurrent rectovaginal fistulas. Surgery. 2001;130(4):753–757. doi:10.1067/msy.2001.116905
- MacRae HM, McLeod RS, Cohen Z, Stern H, Reznick R. Treatment of rectovaginal fistulas that has failed previous repair attempts. Dis Colon Rectum. 1995;38(9):921–925. doi:10.1007/BF02049726
- Ouaissi M, Cresti S, Giger U, et al. Management of recto-vaginal fistulas after prosthetic reinforcement treatment for pelvic organ prolapse. World J Gastroenterol. 2010;16(24):3011–3015. doi:10.3748/wjg.v16.i24.3011
- Ryoo SB, Oh HK, Ha HK, et al. Outcomes of surgical treatments for rectovaginal fistula and prognostic factors for successful closure: a single-center tertiary hospital experiences. Ann Surg Treat Res. 2019;97(3):149–156. doi:10.4174/astr.2019.97.3.149
- Hauch A, Ramamoorthy S, Zelhart M, Dobke M. Refining approaches to surgical repair of rectovaginal fistulas. Ann Plast Surg. 2020;84(5S Suppl 4):S250–S256. doi:10.1097/SAP.0000000000002207
- Mancini M, Righetto M, Modonutti D, et al. Successful treatment of vesicovaginal fistulas via an abdominal transvesical approach: a single-center 50-yr experience. Eur Urol Focus. 2021;7(6):1485–1492. doi:10.1016/j.euf.2020.06.017
- Garg P, Yagnik VD, Kaur B, Menon GR, Dawka S. Role of MRI to confirm healing in complex high cryptoglandular anal fistulas: long-term follow-up of 151 cases. Colorectal Dis. 2021;23(9):2447–2455. doi:10.1111/codi.15695
- Dawka S, Yagnik VD, Kaur B, Menon GR, Garg P. Garg scoring system to predict long-term healing in cryptoglandular anal fistulas: a prospective validation study. Ann Coloproctol. 2022. doi:10.3393/ac.2022.00346.0049
- Huebner M, Rall K, Brucker SY, et al. The rectovaginal septum: visible on magnetic resonance images of women with mayer-rokitansky-kuster-hauser syndrome (Mullerian agenesis). Int Urogynecol J. 2014;25(3):323–327. doi:10.1007/s00192-013-2214-8