Abstract
Background and objective
The difference in efficacy of long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs) for dynamic lung hyperinflation (DLH) in COPD is unclear. The purpose of this study was to elucidate the difference in efficacy of LAMA and LABA alone and the combination thereof for DLH.
Subjects and methods
Thirty stable patients were enrolled and randomly divided into two groups following baseline measurements. One group was treated with 5 μg tiotropium (Respimat inhaler) for 4 weeks following a 4-week treatment with 150 μg indacaterol, while the other group was treated with indacaterol for 4 weeks following a 4-week treatment with tiotropium. For both groups, these treatments were followed by a combination of the two drugs for 4 weeks. Pulmonary function tests, including DLH evaluated by metronome-paced incremental hyperventilation and exercise tolerance evaluated by the shuttle-walk test, were performed at the end of each treatment period.
Results
In total, 23 patients completed this study. Both tiotropium and indacaterol alone significantly increased forced expiratory volume in 1 second, exercise tolerance, and improved health status. Tiotropium significantly improved DLH, but indacaterol did not. The combination therapy resulted in further improvements in lung function and exercise tolerance, but not in DLH.
Conclusion
The efficacy of tiotropium in inhibiting DLH following metronome-paced incremental hyperventilation may be superior to that of 150 μg indacaterol, although the effects on airflow obstruction were the same, and the combination therapy showed further improvement in airflow obstruction, but not in DLH.
Introduction
Dynamic lung hyperinflation (DLH) is an important phenomenon that causes dyspnea and restricts exercise capacity in COPD.Citation1,Citation2 It has been shown that improvements in dyspnea on effort following bronchodilator therapy correlate well with reductions in LH, as indicated by increases in inspiratory capacity (IC).Citation3 We have demonstrated that treatment with tiotropium for 2–3 months resulted in significant improvements in airflow obstruction, lung mechanics, oxygenation, and respiratory impedance in COPD, and it also significantly improved DLH following metronome-paced incremental hyperventilation (MPIH), exercise capacity, and health-related quality of life in emphysema-dominant COPD. However, treatment with salmeterol did not lead to any improvement in DLH.Citation4 It might be suggested that salmeterol does not improve DLH because of the weak bronchodilator action of salmeterol. However, indacaterol is close to a full agonist of the human β2-adrenoceptor, whereas salmeterol displays only partial efficacy. Indacaterol has superior duration of action compatible with once-daily dosing in humans, as well as a fast onset of action, and it is called an ultralong-acting β2-agonist.Citation5 Therefore, a different result for DLH will be obtained compared with results with tiotropium and indacaterol.
Current strategies recommend treatment with long-acting β2-agonist (LABA)/long-acting muscarinic antagonist (LAMA) combination therapy for patients with moderate or more severe COPD.Citation6 Significant improvements in LH represented by IC, functional residual capacity (FRC), and residual volume (RV) were found following treatment with the once-daily tiotropium–olodaterol fixed-dose combination compared with placebo and monotherapies.Citation7 Moreover, the addition of indacaterol to tiotropium provided better effects on not only bronchodilation but also lung deflation (reflected by increased resting IC) compared with tiotropium monotherapy.Citation8 Berton et alCitation9 evaluated DLH via decrease in IC from preexercise levels during a constant-speed tread-mill test to the limit of tolerance. Compared to formoterol monotherapy, the addition of tiotropium to formoterol further improved effort-induced DLH and exercise endurance in patients with moderate–severe COPD. However, little evidence of an additive effect of LABA with LAMA regarding DLH has been demonstrated. The purpose in this study was to determine the superiority of efficacy among the monotherapies of LAMA and LABA and the combination therapy of LAMA and LABA for DLH evaluated by MPIH.
Subjects and methods
Subjects
We recruited 30 symptomatic patients with stable COPD from the outpatient clinic of Shinshu University Hospital. COPD was diagnosed based on clinical history and symptoms and by pulmonary function characterized by irreversible airflow limitation in accordance with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy.Citation6 All subjects had smoking-related COPD without α1-antitrypsin deficiency, and had a smoking history of more than 30 pack-years. There were no current smokers. The patients who had any history of asthma or asthmatic symptoms, walking disability, severe arrhythmia, or heart failure, had suffered from respiratory tract infection or exacerbation of COPD during the preceding 3 months, and had been treated with oral steroids and/or long-term oxygen therapy were excluded from the study. Severity of COPD, assessed according to GOLD strategy, was stage 1 (n=5), stage 2 (n=15), stage 3 (n=6), and stage 4 (n=4). For current therapies, 12 patients were being treated with LAMA, three with LAMA and LABA, four with inhaled corticosteroid (ICS) and LABA, one with LAMA and ICS, and six with LAMA and ICS/LABA. This study was conducted in accordance with International Conference on Harmonisation Good Clinical Practice and the Declaration of Helsinki, and was approved by the institutional research ethics committee of Shinshu University School of Medicine (2101). All patients gave written informed consent to participate. The study was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000027446).
Protocol
This was a random open-label crossover study. Subjects were divided into two groups at random for an accurate evaluation of the influence of the order of drug treatments with tiotropium and indacaterol. The random-allocation sequence was done using sequentially numbered containers. One group was treated with a 5 μg once-daily dosage of tiotropium through a soft-mist inhaler (Respimat) for 4 weeks following a 4-week treatment with a 150 μg once-daily dosage of indacaterol through a dry-powder inhaler, whereas the other group was treated with indacaterol for 4 weeks following a 4-week treatment with tiotropium in the same manner. Finally, both groups were treated with a combination of the two drugs (tiotropium + indacaterol) for 4 weeks. Health status was evaluated using the COPD-assessment test (CAT),Citation10 activity of daily life (ADL) was evaluated using the pulmonary emphysema activity of daily life (P-ADL),Citation11 and pulmonary function tests, including DLH evaluated by MPIH and exercise tolerance evaluated by shuttle-walk test, were performed at baseline and at the end of each treatment period. Pulmonary function tests were performed 2–3 hours after inhalation of the test drug. The primary end point was DLH following MPIH and IC at a rate of 30 or 40 breaths/min (bpm; IC30 or IC40).
Pulmonary function test
Spirometry, FRC lung volume, and lung-diffusion capacity for carbon monoxide (DLCO) were measured using a Chestac 8900 (Chest MI, Tokyo, Japan). FRC was measured using a body box, after which the subject immediately inspired to total lung capacity (TLC) and expired maximally to RV, allowing calculation of lung volume and RV/TLC. For predicted values for forced expiratory volume in 1 second (FEV1) and vital capacity (VC), Japanese local reference dataCitation12 developed by the Japanese Respiratory Society were adopted, and predicted values for DLCO and lung volumes (FRC, RV, and TLC) measured by body plethysmography were determined using the formulae of Nishida et alCitation13 and Boren et al,Citation14 respectively. Respiratory impedance was measured using a MostGraph 01 (Chest MI).
Evaluation of DLH following MPIH
DLH was evaluated by MPIH with incremental increases from ICrest to IC20, IC30, and IC40, in accordance with our previous report.Citation15 IC and VC were measured immediately after breathing at a rate of 20 bpm paced by a metronome for 30 seconds following the measurement of IC and VC at rest. Consequently, the breathing rate was increased to 30 bpm and 40 bpm in 30-second increments, and IC and VC were again measured immediately after MPIH for 30 seconds at each breathing rate. DLH was evaluated by decreases in IC or percentage change from ICrest to IC20 (ΔIC20; IC20 − ICrest), ΔIC30, and ΔIC40.
Statistical analysis
It was calculated that a sample size of 24 patients would have 80% power to detect a difference of 300 mL in mean IC30 or IC40, the primary end point, following treatment, assuming an SD of 500 mL and two-sided significance level of 5%. Assuming a dropout rate of approximately 20%, it was calculated that a total of 30 patients would need to be randomized. Values in the text, figures, and tables represent mean ± standard error of the mean. Repeated-measures analysis of variance was used to test for significant differences at different visits and time points according to each treatment. Comparisons of variables at baseline and at the end of each treatment were performed using paired t-tests. All statistical analyses were performed using Windows-compatible software (StatFlex version 6.0; Artech, Osaka, Japan). P<0.05 was considered significant for all statistical analyses.
Results
Of the 30 registered patients, seven dropped out. One patient dropped out due to incongruity of the throat, one due to cough and exacerbation of dyspnea, and one due to dysuria when they switched to tiotropium. Three other patients dropped out due to exacerbation. One remaining patient refused clinical trial continuation. As a result, 23 patients completed the clinical trial. shows the characteristics and data from the pulmonary function test for patients who completed the trial. There was no meaningful difference between the tiotropium-precedence group (n=11) and the indacaterol-precedence group (n=12) before the clinical trial started.
Table 1 Characteristics at baseline of 23 patients with COPD
CAT scores decreased significantly following each monotherapy and further decreased following combination therapy (). A significant improvement was observed in P-ADL following combination therapy. FEV1 increased significantly following treatment with each monotherapy, to the same level. The combination therapy showed a more significant increase in FEV1. There were no significant changes in lung volume following each treatment. Airway resistance showed significant improvements following tiotropium monotherapy and combination therapy. Among the data obtained from a MostGraph 01, resonant frequency and within-breath changes in reactance at 5 Hz frequency were significantly improved following combination therapy.
Table 2 Results of questionnaires on health status and activity of daily life (ADL) and of the pulmonary function test
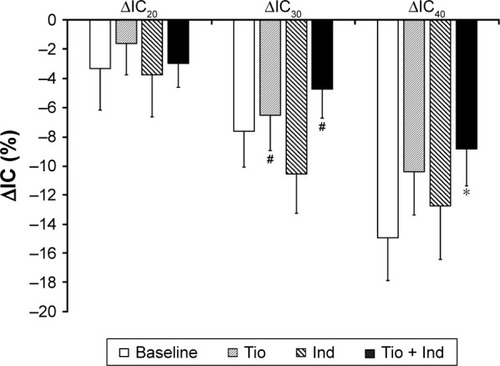
There were no significant changes in any DLH indices following indacaterol monotherapy. However, IC20, IC30, and IC40 values following tiotropium monotherapy and IC30 and IC40 values following combination therapy increased significantly (, ). Furthermore, the decrease in IC from ICrest to IC40 and ΔIC40 was inhibited significantly by combination therapy (). shows the results of the shuttle-walk test. Significant differences were not observed in maximum Borg scale score, lowest peripheral oxygen saturation, maximum pulse rate, or blood pressure at the end of exercise load, but significant increases in walking distance were observed following each monotherapy and patients were able to walk the farthest following combination therapy.
Table 3 Dynamic lung hyperinflation following incremental hyperventilation
Table 4 Exercise tolerance evaluated by shuttle-walk test
Figure 1 Effects of tiotropium (Tio) and indacaterol (Ind) alone and the combination thereof.
Notes: Effect on inspiratory capacity (IC) at rest (ICrest) and IC following metronome-paced incremental hyperventilation at rates of 20, 30, and 40 breaths/min (IC20, IC30, and IC40, respectively). Data expressed as mean ± standard error of mean. *P<0.05, **P<0.01 vs baseline; #P<0.05, ##P<0.01 vs Ind.
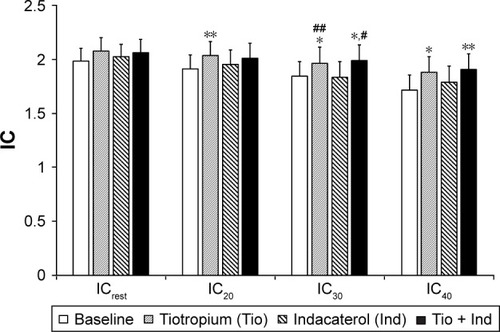
Figure 2 Effects of tiotropium (Tio) and indacaterol (Ind) alone and the combination of thereof.
Abbreviations: IC, inspiratory capacity; ICrest, resting IC; IC20, IC at 20 breaths/min; ΔIC20, change in IC from ICrest to IC20; IC30, IC at 30 breaths/min; ΔIC30, change in IC from ICrest to IC30; IC40, IC at 40 breaths/min; ΔIC40, change in IC from ICrest to IC40.
Discussion
In the present study, tiotropium improved DLH following hyperventilation, but indacaterol did not. In contrast, a similar effect on airflow obstruction was observed. Compared with each monotherapy, combination therapy may be more effective for health status, ADL, airflow obstruction, and exercise tolerance, but not for DLH following MPIH.
The recommended first-line treatment medicine for COPD has been a long-acting bronchodilator, and the efficacy of combination therapy using drugs with different action mechanisms should be determined. The combination therapy of LAMA and LABA for the improvement of pulmonary function has been shown to be superior to that of each monotherapy, and the combination results in an improvement in quality of life and exercise tolerance.Citation16 We have demonstrated that treatment with tiotropium showed a significant improvement in DLH following MPIH, as well as an improvement in pulmonary function and exercise capacity and health-related quality of life, in emphysema-dominant COPD, but the treatment with salmeterol did not show any improvement in DLH.Citation4 We have suggested that the difference in the efficacy for DLH following MPIH may be due to the weak action of the β2-agonist. In this study, the efficacy of an ultralong-acting β2-agonist with more effective action for bronchodilation, indacaterol, was examined. Although indacaterol treatment significantly improved airflow obstruction and exercise tolerance to the same extent as tiotropium, indacaterol could not inhibit DLH following MPIH.
It has been recommended based on consistent evidence that treatment with tiotropium can reduce DLH during or immediately after exercise.Citation17 We also demonstrated that tiotropium significantly reduced DLH following MPIH in the present study, similar to our previously reported result.Citation4 The additive effects of tiotropium with formoterol on effort-related DLH and exercise endurance in patients with moderate–severe COPD were also demonstrated,Citation9 and the advantageous effects were more likely to be found in patients who were severely disabled and functionally responsive to the addition of the cholinergic blockade. However, for the β2-agonist, we demonstrated that treatment with both a short-acting muscarinic antagonist and a short-acting β2-agonist (SABA) significantly increased IC following MPIH. Furthermore, SABA significantly inhibited the decrease in IC following hyperventilation.Citation18 Moreover, the addition of SABA to current therapy, including LAMA, resulted in further improvement in DLH following MPIH, with an improvement in the exercise capacity of patients with moderate–very severe COPD who showed dyspnea on effort despite their current therapy.Citation15 Beeh et alCitation19 examined the effect of 300 μg inhaled once-daily indacaterol on peak and “isotime” exercise IC in patients with COPD in a randomized, double-blind, placebo-controlled, two-period crossover study. Indacaterol showed statistically significant improvements over placebo in peak and isotime IC, along with improvements in resting IC, trough FEV1, dyspnea, and exercise-endurance time. The results in the present study were different from those reported by Beeh et al.Citation19 One possible reason for the difference may have been the difference in method of evaluating DLH from previous reports, which may have led to conflicting results. A second reason may be the different dose used in our study. Beeh et al used inhaled 300 μg once-daily indacaterol, but we used only half this dose and thus did not obtain a sufficient inhibitory effect on DLH. In our country, 150 μg once-daily inhaled indacaterol is approved and is the upper-limit dose. The severity of the COPD of the patients enrolled in the present study was almost the same as that in the report by Beeh et al. In their study, IC at rest and trough FEV1 increased by 182 mL and 145 mL, respectively, on average. However, in the present study, IC at rest and FEV1 increased by only 40 mL and 70 mL, respectively, on average, possibly suggesting that efficacy was insufficient. Kato et al compared the efficacy of inhaled indacaterol at doses of 150 μg, 300 μg, and 600 μg. Inhalation of 150 μg indacaterol caused a significant improvement; however, at the dose of 300 μg, trough FEV1 was further increased and reached its peak.Citation20 In addition, the efficacy of bronchodilation for Japanese individuals has been demonstrated to be equal to Caucasians. The combination therapy resulted in an additive effect on bronchodilation; however, no additive effects were observed on DLH following hyperventilation. These findings may suggest that the lack of efficacy regarding DLH may not be a problem solely due to the dose of indacaterol. It has been demonstrated that LAMA may be superior to LABACitation21 when comparing the effects of LAMA and LABA monotherapy on the exacerbation of COPD. One large difference in action mechanism is the inhibitive effect on mucous production due to differences between LAMA and LABA in their anticholinergic action. Therefore, treatment with tiotropium might reduce mucous hypersecretion at the site of peripheral airways. It may possibly contribute to improvements in air trapping and DLH.
This study is subject to some limitations. The first limitation concerns sample size. Prior to the study, sample size was calculated, and a total of 30 patients needed to be randomized. However, the SDs of IC30 and IC40 at baseline were slightly greater than those predicted and more than 20% of participants dropped out, resulting in a decrease in power to detect a difference. The small sample size of each group and the wide distribution of severity might have affected the results. The second limitation concerns the respiratory rate. With regard to the rate of respiration following hyperventilation, Gelb et alCitation22 reported that in patients with moderate–severe COPD, metronome-timed 20-second hyperventilation at twice the respiratory rate of that at rest reduced IC and was useful in dynamic hyperinflation screening. We evaluated IC at three respiratory rates: 20, 30, and 40 bpm. However, we did not determine the extent of the increase in respiratory rate during exercise. The third limitation concerns the absence of a comparison of our quantitative evaluation following hyperventilation and the conventional evaluation of dynamic hyperinflation during or immediately after exercise loading. This remains to be investigated in the future.
In conclusion, 5 μg once-daily inhaled tiotropium improves DLH following hyperventilation, but 150 μg inhaled once-daily indacaterol does not, although they have a similar effect on airflow obstruction. Additionally, combination therapy may be more effective for health status, ADL, airflow obstruction, and exercise tolerance than each monotherapy, but it is not more effective for DLH following hyperventilation. These findings may be attributed to the insufficient effect of low-dose indacaterol.
Acknowledgments
The authors thank students (M Hanzawa, A Nakagaito, and M Harada) of the Shinshu University School of Health Sciences for help and support. More importantly, the authors and our colleagues thank the patients who participated in this study for their effort and enthusiastic cooperation throughout the study. This work was not supported by any grant or other funding sources.
Disclosure
The authors report no conflicts of interest in this work.
References
- O’DonnellDEBertleyJCChauLKWebbKAQualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanismsAm J Respir Crit Care Med19971551091159001298
- O’DonnellDERevillSMWebbKADynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200116477077711549531
- BelmanMJBotnickWCShinJWInhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19961539679758630581
- FujimotoKKitaguchiYKandaSUrushihataKHanaokaMKuboKComparison of efficacy of long-acting bronchodilators in emphysema dominant and non-dominant COPDInt J Chron Obstruct Pulmon Dis2011621922721660299
- BattramCCharltonSJCuenoudBIn vitro and in vivo pharmacological characterization of 5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-hydroxy-1H-quinolin-2-one (indacaterol), a novel inhaled β2 adrenoceptor agonist with a 24-h duration of actionJ Pharmacol Exp Ther200631776277016434564
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary DiseaseBethesda (MD)GOLD2017
- BeehKMWestermanJKirstenAMThe 24-h lung-function profile of once-daily tiotropium and olodaterol fixed-dose combination in chronic obstructive pulmonary diseasePulm Pharmacol Ther201532535925956072
- MahlerDAD’UrzoABatemanEDConcurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, double-blind comparisonThorax20126778178822544891
- BertonDCReisMSiqueiraACEffects of tiotropium and formoterol on dynamic hyperinflation and exercise endurance in COPDRespir Med20101041288129620580216
- TsudaTSuematsuRKamoharaKDevelopment of the Japanese version of the COPD assessment testRespir Investig2012503439
- GotohYP-ADLJapan Society for Respiratory Care and Rehabilitation, Japanese Respiratory Society, Japanese Association of Rehabilitation Medicine, Japanese Physical Therapy AssociationRespiratory Rehabilitation Manual: Exercise Therapy2nd edTokyoShourinsha2012171
- SasakiENakamuraMKidaKReference values for spirogram and blood gas analysis in Japanese non-smoking healthy adultsJ Jpn Respir Soc200139383399
- NishidaSKambeMSewakeNTakanoMKawaneHPulmonary function in healthy subjects and its prediction–5: pulmonary diffusing capacity in adultsJpn J Clin Pathol197624941947
- BorenHGKoryRCSynerJCThe Veterans Administration Army cooperative study of pulmonary functionAm J Med19664196114
- KitaguchiYFujimotoKKomatsuYHanaokaMHondaTKuboKAdditive efficacy of short-acting bronchodilators on dynamic hyper-inflation and exercise tolerance in stable COPD patients treated with long-acting bronchodilatorsRespir Med201310739440023245993
- FarneHACatesCJLong-acting β2-agonist in addition to tiotropium versus either tiotropium or long-acting β2-agonist alone for chronic obstructive pulmonary diseaseCochrane Database Syst Rev201510CD00898926490945
- O’DonnellDEFlügeTGerkenFEffects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPDEur Respir J20042383284015218994
- FujimotoKYoshiikeFYasuoMEffects of bronchodilators on dynamic hyperinflation following hyperventilation in patients with COPDRespirology200712939917207032
- BeehKMWagnerFKhindriSDrollmannAFEffect of indacaterol on dynamic lung hyperinflation and breathlessness in hyperinflated patients with COPDCOPD2011834034521793716
- KatoMMakitaHUemuraKBronchodilator efficacy of single doses of indacaterol in Japanese patients with COPD: a randomised, double-blind, placebo-controlled trialAllergol Int20105928529320567133
- DecramerMLChapmanKRDahlROnce-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group studyLancet Respir Med2013152453324461613
- GelbAFGutierrezCAWeismanIMNewsomRTaylorCFZamelNSimplified detection of dynamic hyperinflationChest20041261855186015596684

