Abstract
Background
Alpha-1 antitrypsin deficiency (AATD) is a rare genetic condition predisposing individuals to chronic obstructive pulmonary disease (COPD). The treatment is generally extrapolated from COPD unrelated to AATD; however, most COPD trials exclude AATD patients; thus, this study sought to systematically review AATD-specific literature to assist evidence-based patient management.
Methods
Standard review methodology was used with meta-analysis and narrative synthesis (PROSPERO-CRD42015019354). Eligible studies were those of any treatment used in severe AATD. Randomized controlled trials (RCTs) were the primary focus; however, case series and uncontrolled studies were eligible. All studies had ≥10 participants receiving treatment or usual care, with baseline and follow-up data (>3 months). Risk of bias was assessed appropriately according to study methodology.
Results
In all, 7,296 studies were retrieved from searches; 52 trials with 5,632 participants met the inclusion criteria, of which 26 studies involved alpha-1 antitrypsin augmentation and 17 concerned surgical treatments (largely transplantation). Studies were grouped into four management themes: COPD medical, COPD surgical, AATD specific, and other treatments. Computed tomography (CT) density, forced expiratory volume in 1 s, diffusing capacity of the lungs for carbon monoxide, health status, and exacerbation rates were frequently used as outcomes. Meta-analyses were only possible for RCTs of intravenous augmentation, which slowed progression of emphysema measured by CT density change, 0.79 g/L/year versus placebo (P=0.002), and associated with a small increase in exacerbations 0.29/year (P=0.02). Mortality following lung transplant was comparable between AATD- and non-AATD-related COPD. Surgical reduction of lung volume demonstrated inferior outcomes compared with non-AATD-related emphysema.
Conclusion
Intravenous augmentation remains the only disease-specific therapy in AATD and there is evidence that this slows decline in emphysema determined by CT density. There is paucity of data around other treatments in AATD. Treatments for usual COPD may not be as efficacious in AATD, and further studies may be required for this disease group.
Introduction
Alpha-1 antitrypsin deficiency (AATD) is an inherited condition that predisposes patients to chronic obstructive pulmonary disease (COPD)Citation1 albeit with considerable variability of clinical phenotype.Citation2 The spirometric diagnosis of AATD-related COPD and “usual” (unrelated to AATD) COPD is identical, but it manifests at a younger age. Not all subjects develop pulmonary disease and those who do vary in presentation and subsequent decline.Citation3 The first subjects observed with low alpha-1 antitrypsin (AAT) levels were young smokers with basal panacinar emphysema,Citation4 a finding which remains a typical AATD presentation,Citation5,Citation6 though bronchiectasis, neonatal jaundice, liver cirrhosis, and panniculitis may be seen.Citation6–Citation9
The most well-known polymorphisms (protease inhibitor [Pi] Z allele), present in the homozygous state in around 1/5,000 European Caucasians,Citation10 arise from a point mutation in SERPINA1 and result in a change to AAT structure, causing polymerization, accumulation in hepatocytes, and thus reduced circulating level of AAT.Citation11 Homozygous “Z” patients have an AAT level of 1.3–7.7 μM, considerably less than the putative protective threshold of 11 μM typical of PiSZ patients.Citation12 The primary function of AAT is protecting the lung from proteolytic enzymes, primarily neutrophil elastase (NE);Citation13 in deficiency, uninhibited NE can therefore lead to lung damage via quantum proteolysis.Citation14,Citation15
Management of symptomatic lung disease is generally similar to usual COPD, including smoking cessation, inhalers, and pulmonary rehabilitation (PR).Citation16,Citation17 Infusion of plasma-derived AAT (augmentation therapy) to restore physiological levels is the only licensed disease-specific treatment and the only area studied by previous systematic reviews. Its use is variable worldwide, largely due to differing health systems, although controversy over efficacy exists.Citation18,Citation19 A review by Chapman et al included many different study designs, focused on forced expiratory volume in 1 s (FEV1) as an outcome measure, and concluded that augmentation slowed FEV1 decline relative to placebo;Citation18 however, FEV1 has limitations, meta-analysis of varied study designs could have flaws, and major trials have been published since. Cochrane considered only randomized controlled trials (RCTs) and reviewed more outcomes (FEV1, diffusing capacity of the lungs for carbon monoxide [DLCO], computed tomography [CT] density, and quality of life [QoL]), concluding that augmentation was not beneficial, due to a lack of effect on lung function and QoL.Citation19 However, the benefit of measuring lung density by quantitative CT scan analysis was that it relates to mortality in AATD,Citation20,Citation21 subsequently resulting in the review being criticized widely by specialists. As such, a new, more wide ranging review was indicated. In general, COPD meta-analyses demonstrating the impact of pharmacological and nonpharmacological interventions have been published,Citation22,Citation23 but most studies in usual COPD have excluded AATD patients, so the evidence may not be generalizable.
Methods
The systematic review protocol is registered with PROSPERO (CRD42015019354). Standard systematic review methodology was used, aimed at minimizing bias, with reference to the Cochrane Handbook for Systematic Reviews of Interventions.Citation24
Search strategy
The following databases were searched by using no date or language restrictions (Supplementary materials): MEDLINE, MEDLINE In Process and EMBASE (via Ovid), Cochrane Library (Wiley) CENTRAL, CDSR, HTA, EED, and DARE. In addition, Conference Proceedings Citation Index via Web of Science and British Library’s ZETOC were searched for conference proceedings and abstracts. ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials. References of included studies and reviews were checked.
Study selection criteria
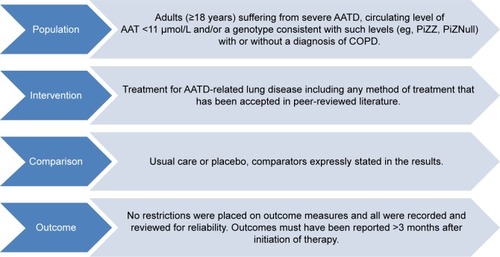
shows the selection criteria. Systematic reviews and primary study designs assessing treatment were eligible provided they included data on ≥10 participants with AATD. Nonsystematic reviews and preclinical studies were excluded. Studies comprising mixed populations were permitted if AATD data were available separately. Any intervention for AATD-related lung disease was eligible for inclusion. The only restriction placed on outcome measures was of reporting >3 months after initiation of therapy, thus limiting to the most clinically relevant studies for this chronic disease. Studies which, for instance, measured solely AAT concentration before and after augmentation dosing were not included, and this criterion ensured such studies were not selected.
Data extraction and assessment of bias
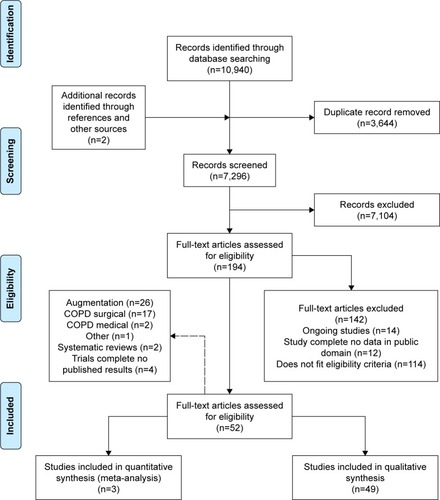
Initial search yields (titles and abstracts) were screened for relevance by two reviewers independently, resolving any disagreement by discussion. Relevant articles were obtained and assessed against the full selection criteria, with translation of non-English language articles if required. Full-text articles were dually reviewed as before, with data extraction and bias assessment completed by one reviewer and checked by another. Missing data were requested from study authors if necessary (Supplementary materials). Reasons for excluding studies were documented and summarized in the PRISMA flow diagram ().Citation25
Figure 2 PRISMA flow diagram.
Abbreviation: COPD, chronic obstructive pulmonary disease.
Evidence synthesis
Most data were suitable only for narrative synthesis, due to heterogeneity of study design and outcomes. Studies were divided into subgroups to reflect four “themes”: AATD-specific, surgical, and medical treatments used in usual COPD, and other treatments. Data from three RCTs of AAT augmentation were meta-analyzed, the study design being sufficiently similar to allow this,Citation26–Citation28 including changes in lung density (measured by quantitative CT scan at least annually), FEV1 % predicted of normal, DLCO, QoL (St Georges Respiratory Questionnaire [SGRQ]), and annualized exacerbation rate (measured at least 6 monthly). Assessment of clinical and methodological heterogeneity was used to determine a fixed or random effect model; fixed effects using mean difference was appropriate in all but DLCO where a standardized mean difference was used. Differences between baseline and follow-up were synthesized and annualized; I Citation2 and τ2 statistics are reported where appropriate.
Results
Following the removal of duplicates, 7,296 records were reviewed with 52 trials, comprising 5,632 participants’ data included in the final analysis (). The two systematic reviews identified were briefly described in the “Introduction” section and justified this new review; hence, they will not be discussed further. The complete study characteristics of the remaining studies are shown in the Supplementary materials.
AATD-specific treatment: augmentation
There were 26 eligible studies using a number of different product brands including Prolastin and Prolastin-C, Aralast, Zemaira, Trypsone, Respitin, and Glassia. Dosing regimes ranged from weekly to monthly with most using 60 mg/kg/week.
RCTs
Three international multisite RCTs were identified,Citation26–Citation28 including a total of 320 participants (58Citation26–180Citation28 per study), conducted over 2–3 years, with optional subsequent open-label elements in two studies.Citation27,Citation28 All studies used CT density change as an outcome, two utilizing it as an experimental measureCitation26,Citation27 and one powering for CT density as the primary outcome (RAPID trialCitation28). Other outcome measures included QoL, spirometry, and gas transfer and COPD exacerbation rates. Two studies used a standard dose (60 mg/kg body weight) intravenous infusion weekly,Citation27,Citation28 while the third used 250 mg/kg infused every 4 weeks.Citation26
Mortality data were only reported by RAPIDCitation28 with one death on treatment and three on placebo. Adverse events (AEs; treatment related or not) were similar in the two studies that reported them;Citation27,Citation28 one did not report AEs.Citation26 The remaining outcomes were possible to meta-analyze and are summarized in and . Heterogenicity was not detected (all I2=0%). In all studies, volume-corrected CT scans at total lung capacity were performed, this being the preferred and most validated scanning method,Citation27,Citation29–Citation31 though three scanning methods were reported in RAPID. Lung density was analyzed by regression in the study by Dirksen et alCitation26 and RAPIDCitation28 with four analysis methods in EXACTLE.Citation27 EXACTLE’s “method 1”Citation27 was used in this meta-analysis as it utilizes the same regression technique as other studies; sensitivity analyses using other EXACTLE methods did not alter the outcome or significance. Annual deterioration in lung density was less on augmentation; difference 0.79 g/L/year (95% confidence interval [CI] 0.29–1.29; P=0.002, ), demonstrating a slower rate of emphysema progression. There was no significant difference in annual FEV1% predicted decline on treatment (0.56% predicted/year [1.14–0.29; P=0.20]). A small nonsignificant difference of −0.11 (−0.33–0.11; P=0.34) in DLCO was observed.
Figure 3 Forest plots of the objective results from meta-analysis of augmentation trials.
Notes: (A) Mean annual change in lung density. (B) Mean FEV1 % predicted. (C) Standardized mean difference in DLCO. Differences in units used for DLCO (mmol/min/kPa and mL/mmHg/min) and the use of percentage change from baseline in RAPID, but annual change in the other studies required the use of a standardized mean difference plot.
Abbreviations: FEV1, forced expiratory volume in one second (L); DLCO, diffusing capacity of the lungs for carbon monoxide; mmol/min/kPa, millimole per minute per kilopascal; mL/mmHg/min, milliliter per millimeter of mercury per minute; SD, standard deviation; CI, confidence interval; df, degrees of freedom; Std, standard; IV, inverse variance.
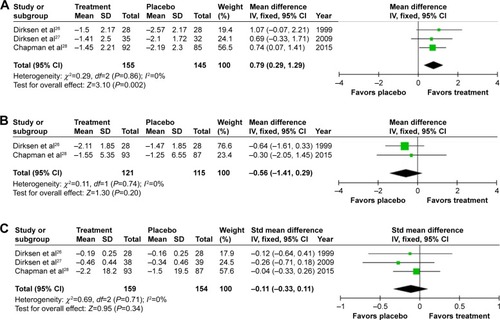
Figure 4 Forest plots for patient-reported outcomes.
Abbreviations: SGRQ, St Georges Respiratory Questionnaire; SD, standard deviation; CI, confidence interval; df, degrees of freedom; IV, inverse variance.
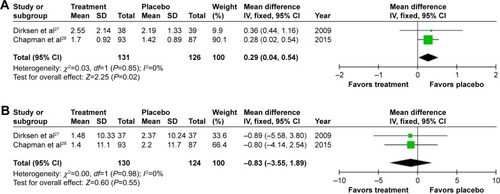
Annual exacerbation rate was not reported in the earliest study,Citation26 but there was a small, statistically significant increase in annual exacerbations on treatment (0.29/year [0.02–0.54; P=0.02], ) when meta-analyzing the other studies.Citation27,Citation28 Small and nonsignificant changes in health status were observed in both groups,Citation27,Citation28 demonstrating greater worsening in SGRQ on placebo 0.83 (−3.55 to 1.89; P=0.55, ).
Observational controlled studies
There were six eligible controlled observational studies, comprising 2,610 participants. AEs and reasons for starting/stopping therapy were reported by one registry; severe events occurred at a rate of 9.5% (69/720 infusions).Citation32
The largest observational study analyzed data from 1,129 patients in the US AATD registry split into three groups “always receiving” (n=390), “partly receiving” (n=357), or “never receiving” (n=382) augmentation.Citation33 Dosing was not standardized with only 51.3% being dosed weekly throughout the study. A survival analysis was conducted, but excluded 81 subjects (55 deaths) due to missing data, such that results could have been biased. Overall mortality was 18.1% (n=204); it was significantly higher for subjects who never received augmentation therapy (as opposed to sometimes or always) when FEV1<50% predicted (P<0.001). Mortality rates were low for other subjects and did not differ between augmentation therapy groups. FEV1 decline was calculated using a slope equation in 927 patients with n=202 excluded due to insufficient data; patients receiving augmentation with mean FEV1 values of 35%–49% predicted had a slower rate of FEV1 decline (−73.7±6.8 vs −93.2±8.9; P=0.03), though this was not seen in the whole group.
Three other studies investigated the effect of AAT augmentation on FEV1 decline.Citation34–Citation36 Seersholm et alCitation34 undertook a nonrandomized surveillance study in two cohorts. A statistically significant difference in FEV1 annual decline was observed (−53 [48–58] vs −75 [63–87] mL/year in treatment vs placebo; P=0.02).Citation34 The other two studies concurred with this result. Wencker et al conducted a pre–post study of augmentation, using inclusion criteria of ≥2 lung function measurements prior to augmentation and two following commencement of therapy within a minimum period of 12 months.Citation36 FEV1 declined significantly slower (−34.3±29.7 vs 49.2±60.8 mL/year, P=0.019) after starting augmentation. Tonelli et al compared 124 augmented PiZZ patients to 40 nonaugmented patients who had a median of two spirometry measurements over a mean follow-up of 41.7±2.6 months.Citation35 Again, FEV1 decline was worse in untreated patients (+10.61±21.4 vs −36.96±12.1 mL/year; P=0.05). All three studies stratified patients to groups by their FEV1 at presentation – FEV1<30%, 30%–65%, and >65% predicted.Citation34–Citation36 Patients with FEV1<30% were consistently observed not to benefit from augmentation in terms of FEV1 decline. Two of the three studies showed those with an FEV1>65% to have statistically significant reductions in FEV1 decline when on augmentation (−122.5±108.4 vs −48.9±54.9 mL/year; P=0.045 and −108.7±17.3 vs −29.2±15.29 mL/year; P=0.0006).Citation35,Citation36 Treatment effect was restricted to patients with FEV1>30% and <65% (−62 [57–67] vs −83 [70–96] mL/year; P=0.04) in one study.Citation34 When FEV1 at commencement of therapy was used to group patients, a statistically significant decrease of rate of decline during treatment was seen if FEV1<30% (53.4±45.3 to 22.1±16.0 mL/year; P<0.0001).Citation36 Subgrouping the FEV1>65% group demonstrated marked benefit in those deemed rapid decliners (FEV1 decline pre- vs postaugmentation −255.7±70.4 vs 52.7±61.3 mL/year; P=0.0016).
The most recent study by Barros-Tizon et alCitation37 was a retrospective medical records review of 127 participants evaluating the effect of augmentation on exacerbation rate. Seventy-five patients had ≥1 exacerbation during the 18 months follow-up required prior to commencement of augmentation. Dosing regimens were not standardized with an average dose of 60.7±3.8 mg/kg/week (Table S1) different from other studies,Citation33 and many patients had missing data. Fewer exacerbations were seen (1.2±1.6 vs 1.0±2.2 pre- vs posttreatment; P<0.01), an effect more marked in those exacerbating previously (2.0±1.6 vs 1.4±2.7; P<0.01).
Controlled studies of differing dose and/or drug manufacturer
Five RCTsCitation38–Citation42 with 176 participants investigated differences between dosing regimens of a productCitation38 or made comparisons between different products.Citation39–Citation42 Campos et alCitation38 used a double-blind crossover study of 60 versus 120 mg/kg/week of Prolastin-C to investigate safety and pharmacokinetics in 30 patients. They reported no increase in AEs, a higher trough serum AAT concentration, and that the higher dose was tolerated well by patients. Four double-blind RCTsCitation39–Citation42 completed investigating pharmacokinetic equivalence between weekly dosing of the new investigational product (Respitin,Citation42 Zemaira,Citation41 Prolastin-C,Citation39 and GlassiaCitation40) and control (60 mg/kg/week Prolastin). All the studies had an optional open-label continuation study for safety data collection (ranging from 8 weeksCitation39 to 2 yearsCitation41) and reported no significant difference in AEs between the investigational product and control. Pharmacokinetic equivalence was reported in all studies with none demonstrating superiority over Prolastin. Stoller et al,Citation42 and Sandhaus et alCitation40 reported no significant differences in lung function parameters between the investigation groups or over the duration of the studies. One death was reported (respiratory arrest related to COPD) in the study by Stocks et al,Citation41 which was in the control arm and considered unrelated to study medication.
Observational uncontrolled studies
Twelve uncontrolled observational studies using intravenous AAT augmentation in 2,526 patients were included, which assessed safety and tolerability,Citation43–Citation45 pharmacokinetics,Citation46–Citation48 pulmonary neutrophilic inflammation,Citation49 longitudinal change in lung function,Citation50–Citation52 and clinical characteristics or recipients.Citation53,Citation54 AAT augmentation was reported as safe and well tolerated (one study drug-related AE out of 555 doses)Citation45 with 26 AEs probably/possibly related to the study drug,Citation43,Citation45,Citation47 largely comprising symptoms commonly observed in infusions of other protein-based products.Citation52 Other adverse reactions reportedCitation43,Citation49 were one widespread skin reaction,Citation49 headache and increased shortness of breath, and hospital treatment for fever, hypotension, and hypoxemia.Citation43 In all, 61 of 2,526 patients died with none attributed to AAT augmentation.Citation46,Citation51,Citation52 Augmentation was again well tolerated and deemed safe in a small study of 60 mg/kg/week versus 240 mg/kg/month.Citation50
Biochemical efficacy at the higher dose given monthly did not demonstrate a protective AAT level in 3 of 16 patients.Citation50 A second study investigating the pharmacokinetics of an alternative dose (Prolastin, 120 mg/kg every 2 weeks) was completed in 23 participants;Citation46 none of the patients maintained target serum AAT levels (>80 mg/dL) and only two maintained serum AAT levels >70 mg/dL from days 7 to 14 suggesting insufficient dosing. Wewers et alCitation47 included 21 patients infused weekly for up to 6 months and demonstrated partial correction of biochemical abnormalities in serum (AAT trough level 126±1 vs 30±1 mg/dL) and pulmonary epithelial lining fluid (AAT 0.46±0.16 vs 1.89±0.17 μM). Similarly, effective concentrations of AAT were reported by two short-term studiesCitation43,Citation44 and one 3-year study.Citation51 A study investigating 18-fluorodeoxyglucose (18FDG) positron emission tomography–CT as a novel noninvasive biomarkerCitation49 had a subgroup of 10 patients with severe AATD. The study demonstrated that 12 weekly infusions of AAT augmentation therapy had no effect on circulating neutrophil 18FDG uptake and activity in the lung.
Pulmonary function was reported in five studies with no significant changes in rates of decline or spirometry being observed.Citation46,Citation47,Citation50–Citation52 An observational registry study where longitudinal FEV1 follow-up was available in 287 patients over 37.8±18.9 months showed significant differences in decline in FEV1<30% predicted and FEV1 30%–65% groups (−35.6±21.3 vs −64.0±26.4 mL; P=0.0008).Citation52
AlphaNet in USA is a not-for-profit health management company, which undertook a prospective study involving 922 members on augmentation, using monthly telephone interviews to collect data including exacerbation history and QoL (SGRQ) at baseline, months 6, and 12.Citation53 Totally, 2,268 exacerbations (mean 2.45±1.3/subject/year) were observed, mostly graded to be of moderate severity. SGRQ did not change by the minimal clinically important difference (MCID) during the 12-month follow-up in any age group, though differences were seen according to age. The same cohort was usedCitation54 to describe exacerbations in more detail. Mean duration was 17.4±11.4 days by symptom-based classification, with a trend for increasing frequency and duration as disease severity increased; Global initiative for chronic Obstructive Lung Disease (GOLD) stage I and II patients had shorter, fewer exacerbations than GOLD stage III and IV (both P<0.05). Annual hospitalization for exacerbations was decreased in 4/14 patients in one study where study entry annual rates were used to assess augmentation; however, the study was insufficiently powered to establish the effectiveness in modifying disease.Citation43
COPD medical management
There were only two eligible studies in AATD using traditional COPD medical management strategies.Citation17,Citation55 The first reported good uptake and efficacy of influenza vaccination in the AlphaNet cohort (n=939),Citation55 with 766 (81.6%) patients receiving vaccination, who then had fewer unscheduled outpatient department visits and critical care admissions (P=0.04 and 0.01, respectively). No statistically significant differences in exacerbation frequency or respiratory outcomes were observed. Another observational study by AlphaNet investigated the use of a multimodal self-management program with a combination of directed patient self-education, organized supervision, and health care provider education (Alpha-1 Disease Management and Prevention Program) in 1,028 participants.Citation17 This 2-year study used data from the first “observational” year as comparator to a second year when the management plan was delivered. A total of 905 participants completed the 2 years demonstrating low attrition. There was strong evidence to support improved compliance in some medications (long-acting beta agonist [LABA] P<0.001, theophylline P=0.01, and systemic steroids P=0.02) and supplementary oxygen (P<0.01) along with reductions in annual exacerbation frequency and duration (P<0.001 and P=0.04, respectively). There were no significant changes to health status.
COPD surgical management
Lung volume reduction (LVR)
Six studies investigated the use of Lung Volume Reduction Surgery (LVRS) in AATD. Five studies (n=71 patients) used an open surgical technique,Citation56–Citation60 and all demonstrated improvements in either physiological measurements or dyspnea. Benefits were inferior and shorter in duration than usual COPD patients in all studies. One small RCT randomized participants to LVRS (n=10) or medical treatment (n=6); higher 2-year mortality (20% vs 0%) occurred in the surgical group, albeit alongside improvements in SGRQ.Citation59 There was one published study using endobronchial valves, which demonstrated their safety in AATD patients with significant benefits in mean FEV1 at 6 months, 1, and 2 years (P=0.0022, 0.0067, and 0.033, respectively).Citation61 The generalizability of this study is not evident as this cohort included strict inclusion criteria including severe heterogeneous emphysema demonstrated by CT scan and scintigraphy, residual volume ≥140%, FEV1 15%–45%, and optimal lobe selection. This resulted in fewer than half of the referrals meeting these criteria.Citation61
Lung transplantation
Eleven studies over 24 years reported 2,146 lung transplants in AATD patients.Citation62–Citation72 Two studies investigated survival after transplantation compared with nontransplantation management; Tanash et alCitation71 observed a survival benefit from transplant (11 [9–14] vs 5 [4–6] years; P=0.006). No significant difference was observed in survival (10.1 vs 8.4 years; P=0.954); however, improvements in total SGRQ (and all domains) were seen at 1 year (P<0.01).Citation72 Six studiesCitation64–Citation69 using retrospective reviews of lung transplant registries (all cause, not specific to AATD) contained sufficient separately reported data on mortality. Two demonstrated superior 10-year survival in AATD patients compared to usual COPD (P=0.04 and P<0.0001, respectively),Citation67,Citation68 but both noted that COPD recipients were often older with greater comorbidity. Conversely, Breen et al observed higher patient survival at 2 years in usual COPD (75.4%±4.4% vs 64.4%±5.4% in AATD); however, insufficient power precluded statistical analysis.Citation64 All other studies showed no difference in long-term survival between AATD- and non-AATD-related COPD.Citation62,Citation65,Citation66,Citation69,Citation70
There were some studies that reported other posttransplant outcomes. A subgroup analysis of patients receiving bilateral lung transplants demonstrated faster posttransplant FEV1 decline in AATD patients (P<0.002).Citation62 Analysis of gastrointestinal complications posttransplant surgery showed that having AATD significantly increased the risk of requiring early postoperative laparotomy (odds ratio 5.74, 95% CI 2.15–15.35), which increased all-cause mortality by 62% (P>0.05).Citation63
Other management
A double-blind, parallel-group RCT of Palovarotene to reduce inflammation and promote structural repair in the lungs failed to demonstrate significant differences in change from baseline for CT density, FEV1, or gas transfer measures and exacerbation.Citation73
Unreported trials
A study of an inhaled AAT augmentation therapy (NCT1217671) is listed as completed, but limited data are currently available.Citation74,Citation75 One registered study (NCT0242385)Citation76 compared two augmentation products in a double-blind, crossover RCT. Only one outcome (AEs) was eligible for inclusion as follow-up for all other outcomes were limited to 35 days; similar safety profile and no serious AEs were seen, but no statistics were available. A second study compared two Prolastin-C levels at 60 and 120 mg/kg to assess safety and pharmacokinetics (NCT1213043).Citation77 No statistical analysis was available but 30 participants completed the study with similar treatment-emergent AEs and safety profile to other studies; no serious AEs occurred. There is currently one study (NCT1983241)Citation78 investigating 60 and 120 mg/kg weekly administrations of Prolastin-C against placebo to determine safety and efficacy using change from baseline in CT lung density as the primary outcome.Citation79
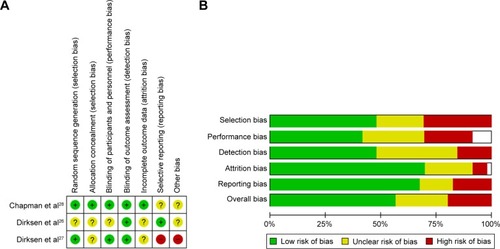
Risk of bias
Risk of bias for the meta-analyzed RCTs is summarized in . Risk of bias in RAPIDCitation28 was low; however, in the two earlier augmentation studies, this was more difficult to assess and generally unclear due to poor reporting, specifically of details such as allocation concealment. The other six RCTs demonstrated low or unclear bias, mostly due to lack of detail in the manuscripts, commonly around blinding. summarizes the risks in the remaining studies. The mixture of RCTs and cohort and observational studies (both prospective and retrospective) led to an overall moderate risk of bias especially in the nonblinded studies. In the controlled or quasi-controlled studies, there was often insufficient reporting of the blinding process and allocation concealment, except in more recent studies, and a trend toward selective outcome reporting.Citation18,Citation36,Citation38 Within the uncontrolled retrospective studies, selection bias was the main issue, with patients being taken from self-selecting groups or registries. Transplant studies from registries suffered from high attrition rates, which may have introduced survivor bias.Citation64–Citation69
Figure 5 Risk of bias in included studies.
Abbreviation: RCT, randomized controlled trial.
Discussion
The available evidence for the management of AATD was largely centered on augmentation, which seemed to benefit emphysema, but with significant deficits in the evidence base for outcomes more typically assessed in COPD studies, such as exacerbation frequency and QoL. Although it is not unreasonable to think that strategies used to treat COPD unrelated to AATD could be extrapolated to AATD patients, there was almost no evidence proving this.
Augmentation
Augmentation was demonstrated to be safe and well tolerated in numerous studies using different products. Investigations into dosing regimes demonstrated few benefits from >60 mg/kg/weekCitation38 (the “standard” dose), and pharmacokinetic equivalence was observed between different manufacturers’ products.Citation40,Citation41
The meta-analysis confirmed that augmentation therapy is able to slow down the progression of severity of emphysema when measured by CT density change compared with placebo (P=0.002). By combining data from up to 320 patients included in three RCTs, it adds to an increasing body of literature demonstrating the usefulness of CT densitometry as a surrogate measure of emphysema and outcome in both AATD and usual COPD.Citation20,Citation80,Citation81 However, the MCID has not yet been established for change in CT density, which would be helpful for interpretation. Meta-analysis also revealed a small but significant increase in annual exacerbation rate on augmentation. This is somewhat counterintuitive and contradicted by studies included in narrative synthesis, which either showed a benefit or no significant difference in exacerbation rate or severity.Citation17,Citation37,Citation54 Increased contact with health care professionals, simply due to attending for infusions, could lead to increased reporting of exacerbations, but this is the opposite result from published observational studies. These findings require caution in their interpretation as none of the studies were powered to detect change in exacerbation rates as an outcome. Studies in usual COPD appropriately powered to detect change in exacerbation frequency often require several thousand patients.Citation82 With advances in technology and a reduction in the cost of digital patient symptom diaries, there are an increasing number of studies using them to collect contemporaneous symptom data, including a recent inhaled augmentation therapy (NCT1217671) trial.Citation75 This has been beneficial in usual COPD trialsCitation83,Citation84 as it avoids recall bias and gives accurate data on severity and duration; hence, the results will be of interest. The Spanish study that focused on exacerbations had issues with missing data and also used variable treatment regimens, such that bias and lack of comparability to RCTs are an issue.Citation37 There are several other reasons why the exacerbations result should be viewed with caution; first, prestudy exacerbation frequency was not reported in the three RCTs. Prior exacerbation rate predicts future exacerbation rate;Citation85,Citation86 hence, if a difference was present pretreatment, it could account for the observation posttreatment. Second, power calculations were not based on detecting change in exacerbation frequency. Nevertheless, this area will be important for further study because faster decline in lung function and significantly increased health care costs occur due to frequent exacerbations in usual COPD,Citation87,Citation88 an effect that might be more marked in AATD due to higher inflammatory burden during events.Citation89
Most observational controlled studies demonstrated some differences in FEV1 decline with recipients of AAT augmentation benefiting over those not receiving therapy.Citation34,Citation35,Citation52 However, such study designs have weaknesses, often relating to differences between treated and untreated groups. For instance, sex, FEV1, and follow-up time differences were seen between treated (German) and untreated (Dutch) patients,Citation34 and age, FEV1, symptoms, inhaled therapies, and oxygen use differed in another studyCitation35 indicating that biases are likely present, which could have influenced results. In most cases, attempts were made to adjust for major differences statistically when comparing groups, and subgroup analyses suggested that benefits did not accrue in patients with FEV1<30% predicted. However, since it has been demonstrated that gas transfer declines more rapidly at this stage,Citation86 it may simply be that different outcome measures were needed to demonstrate any effect. In one study, there was evidence that rapidly declining patients benefited more;Citation52 however, there were only 11 such patients, and the definitions of slow and rapid decline utilized are not widely adopted in practice.
COPD medical management
There was limited evidence about the efficacy of COPD treatments in AATD, and no trials reported the effects of typical treatments such as inhaled bronchodilators (long acting muscarinic antagonist, LABA, etc), steroid combinations (LABA/inhaled corticosteroids [ICS]), or PR. The available evidence was of poor quality, being prone to acquisition and potentially other biases, due to its retrospective, observational design. However, the results for influenza vaccination and self-management were at least suggestive of clinical benefit to AATD patients, thus consistent with the usual COPD literature.Citation22 There is no biological reason to suppose that such interventions would differ in efficacy between usual COPD and AATD.
However, data from the uncontrolled studiesCitation54 and sputum workCitation89 suggest that exacerbations are longer and more pro-inflammatory in AATD, such that treatments targeting their rate (eg, LABA/ICS) might be more beneficial in AATD. Recently, an unfavorable muscle response to exercise has been shown in a small number of AATD patients, compared with usual COPD,Citation90 suggesting that evidence about PR might not be wholly generalizable to AATD either. This is backed up by a study of a 5-week PR program pretransplantation, where AATD patients were observed to have a smaller improvement in 6-min walking test distance compared with usual COPD (47.9 vs 60.6 m).Citation91 However, the PR course was short, and the study was not eligible for inclusion in this review due to short follow-up duration; furthermore, no difference was seen after statistical adjustment for confounders. Hence, uncertainty exists about the value of COPD treatments, and further work is needed.
COPD surgical management
AATD patients receiving LVRS demonstrated benefits, albeit inferior when compared with usual COPD. In the single study, compared to medical care (as in the National Emphysema Treatment Trial study), there were more deaths in surgically treated patients, even though their QoL improved. However, underpowering meant there were no meaningful statistics and the results should be viewed with caution. Higher mortality could reflect differences in the distribution of emphysema in AATD, making LVRS technically more difficult because access to the lung bases is needed.Citation92 One small study demonstrated safety and benefits lasting up to 2 years from endobronchial valves in carefully selected patients – further investigation to reproduce these findings is warranted; however, it is encouraging and suggests that volume reduction might yet be a viable strategy. There is no evidence for endobronchial coiling in AATD yet, though suggestion of benefit in more homogeneous diseaseCitation93 implies they could be valuable.
When compared to usual COPD, AATD lung transplantation recipients had improved survival, but this was potentially confounded by differences in age and comorbidity.Citation68 Earlier onset of severe disease is well documented, and therefore, a survival benefit posttransplant may not be surprising. Two studies comparing AATD patients receiving transplant or notCitation71,Citation72 had conflicting results with respect to survival benefit, despite selection criteria for transplantation being similar in both countries (Sweden and UK). It is possible that more stringent matching procedures in the UK cohortCitation72 explain the difference. Nevertheless, significant health status benefits occurred after transplant indicating that it is appropriate when QoL is poor. However, unlike pulmonary fibrosis where there is unified evidence of survival benefit (in part due to the poor prognosis of disease),Citation94 uncertainty regarding survival benefits should be discussed with patients and is not only unique to AATD but also apparent in usual COPD and may represent the heterogeneity of disease.Citation94 The single study that demonstrated decline in AATD transplant recipients included only five patients and a time frame (2 years) for the calculation of FEV1 decline, which is too short to be accurate; hence, the results may not be reliable.Citation62 Increased posttransplant gastrointestinal complications requiring laparotomy were demonstrated in one studyCitation63 with those patients having a longer intensive care unit stay. There was no statistically significant effect on mortality or duration of mechanical ventilation observed, and the authors note that this was a small single-center retrospective study.
Conclusion
There is good evidence from this systematic review that intravenous augmentation therapy slows decline in emphysema determined by CT density. This supports its use as a surrogate end point and demonstrates that augmentation remains the primary disease-specific therapy. There is paucity of data around other treatments in AATD including potential bias in the selection and reporting of clinical trials. As augmentation therapy is an expensive treatment, a full economic review is needed, and further work on optimizing patient selection for therapy could help rationalize treatment in the UK. All treatments for usual COPD may not be as efficacious in AATD due to important differences in disease process, and studies in specific treatments such as PR need to be appropriately powered to this disease group.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This report is an independent research arising from a Clinical Doctoral Research Fellowship (Ross Edgar), CDRF-2014-05-044, supported by the National Institute for Health Research (NIHR) and Health Education England (HEE). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, Health Education England, or the Department of Health. The review team would like to thank Professor RA Stockley for his collaboration on this review.
Disclosure
Mr Edgar reports grants from HEE and NIHR, during the conduct of the study. This article presents independent research funded by the NIHR. The views expressed are those of the authors and not necessarily those of the NHS, NIHR, HEE, or the Department of Health.
Dr Patel, Mrs Bayliss, and Dr Crossley have nothing to disclose.
Dr Sapey reports grants from NIHR, during the conduct of the study, and grants from Medical Research Council, Alpha-1 Foundation, and British Lung Foundation, outside the submitted work.
Dr Turner reports grants from Grifols Biotherapeutics, Alpha-1 Foundation, outside the submitted work, and The Birmingham AATD Registry has received past funds from CSL Behring for work in AATD, although this was not held by any of the authors.
The authors report no other conflicts of interest in this work.
References
- ErikssonSPulmonary emphysema and alpha1-antitrypsin deficiencyActa Med Scandinavica1964175197205
- StockleyRATurnerAMAlpha-1-Antitrypsin deficiency: clinical variability, assessment, and treatmentTrends Mol Med201420210511524380646
- StockleyRAEdgarRGPillaiATurnerAMIndividualized lung function trends in alpha-1-antitrypsin deficiency: a need for patience in order to provide patient centered management?Int J Chron Obstruct Pulmon Dis201611174527536086
- LaurellCBErikssonSThe serum α1-antitrypsin in families with hypo-α1-antitrypsinemiaClin Chim Acta196511539539814347984
- LaurellC-BErikssonSThe electrophoretic alpha1-globulin pattern of serum in alpha1-antitrypsin deficiency. 1963COPD201310Suppl 13823527532
- NeedhamMStockleyRAAlpha1-Antitrypsin deficiency.3: clinical manifestations and natural historyThorax200459544144515115878
- ErikssonSCarlsonJVelezRRisk of cirrhosis and primary liver cancer in alpha1-antitrypsin deficiencyN Engl J Med1986314127367393485248
- ParrDGGuestPGReynoldsJHDowsonLJStockleyRAPrevalence and impact of bronchiectasis in alpha1-antitrypsin deficiencyAm J Respir Crit Care Med2007176121215122117872489
- SvegerTLiver disease in alpha antitrypsin deficiency detected by screening of 200,000 infantsN Engl J Med197629424131613211083485
- BlancoIde SerresFJFernandez-BustilloELaraBMiravitllesMEstimated numbers and prevalence of PI*S and PI*Z alleles of alpha1-antitrypsin deficiency in European countriesEur Respir J2006271778416387939
- LomasDAEvansDLFinchJTCarrellRWThe mechanism of Z alpha1-antitrypsin accumulation in the liverNature199235763796056071608473
- GreenCEVayalapraSHampsonJAMukherjeeDStockleyRATurnerAMPiSZ alpha-1 antitrypsin deficiency (AATD): pulmonary phenotype and prognosis relative to PiZZ AATD and PiMM COPDThorax2015701093994526141072
- StockleyRANeutrophils and the pathogenesis of COPDChest20021215151S155S12010844
- LiouTGCampbellEJQuantum proteolysis resulting from release of single granules by human neutrophils: a novel, nonoxidative mechanism of extracellular proteolytic activityJ Immunol19961576262426318805666
- CampbellEJCampbellMABoukedesSSOwenCAQuantum proteolysis by neutrophils: Implications for pulmonary emphysema in alpha1-antitrypsin deficiencyJ Clin Invest1999104333734410430615
- StollerJKAboussouanLSA review of alpha1-antitrypsin deficiencyAm J Respir Crit Care Med2012185324625921960536
- CamposMAAlazemiSZhangGWannerASandhausRAEffects of a disease management program in individuals with alpha-1 antitrypsin deficiencyCOPD200961314019229706
- ChapmanKRStockleyRADawkinsCWilkesMMNavickisRJAugmentation therapy for alpha1 antitrypsin deficiency: a meta-analysisCOPD20096317718419811373
- GøtzschePCJohansenHKIntravenous alpha-1 antitrypsin augmentation therapy for treating patients with alpha-1 antitrypsin deficiency and lung diseaseCochrane Database Syst Rev20169CD00785127644166
- GreenCEParrDGEdgarRGStockleyRATurnerAMLung density associates with survival in alpha 1 antitrypsin deficient patientsRespir Med2016112818726874894
- DawkinsPWoodANightingalePStockleyRMortality in alpha-1-antitrypsin deficiency in the United KingdomRespir Med2009103101540154719443188
- JordanREMajothiSHeneghanNRSupported self-management for patients with moderate to severe chronic obstructive pulmonary disease (COPD): an evidence synthesis and economic analysisHealth Technol Assess201519361
- KewKMDiasSCatesCJLong-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysisCochrane Database Syst Rev20143CD01084424671923
- HigginsJPTGreenSCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]The Cochrane Collaboration2011 Available from: http://handbook.cochrane.orgAccessed June 23, 2015
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementPLoS Med200967e100009719621072
- DirksenADijkmanJHMadsenFA randomized clinical trial of alpha1-antitrypsin augmentation therapyAm J Respir Crit Care Med19991605 Pt I1468147210556107
- DirksenAPiitulainenEParrDGExploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiencyEur Respir J20093361345135319196813
- ChapmanKRBurdonJGPiitulainenEIntravenous augmentation treatment and lung density in severe alpha1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trialLancet2015386999136036826026936
- ParrDGDawkinsPAStockleyRAComputed tomography: a new gold standard for the clinical assessment of emphysemaAm J Respir Crit Care Med2006174895417021361
- ParrDGStoelBCStolkJStockleyRAValidation of computed tomographic lung densitometry for monitoring emphysema in alpha1-antitrypsin deficiencyThorax200661648549016537666
- ParrDGDirksenAPiitulainenEDengCWenckerMStockleyRAExploring the optimum approach to the use of CT densitometry in a randomised placebo-controlled study of augmentation therapy in alpha 1-antitrypsin deficiencyRespir Res2009107519678952
- StollerJKFallatRSchluchterMDAugmentation therapy with alpha1-antitrypsin patterns of use and adverse eventsChest200312351425143412740257
- Survival and FEV1 decline in individuals with severe deficiency of alpha1-antitrypsin. The Alpha-1-Antitrypsin Deficiency Registry Study GroupAm J Respir Crit Care Med1998158149599655706
- SeersholmNWenckerMBanikNDoes alpha1-antitrypsin augmentation therapy slow the annual decline in FEV1 in patients with severe hereditary alpha1-antitrypsin deficiency?Eur Respir J19971010226022639387950
- TonelliARRouhaniFLiNSchreckPBrantlyMLAlpha-1-antitrypsin augmentation therapy in deficient individuals enrolled in the Alpha-1 Foundation DNA and Tissue BankInt J Chron Obstruct Pulmon Dis2009444345220054436
- WenckerMFuhrmannBBanikNKonietzkoNWissenschaftliche Arbeitsgemeinschaft zur Therapie von L. Longitudinal follow-up of patients with alpha(1)-protease inhibitor deficiency before and during therapy with IV alpha(1)-protease inhibitorChest2001119373774411243951
- Barros-TizonJCTorresMLBlancoIMartinezMTInvestigators of the rEXA study groupReduction of severe exacerbations and hospitalization-derived costs in alpha-1-antitrypsin-deficient patients treated with alpha-1-antitrypsin augmentation therapyTher Adv Respir Dis201262677822354900
- CamposMAKueppersFStocksJMSafety and pharmacokinetics of 120 mg/kg versus 60 mg/kg weekly intravenous infusions of alpha-1 proteinase inhibitor in alpha-1 antitrypsin deficiency: a multi-center, randomized, double-blind, crossover study (SPARK)COPD201310668769523862647
- StocksJMBrantlyMLWang-SmithLPharmacokinetic comparability of Prolastin-C to Prolastin in alpha1-antitrypsin deficiency: a randomized studyBMC Clin Pharmacol2010101320920295
- SandhausRAStocksJRouhaniFNBrantlyMStraussPBiochemical efficacy and safety of a new, ready-to-use, liquid alpha-1-proteinase inhibitor, GLASSIA (alpha1-proteinase inhibitor (human), intravenous)COPD2014111172523822603
- StocksJMBrantlyMPollockDMulti-center study: the biochemical efficacy, safety and tolerability of a new alpha1-proteinase inhibitor, ZemairaCOPD200631172317175661
- StollerJKRouhaniFBrantlyMBiochemical efficacy and safety of a new pooled human plasma alpha1-antitrypsin, RespitinChest20021221667412114340
- BarkerAFSiemsenFPasleyDD’SilvaRBuistASReplacement therapy for hereditary alpha1-antitrypsin deficiency: a program for long-term administrationChest19941055140614108181327
- SchmidtEWRascheBUlmerWTReplacement therapy for alpha-1-protease inhibitor deficiency in P(i)Z subjects with chronic obstructive lung diseaseAm J Med1988846 A63693289388
- VidalRBarros-TizonJCGaldizJBTolerance and safety of Trypsone: prospective follow-up in alpha-1 antitrypsin deficient subjects with pulmonary emphysemaMinerva Pneumol20104928391
- BarkerAFIwata-MorganIOvesonLRousselRPharmacokinetic study of alpha1-antitrypsin infusion in alpha1-antitrypsin deficiencyChest199711236076139315791
- WewersMDCasolaroMASellersSEReplacement therapy for alpha 1-antitrypsin deficiency associated with emphysemaN Engl J Med198731617105510623494198
- WeberDBeckerMKonietzkoNErgebnis einer 18monati gen Substitutionstherapie bei Patienten mit alpha1-Pi-Mangel und Lungenemphysem [Effect of a 18 month replacement therapy in patients with alpha1-antitrypsin deficiency and lung emphysema]Atemwegs-und Lungenkrankheiten19871312567572 German
- SubramanianDRJenkinsLEdgarRQuraishiNStockleyRAParrDGAssessment of pulmonary neutrophilic inflammation in emphysema by quantitative positron emission tomographyAm J Respir Crit Care Med2012186111125113222837375
- MiravitllesMVidalRTorrellaMBofillJMCotrinaMde GraciaJEvaluacion del tratamiento sustitutivo del enfisema por deficit de alfa-1-antitripsina. [Evaluation of replacement therapy in emphysema caused by alpha 1-antitrypsin deficiency]Arch Bronconeumol19943010479484 Spanish7827760
- SchwaiblmairMVogelmeierCFruhmannGLong-term augmentation therapy in twenty patients with severe alpha-1-antitrypsin deficiency three-year follow-upRespiration199764110159044469
- WenckerMBanikNBuhlRSeidelRKonietzkoNLangzeittherapie des alpha1-Antitrypsin-Mangelassoziierten Lungenemphysems mit Humanem alpha1-Antitrypsin [Long-term treatment of alpha1-antitrypsin deficiency-related pulmonary emphysema with human alpha1-antitrypsin]Pneumologie19985210545552 German9847632
- CamposMAAlazemiSZhangGClinical characteristics of subjects with symptoms of alpha1-antitrypsin deficiency older than 60 yearsChest2009135360060819017884
- CamposMAAlazemiSZhangGExacerbations in subjects with alpha-1 antitrypsin deficiency receiving augmentation therapyRespir Med2009103101532153919464158
- CamposMAAlazemiSZhangGSandhausRAWannerAInfluenza vaccination in subjects with alpha1-antitrypsin deficiencyChest20081331495517908701
- CassinaPCTeschlerHKonietzkoNTheegartenDStamatisGTwo-year results after lung volume reduction surgery in alpha1-antitrypsin deficiency versus smoker’s emphysemaEur Respir J1998125102810329863992
- DauriatGMalHJebrakGFunctional results of unilateral lung volume reduction surgery in alpha1-antitrypsin deficient patientsInt J Chron Obstruct Pulmon Dis20061220120618046897
- FujimotoTTeschlerHHillejanLZabouraGStamatisGLong-term results of lung volume reduction surgeryEur J Cardiothorac Surg200221348348811888768
- StollerJKGildeaTRRiesALMeliYMKarafaMTLung volume reduction surgery in patients with emphysema and alpha-1 antitrypsin deficiencyAnn Thorac Surg200783124125117184672
- TuticMBlochKELardinoisDBrackTRussiEWWederWLong-term results after lung volume reduction surgery in patients with alpha(1)-antitrypsin deficiencyJ Thorac Cardiovasc Surg2004128340841315354100
- HillerdalGMindusSOne-to four-year follow-up of endobronchial lung volume reduction in alpha-1-antitrypsin deficiency patients: a case seriesRespiration201488432032825227936
- BangaAGildeaTRajeswaranJRokadiaHBlackstoneEHStollerJKThe natural history of lung function after lung transplantation for alpha(1)-antitrypsin deficiencyAm J Respir Crit Care Med2014190327428125003824
- BredahlPZemtsovskiMPerchMEarly laparotomy after lung transplantation: Increased incidence for patients with alpha1-anti-trypsin deficiencyJ Heart Lung Transplant201433772773324709270
- BreenTJKeckBHosenpudJDO’ConnellJBWhiteRDailyOPThoracic organ transplants in the United States from October 1987 through December 1991: a report from the UNOS Scientific Registry for Organ TransplantsClin Transpl199233431306709
- BurtonCMMilmanNCarlsenJThe Copenhagen National Lung Transplant Group: survival after single lung, double lung, and heart-lung transplantationJ Heart Lung Transplant200524111834184316297790
- CassiviSDMeyersBFBattafaranoRJThirteen-year experience in lung transplantation for emphysemaAnn Thorac Surg200274516631669 [Discussion] 1669–167012440627
- ChristieJDEdwardsLBAuroraPRegistry of the International Society for Heart and Lung Transplantation: Twenty-fifth Official Adult Lung and Heart/Lung Transplantation Report-2008J Heart Lung Transplant200827995796918765187
- de PerrotMChaparroCMcRaeKTwenty-year experience of lung transplantation at a single center: influence of recipient diagnosis on long-term survivalJ Thorac Cardiovasc Surg200412751493150115116013
- GunesAAboyounCLMortonJMPlitMMaloufMAGlanvilleARLung transplantation for chronic obstructive pulmonary disease at St. Vincent’s HospitalIntern Med J200636151116409307
- InciISchuurmansMEhrsamJLung transplantation for emphysema: impact of age on short-and long-term survivalEur J Cardiothorac Surg201548690690925602056
- TanashHARiiseGCHanssonLNilssonPMPiitulainenESurvival benefit of lung transplantation in individuals with severe alpha1-anti-trypsin deficiency (PiZZ) and emphysemaJ Heart Lung Transplant201130121342134721821433
- StoneHMEdgarRGThompsonRDStockleyRALung transplantation in alpha-1-antitrypsin deficiencyCOPD201613214615226488418
- StolkJStockleyRAStoelBCRandomised controlled trial for emphysema with a selective agonist of the gamma-type retinoic acid receptorEur Respir J201240230631222282548
- International Study Evaluating the Safety and Efficacy of Inhaled, Human, Alpha-1 Antitrypsin (AAT) in Alpha-1 Antitrypsin Deficient Patients with Emphysema2014 Available from: http://ClinicalTrials.gov/show/NCT1217671
- StraussPStolkJMcElvaneyGPiitulainenESeersholmNChapmanKRPhase II/III, double-blind, randomized, placebo-controlled, international study evaluating the safety and efficacy of inhaled, human, alpha-1 antitrypsin (AAT) in alpha-1 antitrypsin deficient patients (AATD) (Abstract)Am J Respir Crit Care Med2014 Meeting AbstractsA5790 Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/464/CN-01038464/frame.htmlAccessed April 21, 2015
- Pharmacokinetic Study of ARALAST (Human Alpha1- PI)2011 Available from: http://ClinicalTrials.gov/show/NCT0242385
- Safety and Pharmacokinetics of Alpha-1 Proteinase Inhibitor in Subjects with Alpha1-Antitrypsin Deficiency2013 Available from: http://ClinicalTrials.gov/show/NCT1213043
- Efficacy and Safety of Alpha1-Proteinase Inhibitor (Human), Modified Process (Alpha-1 MP) in Subjects with Pulmonary Emphysema Due to Alpha1 Antitrypsin Deficiency (AATD)2015 Available from: http://ClinicalTrials.gov/show/NCT1983241
- SorrellsSCamprubiSGriffinRChenJAyguasanosaJSPARTA clinical trial design: Exploring the efficacy and safety of two dose regimens of alpha1-proteinase inhibitor augmentation therapy in alpha1-antitrypsin deficiencyRespir Med2015109449049925727857
- DowsonLJGuestPJHillSLHolderRLStockleyRAHigh-resolution computed tomography scanning in alpha1-antitrypsin deficiency: relationship to lung function and health statusEur Respir J20011761097110411491150
- DowsonLJGuestPJStockleyRALongitudinal changes in physiological, radiological, and health status measurements in alpha1-antitrypsin deficiency and factors associated with declineAm J Respir Crit Care Med200116410 Pt 11805180911734427
- VogelmeierCHedererBGlaabTTiotropium versus salmeterol for the prevention of exacerbations of COPDN Engl J Med2011364121093110321428765
- LeidyNKWilcoxTKJonesPWRobertsLPowersJHSethiSStandardizing measurement of chronic obstructive pulmonary disease exacerbationsAm J Respir Crit Care Med2011183332332920813886
- WaltersEHWaltersJWillsKERobinsonAWood-BakerRClinical diaries in COPD: compliance and utility in predicting acute exacerbationsInt J Chron Obstruct Pulmon Dis2012742743522848156
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- PillaiAPTurnerAMStockleyRARelationship of the 2011 Global Initiative for Chronic Obstructive Lung Disease Strategy to clinically relevant outcomes in individuals with α1-antitrypsin deficiencyAnn Am Thorac Soc201411685986424950156
- AnzuetoALeimerIKestenSImpact of frequency of COPD exacerbations on pulmonary function, health status and clinical outcomesInt J Chron Obstruct Pulmon Dis2009424525119657398
- DonaldsonGCSeemungalTABhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax2002571084785212324669
- HillATCampbellEJBayleyDLHillSLStockleyRAEvidence for excessive bronchial inflammation during an acute exacerbation of chronic obstructive pulmonary disease in patients with alpha(1)-antitrypsin deficiency (PiZ)Am J Respir Crit Care Med199916061968197510588615
- JaroschIGehlertSJackoDDifferent training-induced skeletal muscle adaptations in COPD patients with and without alpha-1 antitrypsin deficiencyRespiration201692533934727686000
- KennKGloecklRSoennichsenAPredictors of success for pulmonary rehabilitation in patients awaiting lung transplantationTransplantation20159951072107725393161
- DaveyCZoumotZJordanSBronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trialLancet201538699981066107326116485
- SciurbaFCCrinerGJStrangeCEffect of endobronchial coils vs usual care on exercise tolerance in patients with severe emphysema: the RENEW randomized clinical trialJAMA2016315202178218927179849
- KotloffRMThabutGLung transplantationAm J Respir Crit Care Med2011184215917121471083