Abstract
Purpose
The degree to which symptoms such as dyspnea affect patients with COPD is individualized. To address the gap between clinical symptom measures and self-perceived disease burden, we investigated the symptom status of adult patients with COPD and followed with an administrative claims analysis of health care resource utilization and costs.
Methods
This was a hybrid US observational study consisting of a cross-sectional patient survey followed by a retrospective analysis of administrative claims data. The primary COPD symptom measures were the modified Medical Research Council (mMRC) Dyspnea scale and the COPD Assessment Test (CAT).
Results
A total of 673 patients completed the survey. Of these, 65% reported mMRC grades 0–1 (low symptomatology) and 35% reported mMRC grades 2–4 (high symptomatology); 25% reported CAT score <10 (low symptomatology) and 75% reported CAT score ≥10 (high symptomatology). More patients with high symptomatology (by either measure) had at least one COPD-related inpatient hospitalization, emergency room visit, physician office visit, or other outpatient services, and filled at least one COPD-related prescription medication vs patients with low symptomatology. COPD-related costs were higher for patients with high symptomatology than patients with low symptomatology. In a multivariate analysis, COPD-related costs were also higher in patients reporting severe symptoms.
Conclusion
Patients with high COPD symptomatology utilized more health care resources and had higher COPD-related health care costs during the 6-month post-survey period than patients with low symptomatology.
Introduction
COPD encompasses a group of preventable respiratory diseases characterized by progressive airflow limitation that is not fully reversible. Symptoms of COPD include chronic cough, exertional dyspnea, expectoration, and wheeze. COPD is also characterized by exacerbations, defined as a worsening of a patient’s respiratory symptoms beyond those expected of normal day-to-day variations, with acute onset, and warranting adjustment of medication.Citation1
The current treatment objectives for stable COPD fall under two categories: relieving and reducing the impact of symptoms, and reducing the risk of adverse health events such as exacerbations, which may affect the patient at a later date. The goal of COPD exacerbation treatment is to minimize the impact of the current exacerbation and to prevent the development of subsequent exacerbations.Citation1 However, guidelines are inconsistently followed and patients may not be treated appropriately.Citation2
Previous research has found that moderate-to-severe exacerbations that require hospitalizations and emergency room (ER) visits are major cost drivers in COPD.Citation3–Citation5 In the Global Initiative for Chronic Obstructive Lung Disease (GOLD) consensus report, it is recommended that COPD is assessed based on the patient’s symptom level, future risk of exacerbations, comorbidities, and severity of airflow limitation.Citation1 Airflow limitation is divided into four severity categories (mild to very severe) based on forced expiratory volume in 1 second. While such a measure may overdiagnose COPD in some elderly patients with normal age-related changes in lung volume, it may also underdiagnose the condition in patients younger than 45 years.Citation6 Furthermore, the degree to which a patient is affected by symptoms, particularly dyspnea, is individualized.Citation7 That is, a level of dyspnea rated as clinically mild may be troublesome to a specific patient, causing patient perception of the condition to be more severe.
To address the gap between clinical symptom measures and patient self-perceived disease burden, we investigated the COPD symptom status of patients and followed with a claims analysis of their health care resource utilization and costs. The aims of this study were to assess patient-reported dyspnea and other COPD symptoms and to determine their association with estimated health care resource utilization and direct costs using administrative claims data.
Methods
Study design
This was a two-part observational study conducted in the US consisting of a cross-sectional patient survey of adults with COPD followed by a retrospective analysis of patients’ administrative claims data.
After completion of the patient surveys that were conducted between October 2011 and January 2012, and after sufficient time had elapsed for full claims capture and adjudication, a database was created consisting of survey respondents’ administrative claims data for the 6-month periods before and after the survey date. A patient-level analytic file for the 6-month pre-and post-survey periods was created and merged with the survey data for analyses of the resource utilization and direct health care costs of survey respondents. The claims analyses occurred after sufficient time had elapsed for full claims adjudication, such that there were administrative claims for the 6-month periods prior to and following the patient survey date.
Patients
The patient survey sample was identified from the Health-Core Integrated Research Database (HIRD), an integrated, de-identified US administrative claims database. The HIRD contains eligibility, medical, and pharmacy claims for approximately 44 million members of 14 commercial health insurance plans across the US. Patients were selected for the survey based on claims submitted from February 1, 2010, to January 31, 2011 (the patient sample list identification period). Eligible patients were aged ≥40 years at the date of the last COPD medical claim within the identification period (the COPD index date), with two or more medical claims with an International Classification of Diseases-Ninth Revision-Clinical Modification (ICD-9-CM) diagnosis code for COPD on different dates of service during the identification period.
Patients were excluded from the study if they had at least one claim during the patient list identification period with an ICD-9-CM diagnosis code for cystic fibrosis, respiratory cancer, pulmonary fibrosis, pneumoconiosis, sarcoidosis, or pulmonary tuberculosis. Additional eligibility and exclusion criteria are included in Supplementary material.
Assessment of data from the HIRD complied with federal and state laws and regulations, including those related to privacy and security of individually identifiable health information. The study protocol and all other patient-related materials were approved by the New England Institutional Review Board. All survey respondents gave full informed verbal consent prior to the survey.
Patient survey
Survey administration
Prenotification letters were sent inviting eligible patients to participate in the study and requesting them to either opt-in or opt-out by telephone. Patients who did not respond to the prenotification letter were contacted by telephone by trained interviewers. Patients who gave verbal consent to participate in the study had the option of completing the one-time survey either by telephone interview or online.
Survey measures
The primary COPD symptom measures were the modified Medical Research Council (mMRC) Dyspnea scaleCitation8–Citation10 and the COPD Assessment Test (CAT).Citation11 Additional secondary measures included the Work Productivity and Activity Impairment (WPAI) scale,Citation12,Citation13 the Clinical COPD Questionnaire (CCQ),Citation14,Citation15 and the Duke Health Profile (DUKE).Citation16
To simplify the analysis and following GOLD recommendations, mMRC and CAT scores were each grouped into two categories of low and high symptomatology:
mMRC grades 0–1 were classified as low dyspnea symptomatology.
mMRC grades 2–4 were classified as high dyspnea symptomatology.
CAT scores 0–9 were classified as low COPD symptomatology.
CAT scores 10–40 were classified as high COPD symptomatology.
Further details of additional secondary measures and other variables can be found in Supplementary material.
Administrative claims analysis
Administrative claims submitted 6 months prior to and following the survey date were extracted from the HIRD for patients who had completed the patient survey. Patient demographics, as well as clinical characteristics (Deyo–Charlson Comorbidity Index [DCI] scoreCitation17 and comorbidities, medications administered, and dyspnea and asthma flags), were assessed using the claims data. In addition, COPD-related health care resource utilization and costs were assessed for the 6-month period prior to and following the survey date. Unique patient identification numbers linked the survey and claims data, and the merged survey/claims data were used for the study analyses.
Statistical analysis
For descriptive analyses, mean, standard deviation (SD), and median were reported for continuous variables. Frequency and percentages were reported for categorical variables. Statistical comparisons between symptom cohorts used bivariate or unadjusted comparisons, in which continuous characteristics were compared using two-sample t-tests and categorical variables were compared using chi-square tests, and used multivariate regression models to determine statistically significant differences in outcomes while adjusting for potential confounding variables. Generalized linear models with a logarithmic link function and gamma distribution determined COPD-related 6-month post-survey period cost drivers. All post-period cost models controlled for pre-survey covariates, including dyspnea and COPD symptom status, age, sex, smoking status, employment status, body mass index (BMI), DCI score, number of COPD maintenance therapy drug classes, home oxygen therapy use, and presence of COPD exacerbations.
All costs are reported in 2012 US dollars. All analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). An a priori α-level of P≤0.05 was considered significant.
Results
Patient disposition
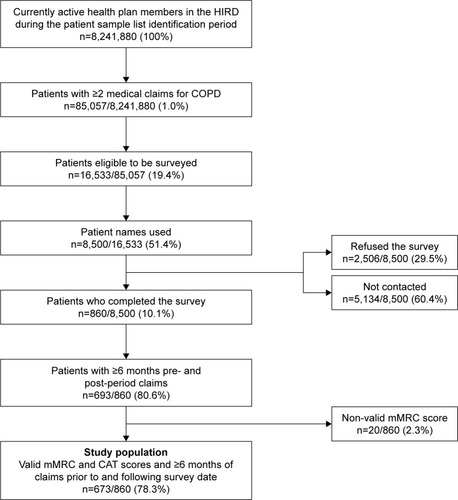
Of the 8,500 patients on the patient list, 860 patients completed the survey and 2,506 patients refused the survey. The remaining 5,134 patients were not contacted due to incorrect contact information, because the maximum number of contact attempts had been reached, or because the target number of completed surveys had been obtained and the survey closed (). After merging the survey data of the 860 respondents with their claims data for the 6-month periods prior to and following the survey date, 693 respondents had at least 6 months of eligibility and claims prior to and following the survey date. Of these people, 673 patients had valid mMRC scores and comprised the study population ().
Figure 1 Sample disposition.
Patient demographics for the study population (N=673) are given in . There were slightly more females (57.8%) and almost half of the population (47.9%) were aged 56–64 years. A large proportion of the population were located in the Midwest (43.8%), and the majority were enrolled in a preferred provider organization (PPO) health plan (64.6%). Approximately half of the population had at least one medical claim for dyspnea during the 12-month patient identification period and the mean (SD) DCI score was 2.65 (2.22).
Table 1 Patient sample demographics
Patient survey results: COPD symptom analyses
mMRC Dyspnea scale
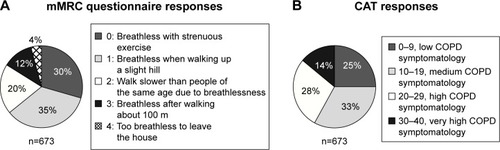
Among the 673 patients, 65% reported mMRC grades 0 or 1, considered mMRC low dyspnea symptomatology and 35% reported mMRC grades 2–4, considered mMRC high dyspnea symptomatology ().
Figure 2 Patient survey COPD symptom results from the (A) mMRC Dyspnea scale and (B) CATa.
Abbreviations: CAT, COPD Assessment Test; mMRC, modified Medical Research Council.
Significant differences between patients with low and high mMRC symptomatology were observed in terms of COPD diagnosis, obesity, employment status, household income, self-reported peripheral vascular disease, and diabetes complications (). No differences between groups were observed for sex, age at the time of the survey, race, educational status, or smoking status.
Table 2 Self-reported patient characteristics by mMRC Dyspnea scale category
Compared with patients with low dyspnea symptoms, a significantly higher percentage of patients with high dyspnea symptoms reported that a physician had told them they had COPD (88.1% vs 69.8%; P<0.0001; ), were diagnosed with COPD at a younger age (52.4 vs 55.2 years; P=0.001; ), and had a longer history of COPD (7.1 years vs 5.0 years; P=0.001; ). Slightly more patients in the high mMRC group had a spirometry test in the last 6 months compared with the low mMRC group (44.5% vs 39.4%; P=0.342; ). Compared with patients with low dyspnea symptoms, a significantly higher percentage of patients with high symptoms were not working (60.2% vs 33.0%; P<0.0001; ), reported a 2010 household income of <$50,000 (61.4% vs 53.6%; P=0.012; ), were obese (BMI >29.9; 48.3% vs 35.7%; P=0.003; ), reported peripheral vascular disease (27.5% vs 11.2%; P<0.0001; ), and reported diabetes complications (8.5% vs 4.4%; P=0.029; ).
COPD Assessment Test
Among the 673 patients, 25% reported CAT scores <10, considered low CAT COPD symptomatology and 75% reported CAT scores ≥10, considered high CAT COPD symptomatology ().
Significant differences between patients with low and high CAT COPD symptomatology were observed in terms of sex, COPD diagnosis, educational status, employment status, household income, hypertension, diabetes, peripheral vascular disease, heart attack, stroke, and a spirometry test in the last 6 months (). No differences between groups were observed for age at the time of the survey, COPD duration, BMI, race, or smoking status.
Table 3 Self-reported patient characteristics by CAT COPD symptom category
Compared with low CAT COPD symptom patients, a significantly higher percentage of patients with high CAT COPD symptoms reported that a physician had told them they had COPD (83.2% vs 55.9%; P<0.0001; ), were diagnosed with COPD at a younger age (53.7 vs 55.9 years; P=0.047; ), were female (60.3% vs 50.3%; P=0.024; ), were not working (46.4% vs 30.5%; P=0.001; ), were less likely to be a college graduate (33.4% vs 43.1%; P=0.039; ), and reported a 2010 household income of <$50,000 (59.1% vs 47.1%; P=0.015; ). More patients in the high CAT COPD symptom group had a spirometry test in the last 6 months (44.1% vs 32.3%; P=0.027; ), reported having hypertension, diabetes, peripheral vascular disease, heart attack, and stroke compared with the low CAT COPD symptom group.
Health care resource utilization and costs
During the 6-month post-survey period, patients with high COPD symptomatology (mMRC scores ≥2 or CAT scores ≥10) utilized more health care resources than patients with low mMRC or CAT scores (). Compared with patients with low COPD symptomatology, a significantly greater percentage of patients with high symptomatology had at least one COPD-related inpatient hospitalization, ER visit, physician office visit, other outpatient service, and filled at least one COPD-related prescription medication.
Figure 3 Unadjusted COPD-related: (A) health care resource utilization and (B) health care costsa in the six-month post-survey period by mMRC or CAT COPD symptomatology.
Abbreviations: CAT, COPD Assessment Test; ER, emergency room; mMRC, modified Medical Research Council.
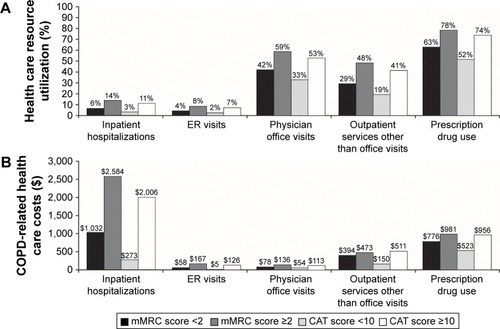
COPD-related health care costs during the 6-month post-survey period were also higher among patients with more severe COPD symptoms (). Inpatient costs were markedly higher for patients with more severe symptoms compared with patients with less severe symptoms (mMRC: $2,584 vs $1,032, P=0.041; CAT: $2,006 vs $273, P<0.001).
Multivariate analyses
Multivariate analyses to determine the drivers of COPD-related costs during the 6-month post-survey period found that after controlling for all other covariates in the model, the estimated COPD-related total costs of patients with high dyspnea symptoms were 46% higher (P=0.025) than for patients with low dyspnea symptoms (). Similarly, the estimated COPD-related total costs of patients with high CAT COPD symptoms were 51% higher than for patients with low CAT COPD symptoms, although this result was not statistically significant ().
Figure 4 Generalized linear model ratios of estimated total COPD-related costs during the 6-month post-survey period of (A) patients with high dyspnea and (B) patients with high CAT COPD symptoms.
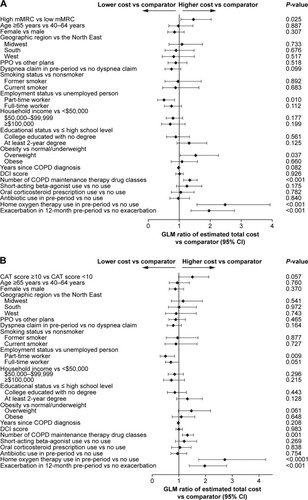
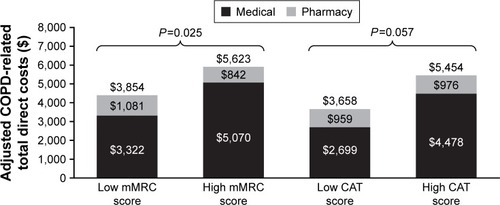
COPD-related costs were higher for patients with high dyspnea symptomatology than those with low dyspnea symptomatology for both unadjusted ($2,003 higher; P=0.015) and adjusted costs ($1,769 higher; P=0.025; ). COPD-related costs were also higher among patients with high COPD symptomatology as measured by CAT scores vs low COPD symptomatology for both unadjusted ($2,707 higher; P<0.0001) and adjusted ($1,769; P=0.057) COPD-related costs (); although after controlling for covariates, the difference was no longer significant. The primary cost driver of both unadjusted and adjusted COPD-related costs was predominantly patient hospitalization costs; although for unadjusted low CAT COPD symptomatology patients, the split between medical and pharmacy costs was approximately even.
Figure 5 Adjusted COPD-related total direct costs in the six-month post-survey period by mMRC Dyspnea scale symptom category and CAT COPD symptom category.
Abbreviations: CAT, COPD Assessment Test; mMRC, modified Medical Research Council.
Patient-reported outcomes (survey)
Significant differences were observed in all patient-reported outcome instrument scale/subscale scores for patients with high symptomatology compared with those with low symptomatology (all P-values of <0.0001, except absenteeism P=0.008; further results are given in Supplementary material).
Discussion
Patients with all severities of COPD are affected by dyspnea, either at rest or during exercise.Citation18 Dyspnea is highly prevalent in patients with COPD ranging from 39.5% to 60.2% in EuropeCitation19 and 70% in the USACitation18 and is a major cause of disability and anxiety.Citation1 Moderate-to-severe dyspnea is associated with a more frequent incidence of exacerbations and a poorer health status and quality of life.Citation19 COPD symptoms, including dyspnea, contribute to disease severity,Citation18 and reducing symptoms, as well as improving health status and reducing the risk of exacerbations, are important goals for the treatment of COPD.Citation1
In this real-world, observational study, we assessed patient perceptions of dyspnea and COPD symptomatology and linked those data to claims data to assess health care resource utilization and costs in the US. We found that patients who rated their dyspnea as more severe (with high mMRC scores [≥2]) or with higher health status impairment (high CAT scores [≥10]) used more health care resources and incurred higher treatment costs than patients who rated their condition as less severe. This was primarily driven by medical costs vs pharmacy costs. As such, these data show that COPD symptoms have a noticeable impact on health care resource utilization and costs. When unadjusted costs were used for the analyses, the cost associated with patients with severe symptoms was greater when assessed using the CAT compared with the mMRC Dyspnea test. However, there was only a significant difference in adjusted costs for patients categorized by mMRC. It is unclear why the cost difference was not significant with CAT; however, this may be due to the multidimensional nature of the CAT, which assesses symptoms and health status, whereas the mMRC scale measures dyspnea only. It has been reported previously that mMRC and CAT do not distinguish symptom groups precisely in the same way.Citation20
Symptoms are the major driving force for physician visits and medication changes.Citation18 Therefore, it is to be expected that symptoms are associated with health care resource use and costs.Citation21 In keeping with these data from a US health care system, worse dyspnea has been shown to be associated with higher health care resource use in Europe, leading to higher primary care and specialist pulmonary consultation annual rates. In addition, more patients with moderate-to-severe dyspnea (mMRC ≥2) had at least one ER visit leading to hospitalization compared with patients with no-to-mild dyspnea (mMRC <2; 26.1% vs 5.8%; P<0.0001). Furthermore, total annual COPD management costs were found to be more than twice as high among patients with moderate-to-severe dyspnea compared with patients with no-to-mild dyspnea (€4,372 vs €2,031; P<0.0001).Citation19 These findings are particularly relevant to payers, as symptoms of COPD are often discounted when coverage decisions are made. Patients with high symptomatology also demonstrated significantly different patient-reported outcome instrument scale/subscale scores compared with those with low symptomatology, indicating that symptomatology impacts on work status, daily activities of living, and productivity.
This study is associated with some limitations. The patient survey results were self-reported and could not be validated; therefore, their accuracy may be subject to self-report and recall biases. Data regarding health care resource use and costs were based on a large US administrative claims database and are therefore subject to the limitations common to administrative claims analyses. Administrative claims are designed for reimbursement, not research, and may contain undetected diagnostic or treatment coding errors or omissions. The study population was identified from the same US administrative claims database and consisted of individuals and/or their dependents with commercial health insurance plans. Consequently, the results may not be generalizable to patients with COPD who are uninsured, covered by government-sponsored Medicare or Medicaid or who live outside the US. Furthermore, many patients with COPD in the US may have been covered by Medicare or Medicaid, and were not included in the analysis. The strengths of the study included the use of the HIRD, which allowed the researchers to link the survey data with respondents’ clinical claims data to allow analysis of health care resource utilization and costs. By integrating multiple data sources, this study capitalized on the strengths of determining COPD symptomatology from the patient’s perspective without needing to rely on patient recall of health care resource utilization and cost. This provided a more comprehensive view of the individual patient, making the results more relevant to managed care providers.
Future studies could build on the results of this study by following patients and reporting health care resource use and costs over a longer period of time, using a broader set of payer databases, examining change in symptoms and claims over time, and cross-checking symptom and claims data with clinical markers for COPD.
Conclusion
This study demonstrated an association between symptoms and outcomes using managed care claims data integrated with patient survey data. Patients with worse dyspnea and higher health status impairment had more comorbidities, particularly cardiovascular disease, higher risk of exacerbation, and higher health care resource utilization and costs.
Acknowledgments
Editorial support (in the form of writing assistance, including editing of the initial draft, assembling tables and figures, collating authors’ comments, and referencing) was provided by Elizabeth Jameson, PhD, at Fishawack Indicia Ltd, UK, and was funded by GSK. This study HO-11-696 was funded by GSK. The present addresses for the authors Jeetvan Patel and Anand A Dalal are Global Health Economics, Amgen, CA, USA and US Health Economics and Outcomes Research, Novartis Pharmaceuticals, NJ, USA, respectively.
Supplementary materials
Methods
Eligibility criteria
Patients were required to have at least 12 months’ continuous enrollment in the health plan prior to the COPD index date, and be currently active, commercially insured health plan members who were eligible to take part in the survey.
Exclusion criteria
Patients who were on the HealthCore or survey vendor “do not call” lists, or who could not understand/speak English were excluded.
Measures
The modified Medical Research Council (mMRC) Dyspnea scale grades the severity of dyspnea according to the degree of breathlessness associated with particular tasks. The scale has been validated and used previously for diagnostic evaluation in clinical trials and also to compare categorizations of dyspnea with staging of disease severity.Citation1–Citation3
The COPD Assessment Test (CAT) measures health status impairment associated with COPD to determine the impact on a patient’s well-being and daily life.Citation4 The eight-item unidimensional measure yields scores ranging from 0 to 40, with higher scores indicating worse COPD-related health status.
The Clinical COPD Questionnaire (CCQ) is a 10-item questionnaire that measures symptom control and functional state in patients with COPD.Citation5,Citation6 Three domain scores are obtained from the CCQ; the symptom score is obtained by dividing the sum of the responses to items 1, 2, 4, and 5 by 4; the functional state score is obtained by dividing the sum of the responses to items 7–10 by 4 and the mental state score is obtained by dividing the sum of the responses to items 3 and 4 by 2. Each domain scores ranges from 0 to 6, with higher scores indicating poorer COPD control.
The Duke Health Profile (DUKE) is a 17-item instrument that assesses an individual’s health and feelings.Citation7 Eleven subscale scores that range from 0 to 100 are reported. For the physical health, mental health, social health, general health, perceived health and self-esteem scores, 100 indicates the best health status and 0 indicates the worst health status. For the anxiety, depression, anxiety-depression, pain and disability scores, the opposite is true and 100 indicates the worst health status and 0 indicates the best health status.
Other survey variables
Other variables collected at the time of the survey included patient demographics (age, sex, race and ethnicity, education and employment status, and 2010 household income level) and clinical characteristics (smoking status; body mass index; history of COPD diagnosis, including duration and age at COPD diagnosis; whether a breathing or spirometry test had been performed in the previous 6 months; and the presence of cardiovascular risk factors).
Results
Patient-reported outcomes (survey)
Patients with high symptomatology were less likely to be working full or part time (mMRC: 37.7% vs 62.2%; CAT: 49.8% vs 65.3%), and reported significantly more time absent from work (mMRC: 4.78% vs 1.47%, P=0.008; CAT: 3.0% vs 0.5%, P=0.004); on-the-job effectiveness was also significantly reduced compared with those with low symptomatology (mMRC: 26.63% vs 11.21%, P<0.0001; CAT: 20.2% vs 2.9%, P<0.0001). These patients also had reduced work productivity attributed to COPD symptoms (mMRC: 28.9% vs 11.9%, P<0.0001; CAT: 21.6% vs 3.5%, P<0.0001) and higher daily activity impairment due to COPD (mMRC: 38.7% vs 14.9%, P<0.0001; CAT: 27.6% vs 5.1%, P<0.0001). Working patients with high symptomatology had significantly higher indirect costs attributable to COPD (mMRC: $6,394 vs $2,671, P<0.0001; CAT: $4,802 vs $784, P<0.0001), CCQ subscale scores, indicating lower COPD control (all P<0.0001), and DUKE subscale scores, indicating worse health status (all P<0.0001).
References
- ManaliEDLyberopoulosPTriantafillidouCMRC chronic dyspnoea scale: relationships with cardiopulmonary exercise testing and 6-minute walk test in idiopathic pulmonary fibrosis patients: a prospective studyBMC Pulm Med2010103220509928
- PaladiniLHodderRCecchiniIBelliaVIncalziRAThe MRC dyspnoea scale by telephone interview to monitor health status in elderly COPD patientsRespir Med201010471027103420116231
- StentonCThe MRC breathlessness scaleOccup Med (Lond)200858322622718441368
- JonesPWHardingGBerryPWiklundIChenWHKline LeidyNDevelopment and first validation of the COPD Assessment TestEur Respir J200934364865419720809
- van der MolenTWillemseBWSchokkerSten HackenNHPostmaDSJuniperEFDevelopment, validity and responsiveness of the Clinical COPD QuestionnaireHealth Qual Life Outcomes200311312773199
- StällbergBNokelaMEhrsPOHjemdalPJonssonEWValidation of the Clinical COPD Questionnaire (CCQ) in primary careHealth Qual Life Outcomes200972619320988
- ParkersonGRBroadheadWETseCKThe Duke Health Profile. A 17-item measure of health and dysfunctionMed Care19902811105610722250492
Disclosure
Jeetvan Patel and Anand A Dalal were employees of GSK at the time of the study and are now employed by Amgen and Novartis Pharmaceuticals, respectively. Judith J Stephenson, Debra Wertz, and Tao Gu are employees of HealthCore, Inc., an independent research organization that received funding from GSK for the conduct of the study. The authors report no other conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung Disease [webpage on the Internet]Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2016) Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016Accessed October 2016
- FitchKIwasakiKPyensonBPlauschinatCZhangJVariation in adherence with Global Initiative for Chronic Obstructive Lung Disease (GOLD) drug therapy guidelines: a retrospective actuarial claims data analysisCurr Med Res Opin20112771425142921599554
- DalalAALiuFRiedelAACost trends among commercially insured and Medicare advantage-insured patients with chronic obstructive pulmonary disease: 2006 through 2009Int J Chron Obstruct Pulmon Dis2011653354222069365
- DalalAARobertsMHPetersenHVBlanchetteCMMapelDWComparative cost-effectiveness of a fluticasone-propionate/salmeterol combination versus anticholinergics as initial maintenance therapy for chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis201161322
- DalalAAShahMLunacsekOHananiaNAClinical and economic burden of patients diagnosed with COPD with comorbid cardiovascular diseaseRespir Med2011105101516152221684731
- HillKGoldsteinRSGuyattGHPrevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary careCMAJ2010182767367820371646
- JonesPLareauSMahlerDAMeasuring the effects of COPD on the patientRespir Med200599suppl BS11S18
- ManaliEDLyberopoulosPTriantafillidouCMRC chronic Dyspnea Scale: relationships with cardiopulmonary exercise testing and 6-minute walk test in idiopathic pulmonary fibrosis patients: a prospective studyBMC Pulm Med2010103220509928
- PaladiniLHodderRCecchiniIBelliaVIncalziRAThe MRC dyspnoea scale by telephone interview to monitor health status in elderly COPD patientsRespir Med201010471027103420116231
- StentonCThe MRC breathlessness scaleOccup Med (Lond)20085822622718441368
- JonesPWHardingGBerryPWiklundIChenWHKline LeidyNDevelopment and first validation of the COPD Assessment TestEur Respir J200934364865419720809
- ReillyMCTannerAMeltzerEOWork, classroom and activity impairment instruments. Validation studies in allergic rhinitisClin Drug Invest199611278288
- StåhlEJanssonSAJonssonACSvenssonKLundbäckBAnderssonFHealth-related quality of life, utility, and productivity outcomes instruments: ease of completion by subjects with COPDHealth Qual Life Outcomes200311812809558
- van der MolenTWillemseBWSchokkerSten HackenNHPostmaDSJuniperEFDevelopment, validity and responsiveness of the Clinical COPD QuestionnaireHealth Qual Life Outcomes200311312773199
- StällbergBNokelaMEhrsPOHjemdalPJonssonEWValidation of the Clinical COPD Questionnaire (CCQ) in primary careHealth Qual Life Outcomes200972619320988
- ParkersonGRBroadheadWETseCKThe Duke Health Profile. A 17-item measure of health and dysfunctionMed Care19902811105610722250492
- DeyoRACherkinDCCiolMAAdapting a clinical comorbidity index for use with ICD-9-CM administrative databasesJ Clin Epidemiol19924566136191607900
- RabeKFImproving dyspnea in chronic obstructive pulmonary disease: optimal treatment strategiesProc Am Thorac Soc20063327027516636097
- PunekarYSMullerovaHSmallMPrevalence and burden of dyspnoea among patients with chronic obstructive pulmonary disease in five European countriesPulmon Ther201625972
- KimSOhJKimYIDifferences in classification of COPD group using COPD assessment test (CAT) or modified Medical Research Council (mMRC) dyspnea scores: a cross-sectional analysesBMC Pulm Med2013133523731868
- SrivastavaKThakurDSharmaSPunekarYSSystematic review of humanistic and economic burden of symptomatic chronic obstructive pulmonary diseasePharmacoeconomics201533546748825663178

