Abstract
The term asthma–COPD overlap syndrome (ACOS) is one of multiple terms used to describe patients with characteristics of both COPD and asthma, representing ~20% of patients with obstructive airway diseases. The recognition of both sets of morbidities in patients is important to guide practical treatment decisions. It is widely recognized that patients with COPD and coexisting asthma present with a higher disease burden, despite the conceptual expectation that the “reversible” or “treatable” component of asthma would allow for more effective management and better outcomes. However, subcategorization into terms such as ACOS is complicated by the vast spectrum of heterogeneity that is encapsulated by asthma and COPD, resulting in different clinical clusters. In this review, we discuss the possibility that these different clusters are suboptimally described by the umbrella term “ACOS”, as this additional categorization may lead to clinical confusion and potential inappropriate use of resources. We suggest that a more clinically relevant approach would be to recognize the extreme variability and the numerous phenotypes encompassed within obstructive airway diseases, with various degrees of overlapping in individual patients. In addition, we discuss some of the evidence to be considered when making practical decisions on the treatment of patients with overlapping characteristics between COPD and asthma, as well as the potential options for phenotype and biomarker-driven management of airway disease with the aim of providing more personalized treatment for patients. Finally, we highlight the need for more evidence in patients with overlapping disease characteristics and to facilitate better characterization of potential treatment responders.
Introduction
The term asthma–COPD overlap syndrome (ACOS) is used to describe patients who were presented with features of both asthma and COPD.Citation1 Terms such as ACOS have arisen to further simplify the classification of patients with COPD and asthma into phenotypes that better describe an individual patient’s disease characteristics; however, a balance must be sought between simplifying terminology versus considering the multiple clusters within the aforementioned diseases as distinct disease entities.Citation2,Citation3 A more clinically relevant approach would be to recognize the extreme variability and the numerous phenotypes encompassed within obstructive airway disease, with various degrees of overlap in individual patients, and to create an awareness that “recognizes” this variability rather than creating an umbrella term, such as ACOS, to “simplify” it. Indeed, oversimplified terminology may result in unnecessary confusion for patients and health-care providers.
Recognition of the considerable variability that exists within obstructive airway diseases is important, because patients with overlapping asthma and COPD are known to experience more frequent exacerbations, poorer quality of life, usually a more rapid decline in lung function, and higher morbidity and mortality than those with either COPD or asthma alone,Citation1,Citation4,Citation5 not unlike what is observed with other comorbidities. As a result, the burden and cost, both direct and indirect, are greater in patients with overlapping asthma and COPD compared with those with either disease individually, with one estimate suggesting that the cost of overlapping asthma and COPD is double than that of asthma alone.Citation6 Increasing awareness within the medical community of the heightened disease burden associated with overlapping morbidities is important and is clearly distinguishable from attempts to redefine patients under umbrella terms such as ACOS.
In this study, we review the current opinion on the identification and treatment of patients with overlapping characteristics of asthma and COPD and provide our commentary on whether there is a real clinical need for another “syndrome” and subcategorization of patients in the already complex environment of airways disease. To achieve this goal, we searched PubMed for relevant citations using search terms, including COPD, asthma, overlap, coexisting, and ACOS.
Epidemiology
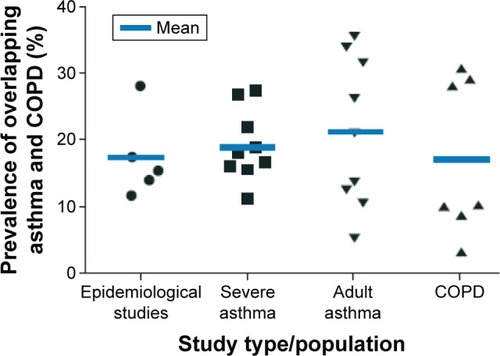
Reliable epidemiological data relating to overlapping asthma and COPD are scarce, partly due to a historical insistence on a clear separation between COPD and asthma and partly due to clinical trial exclusion criteria, which have excluded patients with COPD from asthma trials and vice versa.Citation7,Citation8 The use of spirometric measures, such as forced expiratory volume in 1 second (FEV1), FEV1/forced vital capacity ratio, and degree of reversibility, do not clearly differentiate between asthma and COPD, and may, in addition, contribute to the significant discrepancies that exist in the reported prevalence of overlapping asthma and COPD.Citation9 The Global Initiative for Asthma/Global initiative for chronic Obstructive Lung Disease (GINA/GOLD) guidelines cite epidemiological studies reporting different prevalence rates for overlapping asthma and COPD, with variation by sex and age, which is likely to reflect various sampling methods and definitions used.Citation1,Citation10 The prevalence of overlapping asthma and COPD consistently increases with age, in a similar pattern with the increased prevalence of COPD.Citation8,Citation11,Citation12 In a two-stage multicenter study, in the general population (n=~3,000), the prevalence of overlapping asthma and COPD was 1.6%, 2.1%, and 4.5% in the age groups of 20–44 years, 45–64 years, and 65–84 years, respectively.Citation8 In a more pragmatic approach, Gibson and McDonaldCitation10 have reported a prevalence of overlapping asthma and COPD of ~20% in patients with obstructive airway diseases in the majority of analyses when various study designs were used ().
Figure 1 Prevalence of overlapping asthma and COPD in studies of varying designs in a cluster analysis (following a systematic literature search).
Definitions of obstructive airway disease
Numerous definitions are proposed for distinct airway diseases, including asthma and COPD;Citation13 however, in reality many patients fit the criteria for more than one definition.Citation13 The most recent GINA/GOLD guidelines define ACOS as being characterized by persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD;Citation14,Citation15 however, the guidelines state that it is currently not possible to develop a more specific definition, due to the lack of evidence in relation to the underlying disease mechanisms.Citation1 Guideline development in COPD has placed emphasis on recognizing COPD as a disease where incomplete reversibility of airflow obstruction is the defining characteristic; while for asthma, the emphasis is traditionally based on reversible airway obstruction.Citation1,Citation15 However, these definitions are limited, as not all patients fit “comfortably” within such clear-cut criteria. Indeed, it is recognized that besides patients with COPD, patients with asthma (particularly severe asthma) or overlapping asthma and COPD may also present with fixed airway obstruction.Citation1,Citation16,Citation17 While there is an obvious need for clinical clustering under the well-established asthma and COPD definitions, there is also a requirement to recognize the variability that exists within these definitions, including varying levels of overlap between the two diseases.
For the airway disease clusters identified to date, limited evidence has linked pathological features with clinical patterns or treatment responses.Citation1 The value of novel definitions and clustering should be considered alongside clinical benefit and driven by evidence. There is currently little evidence to guide treatment in each specific phenotype/endotype, thus the requirement for further categorization is questionable, no matter how well intentioned.Citation1,Citation18 Based on the existing definition, patients with ACOS may experience different characteristics to different degrees along the spectrum of those featured in the definitions of asthma and COPD. For instance, a patient with a history of asthma since childhood who developed fixed airflow obstruction after a significant smoking habit most likely differs significantly from a patient with a long-standing diagnosis of COPD and no history of asthma who presents with some “asthmatic” characteristics in elderly age (eg, eosinophilic airway inflammation and/or significant reversibility of airflow limitation). This additional subcategorization and definitions may lead to clinical confusion and inappropriate simplification and resource use and should be carefully considered.
What do we know about the mechanisms underlying ACOS?
For many years, physicians have been aware that asthma and COPD can coexist in some patients and have been treating those patients accordingly.Citation19 However, despite the widespread recognition of some overlap between the two diseases, the mechanisms underlying the overlap between asthma and COPD remain controversial. Two long-standing hypotheses have been proposed in an attempt to highlight the underlying disease mechanisms: the “Dutch hypothesis” proposes that asthma and COPD are manifestations of the same basic disease process, with the former predisposing to the latter during the aging process,Citation20 whereas the “British hypothesis” suggests that asthma and COPD are distinct entities generated by different mechanisms.Citation21 In this hypothesis, it is thought that, in patients with characteristics of both asthma and COPD, both diseases coexist separately in the same individual. Some recent analyses support both hypotheses.Citation22
The complex pathophysiological mechanisms and associated triggers underlying asthma and COPD are well studied and reviewed elsewhere.Citation23,Citation24 In patients with asthma, the “typical” pathogenic processes are known to include mast cell-mediated bronchoconstriction, inflammation due to local antibody production, and eosinophilic inflammation. These complex processes are mediated by a number of different messenger molecules, including histamine, cysteinyl leukotrienes, prostaglandin D2, interleukins (ILs), and chemokines ().Citation25–Citation28 The airflow limitation experienced in a majority of patients with asthma is usually reversible; a diagnosis of persistent airflow limitation is usually only characteristic of patients with severe asthma.Citation1,Citation29 In patients with COPD, the typical pathogenic mechanisms include mucus hypersecretion, alveolar wall destruction, and fibrosis. Again, these complex processes are orchestrated by various cells and messenger molecules, including epithelial cells, macrophages, chemokines, monocytes, neutrophils, T-helper cells, and type 1 cytotoxic cells.Citation25–Citation27 Airflow limitation in patients with COPD is not generally reversible by short-acting β2-agonists alone.Citation1 In patients with overlap between asthma and COPD, the extent of the contribution of the underlying mechanisms of the two diseases may differ significantly between individuals, driven mainly by genetic predisposition, environmental exposure, the initiating condition, and the evolution of the natural history of each patient.
Figure 2 Pathophysiology of asthma, COPD, and overlap.
Abbreviations: TGFβ, tumor growth factor β; TH1, T-helper 1; TC1, type 1 cytotoxic T cells.
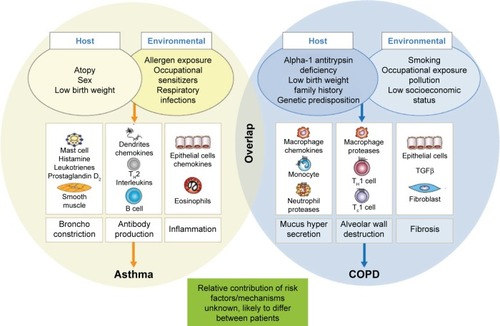
In both asthma and COPD, the pathogenic processes are triggered by interactions between host and environmental factors; the same assumption is made in patients experiencing overlapping asthma and COPD ().Citation30 In addition to the multiple risk factors shown in , evidence suggests that asthma is an independent risk factor for COPD.Citation11,Citation13,Citation31–Citation33 For example, a longitudinal, prospective study of children aged 6–7 years, followed to age 50 years, showed that children with asthma were at considerably greater risk of developing COPD than children without asthma.Citation32 Furthermore, the Tucson cohort study observed that asthmatic subjects were 12.5 times more likely to develop COPD than healthy individuals.Citation33 Finally, the Behavioral Risk Factor Surveillance System Asthma Call-back Survey showed that, in patients with active asthma, the prevalence of physician-diagnosed COPD was higher than in patients with inactive asthma,Citation11 with the potential bias this approach may incorporate. It is likely that some patients with asthma may complicate with COPD, typically following long-standing exposure to cigarette smoke or other environmental noxious particles of gases.Citation13,Citation31
There are conflicting data regarding the genetic component underlying overlapping asthma and COPD. Some analyses have suggested no common genetic component, while others have identified several variants associated with the overlap of COPD and asthma that approach genome-wide significance and may be of relevance; further work in this area is needed.Citation34,Citation35
Clinical and imaging characteristics
In order for a definition of ACOS to be clinically useful, consistency of clinical features that are distinct from asthma and COPD should be apparent. However, it is obvious to experienced clinicians that a patient with childhood asthma who has smoked for several years and has subsequently developed persistent airflow obstruction would significantly differ from a long-standing smoker with no history of airway disease in childhood who developed a syndrome with overlapping characteristics of asthma and COPD in middle age, even though both these patients may present characteristics that would allow for their inclusion under the ACOS umbrella term. Several large studies have assessed the clinical features of patients with overlapping asthma and COPD, including COPDGene (COPD genetic epidemiology) and PLATINO (COPD in five Latin American cities: a prevalence study).Citation34,Citation36 In COPDGene, which was designed to identify genetic factors associated with COPD, patients with overlapping asthma and COPD were younger, more likely to be African-American, smoked less, had greater airway wall thickness, and more gas trapping on expiratory chest CT scans than subjects with COPD alone. In addition, patients with overlapping asthma and COPD experienced more exacerbations and worse quality of life compared with patients with COPD, despite similar lung function.Citation34 Similarly, in PLATINO, a Latin American project for the investigation of airways obstruction, a higher risk of exacerbations was observed in patients with both asthma and COPD compared with COPD alone.Citation36 The PLATINO study also showed that, as could be anticipated by the presence of two respiratory diseases rather than one, patients with overlapping asthma and COPD experience a higher risk of hospitalization, more respiratory symptoms, worse lung function, and generally worse health status compared with patients with COPD alone.Citation36 It is widely recognized today that patients with COPD and coexisting asthma present with a higher disease burden, despite any possible conceptual expectation that the reversible or treatable component of asthma would allow for more effective management and better outcomes.
Airway remodeling occurs in both asthma and COPD. While there are structural similarities in terms of the remodeling between the diseases, the magnitude of change is known to differ. Few studies have accurately assessed the structural changes in patients with overlapping asthma and COPD compared with asthma and COPD alone. Increased airway wall thickness, measured using CT scans, is considered a surrogate measure of airway remodeling and has been observed in patients with overlapping asthma and COPD compared with patients with normal lung function.Citation37 In patients with asthma, increased wall thickness has largely been attributed to inflammation, subepithelial fibrosis, and increased thickness of the smooth muscle.Citation13,Citation36,Citation38,Citation39 Wall thickening is present, but less obvious, in patients with COPD compared with asthma, and the same structural components (epithelium, reticular basement membrane, airway smooth muscle, and mucous glands) are implicated.Citation13 Goblet cell hyperplasia is a feature of the remodeled airway in both patients with asthma and those with COPD.Citation13 Similarly, increased airway wall fibrosis is reported in patients with both asthma and COPD.Citation13 Despite the wide array of features that may be present in patients with coexisting or overlapping asthma and COPD, no specific imaging characteristics of ACOS have been identified to date, probably due to the heterogeneity of this population.
Biomarkers in identification and management
The identification of a biomarker or a panel of inflammatory biomarkers that allows a more precise etiologic diagnosis between asthma and COPD and overlap between the two (if appropriate) would be plausibly the most effective approach for patient classification and tailored treatment.Citation40,Citation41 The GOLD 2015 COPD guidelines provide some guidance in terms of biomarkers for patients with asthma and COPD (), but little is known about patients with overlap between the two diseases.Citation14 In a small study of 44 patients with stable airways disease, sputum neutrophils and total cells were highest in patients with overlapping COPD and asthma and COPD alone compared to those with asthma alone and healthy controls, whereas eosinophil numbers were significantly increased in patients with asthma compared with controls, but not different between asthma and COPD or patients with overlapping asthma and COPD.Citation13 In general, the presence of eosinophilic airways inflammation may be a predictor of steroid responsiveness in patients with asthma, whereas more limited data support this approach in patients with COPD or ACOS.Citation42,Citation43 There is some evidence that eosinophilic airway inflammation is an indicator of potential benefit of inhaled corticosteroid (ICS) therapy, whereas the absence of eosinophilic inflammation may be an indication for non-ICS containing treatment regimens, but further prospective studies in this area are required.Citation44–Citation46
Table 1 Inflammatory biomarkers in the diagnosis of asthma and COPD
The following studies have attempted to include biomarker analyses as diagnostic tools in order to assess the prevalence of overlapping asthma and COPD in patients with respiratory disease. A descriptive study by Tamada et alCitation40 used fractional exhaled nitric oxide (FeNO; 35 ppb cutoff value) as the diagnostic standard of ACOS in a population of patients with COPD, revealing a prevalence of 16%. However, some caution is required in the interpretation of these results, although FeNO has been shown to be a diagnostic biomarker in patients with asthma, data in patients with COPD are less consistent, possibly due to the confounding influence of smoking.Citation47–Citation49 The biomarker profiles of patients with asthma, COPD and overlapping asthma and COPD were assessed in a study by Iwamoto et al.Citation50 The authors found that patients with overlapping asthma and COPD and COPD alone had a similar biomarker profile in plasma surfactant protein A, the soluble form of receptor for advanced glycation of end-products, and sputum myeloperoxidase levels, which differed from the profile in patients with asthma. In addition, elevated levels of sputum neutrophil gelatinase-associated lipocalin were observed in patients with overlapping asthma and COPD versus COPD alone, highlighting potential differences in the underlying mechanisms of these diseases and representing a finding that may warrant further study.Citation50
A study by Ghebre et alCitation22 used sputum cytokine profiling to determine the existence of distinct and overlapping groups of patients with COPD and/or asthma (as defined by the GINA/GOLD guidelines). Three main clusters were identified: Cluster 1 was asthma predominant with evidence of eosinophilic inflammation and increased type 2 T-helper (Th-2) inflammatory mediators; Cluster 2 contained an asthma and COPD overlap group, with predominately neutrophilic airway inflammation and elevated levels of IL-1β and tumor necrosis factor-α and increased bacterial colonization; Cluster 3 was a COPD predominant group with mixed granulocytic airway inflammation and high sputum IL-6 and chemokine ligand 13 levels, and eosinophilic inflammation in some subjects.Citation22 Despite potential implications for stratified management targeting inflammatory pathways, the results of this small study require confirmation in future studies.
Diagnostic approach
The heterogeneous nature of the multiple entities included under the ACOS umbrella makes diagnosis challenging;Citation51 a number of diagnostic approaches have been suggested. The GINA/GOLD documents recommend a stepwise approach, comprising recognition of the presence of a chronic airways disease, syndromic categorization as asthma, COPD or ACOS, confirmation by spirometry and, if necessary, referral for specialized investigations. Although initial recognition and treatment of overlapping asthma and COPD may be made in primary care, referral for confirmatory investigations is encouraged, as outcomes for patients with overlapping disease are often worse than for either disease alone.Citation1 The Australian Asthma Management Handbook recommends pooling features corresponding to asthma and COPD in order to make a diagnosis.Citation52 The Japanese Respiratory Society’s COPD guidelines suggest the following indices for the diagnosis of an asthma component: paroxysmal dyspnea, cough, and wheeze that is worse at night and in the early morning, atopy, and the presence of peripheral blood and/or sputum eosinophilia.Citation53 The Spanish COPD consensus document for asthma–COPD overlap proposes three major and three minor criteria required for a diagnosis (), suggesting that two major criteria or one major and two or more minor criteria are strongly indicative of overlapping asthma and COPD.Citation7 The Czech guidelines are very similar to the Spanish guidelines.Citation54 The differences in diagnostic approaches between guidelines highlight the complexities involved in attempts to further subclassify respiratory disease. It is important to stress that the majority of the aforementioned guidelines and position papers focus on the identification of an asthmatic component in patients with diagnosed COPD, as this is plausibly the most relevant approach for the effective management of individual patients.
Table 2 Major and minor criteria for the identification of the mixed COPD/asthma phenotype
Principles of management
Potential treatment options (pharmacological and nonpharmacological) are listed in ; it should be noted that none of the treatments stated in are specific for the management of patients with overlap of asthma and COPD, and they are all indicated for the management of one or both conditions. The first step in the management of any patient with characteristics of both asthma and COPD is to provide advice on minimizing exposure to risk factors, such as smoking and allergens. The evidence base for the pharmacological treatment of patients with overlap between asthma and COPD is weak, as such patients have historically been excluded from randomized treatment trials; recommendations for management are therefore extrapolated from trials of either disease alone and are opinion led.Citation13 Despite this, there is a common opinion among specialists that patients with characteristics of overlapping asthma and COPD should be considered and treated differently from those with COPD.Citation55,Citation56
Table 3 Potential pharmacological and nonpharmacological treatment options in patients with asthma–COPD overlap
The management of patients with overlapping COPD and asthma requires the aggressive treatment of both conditions, including optimal bronchodilation and the appropriate dose of ICS, adjusted for disease severity. Most consensus papers recommend the use of long-acting beta agonist (LABA)/ICS combination therapy in these patients. In addition, the use of “triple” therapy (long-acting muscarinic antagonist [LAMA], LABA, and ICS) may be considered as an appropriate option in patients with more severe symptoms, especially in the presence of frequent exacerbations.Citation55 It is, however, important to consider that some patients may present reduced response to ICS, especially patients with COPD who continue to smoke or those who smoke and have asthma.Citation57,Citation58 Some evidence suggests that, in asthmatic smokers, leukotriene modifiers and low-dose theophylline may be of benefit;Citation58,Citation59 however, it should be noted that these data were not obtained in patients with overlapping asthma and COPD. Individuals receiving LABA/ICS, but still with poorly controlled asthma, have been shown to benefit from the addition of a LAMA (such as tiotropium),Citation60 although the same would seem intuitive in patients with overlapping asthma and COPD, there is currently no evidence to support this hypothesis.Citation60
As with the selection of any therapy regimen, potential safety and tolerability issues warrant careful consideration. For example, long-term ICS use has been associated with an increased risk of side effects in patients with COPD,Citation61 but it is not known whether the risk in patients with overlapping asthma and COPD is the same.Citation1,Citation61 In patients with asthma, omitting ICS therapy and treating with LABA monotherapy is associated with deteriorating asthma control, increased severity, and increased mortality.Citation62 Although the impact of LABA monotherapy in patients with overlapping asthma and COPD is not known, a cautious approach to monotherapy in these patients may be warranted due to the known increased burden of disease in these patients.Citation62
Although there is, on average, a higher rate of exacerbations in patients with COPD and asthma versus COPD alone, a significant number of patients with COPD also experience frequent exacerbations without the presence of an asthmatic component. It is important to clearly differentiate exacerbating patients with COPD and no asthmatic component, because these patients may benefit more from dual bronchodilation prior to the use of ICS or any other anti-inflammatory therapy.Citation63,Citation64 The efficacy of triple therapy (LABA/LAMA/ICS) in the reduction of exacerbations may have benefits, compared with LABA/LAMA in exacerbating patients with COPD; however, there is a need for prospective clinical trials in this specific population to explore this further.Citation65 Other anti-inflammatory options in exacerbating patients with COPD on effective inhaled therapies include phosphodiesterase-4 inhibitors and macrolides. The results of the Roflumilast and Exacerbations in patients receiving Appropriate Combination Therapy (REACT) study provide some evidence that, in patients with severe COPD with symptoms of chronic bronchitis and at least two exacerbations in the previous year, treatment with roflumilast may reduce exacerbations and hospital admissions, on top of ICS and LABA therapy compared with placebo.Citation66 However, studies comparing this combination to LABA/LAMA/ICS treatment are not available, so it is not clear which combinations are more efficacious. Finally, in patients with COPD with an increased risk of exacerbations, the addition of macrolides to usual treatment has been associated with decreased frequency of exacerbations compared with placebo, although side effects and potential increase in resistance to macrolides should be always considered.Citation67,Citation68
It is clear that, although there is substantial evidence to guide treatment in the majority of patients with asthma and COPD as separate diseases, more research is needed on the effects of different treatments in patients with overlap between the two diseases. The most important first step in the management of such patients is the identification of the predominant disorder that would determine the basis of the appropriate treatment. As with all patients with airways disease, patients with overlapping asthma and COPD, especially those with frequent exacerbations, should be carefully monitored and encouraged to report any deterioration (eg, worsening of cough, wheezing, and dyspnea) following the initiation or change of treatment.Citation69
Challenging the concept of ACOS and a proposed algorithmic approach
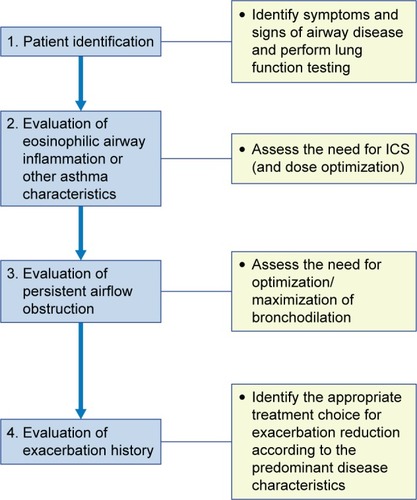
The management of the majority of patients with “pure” COPD or asthma may not present major challenges, as these patients may be successfully treated with established strategies, which work to optimize bronchodilator or anti-inflammatory therapy, respectively. In some patients with more severe or difficult-to-treat forms of either disease, experienced clinicians have little difficulty in incorporating additional treatment options based on specific characteristics, which permit classification into different phenotypes or endotypes. The introduction of an additional collective term with unclear characteristics and nonspecific guidance like ACOS may result in confusion and difficulty in the selection of appropriate treatment options by the busy primary care physician. We strongly believe that the identification of the predominant diagnosis and the potential underlying inflammatory pattern remains central to the proper management of all patients with airways disease. A potential four-step algorithmic approach for patients with overlapping clinical characteristics of asthma and COPD may include the steps outlined in . In this approach, the second step after the identification of patients with airway disease would be the evaluation of eosinophilic airways inflammation (eg, by increased sputum eosinophils or FeNO) or other asthma characteristics (eg, very positive bronchodilator reversibility), which may represent an indication for ICS use and dose optimization. The presence of persistent airflow obstruction (as expressed by the absence of complete reversibility after bronchodilator reversibility testing and/or treatment) may represent an indication for optimization (or maximization) of bronchodilation in appropriate patients (ie, via combination therapy of LAMA and LABA). Finally, the thorough evaluation of exacerbation history can lead to the selection of the most appropriate treatment for exacerbation reduction, according to the predominant disease characteristics, as described in the principles of management section.
Conclusion and the potential future of ACOS
The term ACOS represents a recent “revival” of the longstanding concept of overlap between the two most common airways diseases, COPD and asthma, in some patients. Despite the best of intentions, the complexities of terms such as ACOS are apparent, since the absence of a clear definition and the inclusion of patients with different characteristics under this umbrella term may not facilitate treatment decisions, especially in the absence of clinical trials addressing this heterogenic population.
It is our opinion that a more clinically relevant approach would be to raise awareness of the heterogeneity that exists within current definitions of respiratory disease to facilitate optimal, timely, and individualized treatment for our patients.
Acknowledgments
The authors were assisted in the preparation of the manuscript by Rebecca Douglas, PhD, a professional medical writer contracted to CircleScience, an Ashfield Company, part of UDG Healthcare plc (Tytherington, UK). Medical writing support was funded by Novartis Pharma AG (Basel, Switzerland).
Disclosure
KK has been an employee and shareholder of Novartis Pharma AG since January 1, 2015. In the previous 5 years, he had received honoraria for educational activities and lectures from AstraZeneca, Boehringer Ingelheim, Chiesi, ELPEN, GSK, MSD, Novartis, Takeda, and UCB and has served on advisory boards arranged by AstraZeneca, Chiesi, ELPEN, Novartis, and Takeda. AC and FP are employees and shareholders of Novartis Pharma AG. The authors report no other conflicts of interest in this work.
References
- Global Initiative for Asthma (GINA)Global Initiative for Chronic Obstructive Lung Disease (GOLD) [webpage on the Internet]Diagnosis of diseases of chronic airflow limitation: asthma COPD and asthma – COPD Overlap Syndrome (ACOS) Updated 20152015 Available from: http://goldcopd.org/asthma-copd-asthma-copd-overlap-syndrome/Accessed February 5, 2016
- MiravitllesMSoler-CataluñaJJCalleMSorianoJBTreatment of COPD by clinical phenotypes: putting old evidence into clinical practiceEur Respir J20134161252125623060631
- MilneSKingGGRole of imaging in COPD phenotypingRespirology201520452252325827014
- HardinMSilvermanEKBarrRGCOPDGene InvestigatorsThe clinical features of the overlap between COPD and asthmaRespir Res20111212721951550
- KauppiPKupiainenHLindqvistAOverlap syndrome of asthma and COPD predicts low quality of lifeJ Asthma201148327928521323613
- Gerhardsson de VerdierMAnderssonMKernDMZhouSTunceliOAsthma and chronic obstructive pulmonary disease overlap syndrome: doubled costs compared with patients with asthma aloneValue Health201518675976626409602
- Soler-CataluñaJJCosíoBIzquierdoJLConsensus document on the overlap phenotype COPD-asthma in COPDArch Bronconeumol201248933133722341911
- de MarcoRPesceGMarconAThe coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general populationPLoS One201385e6298523675448
- WurstKEKelly-ReifKBushnellGAPascoeSBarnesNUnderstanding asthma-chronic obstructive pulmonary disease overlap syndromeRespir Med201611011126525374
- GibsonPGMcDonaldVMAsthma-COPD overlap 2015: now we are sixThorax201570768369125948695
- MirabelliMCBeaversSFChatterjeeABActive asthma and the prevalence of physician-diagnosed COPDLung2014192569370024952247
- SorianoJBDavisKJColemanBVisickGManninoDPrideNBThe proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United KingdomChest2003124247448112907531
- GibsonPGSimpsonJLThe overlap syndrome of asthma and COPD: what are its features and how important is it?Thorax200964872873519638566
- Global Initiative for Asthma (GINA) [webpage on the Internet]Global strategy for asthma management and prevention Updated2015 Available from: http://ginasthma.org/2016-gina-report-global-strategy-for-asthma-management-and-prevention/Accessed February 5, 2016
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) [webpage on the Internet]Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease Updated 20162016 Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/Accessed February 5, 2016
- FabbriLMRomagnoliMCorbettaLDifferences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2003167341842412426229
- TashkinDPCelliBDecramerMBronchodilator responsiveness in patients with COPDEur Respir J200831474275018256071
- WeatherallMTraversJShirtcliffePMDistinct clinical phenotypes of airways disease defined by cluster analysisEur Respir J200934481281819357143
- LangePParnerJVestboJSchnohrPJensenGA 15-year follow-up study of ventilatory function in adults with asthmaN Engl J Med199833917119412009780339
- OrieNGMSluiterHJde VriesKTammelingGJWitkopJThe host factor in bronchitisOrieNGMSluiterHJBronchitisAssen, The NetherlandsRoyal Van Gorcum19614359
- BarnesPJAgainst the Dutch hypothesis: asthma and chronic obstructive pulmonary disease are distinct diseasesAm J Respir Crit Care Med20061743240243 discussion 3–416864717
- GhebreMABafadhelMDesaiDBiological clustering supports both “Dutch” and “British” hypotheses of asthma and chronic obstructive pulmonary diseaseJ Allergy Clin Immunol20151351637225129678
- National Heart Lung and Blood Institute (NHLBI)National Institutes of Health (NIH) [webpage on the Internet]Expert panel report 3: guidelines for the diagnosis and management of asthma Full reportWashington, DCUS Department of Health and Human Services2007 Available from: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdlnhtmAccessed January 13, 2014
- TuderRMPetracheIPathogenesis of chronic obstructive pulmonary diseaseJ Clin Invest201212282749275522850885
- PostmaDSRabeKFThe asthma-COPD overlap syndromeN Engl J Med2015373131241124926398072
- SutherlandERMartinRJAirway inflammation in chronic obstructive pulmonary disease: comparisons with asthmaJ Allergy Clin Immunol20031125819827 quiz 2814610463
- BarnesPJImmunology of asthma and chronic obstructive pulmonary diseaseNat Rev Immunol20088318319218274560
- MontuschiPLeukotrienes, antileukotrienes and asthmaMini Rev Med Chem20088764765618537720
- KonstantellouEPapaioannouAILoukidesSPersistent airflow obstruction in patients with asthma: characteristics of a distinct clinical phenotypeRespir Med2015109111404140926412805
- AlshabanatAZafariZAlbanyanODairiMFitzGeraldJMAsthma and COPD overlap syndrome (ACOS): a systematic review and meta analysisPLoS One2015109e013606526336076
- de MarcoRMarconARossiAAsthma, COPD and overlap syndrome: a longitudinal study in young European adultsEur Respir J201546367167926113674
- TaiATranHRobertsMClarkeNWilsonJRobertsonCFThe association between childhood asthma and adult chronic obstructive pulmonary diseaseThorax201469980581024646659
- SilvaGESherrillDLGuerraSBarbeeRAAsthma as a risk factor for COPD in a longitudinal studyChest20041261596515249443
- HardinMChoMMcDonaldMLThe clinical and genetic features of COPD-asthma overlap syndromeEur Respir J201444234135024876173
- SmolonskaJKoppelmanGHWijmengaCCommon genes underlying asthma and COPD? Genome-wide analysis on the Dutch hypothesisEur Respir J201444486087224993907
- MenezesAMMontes de OcaMPérez-PadillaRIncreased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthmaChest2014145229730424114498
- BumbaceaDCampbellDNguyenLParameters associated with persistent airflow obstruction in chronic severe asthmaEur Respir J200424112212815293614
- WardCPaisMBishRAirway inflammation, basement membrane thickening and bronchial hyperresponsiveness in asthmaThorax200257430931611923548
- MontuschiPBarnesPJNew perspectives in pharmacological treatment of mild persistent asthmaDrug Discov Today20111623–241084109121930234
- TamadaTSugiuraHTakahashiTBiomarker-based detection of asthma-COPD overlap syndrome in COPD populationsInt J Chron Obstruct Pulmon Dis2015102169217626491283
- MalerbaMMontuschiPNon-invasive biomarkers of lung inflammation in smoking subjectsCurr Med Chem201219218719622320297
- SmithADCowanJOBrassettKPExhaled nitric oxide: a predictor of steroid responseAm J Respir Crit Care Med2005172445345915901605
- BrightlingCEMcKennaSHargadonBSputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary diseaseThorax200560319319815741434
- PavordIDShawDEGibsonPGTaylorDRInflammometry to assess airway diseasesLancet200837296431017101918805315
- PascoeSLocantoreNDransfieldMTBarnesNCPavordIDBlood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trialsLancet Respir Med20153643544225878028
- PavordIDLettisSLocantoreNBlood eosinophils and inhaled corticosteroid/long-acting beta-2 agonist efficacy in COPDThorax201671211812526585525
- KostikasKMinasMPapaioannouAIPapirisSDweikRAExhaled nitric oxide in asthma in adults: the end is the beginning?Curr Med Chem201118101423143121434851
- KoutsokeraAKostikasKNicodLPFittingJWPulmonary biomarkers in COPD exacerbations: a systematic reviewRespir Res20131411124143945
- SantiniGMoresNShohrehRExhaled and non-exhaled noninvasive markers for assessment of respiratory inflammation in patients with stable COPD and healthy smokersJ Breath Res201610101710226814886
- IwamotoHGaoJKoskelaJDifferences in plasma and sputum biomarkers between COPD and COPD-asthma overlapEur Respir J201443242142923794464
- McDonaldVMSimpsonJLHigginsIGibsonPGMultidimensional assessment of older people with asthma and COPD: clinical management and health statusAge Ageing2011401424921087988
- National Asthma Council (Australia) [webpage on the Internet]Australian Asthma Management Handbook 2014 Available from: http://www.nationalasthma.org.au/handbookAccessed February 5, 2016
- Committee for the Third Edition of the COPD Guidelines of the Japanese Respiratory Society [webpage on the Internet]Guidelines for the Diagnosis and Treatment of COPD (Chronic Obstructive Pulmonary Disease)3rd Edition Available from: http://www.jrs.or.jp/uploads/uploads/files/photos/765.pdf2010
- KoblizekVChlumskyJZindrVChronic Obstructive Pulmonary Disease: official diagnosis and treatment guidelines of the Czech Pneumological and Phthisiological Society; a novel phenotypic approach to COPD with patient-oriented careBiomed Pap Med Fac Univ Palacky Olomouc Czech Repub2013157218920123733084
- MiravitllesMAlcazarBAlvarezFJWhat pulmonologists think about the asthma-COPD overlap syndromeInt J Chron Obstruct Pulmon Dis2015101321133026270415
- MontuschiPMalerbaMSantiniGMiravitllesMPharmacological treatment of chronic obstructive pulmonary disease: from evidence-based medicine to phenotypingDrug Discov Today201419121928193525182512
- MartinRJSzeflerSJKingTSThe predicting response to inhaled corticosteroid efficacy (PRICE) trialJ Allergy Clin Immunol20071191738017208587
- LazarusSCChinchilliVMRollingsNJSmoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthmaAm J Respir Crit Care Med2007175878379017204725
- SpearsMDonnellyIJollyLEffect of low-dose theophylline plus beclometasone on lung function in smokers with asthma: a pilot studyEur Respir J20093351010101719196814
- KerstjensHAEngelMDahlRTiotropium in asthma poorly controlled with standard combination therapyN Engl J Med2012367131198120722938706
- SuissaSPatenaudeVLapiFErnstPInhaled corticosteroids in COPD and the risk of serious pneumoniaThorax201368111029103624130228
- ChowdhuryBADal PanGThe FDA and safe use of long-acting beta-agonists in the treatment of asthmaN Engl J Med2010362131169117120181964
- MontuschiPCiabattoniGBronchodilating drugs for chronic obstructive pulmonary disease: current status and future trendsJ Med Chem201558104131416425587755
- ZhongNWangCZhouXLANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPDInt J COPD201510110151026
- PatalanoFBanerjiDD’AndreaPFogelRAltmanPColthorpePAddressing unmet needs in the treatment of COPDEur Respir Rev20142313333334425176969
- MartinezFJCalverleyPMGoehringUMBroseMFabbriLMRabeKFEffect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trialLancet2015385997185786625684586
- SeemungalTAWilkinsonTMHurstJRPereraWRSapsfordRJWedzichaJALong-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbationsAm J Respir Crit Care Med2008178111139114718723437
- AlbertRKConnettJBaileyWCAzithromycin for prevention of exacerbations of COPDN Engl J Med2011365868969821864166
- LouieSZekiAASchivoMThe asthma-chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerationsExpert Rev Clin Pharmacol20136219721923473596