Abstract
Background and aim
Early detection of COPD may reduce the future burden of the disease. We aimed to investigate whether prescreening with a COPD-6 screening device (measuring FEV1 and FEV6) facilitates early detection of COPD in primary care.
Methods
In primary care, individuals at high risk of COPD (ie, age ≥35 years, relevant exposure, and at least one respiratory symptom) and no previous diagnosis of obstructive lung disease were examined with a COPD-6 screening device. In prioritized order, the criteria for proceeding to confirmatory spirometry were FEV1/FEV6 <0.7, FEV1 <80%pred, or clinical suspicion of COPD regardless of test result (medical doctor’s [MD] decision). Based on spirometry, including bronchodilator (BD) reversibility test, individuals were classified as COPD (post-BD FEV1/FVC <0.70), asthma (ΔFEV1 ≥0.50 L), or no obstructive lung disease.
Results
A total of 2,990 subjects (54% men, mean age 59 years, and mean 28 pack-years) were enrolled, of whom 949 (32%) proceeded from COPD-6 screening to confirmative spirometry based on the following criteria: 510 (54%) FEV1/FEV6 <0.70, 382 (40%) FEV1 <80%pred, and 57 (6%) MD decision. Following confirmative spirometry, the 949 individuals were diagnosed as having COPD (51%), asthma (3%), and no obstructive lung disease (45%). COPD was diagnosed in 487 (16%) of the enrolled subjects in whom confirmative spirometry was performed in 69% based on FEV1/FEV6 <0.7 and in 29% based on FEV1 ≤80%pred.
Conclusion
Prescreening with the COPD-6 device showed acceptable specificity for the selection of subjects for diagnostic spirometry and is likely to be a useful alternative to current practice in primary care.
Introduction
Previous studies have shown that opportunistic screening in primary care enables early diagnosis of chronic obstructive pulmonary disease (COPD).Citation1,Citation2 However, in general, this practice is sparsely implemented. In population-based registry studies, only one third of subjects or less with newly diagnosed COPD had spirometry performed.Citation3–Citation6 Furthermore, the requirement of conventional spirometry has been identified as a barrier to early diagnosis.Citation7 Delayed diagnosis is a missed opportunity for early secondary prevention, most importantly smoking cessation, which could substantially alter the prognosis.Citation8 The demand for screening of an increasing number of subjects has further stressed the need for a feasible procedure.
More than a decade ago, Buffels et al suggested that hand-held spirometers should be made available in primary care.Citation9 FEV6 is a measure of forced expiratory volume in 6 seconds as opposed to a full forced vital capacity (FVC) maneuver. Studies have suggested FEV1/FEV6 measured by spirometry as a valid alternative to FEV1/FVC in screening a high-risk population for COPD.Citation10,Citation11 A meta-analysis based on 11 studies found a sensitivity of 0.89 (95% CI 0.83–0.93) and specificity of 0.98 (95% CI 0.95–0.99) for FEV1/FEV6 in recognition of airway obstruction.Citation12 Several studies have concluded that hand-held screening devices including the COPD-6 and PiKo-6 device are reliable in screening for airway obstruction and selecting subjects for further diagnostic workup.Citation13–Citation20 A recent meta-analysis concluded that the accuracy of FEV1/FEV6 measured by hand-held devices seems to be lower than spirometry, but sufficiently accurate to screen for airway obstruction.Citation21
Existing studies of hand-held screening devices have called for studies conducted in a realistic screening setting. This study aimed to investigate whether prescreening with a COPD-6 device facilitates early detection of COPD in a primary care setting.
Materials and methods
Materials
Denmark has ~3,600 general practitioners (GPs), covering a population of 5.9 million. We aimed to include a representative sample, on a voluntary basis, comprising 180 GPs (corresponding to 5% of Danish GPs) from all over Denmark. Written information about the study as well as the invitation to participate was distributed to all interested GPs by the sponsor’s local representative.
More than 95% of Danish GPs own a spirometer, which was a requirement to participate in this study. Subjects were eligible for enrollment in the study provided they fulfilled the inclusion criteria based on recommendations from the Danish National Board of Health on opportunistic screening for COPD: age ≥35 years, smoker/ex-smoker or other risk exposures for COPD, at least one respiratory symptom (dyspnea, cough, wheeze, sputum, or recurrent respiratory tract infections)Citation31 and had no previous diagnosis of obstructive lung disease or treatment with inhaler medication within the last 12 months. Exclusion criteria were absence of informed consent and inability to perform COPD-6- or spirometry procedure.
Methods
This multicenter study was conducted from March to December 2015. Based on the spirometry guidelines from the Danish Respiratory Society,Citation32 participating GPs were educated in the functions of the COPD-6 (Vitalograph®, Buckingham, UK) and spirometry procedures. The COPD-6 test was repeated three times with the highest values recorded. The device automatically detected blows of poor quality (start too slow, coughing, or <3 seconds duration of blow). Spirometry was performed with at least three forced expiratory maneuvers (and at least two measurements of FEV1 and FVC, differing by <5%). European Community of Steel and Coal reference values for lung function were used.Citation33
The bronchodilator (BD) reversibility tests were performed with 0.4 mg inhaled salbutamol (or equivalent) followed by repeated spirometry 15 minutes later. All procedures were performed in general practice.
Subjects who fulfilled the criteria for inclusion were screened with the COPD-6 device (screening test) and proceeded to diagnostic spirometry with BD reversibility test, based on three criteria in prioritized order: 1) airway obstruction (FEV1/FEV6 <0.70), 2) lung function impairment, that is, FEV1 <80%pred, or 3) medical doctor’s (MD) decision (FEV1/FEV6 close to 0.70 and sustained suspicion of COPD). The prioritized order meant that subjects presenting with both airway obstruction and decreased lung function were categorized into airway obstruction. Based on the findings at the diagnostic spirometry, subjects were categorized as asthma (ΔFEV1 ≥0.50 L), COPD (post-BD FEV1/FVC ratio <0.70), or no obstructive lung disease (remaining individuals).
Data handling and analysis
The aim was to identify at least 500 subjects with COPD and thereby allow for subgroup analyses. Recruitment of 180 GPs was estimated to be sufficient based on, 1) an expected inclusion of at least 30 subjects by each GP, 2) a 15% prevalence of COPD, and 3) an expected dropout rate of 10%.
Data on age, sex, height, body weight, smoking status and/or other risk exposure, pack-years, respiratory symptoms (described above), severity of dyspnea (Medical Research Council scale),Citation34 and results of COPD-6 (FEV1, FEV6) and spirometry (FEV1, FVC, post-BD FEV1, and FVC) were entered into a consolidated web-based database. Body mass index (BMI), FEV1/FEV6, and FEV1/FVC were automatically calculated and recorded.
Statistical analyses were performed by using IBM SPSS version 21 (IBM Corporation, Armonk, NY, USA). Analyses were limited to subjects with complete data. Continuous data were tested for normality and paired t-test was applied if applicable. For continuous non-normal distributed data, Mann–Whitney U test was used. Categorical data were analyzed by Pearson’s chi-squared test. A significance level of 0.05 was set in all analyses.
Ethics
The study was endorsed by the Danish College of General Practitioners. According to the European Federation of Pharmaceutical Industries and Associations code and the Danish Association of the Pharmaceutical Industry, the present study was a non-drug, non-interventional study. Approval from the Danish Scientific Ethics Committee and The Danish Medicines Agency was not mandatory, but they were given all relevant study information. Data handling was approved by the Danish Data Protection Agency.
Results
Characteristics of enrolled subjects
A total of 149 GPs participated in the study, representing a nationwide sample of both large and small clinics. A total of 2,990 subjects were tested with the COPD-6 screening tool and had complete data (). The study population had a mean age of 59 years (range 35–92 years), mean 28 pack-years of smoking, 54% current smokers, and a COPD-6 mean FEV1 of 2.6 L (88%pred) ().
Table 1 Baseline characteristics of all enrolled subjects (n=2,990) and classification according to whether participants were only screened with COPD-6 test (n=2,041) or proceeded to confirmative spirometry (n=949)Table Footnotea
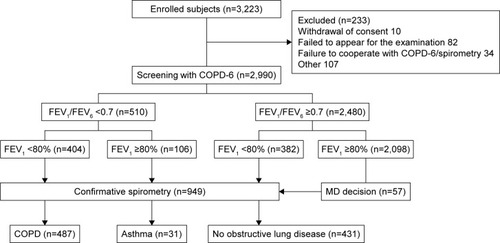
Figure 1 CONSORT diagram of enrolled subjects.
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV, forced expiratory volume; FVC, forced vital capacity; MD, medical doctor’s.
Prevalence of COPD
According to the predefined criteria, confirmative spirometry was indicated in 949 cases (32% of study population) distributed as follows: 510 FEV1/FEV6 <0.7 (with or without FEV1 <80%pred), 382 FEV1 <80%pred, and 57 MD decision. Of the 949 subjects tested with diagnostic spirometry, 487 (51%) fulfilled the criteria for COPD (corresponding to 16% of the study population), 31 (3%) were categorized as having asthma (ΔFEV1 >0.5 L), and 431 (45%) as “no obstructive lung disease” ().
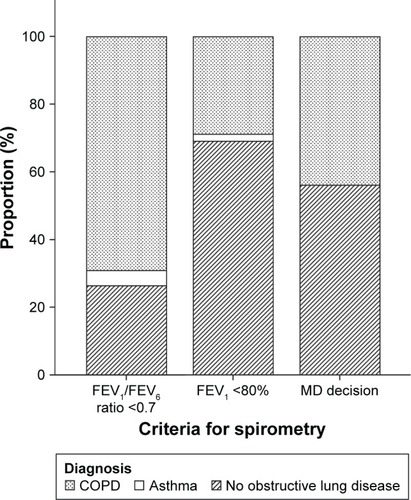
The prevalence of COPD in subjects selected for diagnostic spirometry by criteria FEV1/FEV6 <0.70 was 352/510 (69%) and 110/382 (29%) based on the criteria FEV1 <80% alone ( and ). The prevalence of airway obstruction at the COPD-6 test and at the pre- and post-BD spirometry are given in .
Table 2 Prevalence of airway obstruction (FEV1/FVC <0.70) at the COPD-6 test and at pre- and post-bronchodilator spirometry according to criteria for performing confirmative spirometry
Figure 2 Prevalence of final diagnosis, that is, COPD, asthma, or no obstructive lung disease, according to the three different criteria for proceeding to confirmative spirometry.
Notes: Criteria for, 1) COPD: post-bronchodilator (BD) FEV1/FVC <0.7 and ΔFEV1 <0.5 L at BD reversibility test; 2) asthma: ΔFEV1 ≥0.5 L at BD reversibility test; Non-OLD: not fulfilling criteria 1) or 2). MD decision: MD decision to perform confirmative spirometry based on suspicion of COPD and an FEV1/FVC ratio close to 0.7. Subjects with both FEV1/FEV6 ratio <0.7 and FEV1 <80% were counted in the group “FEV1/FEV6 ratio <0.7”.
Abbreviations: FEV, forced expiratory volume; FVC, forced vital capacity; MD, medical doctor’s; OLD, obstructive lung disease.
Criteria for confirmative spirometry
Applying the single criteria FEV1/FEV6 <0.70 for further diagnostic work-up, 17% of the screened population would have proceeded to confirmative spirometry. However, compared to the combined criteria used in the current study, a diagnosis of COPD would have been missed in 135 (487–352) subjects, corresponding to 27% of all subjects diagnosed with COPD in the study. In total, 439 subjects had an FEV1/FEV6 ≥0.70 at the COPD-6 test, but proceeded to diagnostic spirometry based on FEV1 <80%pred (382) or MD decision (57). Of these subjects, 137 had a post-BD <0.7 and 135 (31%) were diagnosed with COPD ().
For each subject diagnosed with COPD, six COPD-6 tests and two diagnostic spirometry procedures were performed.
Characteristics of subjects screened only with COPD-6 test
Subjects who did not meet criteria for diagnostic spirometry were in comparison to subjects diagnosed with COPD, significantly younger, had less tobacco exposure, and had an overall lower occurrence of respiratory symptoms with significantly less dyspnea, wheezing, and sputum (). As expected, the FEV1 was also on average significantly higher; 2.9 versus 2.0 L (97% vs 69% predicted), respectively.
Characteristics of subjects with COPD compared to other diagnostic groups
Compared to subjects categorized as having asthma, subjects with COPD had significantly more pack-years and lower FEV1 and FVC (). There were no significant differences in symptoms between the two groups, although occurrence of sputum tended to be higher in subjects with COPD.
Table 3 Characteristics of subjects diagnosed with COPD compared to subjects diagnosed with asthma and no obstructive lung disease (no OLD)Table Footnotea,Table Footnoteb
Subjects with COPD, compared to subjects categorized as having no obstructive lung disease, had significant higher age, lower BMI, and higher number of pack-years and had significantly higher occurrence of dyspnea and sputum ().
In the group with no obstructive lung disease, 215 (50%) subjects had FEV1>80%pred. For participants finally classified as having no obstructive lung disease, but having a confirmatory spirometry performed based on FEV1/FEV6 ratio <0.70, 50% had a FEV1/FEV6 ratio close to 0.7 (≥0.65). The mean increase in FEV1 at the BD reversibility test was 0.14 L (0.10).
Detailed spirometry data according to the three criteria for diagnostic spirometry are presented in .
Table 4 Spirometry data grouped by selection criteria for confirmative spirometry
Discussion
Main findings
The study demonstrates that, in a real-life setting, prescreening of high-risk subjects with the COPD-6 device followed by spirometry in selected cases can identify COPD in a high percentage of subjects (16%). Selection of subjects for confirmative spirometry based on a single criteria FEV1/FEV6 <0.70 was found to be insufficient, and the sensitivity was markedly increased primarily by adding the criterion FEV1 <80%pred, and to a lesser extent the criterion MD decision.
Screening with the COPD-6 device showed acceptable specificity for the selection of subjects for diagnostic spirometry.
Interpretation of findings in relation to previously published work
A COPD prevalence of 16% was comparable to findings (17%) reported from a previous study with similar study design, but screening performed only by conventional spirometry.Citation2 A post hoc analysis of data from that study showed that six screening spirometry and 1.4 confirmative spirometry procedures had to be performed per diagnosed subject with COPD.Citation22 Compared to the current study, the COPD-6 device as expected did not reduce the total number of procedures (screening tests plus diagnostic tests), but worked as a replacement for the conventional spirometry screening test.
Six other GP multicenter studies of screening with a COPD-6 device for early detection of COPD were identified. In accordance with the other studies, the vast majority of subjects in the current study had mild to moderate disease. For subjects with available data also on exacerbations, two-thirds belonged to GOLD group A.
One study (Miravitlles et al) had to be disregarded as it is published only in Spanish.Citation18 The two studies by Muller et al and Represas-Represas et al only included subjects if they had respiratory symptoms and exposure to tobacco smoke.Citation14,Citation19 Muller et alCitation19 included 17,856 subjects and found airway obstruction (FEV1/FEV6 <0.7) in 17% of the subjects, and interestingly, a diagnosis of COPD was independently suspected by the MD three times more than indicated by the COPD-6 device. No data from confirmative spirometry was included in the study, thus, direct comparison with our study is not possible. A recent smaller study by Represas-Represas et al included a mixed cohort of 362 subjects from general practice, emergency services, and community pharmacies and found a COPD prevalence of 40% in the general practice cohort (n=167).Citation23 The high prevalence of COPD most likely reflects a much higher tobacco exposure compared to the current study (75% active smokers, mean pack-years 39). An optimal cutoff of FEV1/FEV6 of 0.8 was proposed corresponding to a sensitivity of 92% and specificity of 53%.
Llordes et al included 407 subjects and found a COPD prevalence of 26%.Citation24 The study was similar to our study with regard to smoking exposure and age, and no obvious explanation for the difference in prevalence can be identified based on the available information. Different screening strategies including screening questionnaires and the COPD-6 device were tested, and the latter was found significantly superior with an FEV1/FEV6 cutoff of 0.78 (sensitivity 88% and specificity 72%).
Another multicenter study by Thorn et al including 305 subjects found a COPD prevalence of 25%.Citation17 The higher prevalence of COPD in that study was likely due to inclusion criteria with a minimum smoking exposure of ≥15 pack-years. An FEV1/FEV6 cutoff of 0.73 was suggested, which corresponds to a sensitivity and specificity of 79% and 80%, respectively and at a cutoff of 0.7, the numbers were 53% and 90%, respectively. They concluded that the COPD-6 device may reduce the number of unnecessary spirometry tests, and the results were similar to two other studies, in which an almost similar device (Piko-6, measuring FEV1/FEV6) was validated for the prediction of COPD.Citation15,Citation16 None of the described studies accounted for the occurrence of asthma in the cohort. The present study also differed in the use of a combined criteria for confirmatory spirometry including both FEV1/FEV6 ratio <0.7 and FEV1 <80%pred.
While general screening of all individuals with smoking exposure is not recommended, US Preventive Services Task Force (USPSTF) recently recommended screening of all exposed individuals with respiratory symptoms, which is in concordance with the latest recommendations from GOLD.Citation25,Citation26
Studies of spirometry and the correlation between FEV1/FEV6 ratio and FEV1/FVC have suggested that the cutoff for airway obstruction when using FEV6 should be set at a significantly higher level.Citation11,Citation12,Citation27,Citation28 Accordingly studies of the COPD-6 device show that the best correlation to an FEV1/FVC ratio of 0.7 is an FEV1/FEV6 ratio of 0.73–0.75.Citation14,Citation17,Citation18 However, our COPD-6 device was technically limited to a cutoff value for airway obstruction of 0.7. Thus, the combined criterion for diagnostic spirometry was decided in order to compensate for the risk of insufficient sensitivity. Our results confirmed the issue by showing that although 439 diagnostic spirometry procedures could be spared, 27% of the subjects with COPD in the study would have been missed by the single criterion diagnostic algorithm. Accordingly, of the 439 subjects with a normal COPD-6 test who proceeded to diagnostic spirometry based on the other two criteria, 31% was diagnosed with COPD.
GOLD recommends a simple and operational definition of airway obstruction in primary care (post-BD FEV1/FVC <0.70) and points out the risk of over-diagnosis in the elder- and under-diagnosis of the younger population.Citation26 The MD decision criterion, by which the GP was allowed to perform diagnostic spirometry in subjects with FEV1/FEV6 close to 0.7, was in accordance with GOLD recommendations.
Significant BD reversibility is not uncommon in COPD.Citation29 In the current study, a strict definition of asthma (FEV1 change >0.5 L) was chosen not only to exclude this group of subjects but also because some of these participants may have asthma–COPD overlap.Citation30 As a result, the group of subjects diagnosed as having asthma was small (n=31). As expected, compared to the asthma group, the COPD group had significantly higher tobacco exposure, lower FEV1, and a trend toward a higher occurrence of sputum.
Subjects in the category “no obstructive lung disease” were overall a little younger with less tobacco exposure and less dyspnea and sputum. Half the subjects had normal FEV1 at confirmative spirometry and the majority of the group were overweight or obese, most likely representing subjects without clinically significant lung disease, although some individuals may have asthma despite an only moderate, or no, response to a BD.
Strengths and limitations of this study
The number of GPs enrolled was less than the planned 180 GPs. Nevertheless, the aim of identifying 500 subjects with COPD was largely met (n=487). An acceptable number of potentially eligible subjects (n=233) were excluded for various reasons; further details are given in . The study sample was large and included 149 GPs with a wide geographical representation from across the nation including large and small GP clinics. Thus, the results are very likely to be representative for GP clinics in general, at least in Denmark. The study group had a wide representation of age and an even distribution of sex. A large proportion (more than half) of the study population were current smokers. With regard to the subjects who did not proceed to diagnostic spirometry, the characteristics seem to justify exclusion: younger, less tobacco exposure, less symptoms, and a mean FEV1 predicted of 97%. Furthermore, in order to improve the quality of the COPD-6 and spirometry test, experienced personnel visited the clinics and gave training in performing the procedures.
It limits the study that confirmative spirometry was not conducted on all included patients. Thus, the extent of false-negative COPD-6 procedures could not be examined. However, the number of COPD patients found in the study equals what was expected based on a prior spirometry screening study also in Danish primary care, which indicates few false negatives.Citation22 Furthermore, the possibility of a false-negative COPD-6 test and missed COPD diagnosis was limited to subjects with mild disease (FEV1 >80%). Conducting both COPD-6 and conventional spirometry on all included patients would have conflicted with the secondary aim of the study to test whether the GPs found the COPD-6 device feasible in everyday work. However, by leaving it out, a direct comparison of COPD-6 and conventional spirometry in COPD screening could not be done.
Implications for future research, policy, and practice
As the COPD-6 screening test seems to be less time consuming and easier to perform, our results support the procedure as a favorable alternative which can facilitate the detection of COPD in primary care. The important aspect of the participating GPs’ experience with the COPD-6 screening tool is, therefore, currently being studied.
With focus on the COPD-6 device as a possible quick and handy screening tool, quality of the procedure must not be neglected. Standardization of the procedure is important to ensure reliability and reproducibility, as done in the present study.
Conclusion
The COPD-6 device seems to be a feasible alternative to conventional spirometry for early detection of COPD in general practice. Qualitative evaluation is necessary to fully assess the potential of the device in overcoming current barriers in screening for COPD in high-risk populations. Consensus on the exact criteria for performing confirmative spirometry including the optimal cutoff value for FEV1/FEV6 ratio is needed.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors wish to thank all participating GPs, their staff, and, not least of all, participating individuals.
The study was financially funded by Boehringer-Ingelheim, Denmark. The financial support provided by Boehringer-Ingelheim had no influence on the content of the manuscript. None of the authors received any compensation related to the development of this manuscript.
Disclosure
All the authors are members of the TOPTrack steering committee. The authors report no other conflicts of interest in this work.
References
- YawnBPDuvallKPeabodyJThe impact of screening tools on diagnosis of chronic obstructive pulmonary disease in primary careAm J Prev Med20144756357525241196
- LokkeAUlrikCSDahlRDetection of previously undiagnosed cases of COPD in a high-risk population identified in general practiceCOPD2012945846522643016
- JooMJLeeTAWeissKBGeographic variation of spirometry use in newly diagnosed COPDChest2008134384518347201
- LeeTABartleBWeissKBSpirometry use in clinical practice following diagnosis of COPDChest20061291509151516778268
- HanMKKimMGMardonRSpirometry utilization for COPD: how do we measure up?Chest200713240340917550936
- WiseJCOPD diagnosis must improve, says report by Royal College of PhysiciansBMJ2016355i618427864257
- HaroonSJordanREFitzmauriceDAAdabPCase finding for COPD in primary care: a qualitative study of the views of health professionalsInt J Chron Obstruct Pulmon Dis2015101711171826357469
- JonesRCPriceDRyanDOpportunities to diagnose chronic obstructive pulmonary disease in routine care in the UK: a retrospective study of a clinical cohortLancet Respir Med2014226727624717623
- BuffelsJDegryseJHeyrmanJDecramerMDIDASCO StudyOffice spirometry significantly improves early detection of COPD in general practice: the DIDASCO StudyChest20041251394139915078751
- SwanneyMPJensenRLCrichtonDABeckertLECardnoLACrapoROFEV(6) is an acceptable surrogate for FVC in the spirometric diagnosis of airway obstruction and restrictionAm J Respir Crit Care Med200016291791910988105
- VandevoordeJVerbanckSSchuermansDKartounianJVinckenWFEV1/FEV6 and FEV6 as an alternative for FEV1/FVC and FVC in the spirometric detection of airway obstruction and restrictionChest20051271560156415888828
- JingJYHuangTCCuiWXuFShenHHShould FEV1/FEV6 replace FEV1/FVC ratio to detect airway obstruction? A metaanalysisChest200913599199819349398
- Duong-QuySHua-HuyTMai-Huu-ThanhBDetection precoce de la bronchopneumopathie chronique obstructive post-tabagique au Viet Nam. [Early detection of smoking related chronic obstructive pulmonary disease in Vietnam]Rev Mal Respir200926267274 French19367200
- Represas RepresasCBotana RialMLeiro FernandezVGonzalez SilvaAIdel Campo PerezVFernandez-VillarAValidacion del dispositivo portatil COPD-6 para la deteccion de patologias obstructivas de la via aerea. [Assessment of the portable COPD-6 device for detecting obstructive airway diseases]Arch Bronconeumol201046426432 Spanish20570429
- SichletidisLSpyratosDPapaioannouMA combination of the IPAG questionnaire and PiKo-6(R) flow meter is a valuable screening tool for COPD in the primary care settingPrim Care Respir J201120184189 181 p following 18921597666
- FrithPCrockettABeilbyJSimplified COPD screening: validation of the PiKo-6(R) in primary carePrim Care Respir J201120190198 192 p following 19821597667
- ThornJTillingBLisspersKJorgensenLStenlingAStratelisGImproved prediction of COPD in at-risk patients using lung function pre-screening in primary care: a real-life study and cost-effectiveness analysisPrim Care Respir J20122115916622270480
- MiravitllesMLlorCCalvoEDiazSDiaz-CuervoHGonzalez-RojasNdel FEV1/FEV6 para el diagnóstico de enfermedad pulmonar obstructiva crónica. [Validation of the Spanish version of the Chronic Obstructive Pulmonary Disease-Population Screener (COPD-PS). Its usefulness and that of FEV(1)/FEV(6) for the diagnosis of COPD]Med Clin (Barc)2012139522530 Spanish22015009
- MullerMKoglerHGlaabTWelteTCOPD-Screening in der hausarztlichen Praxis mit einem Lungenfunktions-Schnellmessgerat. [Use of a lung function screening device for identifying patients at risk for COPD in general practice]Pneumologie201266645649 German23132318
- van den BemtLWoutersBCGrootensJDenisJPoelsPJSchermerTRDiagnostic accuracy of pre-bronchodilator FEV1/FEV6 from microspirometry to detect airflow obstruction in primary care: a randomised cross-sectional studyNPJ Prim Care Respir Med2014241403325119686
- HaroonSJordanRTakwoingiYAdabPDiagnostic accuracy of screening tests for COPD: a systematic review and meta-analysisBMJ Open20155e008133
- KjeldgaardPDahlRLokkeAUlrikCSDetection of COPD in a high-risk population: should the diagnostic work-up include bronchodilator reversibility testing?Int J Chron Obstruct Pulmon Dis20151040741425759573
- Represas-RepresasCFernandez-VillarARuano-RavinaAPriegue-CarreraABotana-RialMstudy group of “Validity of COPD-6 in non-specialized healthcare settings”Screening for chronic obstructive pulmonary disease: validity and reliability of a portable device in non-specialized healthcare settingsPLoS One201611e014557126726887
- LlordesMZurdoEJaenAVazquezIPastranaLMiravitllesMWhich is the best screening strategy for COPD among smokers in primary care?COPD20161411927723367
- Guirguis-BlakeJMSengerCAWebberEMMularskiRAWhitlockEPScreening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task ForceJAMA20163151378139327046366
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for Diagnosis, Management and Prevention of COPD2017 Available from: www.goldcopd.comAccessed January 05, 2017
- RosaFWPerez-PadillaRCamelierAEfficacy of the FEV1/FEV6 ratio compared to the FEV1/FVC ratio for the diagnosis of airway obstruction in subjects aged 40 years or overBraz J Med Biol Res2007401615162117906778
- VandevoordeJVerbanckSSchuermansDKartounianJVinckenWObstructive and restrictive spirometric patterns: fixed cut-offs for FEV1/FEV6 and FEV6Eur Respir J20062737838316452596
- AlbertPAgustiAEdwardsLBronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary diseaseThorax20126770170822696176
- NielsenMBarnesCBUlrikCSClinical characteristics of the asthma-COPD overlap syndrome – a systematic reviewInt J Chron Obstruct Pulmon Dis2015101443145426251584
- KOL: Anbefalinger for tidlig opsporing, opfølgning, behandling og rehabilitering (COPD: Recommendations for early detection, monitoring, treatment and rehabilitation)Danish National Board of Health2017 Available from: https://www.sst.dk/da/udgivelser/2017/~/media/8365DCEC9BB240A0BD6387A81CBDBB49.ashxAccessed June 1, 2017
- MadsenFMaltbækNMortensenJPedersenOFLungefunktions-standard; Spirometri og peakflow Lungevolumen Lungediffusionska-pacitet. [Standard for lung function; Spirometry and peak flow Lung volume Lung diffusion capacity]Danish Society of Respiratory Medicine2007 Available from: https://www.lungemedicin.dk/fagligt/klaringsrapporter/5-lfu-standard/file.htmlAccessed November 28, 2016 Danish
- QuanjerPHTammelingGJCotesJESymbols, abbreviations and units. Working Party Standardization of Lung Function Tests, European Community for Steel and CoalEur Respir J Suppl199316851008499056
- BestallJCPaulEAGarrodRGarnhamRJonesPWWedzichaJAUsefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary diseaseThorax19995458158610377201