Abstract
Background
This study was conducted in order to investigate the differences in the respiratory physiology of patients with chronic obstructive pulmonary disease (COPD), asthma-COPD overlap syndrome (ACOS), and asthma with airflow limitation (asthma FL+).
Methods
The medical records for a series of all stable patients with persistent airflow limitation due to COPD, ACOS, or asthma were retrospectively reviewed and divided into the COPD group (n=118), the ACOS group (n=32), and the asthma FL+ group (n=27). All the patients underwent chest high-resolution computed tomography (HRCT) and pulmonary function tests, including respiratory impedance.
Results
The low attenuation area score on chest HRCT was significantly higher in the COPD group than in the ACOS group (9.52±0.76 vs 5.09±1.16, P<0.01). The prevalence of bronchial wall thickening on chest HRCT was significantly higher in the asthma FL+ group than in the COPD group (55.6% vs 25.0%, P<0.01). In pulmonary function, forced expiratory volume in 1 second (FEV1) and peak expiratory flow rate were significantly higher in the asthma FL+ group than in the ACOS group (76.28%±2.54% predicted vs 63.43%±3.22% predicted, P<0.05 and 74.40%±3.16% predicted vs 61.08%±3.54% predicted, P<0.05, respectively). Although residual volume was significantly lower in the asthma FL+ group than in the COPD group (112.05%±4.34% predicted vs 137.38%±3.43% predicted, P<0.01) and the ACOS group (112.05%±4.34% predicted vs148.46%±6.25% predicted, P<0.01), there were no significant differences in functional residual capacity or total lung capacity. The increase in FEV1 in response to short-acting β2-agonists was significantly greater in the ACOS group than in the COPD group (229±29 mL vs 72±10 mL, P<0.01) and the asthma FL+ group (229±29 mL vs 153±21 mL, P<0.05). Regarding respiratory impedance, resistance at 5 Hz and resistance at 20 Hz, which are oscillatory parameters of respiratory resistance, were significantly higher in the asthma FL+ group than in the COPD group at the whole-breath (4.29±0.30 cmH2O/L/s vs 3.41±0.14 cmH2O/L/s, P<0.01 and 3.50±0.24 cmH2O/L/s vs 2.68±0.10 cmH2O/L/s, P<0.01, respectively), expiratory, and inspiratory phases.
Conclusion
Although persistent airflow limitation occurs in patients with COPD, ACOS, and asthma FL+, they may have distinct characteristics of the respiratory physiology and different responsiveness to bronchodilators.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation that is usually progressive according to the Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines.Citation1 Spirometric criterion for airflow limitation is forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio less than 70% after inhalation of bronchodilators, according to the GOLD guidelines.Citation1 However, COPD is not the only disease that shows persistent airflow limitation. Asthma is characterized by chronic inflammation and airway remodeling and may also cause persistent airflow limitation,Citation2 although the patterns of inflammation, the affected structures, and the prime anatomic site at which pathological changes occur are different.Citation3 In the clinical setting, asthma and COPD are among the most commonly encountered chronic lung diseases.Citation1,Citation4 Asthma may be a risk factor for the development of COPD.Citation5 It may therefore be problematic to differentiate asthma from COPD, especially in older patients. It is difficult to clinically differentiate between COPD and asthma in some patients with persistent airflow limitation.Citation6 Moreover, COPD and asthma may overlap and converge in older individuals.Citation7,Citation8 There is increasing clinical recognition of the coexistence of COPD and asthma in individual patients, which results in a clinical syndrome known as asthma-COPD overlap syndrome (ACOS).Citation9–Citation13
The forced oscillation technique (FOT) is a simple method for assessing the oscillatory flow resistance of the respiratory system and has provided important findings in respiratory physiology.Citation14–Citation16 Previous studies have revealed that several parameters evaluated using the FOT may be useful for discriminating between patients with COPD and asthma.Citation17,Citation18 However, there continues to be a lack of information on the differences in pulmonary function, including respiratory impedance, among patients with COPD, ACOS, and asthma with airflow limitation (asthma FL+). This retrospective study was conducted in order to investigate the differences in the respiratory physiology of patients with these chronic obstructive diseases. The aim of the study was to clarify the characteristics of pulmonary functions, including pulmonary impedance, and the findings of chest high-resolution computed tomography (HRCT) of patients with COPD, ACOS, and asthma FL+, and these findings may therefore play a role in developing the diagnostic criteria of ACOS.
Methods
Patients
The medical records for a series of all stable patients with persistent airflow limitation due to COPD, ACOS, or asthma, who were seen at the outpatient clinic of Shinshu University Hospital from April 2011 to October 2015, were retrospectively reviewed to obtain the patients’ clinical data, including the diagnosis, age, sex, body weight, body height, smoking history, laboratory data, and imaging data. All the patients underwent chest HRCT and pulmonary function tests, including respiratory impedance.
The diagnosis of COPD was based on the patients’ clinical history and symptoms, including dyspnea on exertion and pulmonary function characterized by persistent airflow limitation (FEV1/FVC <70% after inhalation of short-acting β2-agonists) in accordance with the GOLD guidelines.Citation1 The patients with COPD who had no history of asthma or asthmatic symptoms were categorized into the COPD group (n=118). The patients with COPD who had experienced asthmatic symptoms, such as episodic breathlessness, wheezing, cough, and chest tightness worsening at night or in the early morning, were categorized into the ACOS group (n=32), in accordance with GOLDCitation1 and Global Initiative for Asthma (GINA) guidelinesCitation4,Citation13 as previously reported.Citation19 All the patients with ACOS had a smoking history of >20 pack-years. The diagnosis of asthma was based on the clinical history and symptoms in accordance with GINA.Citation4 Patients with asthma who showed persistent airflow limitation (FEV1/FVC <70% after inhalation of short-acting β2-agonists) were categorized into the asthma FL+ group (n=27).
A total of 243 patients were included in the initial analysis. Of them, 66 patients were excluded in the final analysis because they had a coexistent diagnosis of other respiratory diseases, such as pulmonary fibrosis (26 patients) or bronchiectasis (two patients) or they did not undergo the FOT measurements (14 patients) or chest HRCT (24 patients). No patient had a respiratory tract infection or an exacerbation of COPD and/or asthma during the preceding 3 months.
This study was approved by the institutional research ethics committee of Shinshu University School of Medicine, and all patients gave their written informed consent to participate.
Pulmonary function tests
Spirometry, measurements of the diffusion capacity of the lung for carbon monoxide (DLco) and closing volume, and a global measure of ventilation heterogeneity (the slope of Phase III of the single breath nitrogen washout test [delta N2]) were performed using a pulmonary function testing system (Chestac-8800; Chest Co., Ltd., Tokyo, Japan). FEV1 was measured before and 20 minutes after inhalation of short-acting β2-agonists (20 μg of procaterol hydrochloride) by aerosol (metered-dose inhaler) with a spacer to evaluate the reversibility of airflow limitation. The functional residual capacity was measured using a body plethysmograph (Body Box; MGC Diagnostics, Ann Arbor, MI, USA), after which the patients immediately inspired to total lung capacity (TLC) and expired maximally to residual volume (RV). The local Japanese reference data,Citation20 developed by the Japanese Respiratory Society, were used to derive the predicted values for FEV1 and vital capacity, while the predicted values for DLco, DLco/alveolar volume, and lung volumes (RV and TLC) measured using the body plethysmograph were determined using the formulas described by Nishida et alCitation21 and Boren et al,Citation22 respectively. Some patients used short-acting β2-agonists as needed to relieve dyspnea but did not use them on the day the pulmonary function tests were performed.
Respiratory impedance was measured using a commercially available multifrequency FOT device (MostGraph-01®) as previously reported,Citation17,Citation23,Citation24 following the standard recommendations.Citation25 We evaluated the resistance at 5 Hz (R5), resistance at 20 Hz (R20), reactance at 5 Hz (X5), resonant frequency (Fres), and low-frequency reactance area (ALX). The oscillatory parameters were expressed at whole-breath, inspiratory, and expiratory phases. The difference between the inspiratory and expiratory phases was calculated for each oscillatory parameter. These FOT measurements were performed prior to the other pulmonary function tests.
The pulmonary function tests were performed by two special technicians according to the American Thoracic Society criteria. Two or three tests were repeated to guarantee repeatability.
Evaluation of the degree of emphysema and bronchial wall thickening
Emphysema was evaluated by HRCT according to the method reported previously.Citation26,Citation27 Briefly, HRCT findings were evaluated at three anatomical levels at full inspiration; near the superior margin of the aortic arch, at the level of the carina, and at the level of the orifice of the inferior pulmonary veins. The low attenuation area (LAA) was visually scored in each bilateral lung field according to the method of Goddard et al.Citation28 Total scores were calculated, and the severity of emphysema was graded as follows: score 0, LAA <5%; score 1, 5%≤ LAA <25%; score 2, 25%≤ LAA <50%; score 3, 50%≤ LAA <75%; and score 4, 75%≤ LAA. Thus, the total emphysema scores ranged from 0 to 24. Bronchial wall thickening (BWT) in all lung fields was graded visually as reported previously:Citation26,Citation27 grade 0, none; grade 1, <50% adjacent pulmonary artery diameter; and grade 2, ≥50% adjacent pulmonary artery diameter. The patients without BWT showed BWT of grade 0, and patients with BWT showed BWT of grade 1 or more. HRCT images were independently analyzed by two pulmonologists with no knowledge of the patients’ clinical status.
Statistical analysis
The values shown in the text, figures, and tables represent mean ± standard error of the mean. The data distribution of the variables in various groups was first assessed using Bartlett’s test. When the data for the variables showed a normal distribution, they were compared using one-way analysis of variance, followed by multiple comparisons according to the Tukey–Kramer method. When the data for the variables did not show a normal distribution, the variables were compared using the Kruskal–Wallis test, followed by multiple comparisons among groups with the nonparametric Tukey–Kramer method. All statistical analyses were performed using Windows-compatible software (StatFlex Version 6.0; Artech, Osaka, Japan). P-values of less than 0.05 were considered to indicate statistical significance in all of the statistical analyses.
Results
Clinical characteristics of each group
shows the age, sex, body mass index, Brinkman index, peripheral eosinophil count, and chest HRCT findings of each group. The proportion of females was significantly higher in the asthma FL+ group than in the other groups. The Brinkman index and LAA score were significantly lower in the asthma FL+ group than in the other groups. Although there were no significant differences in the peripheral eosinophil count among the three groups, it tended to be higher in the ACOS and asthma FL+ groups than in the COPD group. There were significant differences in the LAA score on chest HRCT among the three groups, and the LAA score was significantly higher in the COPD group than in the ACOS group. The prevalence of BWT on chest HRCT was significantly higher in the asthma FL+ group than in the COPD group.
Table 1 Clinical characteristics of patients with COPD, ACOS, and asthma FL+
Pulmonary function tests
FEV1 and peak expiratory flow rate were significantly higher in the asthma FL+ group than in the ACOS group (). RV was significantly lower in the asthma FL+ group than in the other groups, although there were no significant differences in functional residual capacity and TLC. DLco and DLco/alveolar volume were significantly higher in the asthma FL+ group than in the other groups. There were no significant differences in delta N2 and closing volume/vital capacity among the three groups.
Table 2 Pulmonary function of patients with COPD, ACOS, and asthma FL+
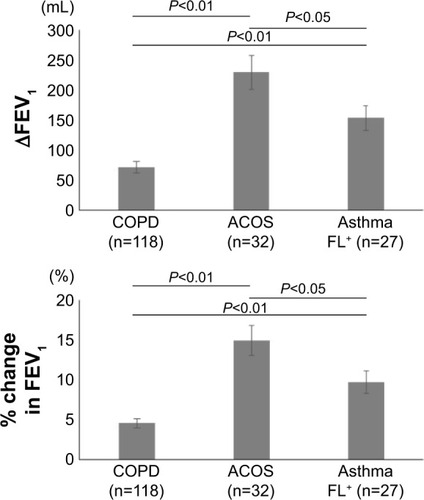
shows the reversibility of airflow limitation in response to short-acting β2-agonists, which was expressed by an increase in FEV1 from the baseline value. The increase in FEV1 was significantly greater in the ACOS group than in the COPD group (229±29 mL [14.90%±1.88% predicted] vs 72±10 mL [4.54%±0.59% predicted], P<0.01) and the asthma FL+ group (229±29 mL [14.90%±1.88% predicted] vs 153±21 mL [9.70%±1.38% predicted], P<0.05). It was also significantly greater in the asthma FL+ group than in the COPD group (153±21 mL [9.70%±1.38% predicted] vs 72±10 mL [4.54%±0.59% predicted], P<0.01).
Figure 1 Response to short-acting β2 agonists in patients with COPD, ACOS, and asthma FL+.
Abbreviations: ACOS, asthma-COPD overlap syndrome; asthma FL+, asthma with airflow limitation; FEV1, forced expiratory volume in 1 second; ∆FEV1, increases in FEV1.
shows respiratory impedance measured using a multifrequency FOT device (MostGraph-01®). At the whole-breath, expiratory, and inspiratory phases, R5 and R20 were significantly higher in the asthma FL+ group than in the COPD group. In the ACOS group, the values of these oscillatory parameters ranged between the values of the asthma FL+ group and the COPD group; however, the differences between the ACOS group and the other groups were not statistically significant.
Table 3 Respiratory impedance in patients with COPD, ACOS, and asthma FL+
Discussion
This is the first report to compare the pulmonary function, including respiratory impedance, of patients with COPD, ACOS, and asthma FL+. We assessed the differences in the respiratory physiology of patients with these obstructive pulmonary diseases, which are commonly encountered chronic lung diseases in the clinical setting. We showed that there were significant differences in several parameters of pulmonary function tests between the COPD group and the asthma FL+ group and between the ACOS group and the asthma FL+ group. There were significant differences among the three groups in the reversibility of airflow limitation in response to short-acting β2-agonists. R5 and R20, which are oscillatory parameters of respiratory resistance, in both inspiratory and expiratory phases were significantly greater in the asthma FL+ group than in the COPD group.
The FOT is theoretically sensitive to peripheral airway functionCitation29,Citation30 and is easy to administer because it is an effort-independent method of evaluating lung mechanics. The FOT parameters of resistance (Rrs) and reactance (Xrs) are parameters of the airway caliber and of the elastic properties of the respiratory system, respectively, and reflect the properties of both small and large airways. In patients with pulmonary obstructive diseases, the oscillatory flow resistance of the respiratory system tends to increase with the degree of airway obstruction, resulting in an increase in Rrs and negative values in Xrs. Xrs is sensitive and specific to the presence of expiratory flow limitation in patients with COPDCitation31 and to change during the recovery from acute exacerbations of COPD.Citation32 The Rrs increased, and Xrs fell to a more negative level in patients with both COPD and asthma in a severity-dependent fashion, regardless of respiratory phase.Citation14–Citation16 Mori et alCitation17 reported that R5, which indicates total airway resistance, is significantly correlated with FEV1, and that it is significantly higher in patients with COPD than in controls, but not than in patients with asthma. Kanda et alCitation18 reported that R5 was significantly higher in both patients with COPD and patients with asthma than in healthy never-smokers, and that R20 was significantly higher in patients with asthma than in patients with COPD and healthy never-smokers. We found that both R5 and R20 were significantly higher in patients with asthma FL+ than in patients with COPD, regardless of respiratory phase, and more than half of the patients with asthma FL+ (55.6%) showed BWT on chest HRCT. Our findings suggest that the asthma FL+ group included patients with more severe asthma, which involved greater degree of airway remodeling. Indeed, previous studies have revealed that patients with more severe asthma had greater airway wall thickening on chest HRCT than those with mild asthma.Citation33,Citation34 On the other hand, although the LAA and airway wall thickening can be observed on the chest HRCT images of many patients with COPD, there are individuals with similar degrees of airflow limitation whose abnormalities appeared to be predominantly related to airway remodeling or whose abnormalities appeared to be predominantly related to a loss of lung parenchyma.Citation33 As a consequence of this heterogeneity in COPD, the mean value of the parameters of airway resistance (R5 and R20) may have been lower in patients with COPD than in patients with asthma FL+ in the present study.
We found that there were no significant differences in delta N2, X5, Fres, and ALX among patients with COPD, ACOS, and asthma FL+. Delta N2 is the preferred parameter, which reflects ventilation heterogeneity, and it increases in a severity-dependent manner in patients with COPD.Citation35 X5, Fres, and ALX are significantly correlated with FEV1 and delta N2 in patients with asthma,Citation24 and Fres is significantly correlated with FEV1 and delta N2 in patients with COPD.Citation17,Citation36 These findings suggest that ventilation heterogeneity exists in patients with COPD, ACOS, and asthma FL+. Furthermore, there was no significant difference in within-breath changes of X5, which suggests the easy collapsibility of small airways during the expiration of the tidal breath, among patients with COPD, ACOS, and asthma FL+. Although previous studies have reported that within-breath changes of X5 discriminated between patients with COPD and asthma,Citation17,Citation18 this may not be applicable to patients with asthma FL+.
We found that there were significant differences in the reversibility of airflow limitation in response to short-acting β2-agonists among patients with COPD, ACOS, and asthma FL+, and the reversibility of airflow limitation was the greatest in patients with ACOS, whereas the prevalence of BWT on chest HRCT tended to be lower in patients with ACOS than in patients with asthma FL+. These findings suggest that patients with ACOS have a lower degree of airway remodeling due to asthma than patients with asthma FL+. Nakano et alCitation33 reported that the airway wall area on chest HRCT increased without a decrease in the luminal area in patients with asthma, whereas the airway luminal area decreased and airway wall area increased in patients with COPD. This different pattern of remodeling may reflect fundamental differences in the inflammatory processes in COPD and asthma and could influence the reversibility of airflow limitation, although the degree of reversibility of airflow limitation in response to bronchodilators in patients with COPD has never been shown to add to the differential diagnosis with asthma.Citation1
There were several limitations in the present study. First, this was a single-center, uncontrolled-design retrospective study with a lack of statistical power due to the small sample size of the asthma FL+ group (n=27). Further prospective studies with larger study populations are required to confirm these results. Second, the assessment of emphysema on chest HRCT was performed according to a visual scoring method, rather than the software-based quantification of the degree of emphysema and BWT. However, the reproducibility of visual scoring was demonstrated in our previous report.Citation27 Third, the definition of ACOS was based on a retrospective evaluation of asthma-related symptoms. It is difficult to clinically differentiate between COPD and ACOS in some patients, because asthma-related symptoms may not only be specific for asthma but may also characterize COPD. Fourth, generalizability is a potential problem in the present study. Shinshu University Hospital, which was the site of patient recruitment, is a major hospital and may have more older patients with high disease severity compared with other general hospitals, which results in a potential selection bias.
Conclusion
Although persistent airflow limitation occurs in patients with COPD, ACOS, and asthma FL+, they may have distinct characteristics of the respiratory physiology and different responsiveness to bronchodilators.
Disclosure
The authors report no conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung Disease [homepage on the Internet]Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease NHLBI/WHO Workshop ReportBethesdaNational Heart, Lung and Blood Institute2001 [Updated 2015]. Available from: http://www.goldcopd.comAccessed January 31, 2016
- VonkJMJongepierHPanhuysenCISchoutenJPBleeckerERPostmaDSRisk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow upThorax200358432232712668795
- JefferyPKRemodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary diseaseProc Am Thorac Soc20041317618316113432
- GINA [homepage on the Internet]Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) [Updated 2015] Available from: http://www.ginasthma.org/Accessed January 31, 2016
- SilvaGESherrillDLGuerraSBarbeeRAAsthma as a risk factor for COPD in a longitudinal studyChest20041261596515249443
- TzortzakiEGProklouASiafakasNMAsthma in the elderly: can we distinguish it from COPD?J Allergy (Cairo)2011201184354321785614
- Diaz-GuzmanEManninoDMAirway obstructive diseases in older adults: from detection to treatmentJ Allergy Clin Immunol2010126470270920920760
- GibsonPGMcDonaldVMMarksGBAsthma in older adultsLancet2010376974380381320816547
- CarolanBJSutherlandERClinical phenotypes of chronic obstructive pulmonary disease and asthma: recent advancesJ Allergy Clin Immunol2013131362763423360757
- KimSRRheeYKOverlap between asthma and COPD: where the two diseases convergeAllergy Asthma Immunol Res20102420921420885905
- LouieSZekiAASchivoMThe asthma-chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerationsExpert Rev Clin Pharmacol2013619721923473596
- NakawahMOHawkinsCBarbandiFAsthma, chronic obstructive pulmonary disease (COPD), and the overlap syndromeJ Am Board Fam Med201326447047723833163
- GINA and GOLD [homepage on the Internet]Diagnosis of Diseases of Chronic Airflow Limitation: Asthma, COPD and Asthma-COPD Overlap Syndrome (ACOS) [updated 2015] Available from: http://www.ginasthma.org/Accessed: January 31, 2015
- OhishiJKurosawaHOgawaHIrokawaTHidaWKohzukiMApplication of impulse oscillometry for within-breath analysis in patients with chronic obstructive pulmonary disease: pilot studyBMJ Open201112e000184
- GrimbyGTakishimaTGrahamWMacklemPMeadJFrequency dependence of flow resistance in patients with obstructive lung diseaseJ Clin Invest1968476145514655653219
- CavalcantiJVLopesAJJansenJMMeloPLDetection of changes in respiratory mechanics due to increasing degrees of airway obstruction in asthma by the forced oscillation techniqueRespir Med2006100122207221916713226
- MoriKShiraiTMikamoMColored 3-dimensional analyses of respiratory resistance and reactance in COPD and asthmaCOPD20118645646322149407
- KandaSFujimotoKKomatsuYEvaluation of respiratory impedance in asthma and COPD by an impulse oscillation systemIntern Med2010491233020045997
- KitaguchiYKomatsuYFujimotoKHanaokaMKuboKSputum eosinophilia can predict responsiveness to inhaled corticosteroid treatment in patients with overlap syndrome of COPD and asthmaInt J Chron Obstruct Pulmon Dis2012728328922589579
- Japanese Society of Chest DiseaseStandards of pulmonary function tests for JapaneseJpn J Respir Dis199331421427
- NishidaOKambeMSewakeNTakanoMKawaneHPulmonary function in healthy subjects and its prediction: 5. Pulmonary diffusing capacity in adultsJpn J Clin Pathol19762411941947
- BorenHGKoryRCSynerJCCallahanRThe Veterans Administration-Army cooperative study of pulmonary function. 2. The lung volume and its subdivisions in normal menAm J Med19664196101
- MoriKShiraiTMikamoMRespiratory mechanics measured by forced oscillation technique in combined pulmonary fibrosis and emphysemaRespir Physiol Neurobiol2013185223524023117106
- ShiraiTMoriKMikamoMRespiratory mechanics and peripheral airway inflammation and dysfunction in asthmaClin Exp Allergy201343552152623600542
- OostveenEMacLeodDLorinoHERS Task Force on Respiratory Impedance Measurements. The forced oscillation technique in clinical practice: methodology, recommendations and future developmentsEur Respir J20032261026104114680096
- KitaguchiYFujimotoKKuboKHondaTCharacteristics of COPD phenotypes classified according to the findings of HRCTRespir Med2006100101742175216549342
- FujimotoKKitaguchiYKuboKHondaTClinical analysis of chronic obstructive pulmonary disease phenotypes classified using high-resolution computed tomographyRespirology200611673174017052301
- GoddardPRNicholsonEMLaszloGWattIComputed tomography in pulmonary emphysemaClin Radiol19823343793877083738
- LutchenKRGillisHRelationship between heterogeneous changes in airway morphometry and lung resistance and elastanceJ Appl Physiol (1985)1997834119212019338428
- ThorpeCWBatesJHEffect of stochastic heterogeneity on lung impedance during acute bronchoconstriction: a model analysisJ Appl Physiol198519978216161625
- DellacàRLSantusPAlivertiADetection of expiratory flow limitation in COPD using the forced oscillation techniqueEur Respir J200423223224014979497
- JohnsonMKBirchMCarterRKinsellaJStevensonRDMeasurement of physiological recovery from exacerbation of chronic obstructive pulmonary disease using within-breath forced oscillometryThorax200762429930617105778
- NakanoYMüllerNLKingGGQuantitative assessment of airway remodeling using high-resolution CTChest20021226 suppl271S275S12475796
- AwadhNMüllerNLParkCSAbboudRTFitzGeraldJMAirway wall thickness in patients with near fatal asthma and control groups: assessment with high resolution computed tomographic scanningThorax19985342482539741365
- GennimataSAPalamidasAKarakontakiFPathophysiology of evolution of small airways disease to overt COPDCOPD20107426927520673036
- MikamoMShiraiTMoriKPredictors of phase III slope of nitrogen single-breath washout in COPDRespir Physiol Neurobiol20131891424623816601

