Abstract
Alzheimer’s disease (AD) is a neurodegenerative disease that mainly occurs in old age and involves progressive cognitive impairment. AD has become a major global issue for public health, with approximately 24 million people currently affected by the disease. Estimates indicted that this number will quadruple by 2050. Because of the high incidence of AD, there is an urgent need to develop new strategies to diagnose and treat AD. Many recent studies have indicated the multiple, yet somewhat controversial, roles of exosomes in AD. Although the underlying mechanisms by which exosomes play a role in AD are still unknown, current evidence suggests that exosomes can carry and spread toxic amyloid-beta, and hyperphosphorylated tau, between cells, and then induce apoptosis, thus contributing to the loss of neurons. In addition, exosomes appear to possess the ability to reduce brain amyloid-beta, and tau hyperphosphorylation, and transfer neuroprotective substances between neural cells. The accumulating data brings hope that the application of exosomes may be helpful for early diagnostics and the identification of new therapeutic targets for AD. Here, we summarized the various roles of exosomes, and how they might relate to the pathogenesis of AD. We also highlight the potential application of exosomes as a therapeutic option in AD therapy.
Introduction
Alzheimer’s disease (AD) is the most prevalent form of dementia, and is accompanied by impaired cognition and behavior in elderly people over 65 years of age. AD affects approximately 24 million people globally, although current estimates indicate that this number is likely to quadruple by 2050.Citation1 AD has several neuropathological hallmarks, including the deposition of β-amyloid (Aβ) peptides in the extracellular matrix between neurons (known as amyloid plaques), the intracellular formation of neurofibrillary tangles (NFTs) arising from the accumulation of hyperphosphorylated tau protein in neurons, neuronal loss, neuroinflammation, and oxidative stress. Due to the high prevalence of AD, and its high economic burden to society, there is significant interest in developing new approaches to treat AD.Citation2,Citation3
Exosomes, a form of nanoscale vesicle, are commonly found in the biological fluids and tissues of the central nervous system, and may carry a small amount of molecular genetic material and proteins that play key roles in intercellular communication.Citation4 This form of vesicle transport may be related to the production, transport, and degradation of toxic proteins in AD.Citation5,Citation6 In cellular and animal models of AD, exosomes have been shown to carry and spread toxic Aβ, and hyperphosphorylated tau, between neural cells, including neurons and glia,Citation7–Citation9 and may then induce cell apoptosis, thus resulting in the loss of neurons.Citation10–Citation12 On the other hand, exosomes may exert positive actions, including the reduction of brain amyloid-beta, or the transfer of neuroprotective substances between neural cells (neurons and glia).Citation13 Since neuron-derived exosomes (NDEs) exist in both cerebrospinal fluid and peripheral blood,Citation14–Citation17 it is possible that targeting changes in the exosomes during the pathogenesis of AD might provide a new alternative approach with which to treat AD. In this review, we discuss the multiple roles of exosomes in AD, particularly the therapeutic strategies that use mesenchymal stem cells (MSCs) to treat AD, and the challenges associated with this practice in clinical scenarios.
Exosomes
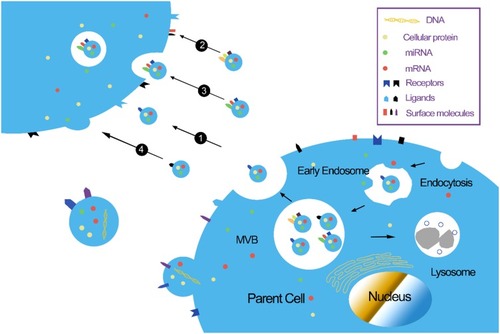
Exosomes are single-lipid membrane vesicles that are secreted by all cell types, with diameters ranging from 30–150 nm.Citation18,Citation19 Small vesicles are produced by the inward budding of the plasma membrane; these vesicles are then fused together to form the early endosome. During the process of endosome formation, proteins, lipids, RNAs, and other substances are enclosed into the lumen, and then accumulated within the late endosome, thus forming multi–vesicular bodies (MVBs); these are subsequently released into the extracellular milieu as “exosomes”.Citation20 Evidence suggests that exosomes act as an important messenger for cellular communication, particularly between cells of the central nervous system.Citation21 Owing to their stable lipid bilayer membrane, exosomes are capable of transferring bioactive molecules (proteins, nucleic acids, and RNAs) between cellsCitation21 (). Because of the exchange of proteins and genetic materials, exosomes not only participate in normal physiological processes, including cell growth, immune regulation, angiogenesis, neuronal communication, and cell migration,Citation22 but also participate in the pathogenesis of various diseases, including AD.Citation23 Our recent study showed that kidney and brain protein (KIBRA), an adaptor-like protein, can regulate the secretion of exosomes through a Rab27A-dependent mechanism, and participate in the progression of AD pathology.Citation24,Citation25 Another of our recent studies indicated that the up-regulation of mammalian target of rapamycin (mTOR) facilitates the release of tau into the extracellular space in an exosome-independent manner in SH-SY5Y cells.Citation26 More recently, research has shown that the mTOR complex 1 (mTORC1) also regulates the release of exosomes through a Rab27A-dependent mechanism. mTORC1 activation inhibits exosome release, while the inhibition of mTORC1 induces the release of exosomes without significantly changing cargo content, thus indicating that mTORC1 controls the release of exosomes, but not formation.Citation27 Due to their ability to cross the blood–brain barrier (BBB),Citation28 it follows that exosomes might represent an important approach for exploring new treatment options for AD.
Figure 1 Biological functions of exosomes. (1) Stimulation of recipient cells by functioning as signal complexes; (2) Transfer of surface receptors or lipids into recipient cells; (3) Delivery of cytoplasmic proteins and nucleic acids via the endocytic pathway; (4) Delivery of cytoplasmic proteins and nucleic acids by membrane fusion.
Markers and Contents in Exosomes
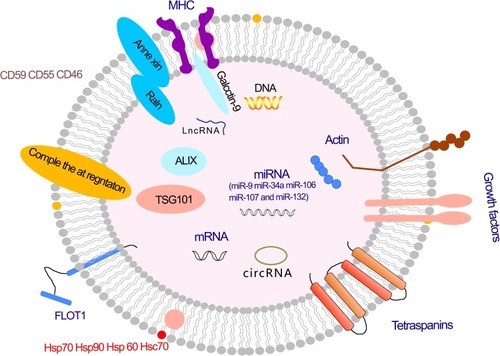
Exosomes are microvesicles that are typically enclosed in a lipid bilayer membrane that is used for transport and serves to protect the luminal cargo against damage from severe extracellular environments.Citation29 The lipid bilayer contains proteins, some of which have been identified as relatively specific exosomal markers, including CD9, Alix, CD63, and TSG101.Citation30 All of these markers, together with CD81, can be used to identify exosomes; otherwise, they could be mistaken for other forms of extracellular vesicles.Citation31 The lipids in exosomes can regulate the exosomal sorting of small RNAs and proteins.Citation32,Citation33 In addition to proteins and lipids, there are a number of other genetic materials found in exosomes, including DNA, mRNA, miRNA, ribosomal RNA (rRNA), circular RNA, and long noncoding RNA (lncRNA).Citation34,Citation35 Exosomes can carry and transmit these genetic materials to play a part in normal physiological processes and diseases.Citation36–Citation38 Exosomes contain RNAs that are involved in many aspects of neurological function, including synaptic transmission, angiogenesis, neurite outgrowth, axonal growth, and neuronal differentiation from neural stem cells.Citation39
In addition to RNA, DNA, and proteins, exosomes have been shown to contain bioactive lipids, such as ceramide, cholesterol, phosphatidylserine, and sphingolipidsCitation40,Citation41 ().
The Role of Exosomes in AD
The Pathogenic Role of Exosomes in AD
Evidence has emerged recently to indicate that exosomes play a harmful role in the aggregation and deposition of specific misfolded proteins, such as Aβ, tau, prions, and α-synuclein; these subnormal proteins are all core features in neurodegenerative disorders.Citation12,Citation42–Citation44 Exosomes have been shown to carry different disease-causing cargos, including proteins, RNA, and miRNA.Citation45 Importantly, exosomes can carry Aβ, tau, prions, and α-synuclein, and can spread pathogenic proteins across the brain.Citation8,Citation46–Citation50 On the other hand, exosomes have generated immense interest after their discovery as mediators for the delivery of important proteins and microRNAs during intercellular communication, suggesting that exosomes may serve as an early biomarker of AD.Citation51,Citation52
Aβ is produced by the successive hydrolysis of amyloid precursor protein (APP) by β-secretase (BACE) and γ-secretase; the β-cleavage of APP mainly occurs in early endosomes.Citation53,Citation54 Soluble amyloid precursor protein beta (sAPPβ), APP, and BACE, have been shown to be co-localized with early endosomal markers (Rab5), and early endosomal antigen-1, in APP mutant HeLa cells by immunofluorescence.Citation53 The accumulated Aβ in MVBs can be released into the extracellular space through exosomes.Citation54 HEK cells expressing APP Swe/Ind were previously shown to be capable of efficiently transferring APP to normal primary neurons by exosomes.Citation55 Plasma exosomes containing AA amyloid oligomers were previously shown to exhibit amyloid-enhancing factor activity in a murine transfer model of AA amyloidosis.Citation56 Plasma NDEs from AD patients induce AD-like neuropathology, including Aβ deposition and tau phosphorylation, in normal mouse brain.Citation57 Meanwhile, in vivo studies have shown that the reduction of exosomes contributes to lower senile plaque deposit in the 5XFAD mouse model, a mouse line that expresses five mutations of familial AD.Citation12 These lines of evidence have confirmed that exosomes promote Aβ aggregation, and accelerate amyloid plaque formation.Citation30 Exosomes might be one of the main mediators participating in the progression of AD neuropathology.Citation58 Accordingly, exosomes from blood,Citation59 CSF,Citation60,Citation61 and cell culturesCitation62 have been shown to contain monomeric Aβ and tau.
Exosomes can not only spread AD pathological proteins; they are also suggested to play a harmful role in impairing neuronal functions by other means in AD. For example, exosome contents have been proposed to induce neuronal apoptosis in astrocytes exposed to amyloid protein, and in 5XFAD mouse models of AD.Citation12,Citation63 Amyloid peptides could activate neutral sphingomyelinase 2 (nSMase2), and induce an increase in the secretion of ceramide-containing exosomes in astrocytes.Citation63 In contrast, these secreted exosomes could be captured by astrocytes, and subsequently cause neural apoptosis.Citation63,Citation64 GW4869, an inhibitor of nSMase2, was shown to reduce Aβ in a mouse model of AD by preventing the secretion of exosomes,Citation12 thus indicating that the ceramide generated by nSMase2 may be critical for the formation of exosomes. Aβ and exosomes were also found to co-localize inside neurons, thus suggesting that exosomes participate in Aβ sorting and oligomerization.Citation65
Tau is a core protein associated with the pathogenesis of AD and is secreted in exosomes.Citation66 Our previous study showed that the up-regulation of mTOR facilitates the release of tau into the extracellular space in an exosome-independent fashion in SH-SY5Y cells.Citation26 Furthermore, a recent study showed that tau protein is released via exosomes from cultured primary neurons, and N2a cells overexpressing different tau constructs or CSF in AD and control subjects.Citation67 These results indicated that intracellular tau is secreted into the extracellular space in an exosome independent manner. Research has also shown that exosomes isolated from CSF samples diagnosed with AD contained higher levels of tau phosphorylation at the epitope Thr-181, indicating that exosomal tau may contribute to abnormal tau phosphorylation.Citation68 Interestingly, tau is phosphorylated at epitope Thr-181, the site that is most enriched in exosomal tau; this epitope therefore represents a specific biomarker for the elevated tau seen in early AD.Citation61,Citation68,Citation69 Another study showed that microglial cells play a significant role in phagocytosis and the secretion of tau in exosomes; the depletion of microglia in two diverse tauopathy mouse models showed that the propagation of tau could be inhibited, and that the inhibition of exosome synthesis reduced the propagation of tau compared with a control group, both in vitro and in vivo.Citation61 Based on these results, exosomes derived from microglia are efficient carriers for spreading tau between neurons. A recent study also showed that exosomes from the CSF of AD patients, containing monomeric and oligomeric tau, can also result in the aggregation of tau in cultured cells.Citation67
Beneficial Actions of Exosomes in AD
Although several studies have shown that exosomes can be harmful for AD, there is a growing body of evidence demonstrating that they also possess beneficial actions that may be of importance in the development of AD. For example, the up-regulation of exosomes containing nSMase2 secretion enhances Aβ uptake in microglia and signifcantly reduces the extracellular levels of Aβ.Citation70 Exosomes from the neuronal genetically modified neuroblastoma cell line (N2a cells) have the capacity to neutralize Aβ-induced disruptions in synaptic plasticity and prevent Aβ-induced neuronal apoptosis.Citation71 In another study, neuroblastoma-derived exosomes were injected into the right hippocampus of AβPP/PS1 transgenic mice; these exosomes were shown to bind to Aβ and subsequently be incorporated into microglia.Citation72 Interestingly, the continuous injection of exogenous exosomes resulted in a marked reduction of Aβ deposition and neurotoxicity in AβPP/PS1 transgenic mice.Citation72 In summary, numerous studies have focused on the neuroprotection functions of exosomes with regard to the reduction and clearance of Aβ.
Among the many approaches adopted to treat AD, stem cell therapy and, particularly, the use of mesenchymal stem cells (MSCs) is receiving significant attention. This is because MSCs are involved in multiple biological processes, including neurogenesis, oligodendrogenesis, axonal connectivity, and myelin formation.Citation73–Citation76 Around eight years ago, a research study showed that the intravenous delivery of MSCs allowed transport across the blood–brain barrier and subsequent migration to sites of neural injury without inducing tumorigenic or immune responses.Citation77 MSCs have also been shown to promote cognitive function in various pathological conditions,Citation78–Citation80 including neuro-regeneration,Citation72 neuroprotection,Citation79 the reduction of Aβ deposits and tau-related cell death,Citation80 and the down-regulation of pro-inflammatory cytokines, such as TNF-a and IL-1β.Citation81 Research attention has re-focused on exosomes recently because a study found that MSCs may exert their therapeutic effects via exosomes;Citation82–Citation84 these observations were made in a range of studies, including the experimental treatment of collagenase-induced osteoarthritis in a mouse model,Citation84 wound healing in diabetic skin ulcerations or extensive burns,Citation85 and limb ischemia in mice.Citation86 Multiple studies have found that MSC-derived exosomes are good candidates for the treatment of AD. Indeed, the co-culture of human adipose-derived mesenchymal stem cells (ADSCs), with N2a cells that overproduced human Aβ, showed reductions in both extracellular and intracellular Aβ levels in N2a cells. These authors also found that exosomes secreted from ADSCs carry enzymatically active neprilysin, the most important Aβ-degrading enzyme in the brain, thus suggesting a potential new treatment for AD.Citation72,Citation87 Hao et al co-cultured injured cortical neurons with ADSCs, and found that ADSCs secreted exosomes that exerted direct neuroprotective effects by inhibiting neuronal cell apoptosis, thus promoting the regeneration and repair of the central nervous system (CNS), and hence restoring bioenergy following energy depletion caused by glutamate excitotoxicity.Citation88 In another study, Ahmed et al demonstrated the detection of neprilysin in exosomes secreted by dental pulp stem cell (DPSC) and that these exosomes were able to degrade Aβ1-42 and to reduce Aβ-induced neurotoxicity in SH-SY5Y neuroblastoma cells in vitro.Citation89 In another study, MSC-derived exosomes were shown to improve learning and memory function in APP/PS1 transgenic mice, reduce Aβ accumulation, and increase the expression of synaptic protein in the brains of APP/PS1 transgenic mice.Citation90,Citation91 Furthermore, treatment with exosomes derived from MSCs were shown to reduce the activation of glial cells, and the levels of inflammatory factors involved in the regulation of the STAT3 and NF-kB pathways.Citation91 A very recent study further showed that the injection of exosomes derived from human umbilical cord mesenchymal stem cells (hucMSCs) into the brain could repair cognitive dysfunction and facilitate the clearance of Aβ deposition in the AβPP/PS1 transgenic mouse model.Citation92 Therefore, MSC-derived exosomes have emerged as an appealing approach for the delivery of therapeutics for AD.
Given the available evidence, it is evident that exosomes may play a vital neuroprotective role in neurodegenerative diseases, including AD, and may, therefore, represent a new therapeutic approach for AD in clinic. summarizes our current understanding of the various roles of exosomes in AD.
Table 1 Multiple Roles of Exosomes in AD
Future Directions: The Clinical Value of Exosomes
Exosomes as Biomarkers
In AD, exosomes can be synthesized and released from brain cells, pass through the BBB, and can be detected in the peripheral blood or in CSF;Citation93 these properties render exosomes as ideal biomarkers to reflect the pathological progress of AD. Changes in the contents of exosomes that are circulating in the blood may serve as early biomarkers for the diagnosis and treatment of AD.Citation94 For example, NDEs have recently been proposed as potential biomarkers for AD; Fiandaca et al found that NDEs in patients with AD showed significantly higher levels of amyloid β 1–42 (Aβ1-42) than those from case controls 1 to 10 years before diagnosis; this test could be developed as a valuable predictor of AD.Citation60 In another paper, Jia et al reported that the levels of Aβ1-42, total tau, p-T181 tau, and p-S396 tau, in NDEs that were isolated from the plasma of AD patients were significantly higher when compared with controls, thus providing a good predictor for disease development during the preclinical stage.Citation95 A recent study also found that extracellular vesicles in the circulatory system can respond to the state of the central nervous system, and that levels of Aβ 42, T-tau, and PT181-tau in NDEs may reflect the pathological changes associated with AD in the brain, and therefore exhibit the same capacity to diagnose AD as those in the CSF.Citation96 In addition, miRNAs, which play significant roles in the regulation of various biological processes, and even the diagnosis of many diseases, are therefore considered as candidates for blood-based exosomal biomarkers for dementia. However, the standardization of exosomes extracted from blood components (plasma or serum) for the development of miRNA biomarkers is complicated.Citation39,Citation97 The literature relating to exosome-associated miRNAs as biomarkers for AD is very limited, and current understanding requires further validation in different cohorts. One of the highlights of existing exosomal research is the characterization of their molecular properties in specific diseases. Extensive attention is now being afforded to exosome-derived biomarkers in the blood of AD biomarker research. However, the development of exosomal biomarkers for AD requires further verification.
Exosomes for Therapeutic Application
Exosomes have the ability to transfer bioactive molecules between cells across the BBB, and can be used as effective natural carriers for the therapeutic delivery of potential-disease modifying molecules.Citation93 Compared with traditional gene therapy candidate vectors such as viruses, polyethylenimine nanoparticles, and liposomes, exosomes have greater advantages in terms of therapeutic efficacy, good levels of safety, a low immune response, and offer the possibility of targeting.Citation97 Exosomes are naturally secreted components of body cells and are widely found in extracellular fluids.Citation18 Exosomes for therapeutic treatment can be obtained from the medium of cultured MSCs with reduced levels of cell immunogenicity.Citation84 The availability of MSCs in a variety of tissues, their ease of isolation, and their significant ability to propagate in vitro have made MSCs desirable potential producers of exosomes.Citation98 The adaptability of these MSCs to genetic modification further enables them to produce exosomes that are rich in the required therapeutic factors. Proteomic analysis of MSC-derived exosomes showed similar immunotolerance characteristics as MSCs,Citation99 further enhancing the importance of these cells as allogeneic and autogenous natural delivery vehicles. Furthermore, it has been suggested that the therapeutic effect of MSCs with regard to targeting pathological regions is exerted via the exosomes that they secrete.Citation100 A recent study has demonstrated that exosomes secreted by MSCs have remarkable migration and homing abilities towards specific areas of neuropathology, and specifically to neurons, and that neuroinflammation plays a vital role in these homing mechanisms.Citation101 These authors also found that 24 hrs after intranasal administration, exosomes derived from MSCs were found to have been transported to the hippocampus, the central region associated with AD.Citation101 Thus, the specific migration and homing abilities of MSC-derived exosomes could pave the way for their use as multifunctional theranostic agents in AD.
Several studies have suggested that exosomes derived from MSCs transfer their therapeutic factors, particularly miRNAs, to recipient cells, thereby altering gene expression and thus promoting a therapeutic response.Citation21,Citation42 Exosomes have also been studied as a delivery platform for encapsulation, or short interfering RNAs (siRNA).Citation90 For example, Alvarez-Erviti et al showed that the delivery of BACE-1 siRNA mediated by exosomes, which specifically targeted β-secretase, resulted in a 60% knockdown of the BACE-1 gene, thus leading to a 55% reduction of Aβ levels in the mouse brain.Citation102 Aβ is derived from the cleavage of Aβ precursor protein (APP) by β- and γ-secretases; MSCs can be genetically modified to secrete exosomes that are enriched with therapeutic factors, such as siRNAs, that specifically target β- and γ-secretase enzymes, such that exosomes can be exploited as valuable nanotechnological approaches that exert vital neuroprotective effects on AD.Citation103,Citation104 This approach has been explored experimentally in an attempt to improve functional recovery after stroke, enhance neurovascular plasticity, and repair injured brain tissue after traumatic brain injury.Citation105–Citation108 Thus, it may be possible to use MSC-derived exosomes as a cell-free therapy for the treatment of AD.Citation109
In addition, the exciting potential of exosomes as therapeutic vehicles lies in drug delivery and development strategies to design and modify both the surface and content of these valuable biological structures. A recent study showed that exosomes derived from curcumin-treated (primed) cells (Exo-cur) can relieve the symptoms of AD by inhibiting the phosphorylation of Tau protein and help to prevent neuronal death both in vitro and in vivo.Citation110 With regard to exosomal-mediated protein delivery, the incorporation of catalase into exosomes (ExoCAT) has further revealed significant neuroprotective effects for Parkinson’s disease in both in vitro and in vivo models.Citation111 In recent years, an exosome-based delivery system developed exosomes for protein loading via optically reversible protein-protein interactions (EXPLORs). This research integrated a reversible protein-protein interaction module that was controlled by blue light, and used an endogenous process for exosome biogenesis; exosomes generated by this process could be readily loaded with cargo.Citation112 Using the EXPLOR technique, most intracellular proteins, including transcription factors, signal transducers, and enzymes, can be efficiently targeted by EXPLOR-based therapeutics.Citation113 Thus, exosomes may hold significant potential to improve targeted drug delivery and neuronal functional recovery in AD therapy.
Challenges and Limitations
Although there is a growing body of literature relating to the rapid development of exosomes derived from MSCs, the therapeutic use of these exosomes remains in its infancy. Many issues need to be resolved before MSC-derived exosomes can be used for clinical treatments. Significant research is still required in order to guarantee the long-term biological safety of these exosomes, and confirm the potential adverse effects and efficacy of exosome administration in patients with AD.Citation114 We also need to know more about the time of administration, the most effective route of administration, including dose-response experiments, before considering MSC-derived exosomes for clinical application.
One of the key issues for the future development of exosomes for clinical application is to scale up the production of appropriate exosomes. The inherent properties of MSCs are advantageous to the large-scale production of exosomes. The large-scale production of clinically normative MSCs for the generation of exosomes is one of the key factors in translating MSC-derived exosomes for clinical application. There are still many problems to be resolved if we are to improve the production of MSC-derived exosomes. The large-scale production of MSC-derived exosomes requires new techniques, and the experimental protocol for extracting exosomes from MSCs still needs to be standardized. However, based on the promising results achieved from preclinical studies thus far, exosome-based therapies are steadily making their way towards clinical application.Citation97,Citation115
Moreover, the precise content of MSC-derived exosomes remains largely unknown because these exosomes contain many molecules that are yet to be identified. The exosome contents have been determined with microarray and proteomics techniques. However, exosome might have different contents according to the origin of the MSCs or their culture conditions. Our understanding of exosome-related therapies is rapidly expanding although exosomes have already been approved for application in several clinical trials. summarizes the advantages and challenges of MSC based therapy and MSC exosome based therapy.
Table 2 Advantages and Challenges of MSC Based Therapy and MSC Exosome Based Therapy
Conclusions
In recent years, exosomes have been gradually viewed as multipotent therapeutic targets and potential biomarkers of various diseases including AD. Thus, the development of genetically modified MSC exosomes might open a novel horizon for therapeutic strategies in AD. In this paper, we have reviewed the possible functions and applications of exosomes in AD. Together, these findings indicate that exosomes have a therapeutic potential for treating AD by enhancing neuroprotection mechanisms and being therapeutic vehicles, and exosomes may be have a vital biomarker role in AD preclinical and clinical studies.
Acknowledgment
This work was supported by Shandong Provincial Natural Science Foundation (grant nos. ZR2019PH017), Science and Technology Project of Jinan city (grant nos. 201805073), and Project funded by China Postdoctoral Science Foundation (grant nos. 2017M622217).
Disclosure
The authors declare no conflicts of interest in this work.
References
- Picançoa L, Ozelaa PF, Britoa M, et al. Alzheimer’s disease: a review from the pathophysiology to diagnosis, new perspectives for pharmacological treatment. Curr Med Chem. 2018;25:3141–3159. doi:10.2174/092986732366616121310112630191777
- Cantarero PD. Economic impact of cognitive impairment and dementia. Rev Esp Geriatr Gerontol. 2017;52:58–60. doi:10.1016/S0211-139X(18)30085-429628039
- Li F, Chen S, Wei C, et al. Monetary costs of Alzheimer’s disease in China: protocol for a cluster-randomised observational study. BMC Neurol. 2017;17(1):15. doi:10.1186/s12883-017-0802-928122529
- Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine. 2013;44:11–19. doi:10.1007/s12020-012-9839-023203002
- Shaimardanova AA, Solovyeva VV, Chulpanova DS, et al. Extracellular vesicles in the diagnosis and treatment of central nervous system diseases. Neural Regen Res. 2020;15(4):586–596. doi:10.4103/1673-5374.26690831638080
- Thompson AG, Gray E, Heman-Ackah SM, et al. Extracellular vesicles in neurodegenerative disease - pathogenesis to biomarkers. Nat Rev Neurol. 2016;12(6):346–357. doi:10.1038/nrneurol.2016.6827174238
- Narasimhan S, Guo JL, Changolkar L, et al. Pathological tau strains from human brains recapitulate the diversity of tauopathies in nontransgenic mouse brain. J Neurosci. 2017;37(47):11406–11423. doi:10.1523/JNEUROSCI.1230-17.201729054878
- Nath S, Agholme L, Kurudenkandy FR, et al. Spreading of neurodegenerative pathology via neuron-to-neuron transmission of beta-amyloid. J Neurosci. 2012;32(26):8767–8777. doi:10.1523/JNEUROSCI.0615-12.201222745479
- Fu H, Hussaini S, Wegmann S, et al. 3D Visualization of the temporal and spatial spread of tau pathology reveals extensive sites of tau accumulation associated with neu ronal loss and recognition memory deficit in aged tau transgenic mice. PLoS One. 2016;11(7):e0159463. doi:10.1371/journal.pone.015946327466814
- Sardar Sinha M, Ansell-Schultz AS, Civitelli L, et al. Alzheimer’ s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018;136:41–56. doi:10.1007/s00401-018-1868-129934873
- Danzer KM, Kranich LR, Ruf WP, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42. doi:10.1186/1750-1326-7-4222920859
- Dinkins MB, Dasgupta S, Wang G, et al. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging. 2014;35:1792–1800. doi:10.1016/j.neurobiolaging.2014.02.01224650793
- Yuyama K, Sun H, Usuki S, et al. A potential function for neuronal exosomes: sequestering intracerebral amyloid-β peptide. FEBS Lett. 2015;589(1):84–88. doi:10.1016/j.febslet.2014.11.02725436414
- Gui Y, Liu H, Zhang L, Lv W, Hu X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget. 2015;6(35):37043–37053. doi:10.18632/oncotarget.v6i3526497684
- Fru¨hbeis C, Fro¨hlich D, Kra¨mer-Albers EM. Emerging roles of exosomes in neuron-glia communication. Front Physiol. 2012;30(3):119.
- Athauda D, Gulyani S, Karnati H, et al. Utility of neuronal-derived exosomes to examine molecular mechanisms that affect motor function in patients with parkinson disease: a secondary analysis of the exenatide-PD Trial. JAMA Neurol. 2019;76(4):420–429. doi:10.1001/jamaneurol.2018.430430640362
- Dubal DB. Neural-derived extracellular vesicles in clinical trials: message in a bottle. JAMA Neurol. 2019;76(4):402–404. doi:10.1001/jamaneurol.2018.432530640380
- Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36(3):301–312. doi:10.1007/s10571-016-0366-z27053351
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi:10.1146/annurev-cellbio-101512-12232625288114
- Kalra H, Drummen G, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17:170. doi:10.3390/ijms1702017026861301
- Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi:10.1038/ncb159617486113
- Fauré J, Lachenal G, Court M, et al. Exosomes are released by cultured cortical neurons. Mol Cell Neurosci. 2006;31:642–648. doi:10.1016/j.mcn.2005.12.00316446100
- Fevrier B, Vilette D, Archer F, et al. Cells release prions in association with exosomes. Proc Natl Acad Sci USA. 2004;101:9683–9688. doi:10.1073/pnas.030841310115210972
- Song L, Tang S, Han XL, et al. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat Commun. 2019;10(1):1639. doi:10.1038/s41467-019-09720-x30967557
- Song L, Tang S, Dong LL, et al. The neuroprotection of KIBRA in promoting neuron survival and against amyloid ß-induced apoptosis. Front Cell Neurosci. 2019;13:137. doi:10.3389/fncel.2019.0013731031595
- Tang Z, Ioja E, Bereczki E, et al. mTor mediates tau localization and secretion: implication for Alzheimer’s disease. Biochim Biophys Acta. 2015;1853(7):1646–1657. doi:10.1016/j.bbamcr.2015.03.00325791428
- Zou W, Lai M, Zhang Y, et al. Exosome release is regulated by mTORC1. Adv Sci (Weinh). 2019;6(3):1801313. doi:10.1002/advs.20180131330775228
- Agnati LF, Guidolin D, Guescini M, et al. Understanding wiring and volume transmission. Brain Res Rev. 2010;64:137–159. doi:10.1016/j.brainresrev.2010.03.00320347870
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–525. doi:10.1038/nrm272819603039
- Keerthikumar S, Gangoda L, Liem M. Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes. Oncotarget. 2015;6:15375–15396. doi:10.18632/oncotarget.v6i1725944692
- Hsu C, Morohashi Y, Yoshimura S, et al. Regulation of exosome secretion by Rab35 and its GTPase activating proteins TBC1D10A-C. J Cell Biol. 2010;189(2):223–232. doi:10.1083/jcb.20091101820404108
- Tytell M, Lasek RJ, Gainer H. Axonal maintenance, glia, exosomes, and heat shock proteins. F1000Research. 2016;5:205. doi:10.12688/f1000research
- De Toro J, Herschlik L, Waldner C, Mongini C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol. 2015;6:203. doi:10.3389/fimmu.2015.0020325999947
- Llorente A, Skotland T, Sylvanne T, et al. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta. 2013;1831(7):1302–1309. doi:10.1016/j.bbalip.2013.04.01124046871
- Yuyama K, Igarashi Y. Physiological and pathological roles of exosomes in the nervous system. Biomol Concepts. 2016;7(1):53–68. doi:10.1515/bmc-2015-003326812803
- Lamichhane TN, Raiker RS, Jay SM. Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enableslimited gene delivery. Mol Pharm. 2015;12(10):3650–3657. doi:10.1021/acs.molpharmaceut.5b0036426376343
- Crescitelli R, Lasser C, Szabo TG, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;12:2.
- Li Y, Zheng Q, Bao C, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–984. doi:10.1038/cr.2015.8226138677
- Iranifar E, Seresht BM, Momen F, et al. Exosomes and microRNAs: new potential therapeutic candidates in Alzheimer disease therapy. J Cell Physiol. 2019;234(3):2296–2305. doi:10.1002/jcp.v234.330191975
- Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi:10.1126/science.115312418309083
- Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89:202–212. doi:10.1016/j.biochi.2006.10.014
- Coleman BM, Hill AF. Extracellular vesicles–Their role in the packaging and spread of misfolded proteins associated with neurodegenerative diseases. Semin Cell Dev Biol. 2015;40:89–96. doi:10.1016/j.semcdb.2015.02.00725704308
- Zheng T, Wu X, Wei X, et al. The release and transmission of amyloid precursor protein via exosomes. Neurochem Int. 2018;114:18–25. doi:10.1016/j.neuint.2017.12.00929277576
- Joshi P, Turola E, Ruiz A, et al. Micro glia convert aggregated amyloid-β into neurotoxic forms through the shedding of microvesicles. Cell Death Differ. 2014;21:582–593. doi:10.1038/cdd.2013.18024336048
- Vella LJ, Hill AF, Cheng L. Focus on extracellular vesicles: exosomes and their role in protein trafcking and biomarker potential in Alzheimer’s and Parkinson’s disease. Int J Mol Sci. 2016;17:173. doi:10.3390/ijms1702017326861304
- Eitan E, Hutchison ER, Marosi K, et al. Extracellular vesicle-associated abeta mediates trans-neuronal bioenergetic and Ca2+-handling deficit in Alzheimer’s disease models. NPJ Aging Mech Dis. 2016;2. doi:10.1038/npjamd.2016.19
- Arellano ZE, Huor A, Leblanc P, et al. Prion strains are differentially released through the exosomal pathway. Cell Mol Life Sci. 2015;72(6):1185–1196. doi:10.1007/s00018-014-1735-825227242
- Polanco JC, Scicluna BJ, Hill AF, Gotz J. Extracellular vesicles isolated from the brains of rTg4510 mice seed Tau protein aggregation in a threshold dependent manner. J Biol Chem. 2016;291(24):12445–12466. doi:10.1074/jbc.M115.70948527030011
- Loov C, Scherzer CR, Hyman BT, Breakefield XO, Ingelsson M. alpha Synuclein in extracellular vesicles: functional implications and diagnostic opportunities. Cell Mol Neurobiol. 2016;36(3):437–448. doi:10.1007/s10571-015-0317-026993503
- Domert J, Rao SB, Agholme L, et al. Spreading of amyloid-beta peptides via neuritic cell-to-cell transfer is dependent on insufcient cellular clearance. Neurobiol Dis. 2014;65:82–92. doi:10.1016/j.nbd.2013.12.01924412310
- Lee S, Mankhong S, Kang JH. Extracellular vesicle as a source of alzheimer’s biomarkers: opportunities and challenges. Int J Mol Sci. 2019;20(7):1728. doi:10.3390/ijms20071728
- You Y, Ikezu T. Emerging roles of extracellular vesicles in neurodegenerative disorders. Neurobiol Dis.2019;130:104512. doi:10.1016/j.nbd.2019.10451231229685
- Tkach M, Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–1232. doi:10.1016/j.cell.2016.01.04326967288
- Xiao T, Zhang W, Jiao B, et al. The role of exosomes in the pathogenesis of Alzheimer’ disease. Transl Neurodegener. 2017;6:3. doi:10.1186/s40035-017-0072-x28184302
- Zheng T, Pu J, Chen Y, et al. Exosomes secreted from HEK293-APP Swe/Ind cells impair the hippocampal neurogenesis. Neurotox Res. 2017;32(1):82–93. doi:10.1007/s12640-017-9713-128321582
- Tasaki M, Ueda M, Ochiai S, et al. Transmission of circulating cell-free AA amyloid oligomers in exosomes vectors via a prion-like mechanism. Biochem Biophys Res Commun. 2010;400:559–562. doi:10.1016/j.bbrc.2010.08.10120807507
- Winston CN, Goetzl EJ, Akers JC, et al. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement. 2016;3:63–72.
- Ngolab J, Trinh I, Rockenstein E, et al. Brain derived exosomes from dementia with Lewy bodies propagate α-synuclein pathology. Acta Neuropathol Commun. 2017;5:46. doi:10.1186/s40478-017-0445-528599681
- Guo JL, Lee VM. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med. 2014;20:130–138. doi:10.1038/nm.345724504409
- Fiandaca MS, Kapogiannis D, Mapstone M, et al. Identifcation of preclinical Alzheimer’s disease by a profle of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. 2015;11:600–607 e601. doi:10.1016/j.jalz.2014.06.00825130657
- Saman S, Kim W, Raya M, et al. Exosome associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fuid in early Alzheimer disease. J Biol Chem. 2012;287:3842–3849. doi:10.1074/jbc.M111.27706122057275
- Rajendran L, Honsho M, Zahn TR, et al. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci USA. 2006;103:11172–11177. doi:10.1073/pnas.060383810316837572
- Wang G, Dinkins M, He Q, et al. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD). J Biol Chem. 2012;287(25):21384–21395. doi:10.1074/jbc.M112.34051322532571
- Edgar JR, Willen K, Gouras GK, et al. ESCRTs regulate amyloid precursor protein sorting in multivesicular bodies and intracellular amyloid-beta accumulation. J Cell Sci. 2015;128:2520–2528. doi:10.1242/jcs.17023326002056
- Pacheco-Quinto J, Clausen D, Pérez-González R, et al. Intracellular metalloprotease activity controls intraneuronal Aβ aggregation and limits secretion of Aβ via exosomes. FASEB J. 2019;33(3):3758–3771. doi:10.1096/fj.201801319R30481490
- Saman S, Lee NC, Inoyo I, et al. Proteins recruited to exosomes by tau overexpression implicate novel cellular mechanisms linking tau secretion with Alzheimer’s disease. J Alzheimers Dis. 2014;40:S47–S70. doi:10.3233/JAD-13213524718102
- Wang Y, Balaji V, Kaniyappan S, et al. The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener. 2017;12(1):5. doi:10.1186/s13024-016-0143-y28086931
- Vanderstichele H, De Vreese K, Blennow K, et al. Analytical performance and clinical utility of the INNOTEST PHOSPHO-TAU181P assay for discrimination between Alzheimer’s disease and dementia with Lewy bodies. Clin Chem Lab Med. 2006;44:1472–1480. doi:10.1515/CCLM.2006.25817163825
- Gomez-Ramos A, Diaz-Hernandez M, Cuadros R, Hernandez F, Avila J. Extracellular tau is toxic to neuronal cells. FEBS Lett. 2006;580:4842–4850. doi:10.1016/j.febslet.2006.07.07816914144
- Yuyama K, Sun H, Mitsutake S, Igarashi Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J Biol Chem. 2012;287(14):10977–10989. doi:10.1074/jbc.M111.32461622303002
- An K, Klyubin I, Kim Y, et al. Exosomes neutralize synaptic-plasticity disrupting activity of Aβ assemblies in vivo. Mol Brain. 2013;6(1):47. doi:10.1186/1756-6606-6-4724284042
- Ding M, Shen Y, Wang P, et al. Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in Alzheimer’s Disease. Neurochem Res. 2018;43(11):2165–2177. doi:10.1007/s11064-018-2641-530259257
- Jiang B, Yan L, Wang X, et al. Concise review: mesenchymal stem cells derived from human pluripotent cells, an unlimited and quality-controllable source, for therapeutic applications. Stem Cells. 2018.
- Bruggeman KF, Moriarty N, Dowd E, et al. Harnessing stem cells and biomaterials to promote neural repair. Br J Pharmacol. 2019;176(3):355–368. doi:10.1111/bph.1454530444942
- Wang SM, Lee CU, Lim HK. Stem cell therapies for Alzheimer’s disease: is it time? Curr Opin Psychiatry. 2018;32(2):105–116. doi:10.1097/YCO.0000000000000478
- Kim SU, Lee HJ, Kim YB. Neural stem cell-based treatment for neurodegenerative diseases. Neuropathology. 2013;33:491–504. doi:10.1111/neup.1202023384285
- Ra JC, Shin IS, Kim SH, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297–1308. doi:10.1089/scd.2010.046621303266
- Kang JM, Yeon BK, Cho SJ, et al. Stem cell therapy for Alzheimer’s disease: a review of recent clinical trials. J Alzheimers Dis. 2016;54:879–889. doi:10.3233/JAD-16040627567851
- Zilka N, Zilkova M, Kazmerova Z, et al. Mesenchymal stem cells rescue the Alzheimer’s disease cell model from cell death induced by misfolded truncated tau. Neuroscience. 2011;193:330–337. doi:10.1016/j.neuroscience.2011.06.08821763758
- Yun HM, Kim HS, Park KR, et al. Placenta-derived mesenchymal stem cells improve memory dysfunction in an abeta1-42-infused mouse model of Alzheimer’s disease. Cell Death Dis. 2013;4:e958. doi:10.1038/cddis.2013.49024336078
- Lee HJ, Lee JK, Lee H, et al. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiol Aging. 2012;33:588–602. doi:10.1016/j.neurobiolaging.2010.03.02420471717
- Leu S, Lin YC, Yuen CM, et al. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J Transl Med. 2010;8:63. doi:10.1186/1479-5876-8-6320584315
- Ikegame Y, Yamashita K, Hayashi S, et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy. 2011;13:675–685. doi:10.3109/14653249.2010.54912221231804
- Zhu Y, Wang Y, Zhao B, et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane derived mesenchymal stem cells for the treat ment of osteoarthritis. Stem Cell Res Ther. 2017;8:64. doi:10.1186/s13287-017-0510-928279188
- Zhang JY, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. doi:10.1186/s12967-015-0417-025638205
- Hu GW, Li Q, Niu X, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenu ate limb ischemia by promoting angiogenesisin mice. Stem Cell Res Ther. 2015;6:10. doi:10.1186/scrt54626268554
- Katsuda T, Tsuchiya R, Kosaka N, et al. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013;3:1197. doi:10.1038/srep0119723378928
- Hao P, Liang Z, Piao H, et al. Conditioned medium of human adipose-derived mesenchymal stem cells mediates protection in neurons following glutamate excitotoxicity by regulating energy metabolism and GAP-43 expression. Metab Brain Dis. 2014;29:193–205. doi:10.1007/s11011-014-9490-y24458787
- Ahmed NEMB, Murakami M, Hirose Y, et al. Therapeutic potential of dental pulp stem cell secretome for treatment: an in vitro study. Stem Cells Int. 2016;2016:8102478. doi:10.1155/2016/810247827403169
- Cui GH, Shao SJ, Yang JJ, et al. Designer self-assemble peptides maximize the therapeutic benefits of neural stem cell transplantation for Alzheimer’s disease via enhancing neuron differentiation and paracrine action. Mol Neurobiol. 2016;53:1108–1123. doi:10.1007/s12035-014-9069-y25586060
- Cui GH, Wu J, Mou FF, et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018;32(2):654–668. doi:10.1096/fj.201700600R28970251
- Yuyama K, Sun H, Sakai S, et al. Decreased amyloid-β pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J Biol Chem. 2014;289(35):24488–24498. doi:10.1074/jbc.M114.57721325037226
- Kanninen KM, Bister N, Koistinaho J, Malm T. Exosomes as new diagnostic tools in CNS diseases. Biochim Biophys Acta. 2016;1862:403–410. doi:10.1016/j.bbadis.2015.09.02026432482
- Cai ZY, Xiao M, Quazi SH, et al. Exosomes: a novel therapeutic target for Alzheimer’s disease? Neural Regen Res. 2018;13(5):930–935. doi:10.4103/1673-5374.23249029863025
- Jia L, Qiu Q, Zhang H, et al. Concordance between the assessment of Ab42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimers Dement. 2019;15(8):1071–1080. doi:10.1016/j.jalz.2019.05.00231422798
- Fernandez-Messina L, Gutierrez-Vazquez C, Rivas-Garcia E, et al. Immunomodulatory role of microRNAs transferred by extracellular vesicles. Biol Cell. 2015;107(3):61–77. doi:10.1111/boc.20140008125564937
- Vakhshiteh F, Atyabi F, Ostad SN. Mesenchymal stem cell exosomes: a two-edged sword in cancer therapy. Int J Nanomed. 2019;14:2847–2859. doi:10.2147/IJN.S200036
- Kassem M, Abdallah BM. Human bone-marrow derived mesenchymal stem cells: biological characteristics and potential role in therapy of degenerative diseases. Cell Tissue Res. 2008;331:157. doi:10.1007/s00441-007-0509-017896115
- Lai RC, Tan SS, Teh BJ, et al. Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int J Proteomics. 2012;971907.22852084
- Liu S, Liu D, Chen C, et al. MSC transplantation improves osteopenia via epigenetic regulation of notch signaling in lupus. Cell Metab. 2015;22(4):606–618. doi:10.1016/j.cmet.2015.08.01826365178
- Perets N, Betzer O, Shapira R, et al. Golden exosomes selectively target brain pathologies in neurodegenerative and neurodevelopmental disorders. Nano Lett. 2019;19(6):3422–3431. doi:10.1021/acs.nanolett.8b0414830761901
- Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341. doi:10.1038/nbt.180721423189
- Ordóñez-Gutiérrez L, Re F, Bereczki E, et al. Repeated intraperitoneal injections of liposomes containing phosphatidic acid and cardiolipin reduce amyloid-β levels in APP/PS1 transgenic mice. Nanomedicine. 2015;11(2):421–430. doi:10.1016/j.nano.2014.09.01525461285
- Markoutsa E, Mourtas S, Bereczki E, et al. Comparison of various types of ligand decorated nanoliposomes for their ability to inhibit amyloid aggregation and to reverse amyloid cytotoxicity. Curr Top Med Chem. 2015;15(22):2267–2276. doi:10.2174/156802661566615060511590226043735
- Xin H, Li Y, Chopp M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front Cell Neurosci. 2014;8:377. doi:10.3389/fncel.2014.0037725426026
- Xin H, Li Y, Cui Y, et al. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–1715. doi:10.1038/jcbfm.2013.15223963371
- Xin H, Li Y, Liu Z, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737–2746. doi:10.1002/stem.140923630198
- Zhang Y, Chopp M, Meng Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856–867. doi:10.3171/2014.11.JNS1477025594326
- Vizoso FJ, Eiro N, Cid S, et al. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18:1852. doi:10.3390/ijms18091852
- Wang H, Sui H, Zheng Y, et al. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3β pathway. Nanoscale. 2019;11(15):7481–7496. doi:10.1039/C9NR01255A30942233
- Haney MJ, Klyachko NL, Zhao Y, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2016;207:18–30. doi:10.1016/j.jconrel.2015.03.033
- Yim N, Ryu S-W, Choi K, et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nat Commun. 2016;7:12277. doi:10.1038/ncomms1227727447450
- Yim N, Choi C. Extracellular vesicles as novel carriers for therapeutic molecules. BMB Rep. 2016;49(11):585–586. doi:10.5483/BMBRep.2016.49.11.17427733233
- Liew LC, Katsuda T, Gailhouste L, et al. Mesenchymal stem cell-derived extracellular vesicles: a glimmer of hope in treating Alzheimer’s disease. Int Immunol. 2017;29(1):11–19. doi:10.1093/intimm/dxx00228184439
- Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287–296. doi:10.1016/j.apsb.2016.02.00127471669