Abstract
Background
The combination of the inhaled muscarinic antagonist umeclidinium (UMEC) with the long-acting β2-agonist vilanterol (VI) has been shown to provide significant improvements in lung function compared with UMEC, VI, or placebo (PBO) in patients with chronic obstructive pulmonary disease (COPD). This study was specifically designed to support these findings by assessing health-related quality of life and symptomatic outcomes in a similar population.
Methods
This was a 12-week multicenter, randomized, double-blind, parallel-group, placebo-controlled study. Eligible patients were randomized 1:1 to receive once-daily UMEC/VI 62.5/25 μg (via ELLIPTA® dry powder inhaler) or PBO for 12 weeks. The primary endpoint was St George’s Respiratory Questionnaire (SGRQ) total score at day 84. Secondary efficacy endpoints included rescue albuterol use (puffs/day) over weeks 1–12 and trough forced expiratory volume in 1 second on day 84. Adverse events were also assessed.
Results
A total of 496 patients were included in the intent-to-treat population in the UMEC/VI (n=248) and PBO (n=248) treatment groups. UMEC/VI 62.5/25 μg provided a significant and clinically meaningful improvement in SGRQ total score at day 84 versus PBO (difference between treatments in SGRQ total score change from baseline: −4.03 [95% confidence interval {CI}: −6.28, −1.79]; P<0.001). UMEC/VI 62.5/25 μg resulted in a statistically significant reduction in rescue albuterol use versus PBO (−0.7 puffs/day [95% CI: −1.1, −0.4]; P<0.001). UMEC/VI 62.5/25 μg provided a significant and clinically meaningful improvement in trough forced expiratory volume in 1 second on day 84 versus PBO (122 mL [95% CI: 71, 172]; P<0.001). The incidence of adverse events was similar between treatments (32% and 30% of patients in the UMEC/VI 62.5/25 μg and PBO groups, respectively).
Conclusion
The results of this study demonstrate that treatment with UMEC/VI 62.5/25 μg provides clinically important improvements in SGRQ and rescue medication use versus PBO in patients with moderate-to-very-severe COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by symptoms of breathlessness, cough, and sputum production and is a major cause of morbidity and mortality globally.Citation1 In addition, COPD has a large impact on quality of life (QoL) and can lead to anxiety, depression, and poor health status.Citation1–Citation4 While lung function endpoints are considered important and conventionally used as primary endpoints in clinical studies of COPD, assessments of health-related QoL (HRQoL) and other patient-reported outcomes provide important information on the benefits of treatment to the patient.Citation5 Patients with worse HRQoL are at risk of shortened survival following an acute COPD exacerbation, and many patients with COPD experience comorbidities, which impact on their HRQoL and survival.Citation1,Citation6,Citation7
Bronchodilators including long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs), are central to the pharmacological management of COPD.Citation1 The combination of the LAMA, umeclidinium (UMEC), and the LABA, vilanterol (VI), is an approved maintenance treatment for COPD in the US, Canada, the EU, and several other countries.Citation8,Citation9 In a previous 24-week, randomized, double-blind, placebo-controlled study, once-daily UMEC/VI 62.5/25 μg demonstrated significantly greater improvements in trough forced expiratory volume in 1 second (FEV1) and 0–6 hours weighted mean FEV1 compared with UMEC 62.5 μg, VI 25 μg, and placebo (PBO).Citation10 UMEC/VI 62.5/25 μg was also associated with an improvement in the St George’s Respiratory Questionnaire (SGRQ) score at day 168 versus PBO, and an increased likelihood of achieving a clinically meaningful improvement in SGRQ score of ≥4 units versus PBO. In addition, UMEC/VI 62.5/25 μg also significantly reduced rescue medication use over 24 weeks versus PBO.
The present study aimed to replicate the therapeutic benefits of once-daily UMEC/VI 62.5/25 μg on HRQoL, as observed in a previous study,Citation10 by investigating the effect of once-daily UMEC/VI 62.5/25 μg on SGRQ score and COPD symptoms (as reflected by rescue medication use). Additionally, lung function was assessed as an objective measure to support the subjective patient-reported outcomes, and to provide additional evidence for the use of UMEC/VI 62.5/25 μg for the maintenance treatment of COPD.
Methods
Study design
This was a 12-week multicenter, randomized, double-blind, parallel-group, placebo-controlled study that took place between September 2014 and March 2015. The study was conducted across 55 study centers in Bulgaria, Germany, Hungary, Romania, the Russian Federation, the Ukraine, and the US (GSK study identifier: 201211: Clinicaltrials.gov identifier: NCT2152605).
The study protocol and written informed consent were reviewed and approved by the Chesapeake Institutional Review Board, as well as each relevant national, regional, or independent ethics committee or institutional review board, in accordance with Good Clinical Practice. The study was conducted in accordance with International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice and all applicable subject privacy requirements, and the ethical principles that are outlined in the Declaration of Helsinki, 2008.Citation11
Patients
Patients eligible for inclusion were ≥40 years of age with a diagnosis of COPD, with a current or prior history of ≥10 pack-years of cigarette smoking at screening, a pre- and post-albuterol (salbutamol) FEV1/forced vital capacity (FVC) ratio of <0.70 and a post-albuterol FEV1 ≤70% of predicted normal values at screening (based on National Health and Nutrition Examination Survey III reference equations).Citation12 Patients also had a score ≥2 on the Modified Medical Research Council Dyspnea Scale at screening.
Exclusion criteria included a current diagnosis of asthma or other known respiratory conditions (α1-antitrypsin deficiency, active tuberculosis, lung cancer, bronchiectasis, sarcoidosis, lung fibrosis, pulmonary hypertension, interstitial lung diseases, or other active pulmonary diseases), hospitalization for COPD or pneumonia within 12 weeks prior to visit 1, lung volume reduction surgery within the 12 months prior to visit 1 and use of long-term oxygen therapy (prescribed for >12 h/day). Patients were also excluded if they had severe hepatic impairment, any rapidly progressing disease or immediate life-threatening illness (eg, cancer), any condition that was likely to affect respiratory function (eg, neurological condition) or an abnormal, clinically significant electrocardiogram finding at screening (atrial fibrillation with rapid ventricular rate >120 beats/min; sustained or nonsustained ventricular tachycardia; second-degree heart block Mobitz type II and third-degree heart block [unless pacemaker or defibrillator had been inserted]; eligibility for all other electrocardiogram findings was at the discretion of the investigator). The use of prohibited medications (Table S1) within the specified time periods also excluded patients from the study.
Treatments
Patients meeting the eligibility criteria at screening (visit 1) completed a 7- to 14-day run-in period, during which albuterol was provided on an as-needed basis. Following the run-in period, eligible patients were randomized 1:1 to receive once-daily UMEC/VI 62.5/25 μg (via ELLIPTA® dry powder inhaler) or matching PBO via ELLIPTA® dry powder inhaler for 12 weeks. Patients were randomized using the Registration and Medication Ordering System, an interactive voice response system. Follow-up clinic visits were scheduled at weeks 4, 8, and 12.
Endpoints
Primary efficacy endpoint
The primary efficacy endpoint was changed from baseline in SGRQ total score at day 84. A reduction in the SGRQ score of 4 units was considered the minimum clinically important difference (MCID) for the comparison of active treatment with PBO.Citation13
Secondary efficacy endpoints
The secondary efficacy endpoints were trough FEV1 on day 84 and rescue albuterol use (puffs/day) over weeks 1–12. An increase of 100 mL in trough FEV1 was considered as the MCID.Citation14
Other endpoints
Other endpoints included the proportion of SGRQ responders at days 28, 56, and 84, and SGRQ total score at days 28 and 56. SGRQ responders were defined as having a total score ≥4 units below baseline, as this is the MCID.Citation13 The percentage of rescue-free days and trough FEV1 at days 28 and 56 were also assessed. Trough FVC at days 28, 56, and 84 were also endpoints.
Safety
Safety assessments included the incidence of adverse events (AEs), including AEs of special interest (including cardiovascular effects, lower respiratory tract infections, and pneumonia), and COPD exacerbations. A COPD exacerbation was defined as an acute worsening of symptoms of COPD requiring the use of any treatment beyond study medication or rescue albuterol. This included use of systemic corticosteroids, antibiotics, and/or emergency treatment or hospitalization.
Statistical analysis
Sample size
The sample size was calculated based on the primary endpoint of SGRQ total score on day 84 and used a two-sample, two-sided t-test with a 5% significance level. A total of 221 patients per treatment arm were required for 90% power to detect a 4-unit difference between treatments in SGRQ total score.
Testing hierarchy
In order to account for multiplicity across primary and secondary endpoints, a step-down closed testing procedure was applied whereby inference for a test in the predefined hierarchy was dependent upon statistical significance having been achieved for previous tests in the hierarchy. The hierarchy consisted of UMEC/VI compared with PBO, performed on the primary and secondary endpoints, in the following order: SGRQ total score at day 84, trough FEV1 at day 84, mean number of puffs/day of rescue medication over weeks 1–12.
Analysis populations
The intent-to-treat (ITT) population comprised all patients randomized to treatment who received at least one dose of study medication in the treatment period.
The primary endpoint of SGRQ total score at day 84 was analyzed using a mixed-effect model repeated measure analysis for the ITT population. This used baseline SGRQ total score, center group, smoking status, day, treatment, day-by-baseline, and day-by-treatment interactions as covariates. Mixed-effect model repeated measure analysis was also performed for trough FEV1 at day 84 and rescue use (mean number of puffs/day) over weeks 1–12. The pre-specified assumptions for the mixed-effect model repeated measure analyses of percentage of rescue-free days were not satisfied; hence, an alternative, nonparametric analysis was performed. No formal statistical analyses were performed on the safety data.
Further details of statistical analyses can be found in the Supplementary materials.
Results
Patient disposition and baseline characteristics
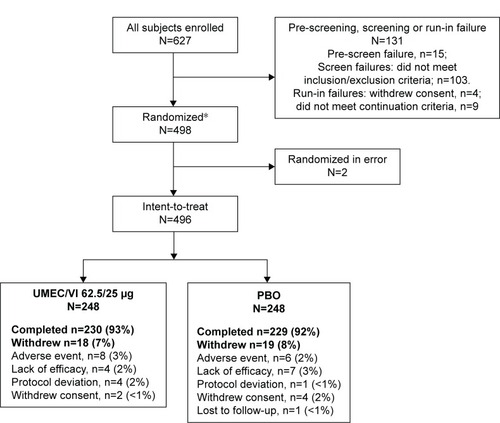
In total, 627 patients were screened; 498 were randomized (249 [50%] in each of the UMEC/VI 62.5/25 μg and PBO groups), and 496 patients were included in the ITT population (248 [50%] in each of the UMEC/VI 62.5/25 μg and PBO groups) (). Overall, 459 (93%) patients completed the study. A total of 18 (7%) and 19 (8%) patients withdrew from the study in the UMEC/VI 62.5/25 μg and PBO groups, respectively. The primary reasons for withdrawal were AEs and lack of efficacy. Patient demographics and baseline characteristics were generally similar between treatment groups (), although there was a greater proportion of patients with Global initiative for chronic Obstructive Lung Disease (GOLD) category D in the UMEC/VI group (n=158 [64%]) compared with the PBO group (n=139 [56%]). At baseline, exacerbation history was similar between treatment groups, with the majority of patients reporting no exacerbations requiring oral corticosteroids, antibiotics, or hospitalization in the previous 12 months (data not shown).
Table 1 Summary of patient demographics and baseline characteristics (ITT population)
Figure 1 Summary of patient disposition.
Abbreviations: PBO, placebo; UMEC, umeclidinium; VI, vilanterol.
Efficacy
SGRQ score
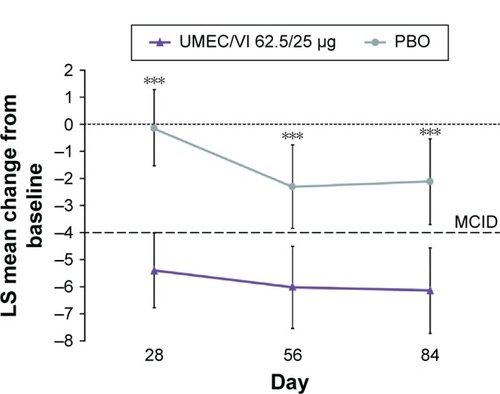
SGRQ total scores at baseline were similar in the UMEC/VI 62.5/25 μg (48.14) and PBO (47.58) groups (). Change from baseline in SGRQ total score had statistically significantly improved at day 84 with UMEC/VI 62.5/25 μg versus PBO (−4.03 [95% confidence interval {CI}: −6.28, −1.79]; P<0.001) (). The improvement was deemed clinically meaningful as it exceeded the MCID of 4 units.Citation13 Statistically significant improvements in SGRQ total score were also observed at days 28 and 56 with UMEC/VI 62.5/25 μg versus PBO ( and ).
Table 2 Summary of SGRQ endpoints (ITT population)
Figure 2 LS mean (95% CI) change from baseline in SGRQ total over time (ITT population).
Abbreviations: CI, confidence interval; ITT, intent-to-treat; LS, least squares; MCID, minimum clinically important difference; PBO, placebo; SGRQ, St George’s Respiratory Questionnaire; UMEC, umeclidinium; VI, vilanterol.
At days 28, 56, and 84, 48%, 51%, and 51% of patients, respectively, were SGRQ responders in the UMEC/VI 62.5/25 μg group, and 29%, 40%, and 40%, respectively, were responders in the PBO group (). This reflects a 69%, 27%, and 31% increase in the number of SGRQ responders with UMEC/VI 62.5/25 μg from PBO at days 28, 56, and 84, respectively. Patients treated with UMEC/VI 62.5/25 μg had statistically significantly higher odds of being an SGRQ responder versus a nonresponder compared with PBO at days 28, 56, and 84 (2.35 [95% CI: 1.58, 3.49]; P<0.001; 1.53 [95% CI: 1.05, 2.23]; P=0.026; 1.61 [95% CI: 1.11, 2.34]; P=0.013, respectively; ).
Lung function
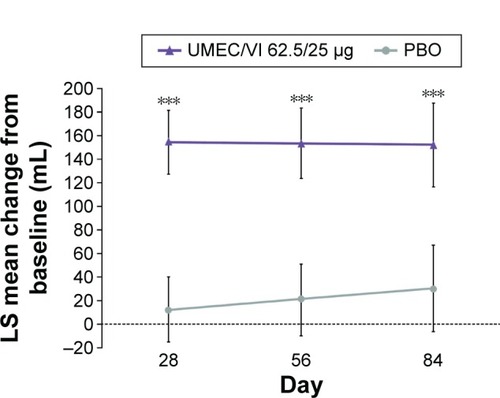
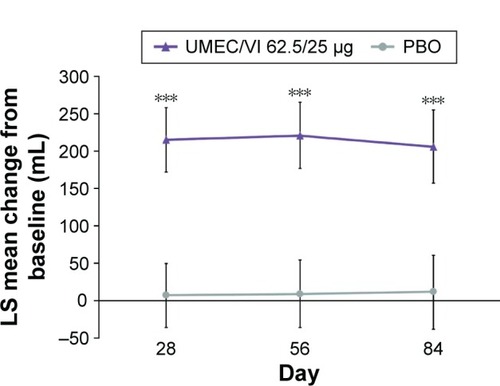
Change from baseline in trough FEV1 had statistically signifi-cantly improved at day 84 with UMEC/VI 62.5/25 μg versus PBO (122 mL [95% CI: 71, 172]; P<0.001) (). The improvement was deemed clinically meaningful as it exceeded the MCID of 100 mL.Citation14 Statistically significant improvements in trough FEV1 were also observed at days 28 and 56 (). Trough FVC had statistically significantly improved on days 28, 56, and 84 with UMEC/VI 62.5/25 μg versus PBO ().
Rescue albuterol use
Rescue albuterol use at baseline was similar in the UMEC/VI 62.5/25 μg (3.4 puffs/day) and PBO (3.8 puffs/day) groups. The mean change from baseline (standard deviation) in rescue albuterol use over weeks 1–12 was −1.4 puffs/day (38.1) and −0.6 puffs/day (30.5) with UMEC/VI 62.5/25 μg and PBO, respectively. UMEC/VI 62.5/25 μg resulted in a statistically significant reduction in rescue albuterol use versus PBO (−0.7 puffs/day [95% CI: −1.1, −0.4]; P<0.001).
At baseline, the proportion of patients with rescue-free days was similar in the UMEC/VI 62.5/25 μg (20.4%) and PBO (23.5%) groups. The mean change from baseline in rescue-free days over weeks 1–12 was 17.8% for UMEC/VI 62.5/25 μg and 2.8% for PBO. The treatment difference in median percentage rescue-free days for UMEC/VI 62.5/25 μg versus PBO was 4.8% (95% CI: 1.22, 11.25; P<0.001).
Safety
The incidence of AEs and serious AEs was similar between treatment groups (). On-treatment AEs occurred in 32% and 30% of patients in the UMEC/VI 62.5/25 μg and PBO groups, respectively; nonfatal serious AEs occurred in 7% and 5%, respectively. The most common AEs reported were headache, 6% and 6%, nasopharyngitis, 5% and 6%, and COPD, 3% and 3%, in the UMEC/VI 62.5/25 μg and PBO groups, respectively ().
Table 3 Summary of AEs (ITT population)
The incidence of special interest AEs was low and was similar between the treatment groups. Cardiovascular effects occurred in 2% of patients in each treatment group. Pneumonia was reported in 2% of patients in the UMEC/VI 62.5/25 μg group and 1% in the PBO group, and bronchitis was reported in none of the patients in the UMEC/VI 62.5/25 μg group and <1% of patients in the PBO group.
Two deaths were reported in the study, none of which were considered to be related to the study medication. Both deaths were in the UMEC/VI 62.5/25 μg treatment group and resulted from paraneoplastic syndrome secondary to lung carcinoma and metastasis, and acute myocardial ischemia.
A smaller proportion of patients experienced COPD exacerbations with UMEC/VI 62.5/25 μg (7%) versus PBO (11%) (). The majority of exacerbations were treated with oral or systemic corticosteroids and antibiotics in both treatment groups (). None of the exacerbations were fatal, and all were reported as having been resolved following treatment.
Discussion
This study demonstrated that UMEC/VI 62.5/25 μg resulted in statistically significant and clinically meaningful improvements from baseline in SGRQ total score on day 84 and all other study visits, and statistically significant and clinically meaningful improvements in SGRQ total score versus PBO were observed on days 28 and 84. The proportion of responders, as assessed by SGRQ total score, was higher with UMEC/VI 62.5/25 μg compared with PBO at day 84. Patients treated with UMEC/VI 62.5/25 μg also had significantly higher odds of being an SGRQ responder (versus a nonresponder) than patients receiving PBO, at all study visits. This treatment benefit was seen despite a large increase in the proportion of responders receiving PBO between weeks 4 and 8 (29%–40%).
These results were corroborated by the reduction in rescue medication use observed over weeks 1–12 for UMEC/VI 62.5/25 μg versus PBO. The improvements observed in the SGRQ, which is a subjective patient-reported outcome, were supported by marked increases in objectively measured airway function (FEV1 and FVC) at all clinic visits with UMEC/VI 62.5/25 μg versus PBO. Patients receiving UMEC/VI 62.5/25 μg demonstrated a similar incidence of AEs compared with patients in the PBO group. These findings were consistent with the previously described safety profile of UMEC/VI 62.5/25 μg.Citation8,Citation9
The results of this 12-week study support the findings of the 24-week study by Donohue et al,Citation10 which also demonstrated that UMEC/VI 62.5/25 μg provided statistically significant and clinically meaningful improvements in SGRQ versus PBO (5.51 units), as well as statistically significant reductions in rescue use versus PBO. In two randomized trials, the combination tiotropium/olodaterol 5/5 μg significantly improved SGRQ versus PBO.Citation15 These improvements exceeded the MCID and were similar to the changes versus PBO observed in the current study.Citation15 Improvements in SGRQ total score have also been reported for other once-daily fixed-dose LAMA/LABA combinations versus PBO, though these were smaller than the MCID of 4.0 units.Citation16,Citation17
In addition, a network meta-analysis of 24 randomized trials by Ismaila et alCitation18 found that on average the LAMA monotherapies tiotropium (2.43 units) and glycopyrronium (3.14 units), both, resulted in improvements versus PBO well below the 4.0-unit MCID for SGRQ at 24 weeks. Similarly, in another network meta-analysis, Cope et alCitation19 reported that twice-daily LABA monotherapy, including formoterol (2.58 unit change) and salmeterol (1.31 unit change), on average resulted in nonclinically relevant improvements in SGRQ versus PBO. The improvements in SGRQ reported here were approximately at or above the MCID. This, combined with the clinically relevant results reported by Donohue et al,Citation10 suggest that dual bronchodilator therapy with LABA/LAMA combinations such as UMEC/VI could result in more consistently improved HRQoL in patients with symptomatic COPD compared with bronchodilator monotherapy.
A limitation of the current study is that UMEC/VI was not compared with other active bronchodilator monotherapy, so comparisons with other active therapies can only be made using historical data and inferences should be made with caution. Additionally, patients in the PBO arm were only permitted to take short-acting β2-agonists and inhaled corticosteroids, even though they had GOLD B or GOLD D COPD. A potential additional limitation of the study was that all patients included were selected to have moderate-to-very-severe breathlessness at study entry, in accordance with guideline recommendations. Although this patient population is the most likely to benefit most from dual therapy, there remains limited data available in less symptomatic patient subgroups with dual bronchodilator therapy. Finally, the current study was of insufficient duration to fully assess the risk of COPD exacerbations, which were assessed only as part of the safety evaluation. Any apparent treatment differences would require confirmation in further studies of a longer duration.
In conclusion, the results of this study demonstrate that treatment with the dual bronchodilator UMEC/VI 62.5/25 μg was well tolerated and provided statistically significant and clinically important improvements in HRQoL versus PBO in patients with symptomatic moderate-to-very-severe COPD.
Acknowledgments
This study (GSK study number: 201211; Clinicaltrials. gov identifier: NCT2152605) was funded by GSK. Palvi Shah (GSK Clinical Statistics, Stockley Park, UK) provided substantial contributions to the study data analysis, data interpretation, and preparation of the manuscript. Medical writing assistance was provided by Joanne Ashworth and Matthew Robinson of Fishawack Indicia Ltd (UK), funding by GSK.
Supplementary materials
Statistical analysis
The sample size calculation used an estimate of residual standard deviation (SD) of 12.63 units, which was the value observed in a previous study evaluating umeclidinium/vilanterol (UMEC/VI) 62.5/25 μg and placebo (PBO).Citation1 With >211 patients per treatment arm, there was >99% power to detect a difference of 100 mL between treatments in trough forced expiratory volume in 1 second with an estimate of residual SD of 220 mL. The study was designed to have 93% power to detect a difference of one puff/day between UMEC/VI and PBO for mean rescue medication use, at the two-sided 5% significance level, using an estimate of residual SD of 3.01 puffs/day over weeks 1–12. These estimates of SD were based on mixed-effect model repeated measures and analysis of covariance analyses from a previous study evaluating UMEC/VI.Citation1 Data for subjects who withdrew prematurely from the study were not explicitly imputed. Hence, to account for an estimated 15% withdrawal rate, 248 patients were planned to be randomized to each of the treatment groups (496 in total).
Table S1 Excluded medications prior to visit 1
Reference
- DonohueJFMaleki-YazdiMRKilbrideSMehtaRKalbergCChurchAEfficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPDRespir Med2013107101538154623830094
Disclosure
TMS is part of a speaker bureau for AstraZeneca and Boehringer Ingelheim, and has received research support from AstraZeneca, Boehringer Ingelheim, Forest Research Institute, GSK, Novartis, Pearl Therapeutics, Theravance and Sunovion. ACD, AC, and WAF are employees of GSK and hold stocks/shares in GSK. DO was an employee of GSK and holder of stock/shares in GSK at the time of the study, but is now employed by Pearl Therapeutics.
References
- GOLDGlobal strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease 2015 Available from: http://www.goldcopd.com/Accessed March 10, 2015
- GudmundssonGGislasonTJansonCDepression, anxiety and health status after hospitalisation for COPD: a multicentre study in the Nordic countriesRespir Med20061001879315893921
- HananiaNAMullerovaHLocantoreNWDeterminants of depression in the ECLIPSE chronic obstructive pulmonary disease cohortAm J Respir Crit Care Med2011183560461120889909
- UrffMvan den BergJWUilSMChavannesNHDamoiseauxRADepression and heart failure associated with clinical COPD questionnaire outcome in primary care COPD patients: a cross-sectional studyNPJ Prim Care Respir Med2014241406625230736
- CazzolaMMacNeeWMartinezFJOutcomes for COPD pharmacological trials: from lung function to biomarkersEur Respir J200831241646918238951
- BarnesPJCelliBRSystemic manifestations and comorbidities of COPDEur Respir J20093351165118519407051
- Garcia-AymerichJGomezFPBenetMIdentification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypesThorax201166543043721177668
- GSKANORO™ ELLIPTA® prescribing information2014 Available from: https://www.gsksource.com/gskprm/htdocs/documents/ANORO-ELLIPTA-PI-MG.PDFAccessed June 1, 2015
- GSKANORO™ ELLIPTA® summary of product characteristics2014 Available from: http://www.medicines.org.uk/emc/medicine/28949#INDICATIONSAccessed June 1, 2015
- DonohueJFMaleki-YazdiMRKilbrideSMehtaRKalbergCChurchAEfficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPDRespir Med2013107101538154623830094
- World Medical AssociationWorld Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects2008 Available from: http://www.wma.net/en/30publications/10policies/b3/17c.pdfAccessed June 1, 2015
- CelliBRMacNeeWATS/ERS Task ForceStandards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paperEur Respir J200423693294615219010
- JonesPWSt George’s Respiratory Questionnaire: MCIDCOPD200521757917136966
- DonohueJFMinimal clinically important differences in COPD lung functionCOPD20052111112417136971
- SinghDFergusonGTBolitschekJTiotropium + olodaterol shows clinically meaningful improvements in quality of lifeRespir Med2015109101312131926320402
- VogelmeierCKardosPHarariSGansSJStengleinSThirlwellJFormoterol mono- and combination therapy with tiotropium in patients with COPD: a 6-month studyRespir Med2008102111511152018804362
- BatemanEDFergusonGTBarnesNDual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE studyEur Respir J20134261484149423722616
- IsmailaASHuismanELPunekarYSKarabisAComparative efficacy of long-acting muscarinic antagonist monotherapies in COPD: a systematic review and network meta-analysisInt J Chron Obstruct Pulmon Dis2015102495251726604738
- CopeSDonohueJFJansenJPComparative efficacy of long-acting bronchodilators for COPD: a network meta-analysisRespir Res20131410024093477