Abstract
Purpose
To systematically review the literature describing the efficacy, effectiveness, and safety of raloxifene for postmenopausal Japanese women with osteoporosis or low bone mass (osteopenia).
Materials and methods
Medline via PubMed and Embase was systematically searched using prespecified terms. Retrieved publications were screened and included if they described randomized controlled trials or observational studies of postmenopausal Japanese women with osteoporosis or osteopenia treated with raloxifene and reported one or more outcome measures (change in bone mineral density [BMD]; fracture incidence; change in bone-turnover markers, hip structural geometry, or blood–lipid profile; occurrence of adverse events; and change in quality of life or pain). Excluded publications were case studies, editorials, letters to the editor, narrative reviews, or publications from non-peer-reviewed journals; multidrug, multicountry, or multidisease studies with no drug-, country-, or disease-level analysis; or studies of participants on dialysis.
Results
Of the 292 publications retrieved, 15 publications (seven randomized controlled trials, eight observational studies) were included for review. Overall findings were statistically significant increases in BMD of the lumbar spine (nine publications), but not the hip region (eight publications), a low incidence of vertebral fracture (three publications), decreases in markers of bone turnover (eleven publications), improved hip structural geometry (two publications), improved blood–lipid profiles (five publications), a low incidence of hot flushes, leg cramps, venous thromboembolism, and stroke (12 publications), and improved quality of life and pain relief (one publication).
Conclusion
Findings support raloxifene for reducing vertebral fracture risk by improving BMD and reducing bone turnover in postmenopausal Japanese women with osteoporosis or osteopenia. Careful consideration of fracture risk and the risk–benefit profile of antiosteoporosis medications is required when managing patients with osteoporosis.
Introduction
Osteoporosis is a major health problem worldwide that is “characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fractures”.Citation1 In Japan, population-based estimates using 2005 data and no age cutoffs suggest that osteoporosis affects between 6.4 million and 11 million people, and that the incidence of osteoporosis increases with age and is significantly greater in women than men.Citation2 Given that Japanese people have the world’s longest life expectancy from birth (currently at 83.7 years for 2010–2015)Citation3 and Japan’s rapidly increasing aged population,Citation4 there is a clear need to reduce the burden of osteoporosis in the coming years.
Fracture is the most serious consequence of osteoporosis. This is primarily because women with osteoporosis have marked deterioration in bone mineral density (BMD) and bone architecture, which results in deterioration in bone strength.Citation5 Of the types of osteoporotic fractures, vertebral fractures are of great concern, because of the risk of subsequent vertebral fractures and the resulting “vertebral fracture cascade”,Citation6 the increased risk of nonvertebral fractures following vertebral fractures,Citation7,Citation8 and the considerable effect vertebral fractures have on pain, health-related quality of life, and mortality rate.Citation9–Citation14 The impact of vertebral fractures is particularly important for Japanese women, because findings in population-based or longitudinal studies that used similar morphometric methods to assess the incidence of vertebral fracture have shown a higher incidence of vertebral fractures in Japanese women than Caucasian women.Citation15–Citation17 Hip fractures resulting from osteoporosis are also a significant burden. In Japan, hip-fracture incidence is expected to increase 68% from 2012 to 2040, with an average hospital cost of US$27,599 for surgical treatment.Citation18
In Japan, therapeutic treatments recommended for osteoporosis include bisphosphonates (eg, risedronate, alendronate), selective estrogen-receptor modulators (eg, raloxifene, bazedoxifene), active vitamin D3 derivatives (eg, alfacalcidol, eldecalcitol), and recombinant parathyroid hormone.Citation19 Bisphosphonates are the most familiar and well-studied of these treatments,Citation19,Citation20 with proven efficacy for vertebral fracture reduction in Japanese patients.Citation21 Of the other treatments, raloxifene, a nonsteroidal benzothiophene derivative of the selective estrogen receptor-modulator class, has been used to treat postmenopausal osteoporosis in Japan since May 2004 (60 mg tablets).Citation19 Raloxifene is a suitable therapy for the treatment of postmenopausal osteoporosis, because the estrogen-like actions of raloxifene in bone averts the imbalance in bone turnover (excess resorption versus formation) caused by postmenopausal estrogen deficiency. In addition, the estrogen-like actions of raloxifene are tissue-specific, because raloxifene does not stimulate mammary or uterine endometrial tissue.Citation22 Compared with placebo, raloxifene has been shown to reduce the relative risk of vertebral fractures by up to 69% in postmenopausal Caucasian women with osteoporosis after 3 years of treatment.Citation23 Additional findings for raloxifene indicate increases in lumbar spine BMDCitation22 and in terms of bone quality, improvements in hip cortical geometry,Citation24,Citation25 and collagen quality by reducing nonenzymatic collagen crosslinks,Citation26 and the maintenance of heterogeneous mineralization in bone.Citation27 Although findings from a post hoc analysis of data from two independent studies indicated that postmenopausal Japanese and Chinese women treated with raloxifene had a lower incidence of vertebral fractures than those treated with placebo,Citation28 the available data describing the effect of raloxifene treatment in postmenopausal Japanese women have not been adequately synthesized. Synthesis and evaluation of these data may provide valuable information for Japanese physicians treating postmenopausal women with osteoporosis.
To evaluate the existing evidence for postmenopausal Japanese women with osteoporosis or low bone mass (osteopenia) treated with raloxifene, we performed a systematic review of the literature. The objective of this review was to examine the efficacy, effectiveness, and safety findings from clinical trials and observational studies of raloxifene and to provide clinical insight into the usefulness of raloxifene for preventing or reducing the risk of subsequent vertebral and nonvertebral fractures in Japan.
Materials and methods
Search strategy
A search for relevant publications was done on May 28, 2013 using the database Medline via PubMed and Embase. The search terms were Japan (Medical Subject Headings [MeSH], Emtree), raloxifene (MeSH, Emtree), Evista, osteoporosis (MeSH, Emtree), fracture (Emtree), fracture*, and bone density (MeSH, Emtree). Search terms were combined using the Boolean operators OR and AND to give the following strategy: Japan AND (raloxifene OR Evista) AND (osteoporosis OR [fracture OR fracture*] OR bone density). The search limits were human species only and publication date from January 1, 1980 onwards.
Study selection
Publications identified in Medline via PubMed and Embase were collated using Endnote X5 (Thomson Reuters, New York, NY, USA). Duplicate publications were discarded, and the remaining publications were screened using prespecified inclusion and exclusion criteria. The title and abstract of each publication were screened initially; the full text of a publication was screened only if screening of the title and abstract was inconclusive. Publications describing randomized controlled clinical trials and observational studies (prospective and retrospective) of postmenopausal women with osteoporosis or osteopenia receiving raloxifene treatment were included if they reported one or more outcome measures. Outcome measures were change in BMD of the lumbar spine, femoral neck, total hip, total neck, or other areas within the hip region; incidence of new vertebral fracture or nonvertebral fracture; change in biochemical markers of bone turnover, hip structural geometry, or blood–lipid profile; occurrence of adverse events (AEs; type, incidence, and severity), in particular venous thromboembolism (VTE), cardiovascular events, stroke, vaginal bleeding, or hot flush; effect on coagulation parameters or breast, uterus, ovary, or reproductive tissues; and change in quality of life or pain.
Publications were excluded if they were case studies, editorials, letters to the editor, narrative reviews, or published in a non-peer-reviewed journal; were multidrug studies that did not include a subanalysis of raloxifene; were multicountry studies that did not include a subanalysis of Japanese participants; were multidisease studies that did not include a subanalysis of participants with osteoporosis or osteopenia; or if participants were on dialysis. The bibliographies of systematic reviews were screened for other potentially relevant publications.
Data extraction and analysis
Data extraction was conducted by one person, and the extracted data were reviewed by all authors. Data extracted were study and participant characteristics (study design, number and mean age of participants, therapy and dose, study duration [ie, number of weeks], disease definition, study objective), and findings for BMD of the lumbar spine, femoral neck, total hip, total neck, or other areas within the hip region (percentage change in BMD from baseline to 52 weeks or BMD at baseline and at 52 weeks), vertebral and nonvertebral fracture incidence, biochemical markers for bone turnover (percentage change in concentration from baseline to 52 weeks or concentration at baseline and at 52 weeks), hip structural geometry parameters (percentage change in parameters from baseline to 52 weeks), blood–lipid profile (percentage change in concentration from baseline to 52 weeks or concentration at baseline and at 52 weeks), AEs (type, incidence, and severity; incidence of VTE, cardiovascular events, stroke, vaginal bleeding, or hot flush) and quality of life and pain (mean change in scores from baseline to 24 weeks).
Results
Literature-search results
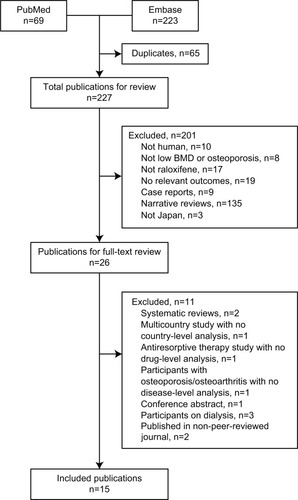
A total of 292 abstracts were retrieved from the search of PubMed and Embase (). Duplicate publications were discarded (n=65), the remaining 227 abstracts screened, and 26 publications selected for full-text review. The main reasons for exclusion were no relevant outcomes reported, raloxifene not included, or study not conducted in humans (). The remaining 15 publications were included for review.
Study and participant characteristics
Of the 15 publications included for review, there were seven randomized controlled trialsCitation29–Citation35 reporting evidence for efficacy and eight observational studiesCitation24,Citation36–Citation42 reporting evidence of effectiveness (). Evidence of safety was reported in 12Citation29–Citation33,Citation35–Citation38,Citation40–Citation42 of the 15 publications. The method of randomization and allocation (eg, randomly generated treatment codes, random self-drawing of prepared sealed envelopes) was described in fourCitation29,Citation32,Citation33,Citation35 of the seven randomized controlled trials. Only the double-blind placebo-controlled trialCitation35 and an open-label randomized controlled trialCitation30 described whether randomization and allocation were blinded. The number of participants enrolled varied from 39 in one randomized controlled trialCitation30 to 7,557 in two postmarketing surveillance observational studies.Citation40,Citation41 The mean age of participants ranged from 63 to 80 years (). On average, most participants had undergone menopause at 50 years of age (data not shown). Most publications (14 of 15) assessed the effects of raloxifene for a minimum of 52 weeks (). A definition for osteoporosis and osteopenia was reported in 14 of the 15 publications, with most publications (n=11) defining osteoporosis and osteopenia according to the Japanese diagnostic criteria year 2000 revisionCitation43 ().
Table 1 Study and participant characteristics
BMD
Findings for BMD were reported in eleven of the 15 publications, and included BMD of the lumbar spine (nine publications),Citation29,Citation31–Citation33,Citation35–Citation38,Citation40 of the femoral neck, total hip, or total neck (six publications),Citation29,Citation32,Citation33,Citation36–Citation38 or of other regions in the hip (five publications).Citation24,Citation33,Citation36,Citation38,Citation39 Findings were reported as the percentage change in BMD from baseline to 52 weeks or BMD at baseline and at 52 weeks in all publications. There were three longer-term studies reporting BMD at 104 weeksCitation24,Citation32,Citation40 and at 156 weeks.Citation40
After 52 weeks of treatment with raloxifene 60 mg/day, lumbar spine BMD increased significantly from baseline in all nine publications reporting findings for lumbar spine BMD, including the randomized placebo-controlled trialCitation35 of raloxifene 60 or 120 mg/day (). In the randomized comparative trials, the increase in lumbar spine BMD for raloxifene was less than that for alendronate (P<0.01),Citation31 more than that for alfacalcidol,Citation29,Citation32 and less thanCitation32 or more thanCitation29,Citation33 that for combination treatment with raloxifene and alfacalcidol ().
Table 2 Studies reporting mean (SD) percentage change in bone mineral density or mean (SD) bone mineral density (g/cm2) of the lumbar spine, femoral neck, total hip, or total neck after 52 weeks of RLX treatmentTable Footnotea
Compared with lumbar spine BMD, the effect of raloxifene 60 mg/day on BMD in the femoral neck, total hip, or total neck () or other regions of the hip (data not shown) was not consistent after 52 weeks of treatment. In the eight publicationsCitation24,Citation29,Citation32,Citation33,Citation36–Citation39 that reported findings for BMD in the femoral neck, total hip, total neck, or other regions of the hip, BMD increased, remained the same, or decreased; few of the increases in BMD were statistically significant.
Fracture incidence
Fracture incidence (vertebral or nonvertebral) was reported in three of the 15 publications, including publications from two randomized controlled trialsCitation31,Citation35 and one observational study.Citation40 However, only the observational study, which was a postmarketing surveillance study, was sufficiently powered to detect the incidence of vertebral fractures.Citation40 Findings from this study suggested that after 36 months of treatment with raloxifene, the incidence of new clinical vertebral and nonvertebral fractures in postmenopausal women is low. Of the 6,967 participants, 36 (0.5%) reported new clinical vertebral fractures and 52 (0.7%) reported new clinical nonvertebral fractures.Citation40 Nearly half of these participants had prevalent fractures: 17 of the 36 participants (47%) with new clinical vertebral fractures and 19 of the 52 participants (37%) with new clinical nonvertebral fractures.
In a smaller randomized placebo-controlled study, few postmenopausal women taking raloxifene (60 mg/day or 120 mg/day) had a new vertebral fracture (0.05%, one of 183, versus placebo 2%, two of 97) or a new nonvertebral fracture (0.05%, one of 183, versus placebo 4%, four of 97) after 52 weeks of treatment.Citation35 In addition, findings from another randomized study suggested that the incidence of vertebral fractures was not significantly different between postmenopausal women taking raloxifene (13.1%, n=61) and alendronate (14.0%, n=61).Citation31
Biochemical markers of bone turnover
Findings for biochemical markers of bone turnover were reported in eleven of the 15 publications: publications from six randomized controlled trialsCitation29–Citation33,Citation35 and five observational studies.Citation36–Citation40 The biochemical markers were alkaline phosphatase or bone-specific alkaline phosphatase (BAP; ten publications), type 1 collagen N-telopeptide (NTx; ten publications), type 1 collagen C-telopeptide (CTx; three publications), osteocalcin (one publication), tartrate-resistant acid phosphatase (one publication), and deoxypyridinoline (one publication) (). Findings were reported as the percentage change in concentration from baseline to 52 weeks or concentration at baseline and at 52 weeks.
Table 3 Studies reporting mean (SD) percentage change in or mean (SD) concentrations for biochemical markers of bone turnover after 52 weeks of RLX treatment
Concentrations of all biochemical markers of bone turnover assessed decreased after 52 weeks of treatment with raloxifene (). The decreases in the biochemical marker concentrations from baseline were statistically significant when statistical significance was reported. When reported, the mean percentage decrease in concentrations after 52 weeks of treatment with raloxifene varied from 10%Citation31 to 38%Citation30 for BAP and 13.5%Citation39 to 35%Citation29,Citation31 for NTx. In the randomized comparative trial of raloxifene and alendronate,Citation31 the mean percentage decreases in serum alkaline phosphatase concentrations after 52 weeks of treatment and urinary NTx concentrations after 12 weeks of treatment were less for raloxifene than alendronate (alkaline phosphatase not significant, NTx P<0.05, ). In the randomized comparative trials of raloxifene and alfacalcidol, the effect of raloxifene on BAP, NTx, and CTx concentrations was more pronounced than that of alfacalcidol after 52 and 104 weeks of treatment,Citation32 and was less pronounced, similar to, or more pronounced than that combination treatment with raloxifene and alfacalcidol ().Citation29,Citation32,Citation33
Hip structural geometry
Findings for the hip structural geometry in the proximal femur were reported in two of the 15 publications, both of which were prospective observational studies.Citation24,Citation39 The hip-structure analysis parameters were the cross-sectional area, mean cortical thickness, section modulus and buckling ratio of the narrow neck, intertrochanter, and shaft regions of the proximal femur; one publication reported the inner diameter,Citation39 and the other reported the outer diameter.Citation24 Findings were reported as the mean (95% confidence interval [CI]) percentage change in parameters from baseline to 52 weeks in both publications and from baseline to 104 weeks in one publication.Citation24
Nearly all hip-structure analysis parameters for the intertrochanter and shaft regions of the proximal femur improved significantly after 52 weeks of treatment with raloxifene. For the intertrochanter and shaft regions, there were significant (P<0.05) increases in the cross-sectional area, mean cortical thickness, and section modulus after 52 weeksCitation24,Citation39 and 104 weeksCitation24 of raloxifene treatment. In addition, there was a significant (P<0.05) decrease in the buckling ratio of the intertrochanter and shaft regions in one publicationCitation39 and in the intertrochanter region in the other publication.Citation24 However, this difference for the intertrochanter region was not significant at 104 weeks.Citation24 In contrast, only a few hipstructure analysis parameters for the narrow neck regions for the proximal femur had improved significantly after 52 weeks of treatment with raloxifene.Citation24 These significant improvements (P<0.05) included increases in the cross-sectional area, section modulus, and outer diameter.Citation24
Blood–lipid parameters
Findings for blood–lipid parameters were reported in five of the 15 publications, including publications from four randomized controlled trialsCitation31,Citation33–Citation35 and one prospective observational study.Citation36 The blood–lipid parameters were total cholesterol (four publications), high-density lipoprotein cholesterol (five publications), low-density lipoprotein (LDL) cholesterol (five publications), and triglycerides (five publications) (). Findings were reported as the percentage change in concentration from baseline to 52 weeks or concentration at baseline and at 52 weeks.
Table 4 Studies reporting mean (SD) percentage change in blood–lipid parameters or mean (SD) blood–lipid parameters after 52 weeks of RLX treatment
In general, the blood–lipid profile of participants had improved after 52 weeks of treatment with raloxifene (). Decreases in the concentrations of both total cholesterol and LDL cholesterol from baseline concentrations were reported in all publications reporting findings of these parameters. These decreases were statistically significant for total cholesterol concentrations in three publicationsCitation31,Citation33,Citation36 and LDL cholesterol concentrations in two publications.Citation31,Citation36 The concentration of high-density lipoprotein cholesterol was significantly increased (P<0.05) in one publication,Citation34 but remained the same in the four other publications (). The concentration of triglycerides either decreased or remained the same ().
In the randomized controlled trial, decreases in total cholesterol concentrations and LDL cholesterol concentrations were significantly greater (P<0.05) for participants receiving raloxifene (60 mg/day and 120 mg/day) than those receiving placebo after 52 weeks of treatment.Citation35 In the randomized comparative trial of raloxifene and alendronate, decreases in LDL cholesterol concentrations were significantly greater (P<0.05) for participants receiving raloxifene than those receiving alendronate after 52 weeks of treatment.Citation31
Safety
Findings for safety variables were reported in 12 of the 15 publications: publications from six randomized controlled trialsCitation29–Citation33,Citation35 and six observational studies.Citation36–Citation38,Citation40–Citation42 Safety variables were the type, incidence, and severity of AEs (four publications) (), study discontinuations resulting from AEs (nine publications) (), stroke risk (one publication),Citation41 and change in markers of coagulation and fibrinolysis (one publication).Citation30 Three publications from one randomized controlled trialCitation34 and two observational studiesCitation24,Citation39 did not report findings for any safety variables.
Table 5 Adverse events (AEs)
Table 6 Study discontinuations
The type, incidence, and severity of AEs were reported in four publications from two randomized controlled trialsCitation29,Citation35 and two observational studies,Citation40,Citation42 both of which were postmarketing surveillance studies (). The safety findings were consistent with those expected for raloxifene use in Japan.Citation44 In the randomized placebo-controlled trial,Citation35 almost half of the participants reported at least one AE, whereas about 10% of the participants in the long-term postmarketing surveillance study reported an AE.Citation40 Few postmenopausal women had hot flushes, leg cramps, breast pain, or vaginal bleeding (when reported) in the randomized trials (). Clinically relevant abnormal changes in breast tissue were reported in one woman taking raloxifene 120 mg/day (inspection and palpation) and in one woman taking placebo (ultrasound examination) in the randomized placebo-controlled trial.Citation35 In addition, clinically relevant abnormal changes in endometrial thickness were reported in two women taking raloxifene 60 mg/day and one woman taking raloxifene 120 mg/day.Citation35 Common AEs reported in the postmarketing surveillance studies were peripheral edema and abdominal discomfort ().
In a postmarketing surveillance study of 6,970 postmenopausal women, the risk of stroke was not significantly increased after 52 weeks of treatment with raloxifene.Citation41 In this study, 23 treatment-emergent stroke cases were reported (crude stroke risk =0.33%). Of these 23 cases, four had a previous history of stroke, nine had risk factors for stroke (eg, hypertension), and ten had no risk factors for stroke. Four women died as a result of stroke.Citation41 In another postmarketing surveillance study of 6,967 postmenopausal women, there were 12 cases of stroke, eight of which were serious, after 156 weeks of treatment with raloxifene.Citation40
Although no VTE events were reported in the randomized placebo-controlled trial, there were eleven cases of VTE, three of which were serious, in the 3-year postmarketing surveillance study (). In another publication, the concentration of plasminogen-activator inhibitor, a marker for the increased risk of VTE, was increased after 52 weeks of treatment with raloxifene.Citation30 This increase in plasminogen activator-inhibitor concentration was noted for participants taking raloxifene in the morning, but not those taking raloxifene in the evening, suggesting that dosing time may have influenced the safety of raloxifene in this study population.
Study discontinuations resulting from AEs were reported in nine publications from six randomized controlled trialsCitation29–Citation33,Citation35 and three observational studies ().Citation36–Citation38 Few participants discontinued treatment because of AEs; leg and limb cramps, and muscle pain were the most common reasons for participants discontinuing raloxifene treatment ().
Quality of life and pain
Findings for quality of life and pain were reported in one publication from a postmarketing surveillance study.Citation42 In this publication, quality of life was assessed using the Short Form (SF)-8 Health Survey, the European Quality of Life Instrument, and the Japanese Osteoporosis Quality of Life Questionnaire, whereas pain was assessed using a visual analog scale and a pain-frequency survey. Findings were reported as the mean (standard deviation) change in scores from baseline to 24 weeks.
Improvement in quality of life and relief from pain was reported after 24 weeks of treatment with raloxifene.Citation42 All scores for the SF-8 domains (general health, physical functioning, role physical, bodily pain, vitality, social functioning, mental health, and role – emotional) improved significantly (P<0.001) from baseline, as did the European Quality of Life Instrument score. Significant improvements (P<0.05) in the total score and the scores of individual domains, except for the recreation/social activities domain, for the Japanese Osteoporosis Quality of Life Questionnaire were also reported. Relief from pain was indicated by a significant decrease (P<0.001) in pain severity (decreased visual analog scale scores) and decreases in the frequency of pain (fewer participants reporting permanent frequent pain).
Discussion
This is the first systematic review describing the efficacy, effectiveness, and safety outcomes of postmenopausal Japanese women with osteoporosis or osteopenia treated with raloxifene. Overall, a broad range of outcomes were reported for raloxifene (eg, BMD, bone turnover, lipid metabolism, AEs) in randomized controlled studies and observational studies, which included postmarketing surveillance studies. Despite the variation in study designs and methods reported, the body of evidence in this systematic review supports the effectiveness of raloxifene in increasing lumbar spine BMD and reducing the incidence of subsequent fracture, is associated with improvements in other healthoutcome measures, and is well tolerated in postmenopausal Japanese women. When reported, lumbar spine BMD increased significantly,Citation29,Citation31–Citation33,Citation35–Citation38,Citation40 and biochemical markers of bone turnover decreased after 52 weeks of treatment with raloxifene.Citation29–Citation33,Citation35–Citation40 However, limited data were available to confirm whether these improvements in bone quality were associated with a reduction in the incidence of vertebral or nonvertebral fracture in postmenopausal Japanese women. The AEs reported in the studies included in this review were consistent with the safety profile of raloxifene use in Japan.Citation44
In bone cells, where postmenopausal estrogen deficiency has caused an imbalance in bone turnover (excess resorption versus formation), raloxifene binds to estrogen receptors and induces conformational changes that are distinct from the binding of estrogen.Citation45 Raloxifene then acts as an agonist to decrease bone resorption and normalize bone turnover, thereby preserving BMD. In the MORE (Multiple Outcomes of Raloxifene Evaluation) study (a pivotal multicenter, international, blinded, randomized, placebo-controlled trial of 7,705 postmenopausal women with osteoporosis from Europe, the Americas, and Oceania),Citation46 raloxifene was shown to increase BMD, improve bone strength, and prevent vertebral fractures, but not to reduce the risk of nonvertebral fractures as a primary outcome.Citation47,Citation48 In our systematic review, the increase in lumbar spine BMD and decrease in biochemical markers of bone turnover in postmenopausal Japanese women support the findings from the pivotal studies of raloxifene conducted in Caucasian populations.Citation47,Citation48 In another publication excluded from our review (because it was published in a non-peer-reviewed journal), the increase in lumbar spine BMD reported for raloxifene was 7.1% at 26 weeks.Citation49 In this study, raloxifene was coadministered with eldecalcitol, an active vitamin D3 analog, which has been shown to enhance the mechanical properties of trabecular and cortical bone by suppressing bone turnover and increasing BMD more than either monotherapy in ovariectomized rats.Citation50 Although in our review there were few head-to-head studies of raloxifene compared with other osteoporosis medications, the data available suggest that the effect of raloxifene on BMD and biochemical markers of bone turnover was not as pronounced as that of alendronate.Citation31 However, it is not clear how these findings translate to any potential differences in the effect of raloxifene on new vertebral fractures, because of the limited length of follow-up (52 weeks) and because this study was not sufficiently powered to assess incidence of vertebral fracture.Citation31
We identified only one publication sufficiently powered to detect vertebral fracture incidence. In this postmarketing surveillance studyCitation40 of Japanese women with osteoporosis treated with raloxifene, the low incidence of vertebral fractures was consistent with findings from the MORE studyCitation47,Citation48 and a post hoc analysis of combined study data from postmenopausal JapaneseCitation35 and Chinese women with osteoporosis.Citation28 Interestingly, the incidence of new clinical nonvertebral fractures (0.7%) was slightly higher than new clinical vertebral fractures (0.5%) in the postmarketing surveillance study.Citation40 This finding may have been due to the criteria used to define new clinical fractures (reported signs or symptoms suggestive of fracture subsequently corroborated by radiographs) that excluded vertebral morphometry, which may have identified more patients with a vertebral fracture. In the post hoc analysis, which was not included in this systematic review because the analysis combined data from both Japanese and Chinese populations, the incidence of new clinical vertebral fractures was significantly lower for postmenopausal Japanese and Chinese women taking raloxifene (60 mg/day or 120 mg/day) than those taking placebo (0 of 289 versus seven of 199 [3.5%], P=0.002).Citation28
Treatments that help improve lumbar spine BMD and bone quality and consequently reduce the incidence of vertebral fracture (which includes preventing or reducing the risk of subsequent vertebral and/or nonvertebral fractures) are important in Japanese populations. This is because the incidence of vertebral fractures in Japanese women appears to be higher than in Caucasian women. In studies using similar morphometric methods, the incidence of vertebral fracture in the Japanese study was about 40 per 1,000 person-years for women in their 70s,Citation15 whereas the incidence in studies of Caucasian women of a similar age are about twofold lower.Citation16,Citation17,Citation51 In another study, the prevalence of vertebral fracture in 70- to- 74-year-old women was greater in Japanese women (248 cases per 1,000) than women of Japanese descent (148 cases per 1,000) or Caucasian women (150 cases per 1,000).Citation52 The higher incidence of vertebral fractures for Japanese women is also apparent compared with women from other Asian countries. The prevalence of vertebral fractures was significantly greater in women aged 65–74 years from Japan than those from Hong Kong, Indonesia, and Thailand.Citation53 Factors specific to the Japanese lifestyle, culture, and ethnicity may influence the risk of fracture in Japanese women.Citation54 For example, BMD is lower in Japanese women than Caucasian women of the same age.Citation43,Citation55 Other factors shown to be possibly associated with vertebral fractures in Japan include weight, age, menstrual history,Citation56 genetic factors,Citation57 bone and calcium metabolism,Citation58 calcium intake,Citation59 and vitamin D levels.Citation60 All of these factors contribute to BMD levels, and thus may indirectly influence the prevalence of vertebral fractures. However, although these other factors may contribute indirectly, future fracture risk in women from Japan can be accurately predicted using age, BMD, and prior vertebral fracture status.Citation61
Findings from this review showed that although proximal femur structural geometry improved with raloxifene treatment,Citation24,Citation39 the effect of raloxifene on the BMD of the femoral neck, total hip, total neck, or other regions of the hip in postmenopausal Japanese women was variable.Citation24,Citation29,Citation32,Citation33,Citation36–Citation39 This variable effect on BMD in the hip region may be explained, at least in part, by participants having different BMD values for the hip region at baseline, because specific BMD values for the hip region were not an inclusion criterion in studies reporting these findings.Citation24,Citation29,Citation32,Citation33,Citation36–Citation39 Hip-structure analysis is a valuable measure of proximal femur geometry and strengthCitation62 that has been used to show age-, ethnic-, and sex-related differences in proximal femur geometry and strength,Citation63–Citation67 as well as the effects of osteoporotic treatments.Citation25,Citation68–Citation71 The findings from the studies that assessed hip structureCitation24,Citation39 suggest that raloxifene may have a beneficial effect on hip-bone quality. However, although this effect may translate to a reduction in the likelihood of hip fracture, there is no published evidence available to show that treatment with raloxifene reduces the incidence of hip fracture in postmenopausal women with osteoporosis.
The safety and tolerability findings in the publications included in this review suggested that raloxifene was well tolerated in most postmenopausal women in Japan. Few postmenopausal women discontinued because of AEs, and few postmenopausal women experienced AEs commonly associated with raloxifene use, such as leg cramps, hot flushes, and peripheral edema.Citation22 The main safety concern of treatment with raloxifene is an increased risk of VTE.Citation22 Although the incidence of VTE in clinical studies of raloxifene is low, findings from the pivotal MORE study, which excluded women with a history of thromboembolic events in the past 10 years, showed that the relative risk of VTE was 3.1 (95% CI 1.5–6.2)Citation46 and of pulmonary embolism was 4.5 (95% CI 1.1–19.5)Citation72 for raloxifene compared with placebo at 36 months. The estimated incidence of deep vein thrombosis in Japanese people is a tenth of that in Caucasian people (42 versus 370–420, respectively, per 1,000,000 people),Citation73 and the findings of this systematic review confirmed the low incidence of VTE in postmenopausal Japanese women taking raloxifene.Citation35,Citation40 In addition, evidence from largescale postmarketing surveillance studies showed that the incidence of stroke or fatal stroke was not different from the general female Japanese population after 1 yearCitation41 or 3 yearsCitation75 of treatment with raloxifene. Although the blood–lipid profile of postmenopausal women taking raloxifene had improved (eg, decreases in both total cholesterol and LDL cholesterol),Citation21,Citation33,Citation35,Citation36 there is no evidence that improved blood–lipid profiles are associated with better cardiovascular outcomes in postmenopausal women at increased risk of coronary heart disease.Citation75
This systematic review retrieved only one publication reporting quality-of-life and pain findings in Japanese women. In this postmarketing surveillance study,Citation42 treatment with raloxifene improved health-related quality-of-life scores and relieved pain. This study is important, because prevalent vertebral fractures can be a major contributor to the health-related quality of life of postmenopausal women with osteoporosis. In particular, multiple vertebral fractures are of concern in Japan, as they are associated with chronic pain and incapacitating spinal deformities, deterioration in activities of daily living, and an increased risk of death.Citation9–Citation14 Specifically, morphometric vertebral fracture in Japanese women is significantly associated with lower health-related quality-of-life scores,Citation76 and this loss of health-related quality of life occurred after incident vertebral fracture.Citation77 Further, in Japan, osteoporosis may also be a significant burden on the patient’s family, who are responsible for providing caregiving support to elderly family members with osteoporosis.
There were several limitations with this systematic review. First, although the publications included in this review reported a broad range of findings for raloxifene (eg, BMD, bone turnover, lipid metabolism, and AEs), these findings were limited by the different methods used and the study quality (ie, there was only one placebo-controlled randomized trial and one randomized trial comparing raloxifene with a bisphosphonate). Second, few publications assessed raloxifene treatment for more than 1 year, despite the increased risks of VTE and stroke with long-term use of raloxifene.Citation75 Third, publications of raloxifene coadministered with active metabolites of vitamin D were included. However, excluding these studies is not clinically appropriate, because active vitamin D3 analogs are widely prescribed in Japan concomitantly with antiresorptive agents to compensate for calcium absorption and inhibit subsequent parathyroid hormone secretion in osteoporosis patients. Fourth, we did not provide a separate analysis of those studies in which raloxifene was coadministered with active metabolites of vitamin D. Although active vitamin D3 analogs are widely prescribed in Japan concomitantly with antiresorptive agents, only threeCitation29,Citation32,Citation33 of the 15 publications included in this review assessed patients taking concomitant raloxifene and active vitamin D3 analogs (alfacalcidol), and all included raloxifene monotherapy treatment groups. Last, although there were no restrictions on language and the bibliographies of retrieved systematic reviews were hand-searched to identify any publications not retrieved in the electronic search, other nonindexed publications and unpublished data were not included.
In conclusion, osteoporosis is a major health problem in the aging population of Japan and is underdiagnosed and undertreated.Citation78 If left untreated, fracture may occur, resulting in considerable pain and decreased health-related quality of life. Findings from this systematic review support the efficacy and effectiveness of raloxifene for preventing or reducing the risk of subsequent vertebral and/or nonvertebral fractures by improving BMD and reducing bone turnover in postmenopausal Japanese women with osteoporosis or osteopenia. Other findings suggest that raloxifene is well tolerated and can improve quality of life. However, these findings should be considered in light of the limitations of the publications and the risk–benefit profile of raloxifene.
Acknowledgments
The authors thank Shuko Nojiri of Eli Lilly Japan K.K. for her contributions on earlier versions of this review. Medical writing assistance was provided by Julie Monk, PhD and Serina Stretton, PhD, CMPP (Certified Medical Publication Professional) of ProScribe Medical Communications, and was funded by Eli Lilly Japan K.K. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP2).
Disclosure
The study was funded by Eli Lilly Japan K.K., the manufacturer of raloxifene and teriparatide. SF has received speaker honoraria and consulting fees from Eli Lilly Japan K.K., Asahi-Kasei, Astellas, Chugai, Daiichi-Sankyo, Eisai, Ono, MDS, and Pfizer. MS is an employee of Eli Lilly Japan K.K.; PGC is an employee of Eli Lilly Australia; JAF and RB are employees of Eli Lilly and Company; all are stock stockholders in Eli Lilly and Company. EH was an employee of Eli Lilly Japan K.K. when this manuscript was developed. EH is currently an employee of Amgen Astellas Biopharma K.K.
References
- [No authors listed]Who are candidates for prevention and treatment for osteoporosis?Osteoporos Int19977116
- YoshimuraNMurakiSOkaHPrevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability studyJ Bone Miner Metab200927562062819568689
- United NationsWorld Mortality Report 2011New YorkUN2012 Available from: http://www.un.org/esa/population/publications/worldmortalityreport2011/World%20Mortality%20Report%202011.pdfAccessed June 13, 2013
- Statistics JapanCurrent population estimates as of October 1, 2012 Available from: http://www.stat.go.jp/english/data/jinsui/2012np/index.htmAccessed June 13, 2013
- MarshallDJohnellOWedelHMeta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fracturesBMJ19963127041125412598634613
- BriggsAMGreigAMWarkJDThe vertebral fracture cascade in osteoporosis: a review of aetiopathogenesisOsteoporos Int200718557558417206492
- KlotzbuecherCMRossPDLandsmanPBAbbottTA3rdBergerMPatients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesisJ Bone Miner Res200015472173910780864
- LindsayRBurgeRTStraussDMOne year outcomes and costs following a vertebral fractureOsteoporos Int2005161788515167988
- IkedaYSudoAYamadaTUchidaAMortality after vertebral fractures in a Japanese populationJ Orthop Surg (Hong Kong)201018214815220808003
- JinbayashiHAoyagiKRossPDItoMShindoHTakemotoTPrevalence of vertebral deformity and its associations with physical impairment among Japanese women: the Hizen-Oshima StudyOsteoporos Int200213972373012195536
- MiyakoshiNHongoMMaekawaSIshikawaYShimadaYItoiEBack extensor strength and lumbar spinal mobility are predictors of quality of life in patients with postmenopausal osteoporosisOsteoporos Int200718101397140317460805
- MiyakoshiNItoiEKobayashiMKodamaHImpact of postural deformities and spinal mobility on quality of life in postmenopausal osteoporosisOsteoporos Int200314121007101214557854
- SuzukiNOgikuboOHanssonTPrevious vertebral compression fractures add to the deterioration of the disability and quality of life after an acute compression fractureEur Spine J201019456757419760437
- TakahashiTIshidaKHiroseDTrunk deformity is associated with a reduction in outdoor activities of daily living and life satisfaction in community-dwelling older peopleOsteoporos Int200516327327915235766
- FujiwaraSKasagiFMasunariNNaitoKSuzukiGFukunagaMFracture prediction from bone mineral density in Japanese men and womenJ Bone Miner Res20031881547155312929946
- European Prospective Osteoporosis Study GroupFelsenbergDSilmanAJIncidence of vertebral fracture in Europe: results from the European Prospective Osteoporosis Study (EPOS)J Bone Miner Res200217471672411918229
- Van der KliftMDe LaetCEMcCloskeyEVHofmanAPolsHAThe incidence of vertebral fractures in men and women: the Rotterdam StudyJ Bone Miner Res20021761051105612054160
- MithalAEbelingPThe Asia-Pacific Regional Audit. Epidemiology, Costs and Burden of Osteoporosis in 2013Nyon, SwitzerlandInternational Osteoporosis Foundation2013 Available from: http://www.iofbonehealth.org/sites/default/files/media/PDFs/Regional%20Audits/2013-Asia_Pacific_Audit_0_0.pdfAccessed August 5, 2014
- Committee of Japanese Guidelines for the Prevention and Treatment of OsteoporosisJapanese Guidelines for the Prevention and Treatment of OsteoporosisTokyoLife Science2011
- HaradaAMatsuiYMizunoMTokudaHNiinoNOhtaTJapanese orthopedists’ interests in prevention of fractures in the elderly from fallsOsteoporos Int200415756056615067499
- IwamotoJSatoYTakedaTMatsumotoHEfficacy of antiresorptive agents for preventing fractures in Japanese patients with an increased fracture risk: review of the literatureDrugs Aging201229319120322372723
- CranneyAAdachiJDBenefit-risk assessment of raloxifene in postmenopausal osteoporosisDrug Saf200528872173016048357
- KanisJAJohnellOBlackDMEffect of raloxifene on the risk of new vertebral fracture in postmenopausal women with osteopenia or osteoporosis: a reanalysis of the Multiple Outcomes of Raloxifene Evaluation trialBone200333329330013678769
- TakadaJMikiTImanishiYEffects of raloxifene treatment on the structural geometry of the proximal femur in Japanese women with osteoporosisJ Bone Miner Metab201028556156720333418
- Uusi-RasiKBeckTJSemanickLMStructural effects of raloxifene on the proximal femur: results from the Multiple Outcomes of Raloxifene Evaluation TrialOsteoporos Int200617457558616392026
- SaitoMMarumoKSoshiSKidaYUshikuCShinoharaARaloxifene ameliorates detrimental enzymatic and nonenzymatic collagen cross-links and bone strength in rabbits with hyperhomocysteinemiaOsteoporos Int201021465566619484165
- BoivinGLipsPOttSMContribution of raloxifene and calcium and vitamin D3 supplementation to the increase of the degree of mineralization of bone in postmenopausal womenJ Clin Endocrinol Metab20038894199420512970287
- NakamuraTLiuJLMoriiHEffect of raloxifene on clinical fractures in Asian women with postmenopausal osteoporosisJ Bone Miner Metab200624541441816937275
- GoraiITanakaYHattoriSIwaokiYAssessment of adherence to treatment of postmenopausal osteoporosis with raloxifene and/or alfacalcidol in postmenopausal Japanese womenJ Bone Miner Metab201028217618419657590
- AndoHOtodaTOokamiHDosing time-dependent effect of raloxifene on plasma plasminogen activator inhibitor-1 concentrations in post-menopausal women with osteoporosisClin Exp Pharmacol Physiol201340322723223323567
- IwamotoJSatoYUzawaMTakedaTMatsumotoHComparison of effects of alendronate and raloxifene on lumbar bone mineral density, bone turnover, and lipid metabolism in elderly women with osteoporosisYonsei Med J29200849111912818306478
- GoraiIHattoriSTanakaYIwaokiYAlfacalcidol-supplemented raloxifene therapy has greater bone-sparing effect than raloxifene-alone therapy in postmenopausal Japanese women with osteoporosis or osteopeniaJ Bone Miner Metab201230334935822130786
- MajimaTKomatsuYShimatsuAEfficacy of combined treatment with raloxifene and alfacalcidol on bone density and biochemical markers of bone turnover in postmenopausal osteoporosisEndocr J200855112713418219181
- HayashiTInaKMaedaMNomuraHThe effects of selective estrogen receptor modulator treatment following hormone replacement therapy on elderly postmenopausal women with osteoporosisNitric Oxide201124419920321513812
- MoriiHOhashiYTaketaniYEffect of raloxifene on bone mineral density and biochemical markers of bone turnover in Japanese postmenopausal women with osteoporosis: results from a randomized placebo-controlled trialOsteoporos Int2003141079380012955333
- MajimaTKomatsuYShimatsuAClinical significance of 1-year treatment with raloxifene on bone and lipid metabolism in Japanese postmenopausal women with osteoporosisEndocr J200754685586217917307
- MajimaTShimatsuAKomatsuYAssociation between baseline values of bone turnover markers and bone mineral density and their response to raloxifene treatment in Japanese postmenopausal women with osteoporosisEndocr J2008b551414818187874
- MajimaTShimatsuASatohNThree-month changes in bone turnover markers and bone mineral density response to raloxifene in Japanese postmenopausal women with osteoporosisJ Bone Miner Metab200826217818418301975
- TakadaJIbaKYoshizakiTYamashitaTCorrelation between a bone resorption marker and structural geometry of the proximal femur in osteoporotic women treated with raloxifeneJ Orthop Surg (Hong Kong)201220220921322933681
- IikuniNHamayaENihojimaSSafety and effectiveness profile of raloxifene in long-term, prospective, postmarketing surveillanceJ Bone Miner Metab201230667468222752125
- UrushiharaHKikuchiNYamadaMYoshikiFMiyauchiARaloxifene and stroke risks in Japanese postmenopausal women with osteoporosis on postmarketing surveillanceMenopause200916597197719357545
- YohKHamayaEUrushiharaHQuality of life in raloxifene-treated Japanese women with postmenopausal osteoporosis: a prospective, postmarketing observational studyCurr Med Res Opin201228111757176623035693
- OrimoHHayashiYFukunagaMDiagnostic criteria for primary osteoporosis: year 2000 revisionJ Bone Miner Metab200119633133711685647
- Eli Lilly JapanEvista [package insert]Kobe, JapanEli Lilly Japan2007
- DutertreMSmithCLMolecular mechanisms of selective estrogen receptor modulator (SERM) actionJ Pharmacol Exp Ther2000295243143711046073
- EttingerBBlackDMMitlakBHReduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) InvestigatorsJAMA1999282763764510517716
- DelmasPDEnsrudKEAdachiJDEfficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trialJ Clin Endocrinol Metab20028783609361712161484
- QuYWongMThiebaudDStockJLThe effect of raloxifene therapy on the risk of new clinical vertebral fractures at three and six months: a secondary analysis of the MORE trialCurr Med Res Opin200521121955195916368046
- KikuchiTEffect of combination therapy with eldecalcitol and raloxifene on bone metabolism in osteoporotic patientsTher Res201233914231429 Japanese
- TakedaSSakaiSShiraishiAKoikeNMiharaMEndoKCombination treatment with eldecalcitol (ED-71) and raloxifene improves bone mechanical strength by suppressing bone turnover and increasing bone mineral density in ovariectomized ratsBone201353116717323232307
- KanisJAJohnellOOdenAThe risk and burden of vertebral fractures in SwedenOsteoporos Int2004151202614593450
- RossPDFujiwaraSHuangCVertebral fracture prevalence in women in Hiroshima compared to Caucasians or Japanese in the USInt J Epidemiol1995246117111778824859
- KwokAWLeungJCChanAYPrevalence of vertebral fracture in Asian men and women: comparison between Hong Kong, Thailand, Indonesia and JapanPublic Health2012126652353122560410
- ItoMNishidaAUetaniMHayashiKOsteoporosis in the Japanese populationSemin Musculoskelet Radiol20015212112611500152
- Centers for Disease Control and PreventionOsteoporosis Available from: http://www.cdc.gov/nchs/data/nhanes/databriefs/osteoporosis.pdfAccessed June 14, 2013
- HuangCRossPDFujiwaraSDeterminants of vertebral fracture prevalence among native Japanese women and women of Japanese descent living in HawaiiBone19961854374428739901
- MoritaAGenetic factors of osteoporosis and fracture and the application of genetic information to the preventive medicineClin Calcium200515813641371 Japanese16062007
- MasakiHMikiTBone and calcium metabolism in elderly womenClin Calcium201121913611367 Japanese21881199
- NakamuraKKurahashiNIshiharaJInoueMTsuganeSCalcium intake and the 10-year incidence of self-reported vertebral fractures in women and men: the Japan Public Health Centre-based Prospective StudyBr J Nutr2009101228529418549509
- OhishiHNakamuraYKishiyaMTohSSpontaneous femoral neck fracture associated with a low serum level of vitamin DJ Orthop Sci201318349649922057737
- FujiwaraSHamayaEGotoWVertebral fracture status and the World Health Organization risk factors for predicting osteoporotic fracture risk in JapanBone201149352052521652001
- BeckTJRuffCBWardenKEScottWWJrRaoGUPredicting femoral neck strength from bone mineral data. A structural approachInvest Radiol19902516182298552
- BeckTJLookerACRuffCBSievanenHWahnerHWStructural trends in the aging femoral neck and proximal shaft: analysis of the Third National Health and Nutrition Examination Survey dual-energy X-ray absorptiometry dataJ Bone Miner Res200015122297230411127194
- BeckTJRuffCBBissessurKAge-related changes in female femoral neck geometry: implications for bone strengthCalcif Tissue Int199353Suppl 1S41S468275379
- BeckTJRuffCBScottWWJrPlatoCCTobinJDQuanCASex differences in geometry of the femoral neck with aging: a structural analysis of bone mineral dataCalcif Tissue Int199250124291739866
- TakadaJBeckTJIbaKYamashitaTStructural trends in the aging proximal femur in Japanese postmenopausal womenBone20074119710217513185
- WangXFDuanYBeckTJSeemanEVarying contributions of growth and ageing to racial and sex differences in femoral neck structure and strength in old ageBone200536697898615869919
- BeckTJLewieckiEMMillerPDEffects of denosumab on the geometry of the proximal femur in postmenopausal women in comparison with alendronateJ Clin Densitom200811335135918495508
- BonnickSLBeckTJCosmanFHochbergMCWangHde PappAEDXA-based hip structural analysis of once-weekly bisphosphonate-treated postmenopausal women with low bone massOsteoporos Int200920691192118830555
- GreenspanSLWymanAHoovenFHPredictors of treatment with osteoporosis medications after recent fragility fractures in a multinational cohort of postmenopausal womenJ Am Geriatr Soc201260345546122316070
- Uusi-RasiKSemanickLMZanchettaJREffects of teriparatide [rhPTH (1–34)] treatment on structural geometry of the proximal femur in elderly osteoporotic womenBone200536694895815878318
- GradyDEttingerBMoscarelliESafety and adverse effects associated with raloxifene: multiple outcomes of raloxifene evaluationObstet Gynecol2004104483784415458908
- KamibayashiJCurrent State of Therapy for Deep Venous Thrombosis and Pulmonary Embolism at Special Centers in JapanStudy Report 55–58TokyoAbnormal Blood Coagulation Study Group, Ministry of Health, Labour and Welfare, Japan1995
- HamayaESowaHRe: Raloxifene and stroke risks in Japanese postmenopausal women with osteoporosis on postmarketing surveillanceMenopause201421110911024281238
- Barrett-ConnorEMoscaLCollinsPEffects of raloxifene on cardiovascular events and breast cancer in postmenopausal womenN Engl J Med2006355212513716837676
- MasunariNFujiwaraSNakataYNakashimaENakamuraTHistorical height loss, vertebral deformity, and health-related quality of life in Hiroshima cohort studyOsteoporos Int200718111493149917541811
- HaginoHNakamuraTFujiwaraSOekiMOkanoTTeshimaRSequential change in quality of life for patients with incident clinical fractures: a prospective studyOsteoporos Int200920569570218836672
- IbaKTakadaJHatakeyamaNUnderutilization of antiosteoporotic drugs by orthopedic surgeons for prevention of a secondary osteoporotic fractureJ Orthop Sci200611544644917013730