Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a common inflammatory lung disease characterized by inflammatory cells activation and production of inflammatory mediators. Methyl-CpG-binding domain protein 2 (MBD2) plays an important role in diverse immunological disorders by regulating immune cell functions, such as differentiation and mediator secretion. However, the role of MBD2 in COPD remains unknown.
Methods
MBD2 protein expression in lung tissues of patients with COPD and cigarette smoke (CS)-exposed mice were evaluated by Western blot and immunohistochemistry. The role of MBD2 in cigarette smoke extract (CSE)-induction of inflammatory mediator expression in the human bronchial epithelial (HBE) cell line was assessed by silencing MBD2 expression in vitro. The involvement of signaling pathways in mediation of inflammation was tested with signaling inhibitors.
Results
Compared with controls, MBD2 expression was distinctly reduced in the bronchial epithelium of both patients with COPD and CS-exposed mice. Moreover, MBD2 expression was decreased in HBE after CSE stimulation in vitro. Moreover, MBD2 knockdown enhanced interleukin (IL)-6 and IL-8 expression in HBE in the presence and absence of CSE treatment by the ERK signaling pathway.
Conclusion
MBD2 protein expression was reduced in the airway epithelium of COPD. In HBE, this reduced expression was associated with increased levels of IL-6 and IL-8 mediated by the ERK pathway. These results suggest that MBD2 could contribute to chronic airway inflammation in COPD.
Introduction
Chronic obstructive pulmonary disease (COPD), characterized by irreversible and progressive expiratory airflow limitation, is a worldwide public health problem that accounts for 5% of total deaths each year and is predicted to be the third leading cause of death by 2020.Citation1–Citation3 Cigarette smoking is the main pathological driver in COPD development by causing aberrant inflammation, protease–anti-protease imbalance, oxidative stress, apoptosis, and accelerated lung aging.Citation4–Citation6 Inflammation, as one of the central features of COPD, causes activation and/or recruitment of immune cells. Human bronchial epithelial (HBE) cells, as the first defensive barrier and major effector in lung tissue, can trigger immune and inflammatory responses by secreting mediators.Citation7 Ongoing inflammation in airways can subsequently activate and/or recruit more immune cells, leading to production of cytokines, chemokines, reactive oxygen species, and enzymes that together contribute to the development and progression of COPD.Citation8 However, the underlying mechanisms of smoking-induced airway inflammation remain largely undefined.
Methyl-CpG-binding domain protein 2 (MBD2) is a member of the MBD protein family that responds to DNA methylation signals.Citation9 MBD2 regulates gene transcription by acting as a methylation-dependent reader that participates in the recruitment of silencing complexes, nucleosome remodeling deacetylases, and histone deacetylases.Citation10,Citation11 Cigarette smoking is associated with elevated serum homocysteine – a well-established risk factor for atherosclerosis.Citation12,Citation13 Yideng et al found that homocysteine could reduce MBD2 protein expression in vascular smooth muscle cells, which suggests that cigarette smoking might regulate MBD2 protein expression.Citation14 Furthermore, accumulating evidence suggests that MBD2 is associated with numerous cancers, as well as several immunological disorders, including systemic lupus erythematosus, autoimmune encephalomyelitis, sclerosis, and dermatomyositis.Citation15–Citation23 MBD2 overexpression was reported to increase interleukin (IL)-10 and TGF-β levels, but reduce the amount of IL-6 in MBD2−/− Treg cells.Citation24 Additionally, MBD2 was recognized with implications in Th1 differentiation through repression of interferon gamma (IFN-γ) expression.Citation25,Citation26 Besides, MBD2 knockdown activated ERK1/2 and p38 MAPK pathways, which are involved in regulating cytokine production.Citation27 Taken together, MBD2 may play an instrumental role in modulating expression of inflammatory mediators.
We hypothesize that MBD2 may contribute to airway inflammation in COPD pathogenesis. The current study was conducted to determine MBD2 expression in the airway epithelium of COPD patients and CS-exposed mice. In in vitro experiments, we analyzed the effects of cigarette smoking on MBD2 protein levels in HBE. Further, we silenced MBD2 expression in HBE to explore the function of MBD2 and the underlying mechanisms involved in modulating the secretion of inflammatory mediators.
Materials and methods
Subjects
Normal lung tissue specimens were obtained from 34 patients with operable nonmalignant and solitary pulmonary nodule, who were recruited from Tongji Hospital, Wuhan, People’s Republic of China. All participants were assigned into three groups: COPD group, non-COPD smoker group, and non-smoker according to their smoking history and pulmonary function. COPD cases were diagnosed before surgery based on a post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity ratio less than 70% according to the criteria of the Global Initiative for Chronic Obstructive Lung Disease. Smokers were patients with smoking history of more than 20 pack-years. Main exclusion criteria were malignant tumors, asthma, severe lung infections, or other obstructive lung diseases. Written informed consent was obtained from all subjects. This study was approved by the Research Ethics Committee of Huazhong University of Science and Technology.
Animals and CS exposure
Six-week-old male C57BL/6 mice were purchased from the Animal Experiment Center of Wuhan University. Mice were housed in sterilized cages with filter tops in specific pathogen-free conditions at Tongji Medical College, Huazhong University of Science and Technology. All animal experiments were approved by the Huazhong University Animal Experiment Ethics Committee and were conducted in accordance with the animal experimentation guidelines of Huazhong University.
Mice were randomly divided into two groups: CS-exposed mice and healthy controls (n=8 per group). CS-exposed mice were exposed to smoke in a PAB-S200 Animal Passive Smoking Exposure System (BioLab Technology Co. Ltd., Beijing, People’s Republic of China) using a modified method of 10 cigarettes for two separate 2.5-h periods per day, 5 days per week for continuously for 8 months.Citation28 Simultaneously, healthy controls were exposed to room air.
Lung function measurement and histological analysis
The lung function of all mice was measured using AniRes 2005 lung function system (Bestlab, Beijing, People’s Republic of China) as previously described.Citation29 Mice were anesthetized intraperitoneally with 90 mg/kg sodium pentobarbital (Servicebio, Wuhan, People’s Republic of China), and a tracheal cannula was inserted via tracheotomy. Then, the mouse was quickly placed supine in a sealed whole-body plethysmograph and connected to a computer-controlled ventilator via a tracheal cannula. The respiratory rate was preset at 90 breaths/min with a tidal volume of 5 mL/kg and the expiration/inspiration time ratio was set at 1.5:1. When all the parameters were stable, lung resistance (RL) was recorded. After the mouse was allowed to acclimatize for at least 30 s, dynamic lung compliance (Cdyn), forced expiratory volume in 0.1 s (FEV0.1s), and ratio of FEV0.1s to forced vital capacity (FEV0.1s/FVC) were evaluated as follows: immediately after the ending of the last expiration of volume history, which was automatically detected by the system, the lungs was inflated to 30 cm H2O tracheal pressure (TLC level) and held for a brief period (0.4 s). Following this, mice was exposed to a negative pressure reservoir (−30 cm H2O), forcing rapid exhalation as quickly as possible.
Bronchoalveolar lavage fluid (BALF) and histological analysis
The right lung was lavaged three times with 0.8 mL saline through the tracheal cannula. The bronchoalveolar lavage fluid (BALF) obtained was centrifuged at 1,000 g for 5 min, and supernatants of the first BALF were stored at −80°C for cytokine measurements. The left lung was not lavaged, but instead was inflated and fixed with 10% formalin at a pressure of 25 cm H2O for 24 h and then embedded in paraffin. Lung tissue sections (5 mm) were stained with hematoxylin–eosin to detect morphological alternations by measuring the mean linear intercept (MLI) under light microscopy.Citation30 Using eight parallel lines drawn across the lung section, MLI was calculated for each sample based on eight random fields at a magnification of 100×. MLI was equal to the ratio of the lengths of cross lines over the total number of intercepts encountered in eight lines per field.
Cell culture and stimulation
Human lung bronchial epithelial cell line (HBE) were obtained from the Wuhan Boster Biotech Co., Ltd. (People’s Republic of China). Cells were maintained in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum at 37°C with 5% CO2. Cell cultures were maintained and stimulated with CSE, which was prepared as previously described.Citation31 Where indicated, cells were pretreated for 1 h with the MAPK signal pathway inhibitors (Selleck) SB-203580 (10 μM), U-0126 (10 μM), or SP-600125 (10 μM), dissolved to the relevant concentration with dimethyl sulfoxide (DMSO).
Cell viability assay
Approximately 3,000 cells were seeded in each well of a 96-well plate (Corning, MA, USA). After treatment, 10 μL of Cell Counting Kit 8 (CCK 8) solution was added, and cells were incubated at 37°C for 3 h according to the manufacturer’s instructions (Dojindo Laboratories, Tokyo, Japan). Optical density (OD) values (measuring wavelength at 450 nm, reference wavelength at 630 nm) were obtained using an EL ×800 Universal Microplate Reader (Bio-Tek Instruments, Inc., VT, USA).
Immunohistochemical analysis
Human lung and mouse lung tissues were stained with rabbit monoclonal MBD2 antibody (1:500, Abcam, Cambridge, UK) and rabbit polyclonal MBD2 antibody (1:100, Santa Cruz Biotechnology, CA, USA), respectively. These proteins were then detected in lung sections with an IgG Streptavidin Biotin Complex kit (Boster, Wuhan, People’s Republic of China) and developed with DAB substrate according to the manufacturer’s protocol (Dako, Glostrup, Denmark). A Nikon Spot image acquisition and processing system (USA) was used for image assessment. The mean integral OD of MBD2 protein staining in the bronchiolar epithelium was measured as follows.Citation32 We used Image-Pro Plus 4.1 professional image analysis software to open all immunohistochemical images from one sample slide. Thereafter, we activated the Magnetic Lasso tool and dragged selection areas only containing bronchial epithelium, then copy and pasted it into a new image. Then, the area of bronchial epithelium and the integral OD of MBD2-positive epithelium were measured. Finally, the mean integral OD of MBD2 protein staining in the bronchial epithelium was the ratio of the integral OD of MBD2-positive epithelium to area of bronchial epithelium. All of the images are assessed with the same parameters.
Western blot analysis
Total proteins were prepared using RIPA lysis supplemented with protease inhibitor cocktail. Equal amounts of protein (40 μg) were subjected to 10% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Germany). Membranes were incubated with the appropriate primary antibody: anti-MBD2 (1:2,000, Santa Cruz Biotechnology, CA, USA), anti-GAPDH antibody (1:4,000, Sungene biotech, Tianjin, People’s Republic of China). After washing with Tris-buffered saline (TBS) containing 0.1% Tween-20, blots were incubated with secondary antibody conjugated to horseradish peroxidase (HRP; 1:4,000, Proteintech, Hubei, People’s Republic of China). The intensity of individual bands was quantified using ImageJ.
SiRNA transfection
MBD2 small interfering RNA (siRNA) and non-targeting negative control siRNA were synthesized by Ruibo Biotechnology, Co. (Guangzhou, People’s Republic of China). The target sequences were as follows: MBD2-siRNA sense 5′-GCGAAACGAUCCUCUCAAU dTdT-3′, MBD2-siRNA anti-sense 3′-dTdT CGCUUUGCUAGGAGAGUUA-5′. SiRNAs were transfected into HBE cells using Lipofectamine 2000 according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA).
RNA isolation and quantitative real-time PCR
Total RNA was extracted using TRIzol (Takara, Dalian, People’s Republic of China). RNA was reversed transcribed into cDNA using a cDNA RT–PCR kit (Takara, Dalian, People’s Republic of China). Then, cDNA was used as a template for quantitating gene expression using a SYBR Green real-time PCR kit (Takara) in an ABI PRISM Fast 7500 system (Applied Biosystems, Foster City, CA, USA). Data were analyzed using the 2−ΔΔCt method. Primers were as follows: β-actin F-5′-GCAAGCAGGACTATGACGAG-3′, β-actin R-5′-CAAATAAAGCCATGCCAATC-3′; IL-6 F-5′-CTGCTGCCTTCCCTGCC-3′, IL-6 R-5′-CCTCTTTGCTGCTTTCACACAT-3′; IL-8 F-5′-TCTGGCAACCCTAGTCTGCT-3′, IL-8 R-5′-AAACCAAGGCACAGTGGAAC-3′; MBD2 F-5′-AAGTGATCCGAAAATCTGGGC-3′, MBD2 R-5′-TGCCAACTGAGGCTTGCT TC-3′; IL-4 F-5′-CCAACTGCTTCCCCCTCTG-3′, IL-4 R-5′-TCTGTTACGGTCAACTCGGTG-3′; IL-10 F-5′-GACTTTAAGGGTTACCTGGGTTG-3′, IL-10 R-5′-TCACATGCGCCTTGATGTCTG-3′; TGF-β F-5′-GGCCAGATCCTGTCCAAGC-3′, TGF-β R-5′-GTGGGTTTCCACCATTAGCAC-3′; TNF-α F-5′-CCTCTCTCTAATCAGCCCTCTG-3′, TNF-α R-5′-GAGGACCTGGGAGTAGATGAG-3′.
Enzyme-linked immunosorbent assay
Concentrations of IL-6 and IL-8 in cell-culture supernatants were measured using IL-6 and IL-8 DuoSet ELISA kits (R&D Systems Europe, Abingdon, UK) according to the manufacturer’s instructions.Citation33 The limits of detection for the IL-6 and IL-8 ELISA kits were 9.38 and 31.3 pg/mL, respectively. Levels of IL-6 and IL-6-inducible CXCL1 (KC) in BALF were measured using commercial mouse IL-6 and KC ELISA kits (R&D Systems Europe, Abingdon, UK). The limits of detection for the mouse IL-6 and KC ELISA kits were 1.8 and 2.0 pg/mL, respectively.
Statistical analysis
In human and mouse sample tests, normally distributed data were presented as mean ± SD and analyzed using Student’s t-test for two groups, or one-way analysis of variance (ANOVA) with Newman–Keuls post hoc test for multiple comparisons. Data which did not meet normal distribution were presented as median with range and evaluated by Mann–Whitney U-test for two groups. In the in vitro experiments, data were analyzed using Student’s t-test for two groups or one-way ANOVA with Newman–Keuls post hoc test for multiple comparisons. The relationship between MBD2 protein expression and proinflammatory cytokines was assessed by using Pearson analysis. All data were analyzed using Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA). P<0.05 was considered statistically significant.
Results
Subject characteristics
The clinical characteristics of subjects are summarized in . Lung tissues were collected from 34 patients. Smoking history was similar among smokers with COPD and without COPD. Compared with non-COPD smokers or nonsmokers, patients with COPD exhibited lower FEV1, FEV1% of predicted, and FEV1/FVC%. No significant differences in age were observed among the groups.
Table 1 Characteristics of study subjects
MBD2 protein expression was decreased in patients with COPD and CS-exposed mice
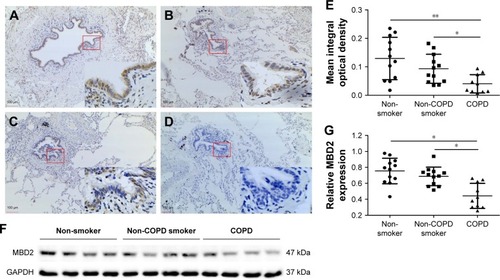
MBD2 protein expression in lung tissues was assessed by immunohistochemistry. Bronchial epithelium showed strong MBD2 protein staining in lung tissues of non-smokers on immunohistochemistry (). Inflammatory cells had moderate immunoreactivity, and there was low or negative MBD2 protein staining in mesenchymal cells (Figure S1). Relative to those of non-COPD smokers (, P<0.05) and non-smokers (, P<0.01), MBD2 protein levels in the epithelium were markedly decreased in patients with COPD (). There was no difference in MBD2 protein expression levels between non-COPD smokers and nonsmokers (). We further analyzed MBD2 protein expression in whole-lung tissue homogenates by Western blot. Consistent with results from immunohistochemistry, the relative expression of MBD2 protein was significantly decreased in patients with COPD, as compared with the other two groups (P<0.05 for both; ).
Figure 1 Decreased expression of MBD2 in patients with COPD.
Abbreviations: ANOVA, analysis of variance; COPD, chronic obstructive pulmonary disease; MBD2, methyl-CpG-binding domain protein 2; IgG Ab, immunoglobulin G antibody.
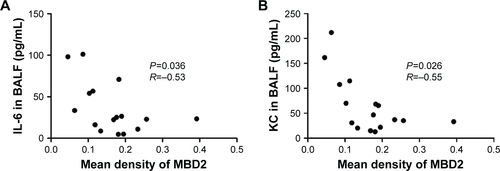
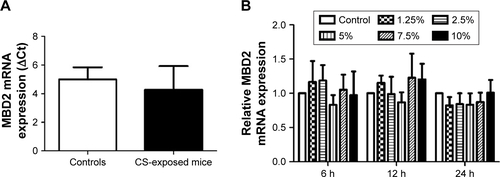
Pulmonary function and MLI were measured to confirm the establishment of a passive smoking model. Compared with controls, forced expiratory volume in 0.1 s (FEV0.1s; P<0.01), ratio of FEV0.1s to forced vital capacity (FEV0.1s/FVC; P<0.01), and dynamic lung compliance (Cdyn; P<0.001) were significantly decreased in CS-exposed mice (), whereas lung resistance (RL) was remarkably increased (P<0.01; ). Moreover, MLI measurement was dramatically elevated in CS-exposed mice (P<0.001; ). Levels of IL-6 and KC in BALF were determined by ELISA. Compared with controls, levels of IL-6 (Figure S2A) and KC (Figure S2B) in BALF were upregulated in CS-exposed mice. These results indicate that CS-exposed mice suffer from airflow limitation, emphysema, and airway inflammation. Then, we examined MBD2 expression in lung tissues of model mice. MBD2 protein expression was significantly downregulated in CS-exposed mice compared with that of controls on evaluation by immunohistochemistry () and by immunoblot (). MBD2 expression in the bronchial epithelium were negatively correlated with IL-6 (P=0.036, R=−0.53, Figure S3A) and KC (P=0.026, R=0.55, Figure S3B) levels in BALF of mice. However, there was no significant difference in MBD2 mRNA levels between the CS-exposed and control groups (Figure S4A).
Figure 2 Lung function and histological measurements in controls and CS-exposed mice.
Notes: Compared with controls, FEV0.1s, FEV0.1s/FVC and Cdyn were significantly decreased in CS-exposed mice (A–C), but RL was elevated (D). Representative lung tissue section stained with hematoxylin–eosin of controls and CS-exposed mice (E), scale bars =100 μM; magnification = ×40. Compared with controls, MLI measurement was significantly increased in CS-exposed mice (F). Data in C and F panels are presented as mean ± SD; data of other panels are presented as median with range, n=8 for controls and n=8 for CS-exposed mice. **P<0.01 and ***P<0.001 represent significant differences compared to controls.
Abbreviations: CS, cigarette smoke; FEV0.1s, forced expiratory volume in 0.1s; FEV0.1s/FVC, ratio of FEV0.1s to forced vital capacity; Cdyn, dynamic lung compliance; RL, lung resistance; MLI, mean linear intercept.
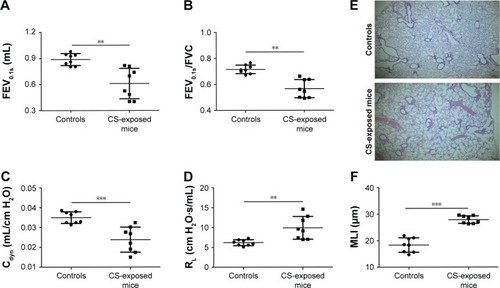
Figure 3 Downregulated MBD2 expression in CS-exposed mice.
Abbreviations: CS, cigarette smoke; MBD2, methyl-CpG-binding domain protein 2; IgG Ab, immunoglobulin G antibody.
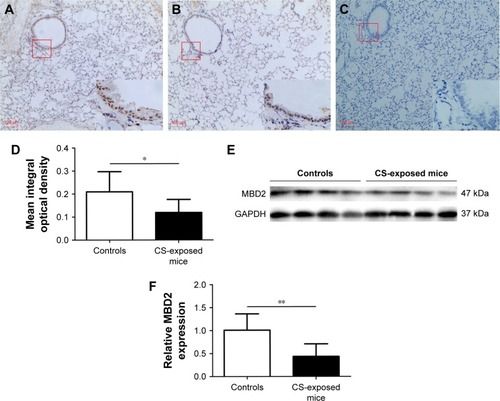
CSE attenuates MBD2 protein expression in HBE
CCK8 cell-activity estimation showed that CSE at concentrations ranging from 1.25% to 10% did not affect cell viability after incubation for 24–72 h ().
Figure 4 CSE attenuated MBD2 expression in HBE cells in vitro.
Abbreviations: ANOVA, analysis of variance; CSE, cigarette smoke extract; HBE, human bronchial epithelial; MBD2, methyl-CpG-binding domain protein 2.
To determine the potential influences of CSE on MBD2 protein expression in vitro, HBE were treated with various concentrations of CSE for 48 h or with 10% CSE for 24, 48, and 72 h. MBD2 protein expression began to significantly decline when cells were treated with 5% CSE (P<0.05) for 48 h, and reached the lowest level at 10% CSE (P<0.001; ). Meanwhile, a time-dependent decrease in MBD2 protein expression was found for HBE treated with 10% CSE (). However, there were no changes in MBD2 mRNA expression in HBE treated with different concentrations of CSE for different time periods (Figure S4B).
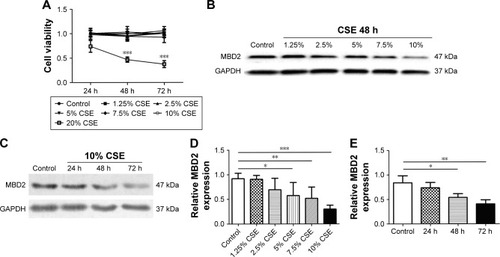
MBD2 knockdown increases cytokine gene expression in HBE
To investigate whether MBD2 participates in modulating cytokines expression in airway epithelium, we silenced the MBD2 gene in HBE using siRNA, and then tested cytokine expression. As expected, after transfecting HBE with MBD2 siRNA (MBD2-siRNA), there was a significant decrease in MBD2 mRNA and protein expression, compared to cells transfected with negative control siRNA (Con-siRNA; ).
Figure 5 MBD2 knockdown increased IL-6 and IL-8 expression in HBE.
Abbreviations: Con-siRNA, negative control siRNA; HBE, human bronchial epithelial; IL, interleukin; MBD2, methyl-CpG-binding domain protein 2.
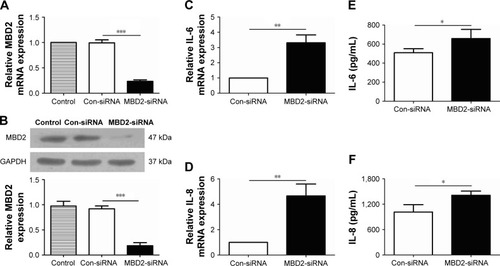
We, next, examined levels of the inflammatory cytokines IL-4, IL-6, IL-8, IL-10, TGF-β, and TNF-α in MBD2-silenced HBE, both at 24 and 48 h after transfection. Compared with Con-siRNA-transfected HBE, mRNA levels of IL-6 (P<0.01 for both) and IL-8 (P<0.05 for 24 h and P<0.01 for 48 h) were significantly increased in MBD2-siRNA-transfected HBE (). However, there were no differences in TGF-β, and TNF-α mRNA between the two groups (data not shown). IL-4 and IL-10 mRNA expression was near or below the detection limit of real-time PCR. Consistent with the results for mRNA levels, IL-6 (P<0.05) and IL-8 (P<0.01) protein expression was significantly elevated in MBD2-knockdown HBE ().
Decreased MBD2 expression enhanced IL-6 and IL-8 secretion promoted by the ERK1/2 pathway in HBE
After MBD2-siRNA transfection for 24 h, HBE were treated with or without 10% CSE for 24 h. The mRNA expression levels of IL-6 (P<0.001) and IL-8 (P<0.05) were markedly increased after 10% CSE simulation (). Similar results were detected in IL-6 (P<0.05) and IL-8 (P<0.05) protein levels (). These data further verified that MBD2 knockdown amplified IL-6 and IL-8 secretion by HBE in a CSE inflammatory milieu.
Figure 6 MBD2 knockdown enhanced ERK1/2 signal pathway-dependent IL-6 and IL-8 secretion by HBE.
Abbreviations: Con-siRNA, negative control siRNA; CSE, cigarette smoke extract; DMSO, dimethyl sulfoxide; HBE, human bronchial epithelial; IL, interleukin; MBD2, methyl-CpG-binding domain protein 2.
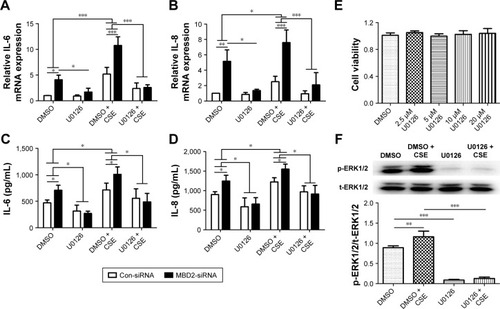
To evaluate the underlying mechanisms by which MBD2 affects cytokine production, we next explored whether MAPK signaling was activated in HBE after MBD2 knockdown by using the inhibitors U-0126, SP600125, and SB239063 to specifically block the ERK1/2, JNK, and p38 MAPK pathways, respectively. CCK8 cell-activity estimation showed that U0126 at concentrations ranging from 2.5 to 20 μM did not affect cell viability after incubation for 72 h (). HBE were preincubated with 10 μM U0126 or vehicle control (DMSO) for 1 h, followed by treatment with or without 10% CSE for 1 h (). As demonstrated in , 10% CSE increased the phosphorylation of ERK1/2 (p-ERK1/2), and U0126 could diminish the CSE-induced activation of ERK1/2 in HBE. Only U-0126, but not SP600125 or SB239063 (data not shown), could significantly inhibit IL-6 and IL-8 expression in MBD2-knockdown HBE, with or without CSE treatment (). Those results indicated that decreased MBD2 levels could enhance secretion of IL-6 and IL-8 via the ERK1/2 pathway.
Discussion
In the present study, we demonstrated for the first time that MBD2 protein expression in airway epithelium from patients with COPD was decreased relative to non-COPD smokers and non-smokers. MBD2 expression was, likewise, distinctly reduced in CS-exposed mice and CSE-stimulated HBE in vitro. As an additional functional verification, we found that MBD2 knockdown promoted HBE to release more IL-6 and IL-8 via ERK signaling. These results indicate that MBD2 might play an instrumental role in modulating airway inflammation associated with COPD.
CS is a complex mixture of more than 4,700 chemical compounds, and represents a major pathogenic factor that has been implicated in numerous diseases, including cancer, cardiovascular diseases, and COPD.Citation34 Cigarette smoking is associated with elevated serum homocysteine, and homo-cysteine was found to reduce MBD2 protein expression in vascular smooth muscle cells, suggesting that cigarette smoking might influence MBD2 protein expression.Citation12–Citation14 Our results showed that MBD2 protein expression was reduced in bronchial epithelium from patients with COPD and in CSE-treated cells in vitro. It was reported that exposure to CSE resulted in no change of MBD2 protein expression in other cells.Citation36 The impact of CS on MBD2 protein expression appear to be cell-type specific. Given that cigarette smoking can affect gene and protein expression in both humans and mice, we further confirmed that MBD2 protein expression was decreased in bronchial epithelium from CS-exposed mice.Citation35 These observations indicated that the decrease in MBD2 protein expression seen in the COPD bronchial epithelium is at least partially due to long-term CS exposure. Interestingly, MBD2 is not the only one of the MBD protein family altered by CSE. Mukhopadhyay showed that MBD3 and MeCP-2 protein were markedly reduced following exposure to CSE in branchial–arch-derived cells, which contribute to the formation of the lip and palate.Citation36
However, we could not detect a distinct difference in MBD2 mRNA expression in lung tissues from CS-exposed mice and in CSE-treated HBE in vitro. Further, Tommasi et al reported no significant alteration of MBD2 mRNA between control mice and those exposed to secondhand smoke.Citation37 In contrast, MBD2 mRNA expression was decreased in subjects who had chronic occupational exposure to benzene, toluene, and xylene, but MBD2 levels were elevated in CD4+ T cells from patients with several autoimmune diseases, including systemic erythematosus lupus, dermatomyositis, and multiple sclerosis.Citation20–Citation22 In addition, Tan et al confirmed a post-transcriptional role for valproate (Sigma-Aldrich, St. Louis, MO, USA) – a drug used to treat seizures – in downregulating MBD2 expression.Citation38 Therefore, CS-induced changes in post-transcriptional regulation might explain the observed alterations in MBD2 protein expression. Post-transcriptional regulation, such as the processing, export, localization, turnover, and translation of mRNAs is an integral part of gene expression and adds substantial complexity to transcriptional control.Citation39 Several miRNAs, including miR-520b, miR-221, and miR-290/371, were reported to regulate MBD2 expression.Citation40–Citation42 However, MBD2 protein-specific changes, such as cleavage, processing, and degradation, were still unknown. As such, further studies are needed to elucidate the underlying mechanisms of MBD2 reduction induced by CS.
MBD2 plays multiple and complex roles in regulating cytokine secretion. Wang et al reported that MBD2 knockdown by siRNA markedly reduced expression of IL-10, CTLA-4, and TGF-β, which are essential for normal Treg function. In a reciprocal study, retroviral transduction of MBD2−/− Treg cells with MBD2 enhanced expression of IL-10, CTLA-4, and TGF-β, but reduced IL-6 expression.Citation24 Cheng et al showed that mRNA levels for TNF-α and MCP-1 were significantly lower in visceral adipose tissues that originated from MBD2−/− mice fed a high-fat diet as compared with that of controls.Citation43 Moreover, researchers demonstrated that MBD2−/− CD4+ T cells manifested a significant increase in IFN-γ and IL-4 secretion, whereas dendritic cells derived from MBD2−/− mice showed a similar capacity to secrete IL-6 and TNF-α.Citation23 So far, there were no reports on the impact of MBD2 on smoking-induced inflammatory responses. Therefore, we used siRNA to knockdown MBD2 in HBE with or without CSE treatment, and found that reduced MBD2, together with a CSE milieu, could synergistically enhance IL-6 and IL-8 secretion. Negative correlation between MBD2 protein expression in the bronchial epithelium of mice and IL-6 as well as KC level in BALF were confirmed. However, there was no difference in the expression of IL-4, IL-10, TGF-β, and TNF-α (data not shown) between MBD2-siRNA HBE and Con-siRNA controls. Impacts of MBD2 on cytokine secretion appear to be cell-type specific. Both IL-6 and IL-8 exert a role in orchestrating inflammation associated with COPD.Citation44 Levels of these cytokines in sputum are related to lung function, disease severity, and clinical outcome of COPD.Citation45–Citation47 Our data implied that the smoking-induced reduction in MBD2 levels could increase secretion of some inflammatory mediators by HBE. Interestingly, this is not the first example of the MBD protein family being involved in regulating the secretion of inflammatory cytokines. Specifically, a deficiency in methyl CpG-binding protein 2 enhances the expression of IL-6 by promoting NF-κB signaling in myeloid-derived cells.Citation48
MAPK signaling possesses critical functions in regulating COPD-related inflammation by activating transcription factors and/or stabilization of post-translational mRNA.Citation49,Citation50 As noted by Rao et al, p38 MAPK and ERK1/2 signals were activated in both MBD2 siRNA-transfected endothelial cells and MBD2−/− mice.Citation27 Consistent with the Rao study, we found that ERK1/2 inhibitors could block increases in cytokine expression caused by knockdown of MBD2 in HBE with or without CSE treatment. These data suggested that MBD2 modulated HBE inflammation probably via ERK1/2 signaling. Several articles demonstrated that the Wnt/β-catenin pathway was downregulated in patients with COPD, CS-exposed mice, and in cellular models.Citation51–Citation53 Guo et al showed that the reduced activity of WNT/β-catenin signaling induced by CSE may promote cytokine production in airway epithelium by p38 MAPK but not by the ERK pathway.Citation52 Although Phesse found that deficiency of MBD2 suppressed the Wnt pathway, we speculated that increased levels of IL-6 and IL-8 expression is independent of Wnt/β-catenin pathway in MBD2-siRNA transfected HBE.Citation54
This study has some limitations. Ideally, primary bronchial epithelial cells from COPD patients, non-COPD smokers, and non-smokers should be used. Instead, we used the HBE cell line for the in vitro studies, and it is unknown whether findings obtained with these cells can be extrapolated to humans. MBD2 expression showed a tendency to decrease in non-COPD smokers compared to non-smokers; however, it failed to reach statistical significance. This might partly be due to the relatively small sample size and the many uncontrolled factors influencing MBD2 protein expression in humans. Moreover, in the future, we should identify genes that MBD2 regulates through direct binding to promoters.
In conclusion, our study demonstrated that MBD2 expression decreased in the lung bronchial epithelium of patients with COPD, and this decrease was associated with increased levels of IL-6 and IL-8 in HBE. MBD2 enhanced the secretion of those inflammatory cytokines via an ERK1/2 signal pathway-dependent mechanism. Therefore, MBD2 might contribute to COPD pathogenesis.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant nos 81570033, 81570047, 81470227, 81370145, and 81370156), National Key Basic Research and Development Program (973 Program, grant no 2015CB553403), Chinese Medical Association Research Project (grant no 2013BAI09B00), and Changjiang Scholars and Innovative Research Team in University (grant no IRT_14R20).
Supplementary materials
Figure S1 Representative immunohistochemical image of cells in non-smokers’ lung tissues.
Notes: The red-boxed area indicates a region of higher magnification. The arrows indicate inflammatory cells and asterisks points to alveolar mesenchymal cells.
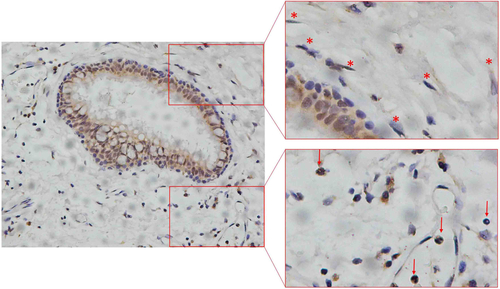
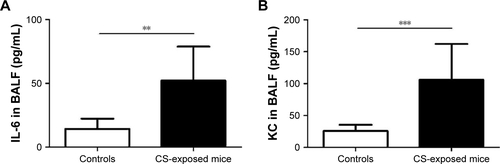
Figure S2 Levels of IL-6 and CXCL1 (KC) in BALF in controls and CS-exposed mice.
Notes: Compared with controls, levels of IL-6 (A) and CXCL1 (KC) (B) in BALF were increased in CS-exposed mice. Data are presented as means ± SD, n=8 for controls and n=8 for CS-exposed mice. P-values were calculated using Student’s t-test; **P<0.01 and ***P<0.001 represent significant differences compared to controls.
Disclosure
The authors report no conflicts of interest in this work.
References
- BarnesPJChronic obstructive pulmonary diseaseN Engl J Med2000343426928010911010
- MurrayCJLopezADAlternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease StudyLancet19973499064149815049167458
- PauwelsRARabeKFBurden and clinical features of chronic obstructive pulmonary disease (COPD)Lancet2004364943461362015313363
- FischerBMPavliskoEVoynowJAPathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammationInt J Chron Obstruct Pulmon Dis2011641342121857781
- ItoKBarnesPJCOPD as a disease of accelerated lung agingChest2009135117318019136405
- PlatakiMTzortzakiERytilaPDemosthenesMKoutsopoulosASiafakasNMApoptotic mechanisms in the pathogenesis of COPDInt J Chron Obstruct Pulmon Dis20061216117118046893
- GaoWLiLWangYBronchial epithelial cells: the key effector cells in the pathogenesis of chronic obstructive pulmonary disease?Respirology201520572272925868842
- BarnesPJShapiroSDPauwelsRAChronic obstructive pulmonary disease: molecular and cellular mechanismsEur Respir J200322467268814582923
- KloseRJBirdAPGenomic DNA methylation: the mark and its mediatorsTrends Biochem Sci2006312899716403636
- BirdAPWolffeAPMethylation-induced repression – belts, braces, and chromatinCell199999545145410589672
- NgHHZhangYHendrichBMBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complexNat Genet1999231586110471499
- DavisJABrownATChenHCigarette smoke increases intimal hyperplasia and homocysteine in a rat carotid endarterectomyJ Surg Res20041211697515313378
- VennABrittonJExposure to secondhand smoke and biomarkers of cardiovascular disease risk in never-smoking adultsCirculation2007115899099517296856
- YidengJJianzhongZYingHHomocysteine-mediated expression of SAHH, DNMTs, MBD2, and DNA hypomethylation potential pathogenic mechanism in VSMCsDNA Cell Biol200726860361117688412
- CheishviliDChikFLiCCSynergistic effects of combined DNA methyltransferase inhibition and MBD2 depletion on breast cancer cells; MBD2 depletion blocks 5-aza-2′-deoxycytidine-triggered invasivenessCarcinogenesis201435112436244625178277
- PontesTBChenESGigekCOReduced mRNA expression levels of MBD2 and MBD3 in gastric carcinogenesisTumour Biol20143543447345324338710
- PulukuriSMRaoJSCpG island promoter methylation and silencing of 14-3-3sigma gene expression in LNCaP and Tramp-C1 prostate cancer cell lines is associated with methyl-CpG-binding protein MBD2Oncogene200625334559457216786000
- SansomOJBergerJBishopSMHendrichBBirdAClarkeARDeficiency of Mbd2 suppresses intestinal tumorigenesisNat Genet200334214514712730693
- SapkotaYRobsonPLaiRCassCEMackeyJRDamarajuSA two-stage association study identifies methyl-CpG-binding domain protein 2 gene polymorphisms as candidates for breast cancer susceptibilityEur J Hum Genet201220668268922258532
- BaladaEOrdi-RosJSerrano-AcedoSMartinez-LostaoLVilardell-TarrésMTranscript overexpression of the MBD2 and MBD4 genes in CD4+ T cells from systemic lupus erythematosus patientsJ Leukoc Biol20078161609161617360956
- FagonePManganoKDi MarcoRExpression of DNA methylation genes in secondary progressive multiple sclerosisJ Neuroimmunol2016290666926711572
- LeiWLuoYLeiWAbnormal DNA methylation in CD4+ T cells from patients with systemic lupus erythematosus, systemic sclerosis, and dermatomyositisScand J Rheumatol200938536937419444718
- ZhongJYuQYangPMBD2 regulates TH17 differentiation and experimental autoimmune encephalomyelitis by controlling the homeostasis of T-bet/Hlx axisJ Autoimmun2014539510424934598
- WangLLiuYHanRMbd2 promotes foxp3 demethylation and T-regulatory-cell functionMol Cell Biol201333204106411523979593
- HutchinsASArtisDHendrichBDBirdAPScottPReinerSLCutting edge: a critical role for gene silencing in preventing excessive type 1 immunityJ Immunol200517595606561016237047
- HutchinsASMullenACLeeHWGene silencing quantitatively controls the function of a developmental trans-activatorMol Cell2002101819112150909
- RaoXZhongJZhangSLoss of methyl-CpG-binding domain protein 2 enhances endothelial angiogenesis and protects mice against hind-limb ischemic injuryCirculation2011123252964297421670230
- SeimetzMParajuliNPichlAInducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in miceCell2011147229330522000010
- WuHYangSWuXInterleukin-33/ST2 signaling promotes production of interleukin-6 and interleukin-8 in systemic inflammation in cigarette smoke-induced chronic obstructive pulmonary disease miceBiochem Biophys Res Commun2014450111011624866242
- ThurlbeckWMMeasurement of pulmonary emphysemaAm Rev Respir Dis19679557527645337140
- XuYLiHBajramiBCigarette smoke (CS) and nicotine delay neutrophil spontaneous death via suppressing production of diphosphoinositol pentakisphosphateProc Natl Acad Sci U S A2013110197726773123610437
- LappasMOdumetseTLRileyCPre-labour fetal membranes overlying the cervix display alterations in inflammation and NF-kappaB signalling pathwaysPlacenta20082912995100218952281
- WangMGaoPWuXImpaired anti-inflammatory action of glucocorticoid in neutrophil from patients with steroid-resistant asthmaRespir Res201617115327852250
- StedmanRLThe chemical composition of tobacco and tobacco smokeChem Rev19686821532074868017
- MorissetteMCLamontagneMBérubéJCImpact of cigarette smoke on the human and mouse lungs: a gene-expression comparison studyPLoS One201493e9249824663285
- MukhopadhyayPGreeneRMPisanoMMCigarette smoke induces proteasomal-mediated degradation of DNA methyltransferases and methyl CpG-/CpG domain-binding proteins in embryonic orofacial cellsReprod Toxicol20155814014826482727
- TommasiSZhengABesaratiniaAExpression of epigenetic modifiers is not significantly altered by exposure to secondhand smokeLung Cancer201590359860326525280
- TanNNTangHLLinGWEpigenetic downregulation of Scn3a expression by valproate: a possible role in its anticonvulsant activityMol Neurobiol20165442831284227013471
- MataJMargueratSBählerJPost-transcriptional control of gene expression: a genome-wide perspectiveTrends Biochem Sci200530950651416054366
- CuiSLiuLWanTJiangLShiYLuoLMiR-520b inhibits the development of glioma by directly targeting MBD2Am J Cancer Res20177715281539 eCollection 201728744402
- HeSLaiRChenDDownregulation of miR-221 inhibits cell migration and invasion through targeting methyl-CpG binding domain protein 2 in human oral squamous cell carcinoma cellsBiomed Res Int2015201575167226788506
- CaoYGuoWTTianSmiR-290/371-Mbd2-Myc circuit regulates glycolytic metabolism to promote pluripotencyEMBO J201534560962325603933
- ChengJSongJHeXLoss of Mbd2 protects mice against high-fat diet-induced obesity and insulin resistance by regulating the homeostasis of energy storage and expenditureDiabetes201665113384339527554473
- DecramerMRennardSTroostersTCOPD as a lung disease with systemic consequences – clinical impact, mechanisms, and potential for early interventionCOPD20085423525618671149
- AgustíAEdwardsLDRennardSIEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) InvestigatorsPersistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotypePLoS One201275e3748322624038
- FranciosiLGPageCPCelliBRMarkers of disease severity in chronic obstructive pulmonary diseasePulm Pharmacol Ther200619318919916019244
- GaoPZhangJHeXHaoYWangKGibsonPGSputum inflammatory cell-based classification of patients with acute exacerbation of chronic obstructive pulmonary diseasePLoS One201385e5767823741289
- O’DriscollCMLimaMPKaufmannWEBresslerJPMethyl CpG binding protein 2 deficiency enhances expression of inflammatory cytokines by sustaining NF-kB signaling in myeloid derived cellsJ Neuroimmunol2015283232926004152
- KrachtMSaklatvalaJTranscriptional and post-transcriptional control of gene expression in inflammationCytokine20022039110612453467
- RahmanIOxidative stress in pathogenesis of chronic obstructive pulmonary disease: cellular and molecular mechanismsCell Biochem Biophys200543116718816043892
- WangRAhmedJWangGDown-regulation of the canonical Wnt β-catenin pathway in the airway epithelium of healthy smokers and smokers with COPDPLoS One201164e1479321490961
- GuoLWangTWuYWNT/β-catenin signaling regulates cigarette smoke-induced airway inflammation via the PPARδ/p38 pathwayLab Invest201696221822926322419
- KneidingerNYildirimAÖCallegariJActivation of the WNT/β-catenin pathway attenuates experimental emphysemaAm J Respir Crit Care Med2011183672373320889911
- PhesseTJParryLReedKRDeficiency of Mbd2 attenuates Wnt signalingMol Cell Biol200828196094610318644872