?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The purpose of this study was to assess the cost-effectiveness of bazedoxifene and raloxifene for prevention of vertebral and nonvertebral fractures among postmenopausal Spanish women aged 55–82 years with established osteoporosis and a high fracture risk.
Methods
A Markov model was developed to represent the transition of a cohort of postmenopausal osteoporotic women through different health states, ie, patients free of fractures, patients with vertebral or nonvertebral fractures, and patients recovered from a fracture. Efficacy data for bazedoxifene were obtained from the Osteoporosis Study. The perspective of the Spanish National Health Service was chosen with a time horizon of 27 years. Costs were reported in 2010 Euros. Deterministic results were presented as expected cost per quality-adjusted life-year (QALY), and probabilistic results were represented in cost-effectiveness planes.
Results
In deterministic analysis, the expected cost per patient was higher in the raloxifene cohort (€13,881) than in the bazedoxifene cohort (€13,436). QALYs gained were slightly higher in the bazedoxifene cohort (14.56 versus 14.54). Results from probabilistic sensitivity analysis showed that bazedoxifene has a slightly higher probability of being cost-effective for all threshold values independent of the maximum that the National Health Service is willing to pay per additional QALY.
Conclusion
Bazedoxifene was shown to be a cost-effective treatment option for the prevention of fractures in Spanish women with postmenopausal osteoporosis and a high fracture risk. When comparing bazedoxifene with raloxifene, it may be concluded that the former is the dominant strategy.
Introduction
Osteoporosis is a frequently occurring disease in postmenopausal women, characterized by low bone mass and microarchitectural deterioration of bone tissue, resulting in increased bone fragility and fracture risk.Citation1,Citation2 Osteoporotic fractures commonly occur at the hip, spine, and forearm, with vertebral fractures being the most frequent.Citation3 Of all patients who sustain a vertebral fracture, it is estimated that 20% will suffer a new vertebral fracture within 1 year.Citation4 Of all osteoporotic fractures, hip fractures are the most serious, with an elevated mortality risk as well as a high hospital burden in Spain.Citation5
Osteoporosis has been a growing economic issue due to the increased number of fractures during the last 20 years, combined with the development of novel agents for the prevention and treatment of osteoporosis.Citation6 Aside from the economic consequences, osteoporosis also has a negative impact on quality of life for the affected individual.Citation7 The high impact of these socioeconomic consequences makes osteoporosis a high priority health problem.
Over the last decade, various new treatments for the prevention of osteoporotic fractures have been developed and approved. Although existing therapies for postmenopausal osteoporosis have been shown to be effective, they may not be appropriate for all women because of concerns related to safety and/or tolerability.Citation8,Citation9 One of the currently available therapies is raloxifene, a selective estrogen receptor modulator (SERM) that has been shown to reduce the risk of vertebral fractures in postmenopausal women.Citation10 Another selective estrogen receptor modulator, bazedoxifene, has been shown to prevent bone loss and to decrease bone turnover, with a favorable endometrial, ovarian, and breast safety profile in a 2 year, Phase III study of postmenopausal women at risk for osteoporosis.Citation11–Citation13 A 3 year, global Phase III study in osteoporotic women aged 55 years, ie, the Osteoporosis Study,Citation14 compared bazedoxifene with placebo and raloxifene. Bazedoxifene and raloxifene both reduced the risk of new vertebral fractures compared with placebo. In a post hoc subgroup analysis of patients at higher risk, bazedoxifene significantly reduced the risk of nonvertebral fractures compared with placebo and raloxifene.Citation14 Higher-risk patients were defined as women with a femoral neck T score ≤–3.0 and/or at least a moderate to severe vertebral fracture or multiple mild vertebral fractures. Many participants in the Osteoporosis Study participated in a 2 year extension study in which bazedoxifene showed sustained efficacy in preventing fractures over 5 years of therapy.Citation15
Approximately two million women were estimated to have osteoporosis in Spain in 2010.Citation16 It is important to evaluate both clinical and economic implications with the introduction of a new treatment, given that treating this population is associated with a high socioeconomic burden. Clinical aspects are normally investigated in clinical trials within a controlled setting and a limited time frame. In the case of osteoporosis, economic modeling is necessary to study the long-term consequences of fracture risk reduction beyond the time frames of clinical trials.
In Spain, several studies have investigated the socioeconomic impact of treatment of osteoporosis to the Spanish National Health Service, as well as for patients.Citation17–Citation20 Cost-effectiveness analyses of osteoporosis vary considerably between countries.Citation18 Different tools are being used to estimate fracture risk, which can significantly impact the cost-effectiveness of treatment. A recent cost-effectiveness analysis comparing bazedoxifene with placebo used the FRAX® tool (World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, Sheffield, UK) that provides fracture probabilities for specific populations.Citation18 Although FRAX can be used to predict the probability of hip or other major osteoporotic fractures, the criteria should not be generalized to other countries having different fracture incidence rates and health care costs.Citation21 Therefore, when comparing the cost-effectiveness of bazedoxifene with raloxifene in Spanish women with osteoporosis, it is important to take into account that the incidence of fractures is different between southern European countries and countries in the Scandinavian region.Citation22,Citation23 The objective of this study was to compare the cost-effectiveness of bazedoxifene and raloxifene in the prevention of vertebral and nonvertebral fractures in women diagnosed with osteoporosis. The analysis is based on the Osteoporosis StudyCitation14 and applied to the Spanish setting.
Materials and methods
Model specifications
The computer simulation model in Microsoft® (Microsoft Corporation, Redmond, WA, USA) Excel used to calculate cost-effectiveness was an updated Markov model that has been used previously to estimate the cost-effectiveness of bazedoxifene incorporating the FRAX algorithm from a European perspective.Citation18 The model represented the transition of a cohort of postmenopausal women with osteoporosis and aged 55 years through various health states with occurrence of events based on yearly probabilities. The starting age was based on women recruited for a 3 year clinical study of bazedoxifene.Citation14 The analysis was performed from the health care perspective, following all patients from initiation of treatment until they were 82 years of age and had received bazedoxifene or raloxifene for this 27-year time period. It was assumed that no patient discontinued treatment because of adverse effects.
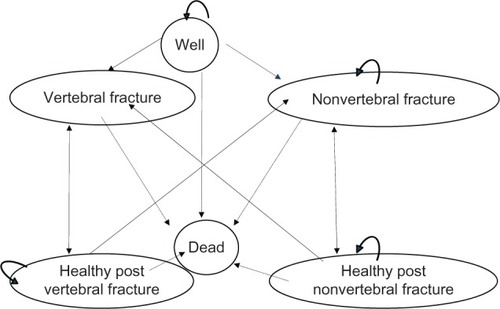
The model consisted of six health states. All patients began in the “well health” or “no event state”. In each cycle, a patient had a probability of sustaining a fracture, remaining healthy, or dying. After 1 year in any fracture state, the patient had a risk of sustaining a new fracture or dying. If a patient died, she would move to the dead-health state and remain there for the rest of the simulation. After 1 year, the patient moved to the corresponding post-fracture state if no additional fracture occurred. The patient would automatically remain in the post-fracture state (shown as a circular arrow in ) if she did not die or sustain a new fracture. Fractures could be vertebral or nonvertebral, with half consisting of hip fractures and half consisting of wrist fractures. After a nonvertebral fracture, it was possible to sustain a vertebral fracture or another nonvertebral fracture.
Target patient groups, efficacy, and side effects
The Osteoporosis StudyCitation14 was a 3-year, randomized, doubleblind, placebo-controlled and active-controlled trial including 7,492 healthy postmenopausal osteoporotic women aged 55–82 years. All women were at least 2 years postmenopausal and had osteoporosis. Osteoporosis was defined as low bone mineral density with a T score between −2.5 and −4.0, or radiographically confirmed vertebral fractures and lumbar spine and femoral neck bone mineral density T scores not worse than −4.0. Women were excluded if they had diseases that may affect bone metabolism, conditions that could interfere with bone mineral densitometry, pathologic vertebral fractures, vasomotor symptoms requiring treatment, or serious conditions (endometrial hyperplasia or carcinoma, abnormal vaginal bleeding, malignancy within 10 years of the study, endocrine disorders requiring treatment, or untreated malabsorption disorders). Women with an active or past history of deep vein thrombosis, pulmonary embolism, or retinal vein thrombosis were also excluded, as were subjects with elevated fasting total cholesterol or triglyceride levels (≥310 mg/dL or ≥300 mg/dL, respectively). Use of androgens, systemic estrogen (except for estriol 2.0 mg/day), topical estrogen (more than three times per week), progestagens, selective estrogen receptor modulators, bisphosphonates, calcitonin, parathyroid hormone, and cholecalciferol (>50,000 IU/week) was prohibited within 6 months of screening.
Subjects were assigned to treatment using a computerized randomization/enrolment system, which assigned unique randomization and package numbers. Randomization was stratified by prevalent vertebral fracture status to ensure a similar distribution of subjects with and without vertebral fractures across the treatment groups.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the clinical ethics research committee or institutional review board at each institution.
Patients were randomly assigned to each treatment group and received at least one dose of study medication, ie, bazedoxifene 20 mg daily (n = 1886), bazedoxifene 40 mg daily (n = 1872), raloxifene 60 mg daily (n = 1849), or placebo (n = 1885) for 36 months. From the total number of eligible patients, the proportion of patients completing the study was 66% for those receiving bazedoxifene 20 mg or 40 mg daily, 68% for those receiving bazedoxifene 60 mg daily, and 67% for those receiving placebo. Approximately 56% of participants in each treatment group had at least one vertebral fracture at baseline, and the majority had one mild vertebral fracture. The base-case populations in this study for the comparison of bazedoxifene and raloxifene were based on a subgroup of high-risk patients with a T score ≤−3.0 or at least one moderate fracture or multiple mild vertebral fractures. Patients receiving bazedoxifene 20 mg daily or raloxifene 60 mg daily were compared.
For osteoporotic patients without fractures, a relative risk reduction for vertebral fractures of 35% (95% confidence interval [CI] 0.32–1.30) was seen in patients treated with bazedoxifene versus 41% (95% CI 0.29–1.21) for those treated with raloxifene (). Relative risk reductions were 45% (95% CI 0.32–0.94) for bazedoxifene versus 43% (95% CI 0.34–0.97) for raloxifene in patients with previous vertebral fractures (). No differences in the incidence of nonvertebral fractures were observed between either treatment in women without prior fractures, although the reduced relative risk in high-risk patients with previous fractures was 46% with bazedoxifene and 8% with raloxifene.
Table 1 Transition probabilities for bazedoxifene 20 mg/day and raloxifene 60 mg/day
Bazedoxifene and raloxifene were associated with a number of adverse events, including leg cramps, venous thrombolytic events such as deep vein thrombosis, and breast cysts/fibrocystic breast disease.Citation14 To account for these adverse events, costs and utilities for each health state were corrected based on their incidences. The incidence of leg cramps was significantly different between the groups, with an incidence of 10.9% on bazedoxifene versus 11.7% on raloxifene (P < 0.01). The incidence of deep vein thrombosis was 0.4% in both groups and the incidence of breast cysts/fibrocystic breast disease was 0.7 in the bazedoxifene group and 1.7% in the raloxifene group (P < 0.05).
Incidence and fracture risk
Country-specific and age-specific normal population incidences were used when possible. A vertebral fracture can be classified as a clinical fracture (ie, symptomatic fractures that come to clinical attention) or as a morphometric fracture, which includes all fractures, both symptomatic and asymptomatic. The morphometric definition of a fracture was used for this study because it provided more specific incidence data, with an age-standardized incidence ratio of 10.2 (95% CI 4.7–15.7) per 1000 habitants for the female southern European population because clinical fracture data were lacking.Citation23
Incidence rates for nonvertebral fractures (ratio 24.2 [95% CI 21.70–26.70]) nonvertebral fractures per 1,000 female inhabitants) were obtained from Marín et alCitation24 and consisted mostly of wrist fractures (36.7%) and hip fractures (14.9%). Population fracture incidence rates were adjusted to reflect the risk in each treatment group.
The probability of having a new fracture, a second fracture, or remaining healthy was determined by the relative risk of vertebral or nonvertebral fractures affected by treatment with bazedoxifene or raloxifene based on the Osteoporosis StudyCitation14 ().
Mortality
Age-specific normal population mortality rates were obtained from the Spanish National Statistics Agency.Citation25 These were adjusted in the model to take into account mortality associated with fractures.Citation18 In this analysis, we derived estimates of the excess mortality after vertebral fractures from a study based on Spanish patients which showed an increase in mortality of 20%–34% within 5 years of the fracture.Citation26 The relative risk in the year after a vertebral fracture was estimated at 5.4 and was similar in subsequent years. The relative risk of mortality in the year after a nonvertebral fracture was 20.Citation27 The relative risk of excess mortality in the years subsequent to a nonvertebral fracture were estimated at 30, mostly attributable to hip fractures, although there are studies which claim there is little or no relationship between comorbid conditions and post-fracture mortality.Citation17 Based on this study, a relative risk of 10 was assumed for patients who sustained a nonvertebral fracture in subsequent years, because these not only included hip fractures but also wrist fractures.
Quality of life
Utility weights were derived from a global longitudinal study of 57,141 postmenopausal osteoporotic women aged 55 years and older that examined health-related quality of life in women who sustained fractures and the effect of fracture location on their quality of life.Citation28 Utility values were elicited using the EQ-5D® and Short-Form 36 subscales mapped to a country-specific preference-based value. The reduction in quality of life after a vertebral fracture was 38% lower than that observed in a healthy individual. Reduction in quality of life after a nonvertebral fracture estimated based on reductions for hip and wrist fractures was 39%, of which 55% was caused by hip fractures. Reduction in quality of life in the years following a vertebral fracture was 9% lower than that of a healthy individual. A 6% reduction in quality of life was estimated for hip and wrist fractures in the years following a nonvertebral fracture.
Venous thrombolytic events, primarily deep vein thrombosis, were assumed to be associated with a 10% utility loss per year based on assumptions in previous publications.Citation29,Citation30 No appropriate estimate was found for utility loss due to leg cramps and breast cysts/fibrocystic breast disease. A similar 10% decrease in quality of life was assumed for leg cramps and breast cysts/fibrocystic breast disease as for deep vein thrombosis in all health states. Based on the incidence rates of adverse events for both treatments, utilities were corrected for the decrease in quality of life associated with adverse events ().
Table 2 Utilities
Costs
Treatment costs for osteoporosis consisted of drug costs, diagnostic and follow-up tests, and physician visits. Costs were represented in 2010 Euros and discounted according to health economic guidelines, resulting in a 3% discount for costs and benefits.Citation31 Drug tariffs were derived from a Spanish drug cost database.Citation32 Drug costs for bazedoxifene were assumed to be similar to those for raloxifene. Monitoring of treatment for osteoporosis was estimated to include annual physician visit and annual bone mineral density measurement, based on other studies and expert opinion.Citation33,Citation34
Event-related fracture resource utilization was obtained by expert consultation. Vertebral fractures were assumed to be associated with 2 days of hospitalization. Outpatient treatment comprised of two imaging procedures, three specialist visits, and concomitant medication such as analgesics over 90 days. Vertebral fracture costs resulted in approximately €3878 per event.
Nonvertebral fracture costs were assumed to consist of 50% hip fractures and 50% wrist fractures. Hip fractures were associated with 15 hospitalization days and similar outpatient treatment to that for vertebral fractures, including additional rehabilitation costs during a 40-day period. Wrist fractures included four hospitalization days, surgery costs, and outpatient treatment similar to that for hip fractures, with one less imaging procedure. Nonvertebral fracture costs were estimated at €7,478 per event ().
Table 3 Osteoporosis treatment and fractures: resource utilization in units and costs
Resource utilization associated with the treatment of adverse events such as leg cramps, deep vein thrombosis, and breast cysts/fibrocystic breast disease, was added to all health states based on the treatment-related incidence and expert validation (). Treatment of leg cramps and breast cysts/fibrocystic breast disease was associated with one diagnostic test and one specialist physician visit per year. Management of deep vein thrombosis included several diagnostic tests, a specialist physician visit, and use of concomitant medication.
Table 4 Adverse events: resource utilization in units and costs
Analyses
In this study, quality-adjusted life-years (QALYs) gained was included as an effectiveness measure to allow us to compare the value of the interventions across different disease states. The incremental cost-effectiveness ratio (ICER), which is a measure of the cost per QALY gained in this study, is defined as:
where ΔC is the difference in cost between treatment with bazedoxifene and raloxifene and ΔE is the difference in effectiveness (QALYs) between each treatment. The ICER could be computed from the main outputs, cost, and QALYs in this model.
The variation in effects, in terms of both reduced fracture risk and utilities, as well as direct health care costs, was included in the probabilistic sensitivity analysis, which was done using statistical distributions to capture parameter uncertainty. We used beta and gamma distributions for probabilities and costs, respectively. The results from 1000 cohort iterations were presented as cost-effectiveness acceptability curves and as a scattered plot in the incremental cost-effectiveness plane.
Results
The base-case analysis consisted of postmenopausal women with established osteoporosis aged 55 years. Health care costs for treatment of osteoporosis and fractures per patient were similar for both treatment groups but, corrected for the incidence of adverse events, resulted in a slightly higher event cost for raloxifene than for bazedoxifene ().
Table 5 Annual cost per health state
Deterministic results using a 27-year horizon showed that the expected cost per patient was higher in the raloxifene cohort (€13,436) than in the bazedoxifene cohort (€13,381, ). The estimated gain in QALYs was slightly higher in the bazedoxifene cohort than in the raloxifene cohort (14.56 versus 14.54). The ICER showed bazedoxifene to be the dominant treatment strategy, being less costly (by €444) and more effective (+0.03 QALYs) compared with raloxifene.
Table 6 Total cost, incremental costs, QALY, QALYs gained, and ICER
Sensitivity analysis
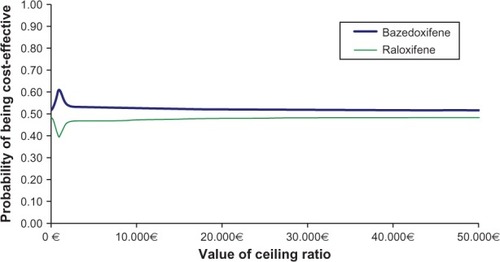
The probabilistic analysis showed a large variation in both costs and effects when introducing uncertainty around the input parameters. Cost-effectiveness acceptability curves showed that treatment with bazedoxifene had a higher probability of being cost-effective than treatment with raloxifene using alternative values up to €50,000 for the maximum willingness to pay for an additional QALY gained by the National Health Service (). If taking into account the commonly, albeit not officially, accepted willingness-to-pay threshold of €30,000 for a QALY in the health care sector in Spain,Citation35 bazedoxifene is a cost-effective option.
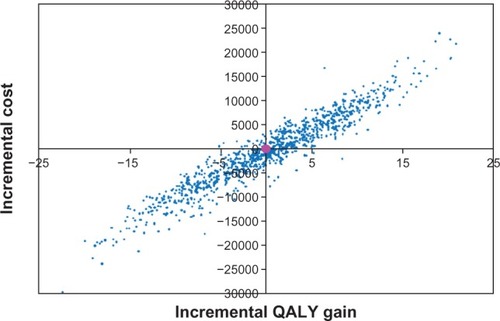
The mean incremental QALY and cost gain amounted to 0.16 and −€428, respectively, which showed that bazedoxifene was the dominant treatment strategy (). The incremental costs were scattered on both sides of the x axis, indicating that bazedoxifene generates cost savings (52% of observations were below the x axis). Fifty-one percent of the observations were located on the right of the y axis, indicating observations where the gain in QALYs was higher for bazedoxifene. According to the probabilistic sensitivity analysis, bazedoxifene generated greater health benefit in terms of QALYs gained, but at less cost.
Discussion
This study investigated the cost-effectiveness of bazedoxifene compared with raloxifene in postmenopausal Spanish women with osteoporosis using effectiveness data from the Osteoporosis Study.Citation14 The results of this study indicate that bazedoxifene was the dominant treatment strategy compared with raloxifene for the prevention of vertebral and nonvertebral fractures in high-risk postmenopausal osteoporotic women aged 55–82 years.
Probabilistic sensitivity analysis that accounted for parameter uncertainty confirmed the deterministic results. Treatment with bazedoxifene demonstrated a higher probability of being cost-effective than treatment with raloxifene up to a maximum of €50,000 for willingness to pay for an additional QALY gained.
Although no guidelines are available in Spain to determine whether an intervention can be considered cost-effective, a nonofficial threshold of €30,000 for a QALY is considered acceptable, and compares favorably with other medical and surgical procedures.Citation35 When this threshold is taken into account, bazedoxifene was a cost-effective treatment option compared with raloxifene.
Any conclusions from this study need to be placed into the context of assumptions made for this model. Important issues to consider are the epidemiology, morbidity, and mortality associated with vertebral and nonvertebral fractures, as well as adverse events arising from both treatments. These issues have been addressed as much as possible by assuming conservative scenarios or by including a probabilistic sensitivity analysis.
The general conclusions of this study are primarily based on vertebral and nonvertebral fracture outcomes and the effect of adverse events associated with both treatments. From our results, it is apparent that the effect of treatment on fracture risk and adverse events related to both treatments are important drivers for cost-effectiveness.
In the base case, treatment effects for the prevention of vertebral fractures and nonvertebral fractures with or without previous fractures were based on a head-to-head comparison of bazedoxifene with raloxifene.Citation14 Relative risk reductions for vertebral fractures were higher for the raloxifene cohort, although relative risks were lower for patients in the bazedoxifene cohort who had sustained earlier vertebral and nonvertebral fractures. Differences in relative risk reduction for nonvertebral fractures after prior fractures were larger and more favorable for bazedoxifene. No treatment effect was assumed for nonvertebral fractures in patients without fractures because the fracture incidence did not differ significantly from placebo.Citation14 Similar results were found comparing raloxifene with placebo in the Multiple Outcomes of Raloxifene Evaluation study.Citation10 If effects on nonvertebral fractures in patients without prior fractures were included, these could further improve cost-effectiveness.
Adverse events associated with both treatments were obtained from the Osteoporosis Study.Citation14 The main effect observed was a decrease in quality of life for affected patients and associated treatment costs incurred by patients. Similar findings for loss of quality of life because of adverse events were reported in studies of raloxifene.Citation10,Citation36 An increased incidence of venous thrombolytic events, primarily deep vein thrombosis, was observed in the bazedoxifene and raloxifene groups, a finding consistent with that reported in earlier studies.Citation36,Citation37 Further, bazedoxifene was associated with a lower incidence of breast cyst/fibrocystic breast disease compared with raloxifene. All adverse events were assumed to cause a 10% decrease in quality of life in the first year and subsequent years because appropriate estimates for utility loss were lacking in the literature. When the utilities were corrected for decrease in quality of life, the QALY gain was higher for the bazedoxifene cohort, leading to better cost-effectiveness. Other estimates of decrease in quality of life could influence cost-effectiveness ratios.
The incidence of breast cancer in the study reported by Silverman et alCitation14 was low for bazedoxifene and raloxifene, and no significant differences were observed in the incidence of breast cancer between the treatment groups. In the same study,Citation14 treatment with bazedoxifene was associated with fewer cases of breast cancer than treatment with raloxifene over a period of 3 years, although these results were not significant. These results contrast with previous reports that raloxifene is associated with a reduction in breast cancer risk.Citation37–Citation39 Although different studies, as mentioned before, report possible effects of bazedoxifene and raloxifene on risk of breast cancer, any decrease in quality of life due to breast cancer for the second and following years after having breast cancer, as reported by Zethraeus et al,Citation40 has not been included in this model. Including decrease in quality of life because of breast cancer, might affect the cost-effectiveness ratio, and would improve for bazedoxifene based on the lower number of cases observed, as was seen in the study reported by Silverman et al.Citation14
An important strength of this study is that data on incidence of events, post-event mortality, and costs were country-specific. Apart from its strengths, there were also several limitations to the study. We only included patients who sustained a vertebral or nonvertebral fracture, and there were no data included for patients who could have sustained multiple fractures simultaneously. Therefore, the effect of multiple fractures in terms of costs and quality of life could not be determined.
Regarding data on quality of life, a limitation of this study was the lack of references for loss of quality of life as a result of adverse events, such as leg cramps and breast cysts/fibrocystic breast disease. Decrease in quality of life because of deep vein thrombosis was based on assumptions made in previous studies,Citation28,Citation29 although supportive evidence was lacking.
The effects of poor adherence and persistence were not investigated in this study. Adherence tends to be higher in clinical trials than in clinical practice. Although data on adherence are available for raloxifene,Citation41 no data outside of clinical trials are available for bazedoxifene. Overall adherence with treatment for osteoporosis has been shown to be poor.Citation42,Citation43 As a consequence of nonoptimal persistence, the number of fractures avoided could be reduced, results in less QALY gain for the treatment population. Another effect is the reduction in intervention costs when treatment is stopped before the planned treatment duration. Therefore, less persistence could lead to less effectiveness, which might be compensated for somewhat by lower intervention costs, meaning persistence is likely to have a small effect on cost-effectiveness ratios, which is in line with the results of Jonsson et al.Citation44
Whether bazedoxifene is a cost-effective treatment depends largely on the probability of having a nonvertebral fracture, sustaining a subsequent nonvertebral fracture, and decreased quality of life due to adverse events, as well as the amount the Spanish National Health Service is willing to pay for a QALY gained. Bazedoxifene compared with raloxifene in this study was shown to fall below the threshold of €30,000 for an intervention that demonstrates typical benefits in Spain. It is important to recognize that the present study was undertaken in a Spanish setting and that the results are not automatically applicable elsewhere, given that fracture risk, mortality, and costs may differ from country to country.
Conclusion
Bazedoxifene was shown to be a cost-effective treatment option for the prevention and treatment of fractures in postmenopausal osteoporotic women with a high fracture risk in Spain. When comparing bazedoxifene with raloxifene, it may be concluded that bazedoxifene is the dominant treatment strategy. Results of probabilistic sensitivity analysis show that the choice of the optimal strategy of bazedoxifene is independent of the maximum that the Spanish National Health Service is willing to pay per additional QALY. Bazedoxifene demonstrated a slightly higher probability of being cost-effective for all threshold values.
Disclosure
Nuria Pérez-Álvarez and Lisette Kaskens are employees of BCN Health Economics and Outcomes Research, Barcelona, Spain, a consultancy hired by Pfizer Inc, to develop the economic model and the manuscript. Josep Darbà was involved as an external advisor hired by Pfrizer Inc. from the Universitat de Barcelona and responsible for the development, review of the model, comments and review of the manuscript. Susana Holgado-Pérez reports no conflict of interest in this work. Jill Racketa was an employee of Pfizer Inc, at the time of this study, and Javier Rejas is an employee of Pfizer SLU. The authors wish to thank Roger Lou, a former employee of Pfizer Inc, for his assistance in the logistic part of the study and comments and review of the manuscript. Additional editorial support was provided by Bo Choi of MedErgy and funded by Pfizer Inc.
References
- NIH Consensus Development PanelOsteoporosis prevention, diagnosis, and therapyJAMA200128578579511176917
- Koda-KimbleMAYoungLYAlldredgeBKApplied Therapeutics Clinical Use of Drugs9th edPhiladelphia, PAWolters Kluwer Health/Lippincott Williams & Wilkins2009
- SosaMGómezde Tejada MJHernándezHernández DConcepto, clasificación, factores de riesgo y clínica de la osteoporosis. [Concept, classification, risk factors and clinics of osteoporosis]Rev Esp Enf Metab Oseas200110711 Spanish
- MeltonLJIIIAtkinsonEJCooperCO’FallonWMRiggsBLVertebral fractures predict subsequent fracturesOsteoporos Int19991021422110525713
- BouzaCLópezTPalmaMAmateJMHospitalised osteoporotic vertebral fractures in Spain: analysis of the national hospital discharge registryOsteoporos Int20071864965717221295
- CooperCCampionGMeltonLJIIIHip fractures in the elderly: a world-wide projectionOsteoporos Int199222852891421796
- Sociedad Española de Investigaciones Óseas y Metabolismo Mineral (SEIOMM) Osteoporosis postmenopáusica. Guía de práctica clínica. [Spanish Society for Bone Research and Mineral Metabolism. Postmenopausal osteoporosis. Clinical practice guideline] 2003Rev Clin Esp20032031049650614563246
- MacLeanCNewberrySMaglioneMSystematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosisAnn Intern Med200814819721318087050
- LewieckiEMEmerging drugs for postmenopausal osteoporosisExpert Opin Emerg Drugs20091412914419249985
- EttingerBBlackDMMitlakBHMultiple Outcomes of Raloxifene Evaluation (MORE) InvestigatorsReduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trialJAMA199928263764510517716
- ArunBAnthonyMDunnBThe search for the ideal SERMExpert Opin Pharmacother2002368169112036407
- MillerPDChinesAAChristiansenCEffects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled studyJ Bone Miner Res20082352553518072873
- PinkertonJ VArcherDFUtianWHBazedoxifene effects on the reproductive tract in postmenopausal women at risk for osteoporosisMenopause2009161102110819546825
- SilvermanSLChristiansenCGenantHKEfficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trialJ Bone Miner Res2008231923193418665787
- de VilliersTJChinesAAPalaciosSSafety and tolerability of bazedoxifene in postmenopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled phase 3 trialOsteoporos Int20112256757620535606
- Del PinoMontes JEpidemiología de las fracturas osteoporóticas: las fracturas vertebrales y no vertebrales. [Epidemiology of osteoporotic fractures: Vertebral fractures and non-vertebral fractures]Rev Osteoporos Metab Miner20102S8S12 Spanish
- StromOBorgstromFSenSSCost-effectiveness of alendronate in the treatment of postmenopausal women in 9 European countries – an economic evaluation based on the fracture intervention trialOsteoporos Int2007181047106117333449
- BorgstromFStromOKlemanMCost-effectiveness of bazedoxifene incorporating the FRAX(R) algorithm in a European perspectiveOsteoporos Int20112295596520532482
- Instituto de Salud Carlos IIIAnálisis coste-utilidad de los tratamientos farmacológicos para la prevención de fracturas en mujeres con osteoporosis en ESPAÑA. [Cost-utility analysis of pharmaceutical treatments for the prevention of fractures in women with osteoporoisis in Spain]Informe Público de Evaluación de Tecnologías Sanitarias IPE 63/2010 [Public report for the Evaluation of Health Technologies IPE 63/2010] Available from: http://www.isciii.es/htdocs/publicaciones/documentos/63Accessed May 13, 2011 Spanish
- DarbaJRestovicGKaskensLPatient preferences for osteoporosis in Spain: a discrete choice experimentOsteoporos Int2011221947195420838770
- Sanfelix-GenovesJPeiroSSanfelix-GimenoGDevelopment and validation of a population-based prediction scale for osteoporotic fracture in the region of Valencia, Spain: the ESOSVAL-R studyBMC Public Health20101015320334639
- IsmailAAPyeSRCockerillWCIncidence of limb fracture across Europe: results from the European Prospective Osteoporosis Study (EPOS)Osteoporos Int20021356557112111017
- FelsenbergDSilmanAJLuntMIncidence of vertebral fracture in Europe: results from the European Prospective Osteoporosis Study (EPOS)J Bone Miner Res20021771672411918229
- MarínFGonzalez-MaciasJMoyaRFragility nonspinal fractures in a cohort of 5,201 women aged 65 years and older during a 3-year follow-upMed Clin (Barc)2006127401404 Spanish17020682
- Ministerio de Sanidad, Política Social eIgualdad (MSPI)Series 1981–2007 Mortalidad por causa de muerte, España y comunidades autónomas. [Series 1981–2007: Mortality by mortality cause, Spain and Autonomous Regions] Available from: http://www.msc.es/estadEstudios/estadisticas/estadisticas/estMinisterio/mortalidad/seriesTablas.htmAccessed April 26, 2011 Spanish
- Agència d′Informació, Avaluació i Qualitat en SalutGuía de Práctica Clínica sobre Osteoporosis y Prevención de Fracturas por Fragilidad. 2010. Guías de Práctica Clínica en el SNS: AATRM Nº 2007/02. [Agency of Information, Evaluation and Quality in Health. Clinical practice guideline on osteoporosis and prevention of fragility fractures. 2010. Clinical guidelines of the NHS: AATRM Nº 2007/02] Available from: http://www.gencat.cat/salut/depsan/units/aatrm/pdf/gpc_osteoporosi_aatrm2010_vcompleta.pdfAccessed May 13, 2011 Spanish
- Sociedad Navarra de Medicina de Familia y Atención PrimariaDocumento para el Manejo de la Osteoporosis en Atención Primaria (Actualización Diciembre 2006). [Navarran Society of Family Medicine and Primary Care. Report for the management of osteoporosis in Primary Care (updated December 2006)] Available from: http://www.guiasalud.es/GPC/GPC_363.pdfAccessed April 29, 2011 Spanish
- AdachiJDAdamiSGehlbachSImpact of prevalent fractures on quality of life: baseline results from the global longitudinal study of osteoporosis in womenMayo Clin Proc20108580681320634496
- SobockiPLekanderIBorgstromFStromORunesonBThe economic burden of depression in Sweden from 1997 to 2005Eur Psychiatry20072214615217194573
- ZethraeusNBorgstromFJonssonBKanisJReassessment of the cost-effectiveness of hormone replacement therapy in Sweden: results based on the Women’s Health Initiative randomized controlled trialInt J Technol Assess Health Care20052143344116262965
- PintoJLSanchezFMétodos para la evaluación económica de nuevas prestaciones. [Methods for the economic evaluation of new services]. Editado por: Centre de Recerca en Economía i Salut – Cres y Ministerio de Sanidad y Consumo, España [Edited by: Centre for Economics and Health - Cres and Ministry of Health and Consumption, Spain] Available from: http://www.msc.esAccessed April 29, 2011 Spanish
- Vademecum.es. [Drug cost database] Available from: http://www.vademecum.es/Accessed April 29, 2011 Spanish
- BorgstromFJonssonBStromOKanisJAAn economic evaluation of strontium ranelate in the treatment of osteoporosis in a Swedish setting: based on the results of the SOTI and TROPOS trials. [Methods for the economic evaluation of new services]Osteoporos Int2006171781179317009083
- JonssonBChristiansenCJohnellOHedbrandtJCost-effectiveness of fracture prevention in established osteoporosisOsteoporos Int199551361427599450
- SacristánJAOlivaJdel LlanoJPrietoLPintoJLWhat is an efficient health technology in Spain?Gac Sanit200216334343 Spanish12113733
- CummingsSREckertSKruegerKAThe effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene EvaluationJAMA19992812189219710376571
- GradyDEttingerBMoscarelliESafety and adverse effects associated with raloxifene: multiple outcomes of raloxifene evaluationObstet Gynecol200410483784415458908
- Barrett-ConnorEMoscaLCollinsPEffects of raloxifene on cardiovascular events and breast cancer in postmenopausal womenN Engl J Med200635512513716837676
- VogelVGCostantinoJ PWickerhamDLEffects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trialJAMA20062952727274116754727
- ZethraeusNJohannessonMJonssonBA computer model to analyze the cost-effectiveness of hormone replacement therapyInt J Technol Assess Health Care19991535236510507194
- ZillerVWetzelKKyvernitakisISeker-PektasBHadjiPAdherence and persistence in patients with postmenopausal osteoporosis treated with raloxifeneClimacteric20111422823520964548
- SirisESHarrisSTRosenCJAdherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databasesMayo Clin Proc2006811013102216901023
- PapaioannouAIoannidisGAdachiJDAdherence to bisphosphonates and hormone replacement therapy in a tertiary care setting of patients in the CANDOO databaseOsteoporos Int20031480881314523610
- JonssonBStromOEismanJACost-effectiveness of denosumab for the treatment of postmenopausal osteoporosisOsteoporos Int20112296798220936401
- ChristodoulouCCooperCWhat is osteoporosis?Postgrad Med J20037913313812697910
- NavesMaz-LopezJBGomezCRodriguez-RebollarARodriguez-GarciaMCannata-AndiaJBThe effect of vertebral fracture as a risk factor for osteoporotic fracture and mortality in a Spanish populationOsteoporos Int20031452052412730754
- Hospit al Lluís Alcanyis, Memoria 2000. [Lluís Alcanyis hospital, Note 2000] Available from: http://www.ctv.es/USERS/vgisbertj/home.htmlAccessed May 3, 2011 Spanish
- Hospital de la Esperanza, Tarifario 1995, Hospital de la Esperanza, Barcelona, 1995. [Esperanza Hospital, Tariffs 1995 of Hospital Esperanza, 1995] Spanish
- Instituto Nacional de la Salud, Resultados de la gestión analítica en los hospitales del INSALUD GECLIF 2000. [National Health Institute, Results of the analytical management in hospitals of INSALUD GECLIF 2000]Subdirección General de Coordinación Administrativa, Hospitales INSALUDMadrid2001 Spanish
- FinnernH WSykesD PThe hospital cost of vertebral fractures in the EU: estimates using national datasetsOsteoporos Int20031442943612730759
- CernudaCCoste de consultas externas: visitas y pruebas. [Costs of external visits: visits and tests] Todo Hospital, nº 153 Enero/Febrero1999H. Universitario Josep TruetaGirona1999 Spanish
- Diari Oficial de la Generalitat de Catalunya. Departament de Sanitat i Seguretat Social. Orden de 29 de Septiembre de 1997; núm. 2504. [Official Journal of the Generality of Catalonia. Department of Health and Social Security. Order of 29th of September 1997] Spanish
- Diari Oficial de la Generalitat de Catalunya. Institut Català de la Salut. Departament de Salut. RESOLUCIÓN SLT/383/2009, de 21 de enero, sobre la revisión de precios públicos correspondientes a los servicios sanitarios que presta el Instituto Catalán de la Salud. [Official Journal of the Generality of Catalonia. Catalonian department of health. Department of Health. Resolution SLT/383/2009, 21st of January, concerning the review of public tariffs corresponding to health services by the Catalan Health Institute]Febrero2009 núm. 5325. Available from: https://www.gencat.cat/eadop/imagenes/5325/09042029.pdfAccessed March 3, 2011 Spanish
- Boletín Oficial De La Rioja. Consejería de Salud. Resolución del Consejero de Salud por la que se dispone la publicación de las tarifas por servicios sanitarios prestados a particulares en los centros del Servicio Riojano de Salud. [Official Journal of the Rioja. Health council. Resolution by the health council by which it disposes over the publication of tariffs for health services to patients in Riojan health centers]Febrero2009 núm. 27. Available from: http://www2.larioja.org/pls/dad_user/G04.texto_integro?p_cdi_accn=443-230928Accessed March 3, 2011 Spanish