Abstract
Purpose
People with COPD have cognitive dysfunction, which is greater in those hospitalized for exacerbations than in stable outpatients. We tested the hypothesis that cognitive dysfunction at exacerbation is a disease-specific feature of COPD, rather than a nonspecific feature of hospitalization for acute illness, by comparing cognition between patients hospitalized for acute COPD exacerbations and those with worsening heart failure (HF).
Patients and methods
A total of 40 hospital inpatients were recruited, 20 patients with COPD exacerbations and 20 patients with congestive or left-sided HF. Exclusion criteria included previous stroke, known neurological disease, and marked alcohol excess. Participants completed the Montreal cognitive assessment (MoCA) and Hospital Anxiety and Depression Scale (HADS) and underwent spirometry and review of clinical records.
Results
Age (mean±SD, COPD 73±10; HF 76±11 years), acute illness severity (Acute Physiology and Chronic Health Evaluation [APACHE]-II, COPD 15.4±3.5; HF 15.9±3.0), comorbidities (Charlson index, COPD 1.3±1.9; HF 1.6±1.5), and educational background were similar between COPD and HF groups. MoCA total was significantly lower in COPD than in HF (COPD 20.6±5.6; HF 24.8±3.5, P=0.007); however, significance was lost after correction for age, sex, and pack year smoking history. When compared with HF patients, the COPD cohort performed worse on the following domains of the MoCA: visuospatial function (median [IQR], COPD 0 [1]; HF 2 [1], P=0.003), executive function (COPD 2 [1]; HF 3 [1], P=0.035), and attention (COPD 4 [3]; HF 6 [2], P=0.020). Age (P=0.012) and random glucose concentration (P=0.041) were associated with cognitive function in whole group analysis, with pack year smoking history reaching borderline significance (P=0.050).
Conclusion
Total MoCA score for COPD and HF indicated that both groups had mild cognitive impairment, although this was greater in people with COPD. Mechanisms underlying the observed cognitive dysfunction in COPD remain unclear but appear related to blood glucose concentrations and greater lifetime smoking load.
Introduction
Cognitive dysfunction is common in people with COPD. In a recent systematic review, cognitive impairment was reported to affect one in three COPD patients.Citation1 Impaired cognition in people with COPD is associated with reduced treatment adherence, impaired performance in daily activities, and increased mortality. For example, in an observational study of 157 patients referred for pulmonary rehabilitation, patients with cognitive impairment were twice as likely to fail to complete the program as those without.Citation2 In a systematic review of 13 studies assessing the impact of cognitive impairment on self-management in COPD, cognitive dysfunction was associated with an increased need for assistance in activities of daily living, reduced symptom recall, and increased errors in inhaler technique.Citation3 Understanding what drives cognitive impairment in people with COPD is therefore important in order to identify factors that could be modified to improve health outcomes.
Mild cognitive impairment refers to a decline in cognition, which presents as the intermediary stage between normal cognitive aging and dementia, independent of educational level and not severe enough to interfere with daily activities.Citation4 Longitudinal studies have reported that one-half of individuals who meet the criteria for mild cognitive impairment develop dementia within 3 years.Citation5
While the mechanisms that underlie cognitive impairment in COPD are not completely understood and are likely to be multifactorial, COPD-specific factors have been proposed.Citation6 These include hypoxemia,Citation7 disease severity,Citation8 reduced physical activity,Citation9 and exacerbations.Citation10,Citation11 Hospitalization due to the exacerbations of COPD in particular appears to be a high risk for cognitive dysfunction. A study comparing 30 COPD patients hospitalized with exacerbations, 50 stable COPD patients, and 30 age-matched healthy controls found that 50% of hospitalized patients had impaired processing speed compared to 24% of stable COPD patients and 3% of controls.Citation11 Moreover, hospitalized patients deemed medically fit for discharge had significant deficits in cognitive function, which had not improved 3 months after discharge.Citation11 In a 2-year prospective cohort study of patients hospitalized with COPD exacerbations, baseline assessment of cognitive function was significantly worse in those who died during follow-up than in those who survived.Citation12 What remains unclear is whether the cognitive dysfunction seen in hospitalized COPD patients is due to a COPD-specific effect or rather a nonspecific effect of acute illness and hospitalization itself.
The hypothesis underlying this study is that patients hospitalized due to COPD exacerbations are more likely to have cognitive impairment than those hospitalized with decompensation of other chronic illnesses, due to COPD-specific factors. To test this hypothesis, we compared the prevalence of cognitive impairment in hospitalized patients with COPD exacerbations and decompensated heart failure (HF) and investigated the relationship between cognitive impairment and clinical characteristics within disease groups.
Methods
Design
This was a prospective case–control study. All participants gave written informed consent. The study was approved by the West Midlands Research Ethics Committee (13/WM/0434).
Patient selection
Patients admitted to hospital with the exacerbations of COPD were recruited and compared with patients hospitalized with decompensated HF. HF patients were selected as a control group for comparison with COPD patients because a similarly high prevalence of cognitive impairment has been reported in both diseases.Citation1 They were also expected to be comparable in terms of age and comorbidities.
Inclusion criteria
COPD patients were identified on admission with a physician diagnosis of an acute exacerbation of COPD. Participants had symptoms of exacerbation (cough, sputum, and breathlessness), a significant smoking history (>10 pack years), and obstructive spirometry (ratio of FEV1 to FVC <0.7).
HF patients were identified on admission with a physician diagnosis of decompensated HF. Participants had edema and/or dyspnea, a prior diagnosis of HF, evidence of reduced ejection fraction, and/or diastolic dysfunction on echocardiogram.
Exclusion criteria
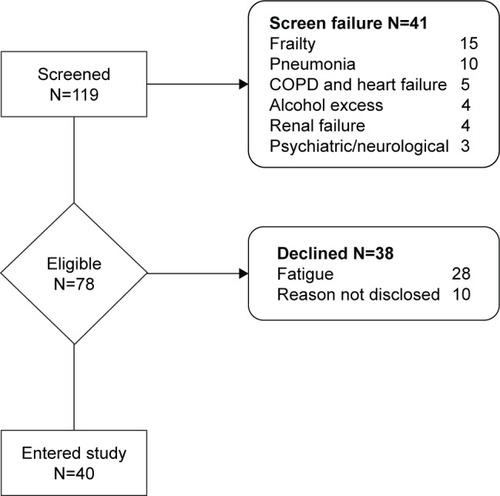
Patients were excluded if they had combined COPD and HF, asthma, pneumonia, past medical history of stroke, neurological disorder, psychiatric disorder, marked alcohol excess (male >56 U/week, female >42 U/week), or severe renal failure (>500 μmol/L creatinine) ().
Clinical measures
Two examiners performed the clinical exams to ensure coverage of the patients who came in as emergencies. Both examiners were trained to perform research techniques by the same clinical research coordinator. Both examiners assessed patients from both groups.
Evaluation of comorbidities and airflow limitation
Comorbidities were measured using the Charlson comorbidity index.Citation13 Anxiety and depression were assessed using the Hospital Anxiety and Depression Scale (HADS, maximum score 42).Citation14 The HADS is split into anxiety (21 points) and depression (21 points) subscales, and the scores are categorized as follows: 0–7 points, normal; 8–10 points, mild; 11–14 points, moderate; and 15–21 points, severe. Illness severity was measured using the Acute Physiology and Chronic Health Evaluation (APACHE-II, maximum score 71).Citation15 The burden of COPD symptoms was measured using the COPD assessment test (CAT).Citation16 The CAT scores are characterized as follows: 0–10 points, mild; 11–20 points, moderate; 21–30 points, severe; and 31–40 points, very severe, clinical impact.
Airflow limitation was measured according to standardized spirometry guidelinesCitation17 using the EasyOneTM World-spirometer device (NDD Medical Technologies). Absolute FEV1, FVC, FEV1:FVC, and percentage predicted measurements were obtained.
Cognitive status
The presence of delirium was assessed with the confusion assessment method (CAM),Citation18 a bedside diagnostic tool that consists of nine operationalized criteria from the Diagnostic and Statistical Manual of Mental disorders (DSM-III-R). The Montreal cognitive assessment (MoCA)Citation19 was used to assess the following cognitive domains: visuoconstructional skills, executive function, naming, delayed recall, attention, language, abstraction, and orientation. MoCA was scored out of a total of 30 points, and a cutoff of less than 26 was considered indicative of mild cognitive impairment.
Statistical analyses
Continuous data were described using mean ± SD, and categorical data were described as number (%) participants. Normally distributed variables were compared using the independent t-test and non-normally distributed variables by Mann–Whitney U tests. Categorical variables were compared using Chi-squared tests. Analysis of covariance (ANCOVA) was used to control for age, sex, and pack year smoking history, during the comparison of normally distributed continuous variables. Group-specific associations between MoCA and clinical measures were tested using Pearson’s correlation and corrected for age and sex. Pack year smoking history was not corrected in the correlational analysis in order to investigate its effect. Where values were not normally distributed they were log-transformed before correlations were performed. Variables, which were significantly associated with MoCA, were further analyzed using ANCOVA. The ANCOVA model tested for the following main effects: dependent variable, MoCA total; fixed factors, group (COPD/HF); covariates, age, sex, random glucose concentration, and pack year smoking history. The following interactions were also assessed – group by random glucose concentration and group by pack year smoking history. All statistical analyses were performed using IBM SPSS® (version 21.0).
Results
Patient demographics
A total of 20 COPD patients and 20 patients with HF were recruited. Demographics and clinical characteristics are compared in .
Table 1 Clinical and demographic characteristics of participants
COPD and HF patients have similar comorbidities and acute illness severity
COPD patients and HF patients were well-matched for age, comorbidities (Charlson comorbidity index), and acute illness severity (APACHE-II score). As expected, COPD patients had worse airflow obstruction, greater smoking pack years, and more respiratory symptoms (CAT) and were more likely to be receiving bronchodilators (beta-2 agonists and anticholinergics) and either inhaled or systemic corticosteroids. Conversely, HF patients were more likely to be taking cardiac medications such as diuretics, angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, and aldosterone antagonists. Interestingly, despite the differences in underlying diagnosis, both groups had elevated systemic inflammatory markers (C-reactive protein [CRP]). COPD patients had a higher circulating white blood count, which in the absence of differences in CRP is most likely due to systemic corticosteroid treatment. The groups were well-matched for other factors that might affect cognition, including alcohol consumption and sedative medication. COPD patients were significantly more anxious, but not more depressed, than patients with HF (). A total of 50% of people with HF and only 10% of people with COPD had diabetes mellitus (P=0.014). Random blood glucose did not differ between groups.
Cognitive impairment is common in COPD patients even when delirium is infrequent
A total of 15% of the patients in the COPD group showed evidence of delirium, compared to no patients in the HF group. The MoCA score was 4 points (95% CI 1–7) lower in COPD patients than in people with HF (mean ± SD, COPD 20.6±5.6; HF 24.8±3.5, P=0.007). Statistical significance was lost after controlling for age, sex, and smoking pack year history (P=0.292). Using the MoCA score <26 points as suggestive of cognitive impairment, 90% of people in the COPD group had cognitive impairment compared to 55% of people in the HF group. Reviewing MoCA sub-domains, COPD patients scored significantly lower in the cognitive domains of visuospatial function, executive function, and attention. Naming, language, abstraction, delayed recall, and orientation were not significantly different between the groups ().
Table 2 Cognitive assessment
In COPD patients, poor cognitive performance is associated with greater smoking history but not lung function or oxygen saturation
In the COPD group, MoCA score was inversely correlated with smoking pack year history (). There was no association between MoCA score and inflammatory markers (white blood cell count and CRP), hypoxemia (oxygen saturations), lung function (FEV1% predicted), illness severity (APACHE-II, CAT), anxiety and depression (HADS), or random glucose concentration.
Table 3 Correlations with MoCA in COPD
In HF patients, poor cognitive performance was associated with higher random blood glucose concentration
In people with HF, MoCA score was negatively correlated with random glucose concentration (). There was no association between MoCA score and inflammatory markers (white blood cell count and CRP), hypoxemia (oxygen saturations), lung function (FEV1% predicted), smoking pack years, illness severity (APACHE-II, CAT), or anxiety and depression (HADS).
Table 4 Correlations with MoCA in heart failure
In the whole group, age, random glucose concentration, and pack year smoking history were independently associated with cognitive function
The relationship between MoCA and underlying diagnosis (COPD or HF) was explored using an ANCOVA (). Group allocation, age, and sex were included in the model, along with factors that individually correlated with the MoCA in COPD and HF (smoking pack year history and random glucose concentration, respectively, and ). In this model, diagnosis was not a significant determinant of cognitive function. However, age (P=0.012), random glucose concentration (P=0.041), and pack year smoking history (P=0.050 – borderline statistical significance) were significantly associated with cognitive function.
Table 5 ANCOVA model of relationships with MoCA
Discussion
The aim of this study was to determine if cognitive impairment was more prevalent in people hospitalized with COPD exacerbations than in people hospitalized due to decompensated HF. We found that patients with an acute exacerbation of COPD on average scored 4 points worse on the MoCA and were significantly more likely to have cognitive impairment, defined as MoCA <26, than those with decompensated HF. Statistical differences in cognitive function between groups did not survive adjustment for age, sex, and pack year smoking history. ANCOVA in the whole group found that age, random glucose concentration, and pack year smoking history, but not underlying diagnosis (COPD or HF), were independent determinants of cognitive function.
Our findings of significant cognitive impairment in COPD patients hospitalized with exacerbations are consistent with other studies. Dodd et alCitation11 reported that people hospitalized for COPD have greater cognitive impairment than stable outpatients with COPD and age-matched controls. That study is not directly comparable to ours as hospitalized patients in the Dodd study were at the point of discharge. López-Torres et alCitation20 reported a mean MoCA total score of 19.28±2.08 points in 48 patients hospitalized for acute exacerbation of COPD at admission, which is similar to the MoCA total of COPD patients in our study at 20.6±5.6 points. Furthermore, consistent with our work, visuospatial function, executive function, and attentional deficits have previously been reported in COPD.Citation6,Citation21 Our study extends the findings of previous investigations in that we show that cognitive impairment in hospitalized COPD patients is greater than that in a hospitalized comparator group with decompensated HF.
We explored potential reasons underlying differences in cognition between hospitalized patients with COPD exacerbations or decompensated HF. Pack year smoking history differed markedly between the groups and was associated with cognitive dysfunction in COPD patients, independent of age and sex. In COPD, smoking load is significantly associated with more severe lung diseaseCitation22,Citation23 and increased risk of hospitalization.Citation24 Smoking is also a well-recognized cause of vascular disease,Citation25 which can impair cerebral perfusion, altering cognition.Citation26 Moreover, cigarette smoke is thought to contain particulates which have a direct neurotoxic effect.Citation27 Greater smoking history could therefore contribute both to an increased risk of hospitalization and to the development of cognitive impairment, explaining the association. Smoking history of >10 pack years was an inclusion criterion for the COPD group and not for the HF group, who comprised people with HF from diverse etiology. The relatively light smoking load in those with HF could be considered less deleterious to cognitive function, which could explain why correcting for its effect removed the significant difference in MoCA total between the groups, despite patients with HF being older. ANCOVA showed that smoking pack year history marginally missed significance as a predictor of cognitive impairment, possibly due to a lack of statistical power and that this was not a group dependent effect. This suggests that cognitive impairment is not COPD specific but a smoking-specific effect.
Random glucose concentration was inversely correlated with MoCA in HF but not in COPD patients. In HF patients, elevated random glucose concentration could merely have been a marker for underlying diabetes, which affected 50% patients, and is independently associated with cognitive impairment.Citation28 Mean random glucose concentration was the same in COPD patients as in HF patients, despite only 10% COPD patients having diagnosed diabetes mellitus. Elevated blood glucose is common during COPD exacerbations,Citation29 at least in part due to corticosteroid therapy.Citation30 Despite there being no significant difference in random glucose concentrations between groups, the ANCOVA revealed that random glucose concentration predicted cognitive function and that this was not diagnosis dependent. This is consistent with a larger study by Crane et al who reported that hyperglycemia may be a risk factor for dementia.Citation31 Previous studies have proposed that “overspill” of inflammation from the lungs of COPD patients into the systemic circulationCitation32 may promote a neuro-inflammatory response.Citation33 This could be particularly marked during exacerbations and drive cognitive impairment. In the present study, lack of differences in systemic inflammation (CRP) between groups and lack of associations of inflammatory markers (CRP, white cell count) with cognitive function indicated that cognitive impairment in this hospitalized COPD group was not explained by inflammation. White cell count did differ between groups, but in the absence of a difference in serum CRP, this is most likely explained by corticosteroid treatment for COPD exacerbation. Corticosteroids can have neuropsychiatric side effects. However, we have previously shown that the more marked cognitive impairment seen in hospitalized COPD patients does not resolve with recovery and cessation of corticosteroids.Citation11 Several studies have reported a relationship between hypoxia and cognitive dysfunction in COPD.Citation7,Citation34,Citation35 However, these studies are limited by variation in the definition of hypoxemia between studies, correlations are often weak, and most evidence relates to non-hospitalized COPD patients.Citation6 Although in our study, oxygen saturations were lower in COPD than HF patients, there was no association between oxygen saturations and cognitive impairment.
Limitations
Studies in hospital inpatients with acute illness are difficult to conduct because recruitment depends on unpredictable hospital admissions and patients can be too unwell to participate when ill enough to be eligible. In this context, we were able to recruit 20 patients in each group. The small size of this study means that our results need to be interpreted with caution as there is a risk of type 2 error due to insufficient power. We selected people with HF as “controls” with a chronic disease other than COPD prone to hospitalization for exacerbations. COPD and HF affect people at similar ages and are associated with a similar background prevalence of cognitive impairment.Citation1 Despite this, there were other significant differences between groups, particularly in smoking and diabetes. Matching for these characteristics would have been desirable, although difficult.
Premorbid cognition was not measured in this study, which is known to be a major determinant of cognitive function in terms of brain reserve,Citation36 so it is unclear how this might have influenced our analysis.
Cognitive impairment was measured using the MoCA. As a brief screening instrument, the MoCA has clinical utility as a general measure of cognitive impairment. Although we have analyzed the subdomains of the MoCA, which indicate that COPD patients have impairments in visuospatial function, executive function, and attention, an in-depth battery of neuropsychological tests would be required to assess this fully.
Associations between the MoCA and clinical characteristics gave some clues as to the mechanisms underlying cognitive impairment in hospitalized COPD patients. However, as this is a cross-sectional study it is not possible to attribute direction of causation.
Finally, as two examiners performed the clinical exams, inter-rater variability should have been calculated to determine the consistency and reliability of the scoring. Despite this, both examiners assessed patients from both patient groups so there was no opportunity for systematic bias to occur.
Cognitive impairment has a significant clinical impact for COPD patients. As patients hospitalized with COPD exacerbations appear particularly vulnerable to cognitive impairment, they may be the group who would benefit most from targeted interventions. Our study found that COPD patients performed significantly worse on the visuospatial, executive function and attention domains of the MoCA, compared to HF patients. Lezak et alCitation37 described executive function as the ability to formulate goals, plan achievement, and perform behaviors effectively. Impairment in this area can result in inability to utilize and adhere to medication adequately as well as inability to perform other aspects of personal management. Visuospatial function involves the perception of orientation, spatial location, direction, and distance. Impairments in this area can lead to the inability to plan a route or leaving home and getting lost. Attention requires appropriate allocation of processing resources; therefore, dysfunction in this area would result in difficulty concentrating on tasks.Citation38 Raising awareness of the existence of cognitive impairment, using a reliable tool, facilitated with support groups and health care contacts, may be the route to explore to ensure improved adherence to therapy and perhaps to reduce hospitalizations.
Conclusion
COPD patients hospitalized for exacerbations have marked cognitive impairment, which is not explained by hospitalization. Cognitive impairment is associated with pack year smoking history, rather than systemic inflammation or hypoxia, indicating that smoking could be an important underlying mechanism. In hospitalized patients with either COPD or HF, random blood glucose was associated with cognitive impairment, indicating a potential contributing role for diabetes. Awareness of the prevalence of cognitive impairment in hospitalized patients with COPD has importance for treatment adherence and discharge planning to reduce readmissions.
Disclosure
JWD reports a British Lung Foundation research grant, personal fees from Boerhinger Ingelheim, and non-financial support from NAPP Pharmaceutical, and DRB reports a National Institute for Health Research academic grant, outside the submitted work. PWJ is also employed as a Global Medical Expert by GlaxoSmithKline. The other authors report no conflicts of interest in this work.
References
- YohannesAMChenWMogaAMLeroiIConnollyMJCognitive impairment in chronic obstructive pulmonary disease and chronic heart failure: a systematic review and meta-analysis of observational studiesJ Am Med Dir Assoc2017185451.e14551.e11
- CleutjensFAHMSpruitMAPondsRWHMThe impact of cognitive impairment on efficacy of pulmonary rehabilitation in patients with COPDJ Am Med Dir Assoc201718542042628108209
- BairdCLovellJJohnsonMShiellKIbrahimJEThe impact of cognitive impairment on self-management in chronic obstructive pulmonary disease: a systematic reviewRespir Med201712913013928732820
- PetersenRCSmithGEWaringSCIvnikRJTangalosEGKokmenEMild cognitive impairment: clinical characterization and outcomeArch Neurol199956330330810190820
- GauthierSReisbergBZaudigMInternational Psychogeriatric Association Expert Conference on mild cognitive impairmentMild cognitive impairmentLancet200636795181262127016631882
- DoddJWGetovSVJonesPWCognitive function in COPDEur Respir J201035491392220356988
- GrantIHeatonRKMcSweenyAJAdamsKMTimmsRMNeuropsychologic findings in hypoxemic chronic obstructive pulmonary diseaseArch Intern Med19821428147014767103628
- KleinMGauggelSSachsGPohlWImpact of chronic obstructive pulmonary disease (COPD) on attention functionsRespir Med20101041526019748260
- SinkKMEspelandMACastroCMLIFE Study InvestigatorsEffect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the life randomized trialJAMA2015314878179026305648
- TulekBAtalayNBYildirimGKanatFSüerdemMCognitive function in chronic obstructive pulmonary disease: relationship to global initiative for chronic obstructive lung disease 2011 categoriesRespirology201419687388024935516
- DoddJWCharltonRAvan den BroekMDJonesPWCognitive dysfunction in patients hospitalized with acute exacerbation of COPDChest2013144111912723349026
- AlmagroPCalboEOchoa de EchagüenAMortality after hospitalization for COPDChest200212151441144812006426
- CharlsonMEPompeiPAlesKLMacKenzieCRA new method of classifying prognostic comorbidity in longitudinal studies: development and validationJ Chronic Dis19874053733833558716
- ZigmondASSnaithRPThe hospital anxiety and depression scaleActa Psychiatr Scand19836763613706880820
- KnausWAZimmermanJEWagnerDPDraperEALawrenceDEAPACHE-acute physiology and chronic health evaluation: a physiologically based classification systemCrit Care Med1981985915977261642
- JonesPWHardingGBerryPWiklundIChenWHKline LeidyNDevelopment and first validation of the COPD Assessment TestEur Respir J200934364865419720809
- MooreVCSpirometry: step by stepBreathe201283232240
- InouyeSKvan DyckCHAlessiCABalkinSSiegalAPHorwitzRIClarifying confusion: the confusion assessment method. A new method for detection of deliriumAnn Intern Med1990113129419482240918
- NasreddineZSPhillipsNABédirianVThe Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairmentJ Am Geriatr Soc200553469569915817019
- López-TorresIValenzaMCTorres-SánchezICabrera-MartosIRodriguez-TorresJMoreno-RamírezMPChanges in cognitive status in COPD patients across clinical stagesCOPD201613332733226667660
- Antonelli-IncalziRCorsonelloATrojanoLScreening of cognitive impairment in chronic obstructive pulmonary diseaseDement Geriatr Cogn Disord200723426427017351318
- RiescoJAAlcázarBTriguerosJACampuzanoAPérezJLorenzoJLActive smoking and COPD phenotype: distribution and impact on prognostic factorsInt J Chron Obstruct Pulmon Dis2017121989199928740378
- ZhangJLinXFBaiCXComparison of clinical features between nonsmokers with COPD and smokers with COPD: a retrospective observational studyInt J Chron Obstruct Pulmon Dis20149576324426780
- Montserrat-CapdevilaJGodoyPMarsalJRBarbéFPredictive model of hospital admission for COPD exacerbationRespir Care20156091288129426286737
- DurazzoTCMeyerhoffDJNixonSJChronic cigarette smoking: implications for neurocognition and brain neurobiologyInt J Environ Res Public Health20107103760379121139859
- GuptaNSimpkinsANHitomiEDiasCLeighRNIH natural history of stroke investigatorsWhite matter hyperintensity-associated blood-brain barrier disruption and vascular risk factorsJ Stroke Cerebrovasc Dis201827246647129100854
- SwanGELessov-SchlaggarCNThe effects of tobacco smoke and nicotine on cognition and the brainNeuropsychol Rev200717325927317690985
- ZillioxLAChadrasekaranKKwanJYRussellJWDiabetes and Cognitive ImpairmentCurr Diab Rep20161698727491830
- BakerEHJanawayCHPhilipsBJHyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary diseaseThorax200661428428916449265
- WaltersJAWaltersEHWood-BakerROral corticosteroids for stable chronic obstructive pulmonary diseaseCochrane Database Syst Rev20053CD005374
- CranePKWalkerRHubbardRAGlucose levels and risk of dementiaN Engl J Med2013369654054823924004
- DecramerMRennardSTroostersTCOPD as a lung disease with systemic consequences – clinical impact, mechanisms, and potential for early interventionCOPD20085423525618671149
- BarnesPJChronic obstructive pulmonary disease: effects beyond the lungsPLoS Med201073e100022020305715
- HuppertFAMemory impairment associated with chronic hypoxiaThorax198237118588607164006
- StussDTPeterkinIGuzmanDAGuzmanCTroyerAKChronic obstructive pulmonary disease: effects of hypoxia on neurological and neuropsychological measuresJ Clin Exp Neuropsychol19971945155249342687
- LivingstonGSommerladAOrgetaVDementia prevention, intervention, and careLancet2017390101132673273428735855
- LezakMDHowiesonDBLoringDWHannayHJFischerJSNeuropsychological Assessment4th edNew York: NY, USOxford University Press2004
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders5th edArlington, VAAmerican Psychiatric Association2013