Abstract
Background
Compelling evidence has shown that diabetic metabolic disorder plays a critical role in the pathogenesis of Alzheimer’s disease, including increased expression of β-amyloid protein (Aβ) and tau protein. Evidence has supported that minocycline, a tetracycline derivative, protects against neuroinflammation induced by neurodegenerative disorders or cerebral ischemia. This study has evaluated minocycline influence on expression of Aβ protein, tau phosphorylation, and inflammatory cytokines (interleukin-1β and tumor necrosis factor-α) in the brain of diabetic rats to clarify neuroprotection by minocycline under diabetic metabolic disorder.
Method
An animal model of diabetes was established by high fat diet and intraperitoneal injection of streptozocin. In this study, we investigated the effect of minocycline on expression of Aβ protein, tau phosphorylation, and inflammatory cytokines (interleukin-1β and tumor necrosis factor-α) in the hippocampus of diabetic rats via immunohistochemistry, western blotting, and enzyme-linked immunosorbent assay.
Results
These results showed that minocycline decreased expression of Aβ protein and lowered the phosphorylation of tau protein, and retarded the proinflammatory cytokines, but not amyloid precursor protein.
Conclusion
On the basis of the finding that minocycline had no influence on amyloid precursor protein and beta-site amyloid precursor protein cleaving enzyme 1 which determines the speed of Aβ generation, the decreases in Aβ production and tau hyperphosphorylation by minocycline are through inhibiting neuroinflammation, which contributes to Aβ production and tau hyperphosphorylation. Minocycline may also lower the self-perpetuating cycle between neuroinflammation and the pathogenesis of tau and Aβ to act as a neuroprotector. Therefore, the ability of minocycline to modulate inflammatory reactions may be of great importance in the selection of neuroprotective agents, especially in chronic conditions like diabetes and Alzheimer’s disease.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder that is characterized by progressive cognitive impairment. Histopathological hallmarks mainly indicate extracellular amyloid peptide deposition in neuritic plaques and intracellular deposits of hyperphosphorylated tau. Glucose metabolic disorders are probably an initiating factor.Citation1,Citation2 A variety of mechanisms have been postulated in the risk of AD and diabetes mellitus (DM).Citation3,Citation4 Some scholars have even proposed that AD is type 3 diabetes.Citation5,Citation6 However, the relationship between DM and the process of AD remains elusive.
Minocycline, a tetracycline derivative, has a neuroprotective capability by limiting inflammation and oxidative stress.Citation7,Citation8 Previously, we have found that minocycline can downregulate β-amyloid protein (Aβ) in the hippocampus, caused by diabetic metabolic disorder through inhibition of nuclear factor-κB pathway activation.Citation9 In addition, several studies from our group have also evidenced that minocycline improves cognitive impairment triggered by both cerebral ischemia and diabetic metabolic disorder.Citation9–Citation11 It is well accepted that the neuroinflammation induced by diabetic disorder plays an important role in Aβ production and the hyperphosphorylation of tau.Citation12,Citation13 To further evaluate the neuroprotective role of minocycline in the diabetes related neurodegenerative process of AD, the present study was implemented to detect levels of tau and Aβ proteins in the brain of a DM model established by high fat diet and intraperitoneal injection of streptozocin (STZ), and observe the effects of minocycline on the inflammatory markers and levels of tau and Aβ proteins. These results noted that minocycline decreased the levels of the inflammatory markers and Aβ, and inhibited the hyperphosphorylation of tau in the hippocampus of rats with DM. This study suggests that minocycline can reduce neuroinflammation, and then lowers the hyperphosphorylation of tau and the level of expression of Aβ.
Methods
Animals and minocycline administration
Thirty-six Sprague-Dawley rats (10-month-old, female, body weight 200–250 g from the Third Military Medical University for Animal Field Hospital, Chongqing, People’s Republic of China) were fed with high fat and high sugar for 2 months (food composition: 10% lard, 20% sucrose, 2.5% cholesterol, bile salt 1%, 6.5% conventional food) to induce the onset of insulin resistance,Citation14 and the diabetes model was established by 50 mg/kg intraperitoneal injection of streptozocin (STZ) (Sigma Chemical Co, St Louis, MO, USA) after the 2 month high fat and high sugar feed.Citation9,Citation15 Animals were randomly divided into a control model group (subdivided into 4, 6, and 8 weeks after establishment of diabetes, with 6 animals per subgroup) and a minocycline intervention group (subdivided into 4, 6, and 8 weeks after minocycline intervention, respectively, with 6 animals per subgroup). Minocycline was administered by gavage on the same days as the STZ injection. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No 85–23, revised 1996).Citation16 The animal experiments were performed according to internationally followed ethical standards and approved by the research ethics committee of Chongqing Medical University, Chongqing, People’s Republic of China.
Minocycline (100 mg/capsule, made by China Huishi Pharmaceutical Limited Company, People’s Republic of China) was dissolved into 0.5 mg/mL density with saline. The animals in the minocycline intervention group were executed through gavage via the stomach by 50 mg/kg/day of minocycline. Diabetic rats were administered the same volume of buffer by douche via the stomach. The minocycline dosage was determined according to the previous studies.Citation9,Citation17 Minocycline was administered by gavage 2 days after STZ injection.
Enzyme-linked immunosorbent assay (ELISA)
A 500 pg/mL solution was prepared as manufacturer’s instruction and a standard curve created using computer software (Microsoft Excel [version 2010], Microsoft, Redmond Washington, USA) capable of generating a four parameter logistic curve fit. Rat hippocampus tissues were dissected and homogenized in tissue protein extraction reagent buffer (Biosource International, Inc, Camarillo, CA, USA). The analysis of Aβ by ELISA was conducted. Aβ inhibitors and 4-benzenesulfonyl fluoride hydrochloride (Sigma Chemical Co,) were added to tissue lysates to prevent degradation of Aβ. The concentration of Aβ40/42 was measured using the Aβ40 or Aβ42 Colorimetric ELISA kit (Biosource International, Inc) according to the manufacturer’s instruction.
Western blotting
Samples were collected in the presence of protease inhibitors. After homogenization, the lysates were centrifuged at 100,000 × g, and the supernatants were saved for western blot. Equal amounts of lysates were subject to sodium dodecyl sulfate polyacrylamide gel electrophoresis (Tris-glycine mini gel; 1:2500; Biosource International, Inc) and western blot analysis using antibodies specific for the following: interleukin (IL)-1β, tumor necrosis factor (TNF)-α, Aβ precursor protein (APP), and tau proteins. The optical densities of the specific bands were measured by image analysis software (HPIAS 2000, Tongji Qianping Company, Wuhan, People’s Republic of China).
Immunohistochemical assay
Avidin-Biotin Complex (ABC) method is a standard IHC method and a widely used technique for immunhistochemical staining. Avidin, a large glycoprotein, can be labeled with peroxidase or fluorescein and has a very high affinity for biotin. Biotin, a low molecular weight vitamin, can be conjugated to a variety of biological molecules such as antibodies. The second antibody, a goat anti-mouse immunoglobulin (Ig) labeled with biotin, was from Vector Laboratories, Burlingame CA, USA. The intensity of staining for each of two hundred cells was adjusted. Five grades distinguished the degrees of staining, which represented five reaction coefficients, respectively. The five products of every coefficient and the corresponding cell number were added up, which resulted in the value of a positive score. All slides were measured in duplicate. Those samples with a score over 10, or frequency over 5%, were considered as positive.
Statistical analysis
All statistical data were analyzed via the SPSS software for Windows 2000 (SPSS, Inc, Chicago, IL, USA). One-way analysis of variance was employed. A standard curve was used for analysis of ELISA results. P < 0.05 was considered as statistically significant.
Results
Minocycline decreased expression of Aβ but not APP
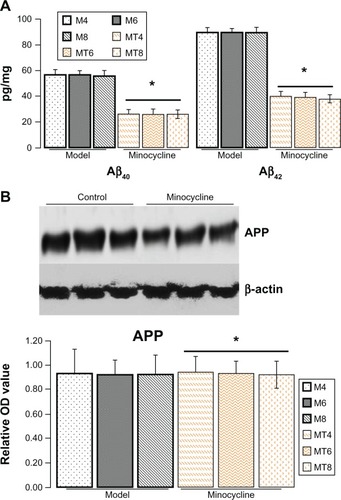
The ELISA results showed that the Aβ40 levels were significantly decreased from 56.43 ± 7.03 pg/mg in the model animals to 26.03 ± 6.13 pg/mg of lysate in the minocycline administration (P = 0.0001), and Aβ42 levels from 89.45 ± 9.28 pg/mg to 39.04 ± 6.03 pg/mg of lysates (P = 0.0003) (). However, the results by immunostaining indicated that minocycline intervention had no effect on APP ().
Figure 1 Assay of Aβ40/42 and amyloid precursor protein.
Abbreviations: APP, amyloid precursor protein; M4, M6, M8: 4, 6, and 8 weeks after establishment of diabetes; MT4, MT6, MT8: 4, 6, and 8 weeks after minocycline intervention; OD, optical density.
Minocycline decreased phosphorylation of tau
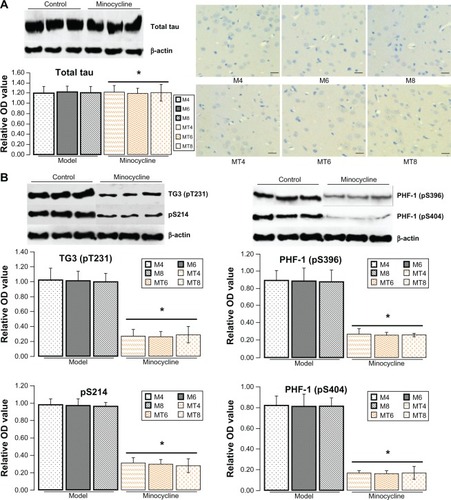
The previous results showed that minocycline restrained expression of Aβ that occurs in the central nervous system during abnormal glucose metabolism. To further explore the neuroprotective mechanism of minocycline in a diabetic state, total and phosphorylated tau proteins (a molecular marker of AD) were tested by western blotting or immunohistochemistry. The levels of total tau protein by western blotting or immunohistochemistry showed no significant change between control and the minocycline intervention group (). The levels of phosphorylated tau proteins, including pre-tangle marker phospho-tau antibody TG3 (pT231), intraneuronal tangle marker phospho-tau protein (Ser214, pS214), and extracellular tangle marker PHD finger protein-1 ([PHF-1] pS396/pS404), significantly decreased after minocycline treatment (P = 0.0001), when compared with control model animals ().
Figure 2 Assay for tau proteins.
Abbreviations: OD, optical density; M4, M6, M8: 4, 6, and 8 weeks after establishment of diabetes; MT4, MT6, MT8: 4, 6, and 8 weeks after minocycline intervention; PHF-1, PHD finger protein-1.
Minocycline downregulated IL-1β and TNF-α
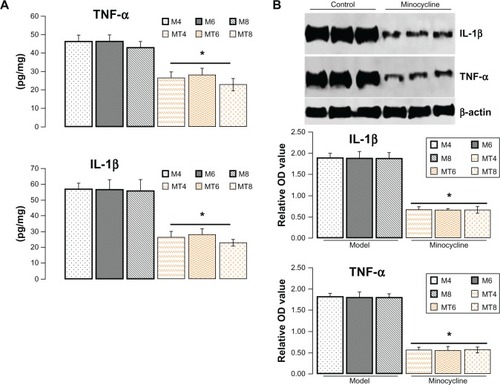
Increasing research studies have supported that diabetes induced neuroinflammation plays a crucial role in tau and Aβ pathogenesis.Citation12,Citation18,Citation19 Whether such neuroprotective effects of minocycline on diabetic metabolism disorder are through inhibiting inflammation is still unclear. To clarify the academic hypothesis, the proinflammatory cytokines, IL-1β and TNF-α, were detected here by ELISA and western blotting. The results by ELISA showed that IL-1β levels significantly decreased from 56.32 ± 6.02 pg/mg in control model animals to 25.48 ± 6.35 pg/mg of lysates in the minocycline treatment group (P = 0.0005), while TNF-α levels were reduced from 42.43 ± 6.62 pg/mg in control model animals to 23.44 ± 6.52 pg/mg in the minocycline treated animals (P = 0.0001) (). Similar to the previous ELISA results, the levels of IL-1β and TNF-α, as measured by western blotting, were distinctly lower after minocycline treatment (P = 0.0001) when compared with control model animals ().
Figure 3 Assay of protein level of interleukin-1β and tumor necrosis factor-α.
Abbreviations: IL-1β, interleukin-1β; OD, optical density; TNF-α, tumor necrosis factor-α; M4, M6, M8: 4, 6, and 8 weeks after establishment of diabetes; MT4, MT6, MT8: 4, 6, and 8 weeks after minocycline intervention.
Discussion
STZ, a powerful alkylating agent used to develop animal models to study diabetes and associated complications, is particularly toxic to the insulin-producing beta cells of the pancreas in mammals via interfering with glucose transport and impacting glucokinase function. Diabetic models will be successfully established, which occurred with cataract, hyperglycemia, uric acid, diabetic nephropathy, and a blood glucose level equal to or above 150 mg/dL were identified as diabetic while the blood glucose level of normal rats varied between 50 to 135 mg/dL.Citation18,Citation19 Diabetic animals were continually given a high fat and high sugar diet. This study demonstrated that minocycline inhibited tau phosphorylation and decreased Aβ protein to maintain neural function, and attenuated neuroinflammation under diabetic metabolism disorder, indicating the neuroprotective role of minocycline in AD via inhibiting neuroinflammation.
Minocycline, an antibiotic that effectively crosses the blood–brain barrier, has been found to have significant neuroprotective effects on cerebral ischemia disorders,Citation20,Citation21 demyelinating diseases,Citation22,Citation23 and especially in neurodegenerative disorders including amyotrophic lateral sclerosis,Citation24,Citation25 AD,Citation26 Huntington’s disease,Citation27,Citation28 and Parkinson’s disease.Citation26,Citation29 Previously, the results from our group have demonstrated that minocycline improved cognitive impairment and retarded Aβ generation in the hippocampus caused by diabetic metabolic disorder, through inactivating the nuclear factor-κB pathway.Citation9 Considering the critical role of tau and Aβ pathology in the progress of cognitive impairment,Citation30,Citation31 it is possible that minocycline improves cognitive impairment via limiting tau hyperphosphorylation and Aβ pathology. On the basis of the finding that minocycline had no influence on beta-site amyloid precursor protein cleaving enzyme 1 in diabetic disorder, which determines the speed of Aβ generation,Citation9 it seems that minocycline has no modification on APP, since the critical role in Aβ generation is through modulating APP processing, and minocycline has no influence on APP level in this study.
Literature has shown that the two classical histopathological hallmarks include amyloid plaques (extracellular Aβ deposition) and neurofibrillary tangles (intracellular deposits of hyperphosphorylated tau protein) in AD.Citation32–Citation35 The hyperphosphorylation of tau plays an important role in the progress of AD. This study detected the levels of phosphorylated tau proteins, including pre-tangle marker phospho-tau antibody TG3 (pT231),Citation34,Citation35 intraneuronal tangle marker phospho-tau protein (Ser214, pS214), and the extracellular tangle marker PHF-1 (pS396/pS404).Citation36,Citation37 The results from this research found that minocycline strongly limited the phosphorylation of tau protein, including TG3 (pT231), pS214, and PHF-1 (pS396/pS404), but not total tau. These data implicate that one of the neuroprotective roles of minocycline is via the suppression of the tau phosphorylation.
A variety of research data supports that minocycline acts as the neuroprotector via inhibiting brain inflammation,Citation9,Citation10 astrocyte reactivation,Citation38 microglia activation,Citation39–Citation41 oxidative stress,Citation9,Citation38 apoptosis,Citation9 and extracellular matrix degradation.Citation42 Two common pathophysiological mechanisms of diabetic damage include oxidative stress and inflammation. Increasing evidence suggests that TNF-α and IL-1β (the inflammation markers) participate in the progression of brain injury from diabetes.Citation43,Citation44 To further explain the neuroprotective mechanisms of minocycline, TNF-α and IL-1β were measured. The decrease of TNF-α and IL-1β evoked by minocycline occurred in this investigation. Accordingly, minocycline decreased tau hyperphosphorylation and downregulated Aβ protein by restraining neuroinflammation from diabetes, while inflammation contributes to both the hyperphosphorylation of tau and Aβ generation.
Neuroinflammation may be responsible for neurodegeneration in vulnerable regions such as the hippocampus.Citation45,Citation46 There is growing evidence that supports a prominent role of inflammation in the development of AD.Citation47 An important feature of AD is that the hyperphosphorylation of tau and Aβ deposit may trigger an active, self-perpetuating cycle of chronic neuroinflammation which serves to further promote the hyperphosphorylation of tau and Aβ generation.Citation48,Citation49 Therefore, minocycline may lower the self-perpetuating cycle in the pathogenetic cascade of neurodegeneration in AD, indicating that minocycline aims at both mechanisms to be a beneficial agent in the prevention and treatment of AD.
This study has evaluated the influence of minocycline on tau protein and Aβ protein under diabetic disorder. Minocycline decreases the hyperphosphorylation of tau and Aβ production by inhibiting the process of neuroinflammation. Minocycline may also lower the self-perpetuating cycle between neuroinflammation and the pathogenesis of tau and Aβ to act as a neuroprotector, maintain neural function, and improve behavioral deficits under diabetic disorder. Accordingly, the ability of minocycline to modulate the inflammatory reaction may be of great importance in the selection of neuroprotective agents, especially in chronic procedures like AD. Hence, further studies exploring the effect of minocycline on these mechanisms may lead to better understanding and improvement of the role of treatment.
Acknowledgments
This study was supported in part by the Animal Center of Chongqing Medical University and research projects from the Ministry of Civil Affairs, People’s Republic of China (2008-47-3-02).
Disclosure
The authors report no conflicts of interest in this work.
References
- WangKCWoungLCTsaiMTLiuCCSuYHLiCYRisk of Alzheimer’s disease in relation to diabetes: a population-based cohort studyNeuroepidemiology20123823724422572745
- TakedaS[Pathological interaction between diabetes mellitus and Alzheimer’s disease.]Nihon Shinkei Seishin Yakurigaku Zasshi201232239244 Japanese23373309
- KimBBackusCOhSFeldmanELhyperglycemia-induced tau cleavage in vitro and in vivo: a possible link between diabetes and Alzheimer’s diseaseJ Alzheimers Dis20133472773923254634
- BartlJMonoranuCMWagnerAKKolterJRiedererPGrunblattEAlzheimer’s disease and type 2 diabetes: two diseases, one common link?World J Biol Psychiatry20131423324022332892
- PilcherHAlzheimer’s disease could be “type 3 diabetes”Lancet Neurol2006538838916639835
- de la MonteSMTongMLester-CollNPlaterMJrWandsJRTherapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer’s diseaseJ Alzheimers Dis2006108910916988486
- ZhangZYZhangZFauserUSchluesenerHJImproved outcome of EAN, an animal model of GBS, through amelioration of peripheral and central inflammation by minocyclineJ Cell Mol Med20091334135118400050
- KuangXScofieldVLYanMStoicaGLiuNWongPKAttenuation of oxidative stress, inflammation and apoptosis by minocycline prevents retrovirus-induced neurodegeneration in miceBrain Res2009128617418419523933
- CaiZZhaoYYaoSBin ZhaoBIncreases in beta-amyloid protein in the hippocampus caused by diabetic metabolic disorder are blocked by minocycline through inhibition of NF-kappaB pathway activationPharmacol Rep20116338139121602593
- CaiZYYanYChenRMinocycline reduces astrocytic reactivation and neuroinflammation in the hippocampus of a vascular cognitive impairment rat modelNeurosci Bull201026283620101270
- CaiZYYanYSunSQMinocycline attenuates cognitive impairment and restrains oxidative stress in the hippocampus of rats with chronic cerebral hypoperfusionNeurosci Bull20082430531318839024
- GranicIDolgaAMNijholtIMvan DijkGEiselULInflammation and NF-kappaB in Alzheimer’s disease and diabetesJ Alzheimers Dis20091680982119387114
- de la MonteSMBrain insulin resistance and deficiency as therapeutic targets in Alzheimer’s diseaseCurr Alzheimer Res20129356622329651
- DongSFHongYLiuMBerberine attenuates cardiac dysfunction in hyperglycemic and hypercholesterolemic ratsEur J Pharmacol201166036837421458442
- ReedMJMeszarosKEntesLJA new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated ratMetabolism2000491390139411092499
- ClarkJDGebhartGFGonderJCKeelingMEKohnDFSpecial Report: The 1996 Guide for the Care and Use of Laboratory AnimalsILAR J1997381414811528046
- RoulstonCLLawrenceAJWiddopREJarrottBMinocycline treatment attenuates microglia activation and non-angiotensin II [125I] CGP42112 binding in brainstem following nodose ganglionectomyNeuroscience20051351241125316165304
- OzakiKSanoTTsujiNMatsuuraTNaramaIInsulin-induced hypoglycemic peripheral motor neuropathy in spontaneously diabetic WBN/Kob ratsComp Med20106028228720819377
- TzeWJTaiJWongFCDavisHRStudies with implantable artificial capillary units containing rat islets on diabetic dogsDiabetologia1980195415456780399
- BhattacharyaPPandeyAKPaulSMinocycline and magnesium in combination may be a good therapeutic intervention for cerebral ischemiaMed Hypotheses2011771129113121985758
- MartinABoisgardRKassiouMDolleFTavitianBReduced PBR/TSPO expression after minocycline treatment in a rat model of focal cerebral ischemia: a PET study using [(18)F]DPA-714Mol Imaging Biol201113101520383592
- ChenXMaXJiangYPiRLiuYMaLThe prospects of minocycline in multiple sclerosisJ Neuroimmunol20112351821565409
- DefauxAZurichMGHoneggerPMonnet-TschudiFMinocycline promotes remyelination in aggregating rat brain cell cultures after interferon-gamma plus lipopolysaccharide-induced demyelinationNeuroscience2011187849221549181
- ZhangWNarayananMFriedlanderRMAdditive neuroprotective effects of minocycline with creatine in a mouse model of ALSAnn Neurol20035326727012557297
- GordonPHMooreDHMillerRGEfficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trialLancet Neurol200761045105317980667
- BlumDChtartoATenenbaumLBrotchiJLevivierMClinical potential of minocycline for neurodegenerative disordersNeurobiol Dis20041735936615571972
- BantubungiKJacquardCGrecoAMinocycline in phenotypic models of Huntington’s diseaseNeurobiol Dis20051820621715649711
- YongVWWellsJGiulianiFCashaSPowerCMetzLMThe promise of minocycline in neurologyLancet Neurol2004374475115556807
- DuYMaZLinSMinocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s diseaseProc Natl Acad Sci USA200198146691467411724929
- AmlienIKFjellAMWalhovdKBMild cognitive impairment: cerebrospinal fluid tau biomarker pathologic levels and longitudinal changes in white matter integrityRadiology201326629530323151827
- ParnettiLChiasseriniDEusebiPPerformance of abeta1–40, abeta1–42, total tau, and phosphorylated tau as predictors of dementia in a cohort of patients with mild cognitive impairmentJ Alzheimers Dis20122922923822232006
- LomoioSScheriniENecchiDBeta-amyloid overload does not directly correlate with SAPK/JNK activation and tau protein phosphorylation in the cerebellar cortex of Ts65Dn miceBrain Res2009129719820619703431
- MohandasERajmohanVRaghunathBNeurobiology of Alzheimer’s diseaseIndian J Psychiatry200951556119742193
- PaponMAEl KhouryNBMarcouillerFDeregulation of protein phosphatase 2A and hyperphosphorylation of tau protein following onset of diabetes in NOD miceDiabetes20136260961722961084
- WangYChengZQinWJiaJVal97 Leu mutant presenilin-1 induces tau hyperphosphorylation and spatial memory deficit in mice and the underlying mechanismsJ Neurochem201212113514521929538
- AugustinackJCSchneiderAMandelkowEMHymanBTSpecific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s diseaseActa Neuropathol2002103263511837744
- LiuYSuYWangJRapamycin decreases tau phosphorylation at Ser214 through regulation of cAMP-dependent kinaseNeurochem Int20136245846723357480
- KellerAFGravelMKrizJTreatment with minocycline after disease onset alters astrocyte reactivity and increases microgliosis in SOD1 mutant miceExp Neurol2011228697921168408
- GiulianiFHaderWYongVWMinocycline attenuates T cell and microglia activity to impair cytokine production in T cell-microglia interactionJ Leukoc Biol20057813514315817702
- SiQCosenzaMKimMOA novel action of minocycline: inhibition of human immunodeficiency virus type 1 infection in microgliaJ Neurovirol20041028429215385251
- TikkaTFiebichBLGoldsteinsGKeinanenRKoistinahoJMinocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microgliaJ Neurosci2001212580258811306611
- YenariMAXuLTangXNQiaoYGiffardRGMicroglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitroStroke2006371087109316497985
- ParthsarathyVHolscherCThe type 2 diabetes drug liraglutide reduces chronic inflammation induced by irradiation in the mouse brainEur J Pharmacol2013700425023276669
- CelikSErdoganSCaffeic acid phenethyl ester (CAPE) protects brain against oxidative stress and inflammation induced by diabetes in ratsMol Cell Biochem2008312394618265948
- HurleyLLTizabiYNeuroinflammation, neurodegeneration, and depressionNeurotox Res20132313114422895696
- PintadoCGavilanMPGavilanELipopolysaccharide-induced neuroinflammation leads to the accumulation of ubiquitinated proteins and increases susceptibility to neurodegeneration induced by proteasome inhibition in rat hippocampusJ Neuroinflammation201298722559833
- CraftJMWattersonDMVan EldikLJHuman amyloid beta-induced neuroinflammation is an early event in neurodegenerationGlia20065348449016369931
- CaiZZhaoBRatkaAOxidative stress and beta-amyloid protein in Alzheimer’s diseaseNeuromolecular Med20111322325021901428
- TexelSJMattsonMPImpaired adaptive cellular responses to oxidative stress and the pathogenesis of Alzheimer’s diseaseAntioxid Redox Signal2011141519153420849373
