Abstract
Background
COPD is a highly heterogeneous disease. Potential biomarkers to identify patients with COPD who will derive the greatest benefit from inhaled corticosteroid (ICS) treatment are needed. Blood eosinophil count can serve as a predictive biomarker for the efficacy of ICS treatment. The aim of this systematic review and meta-analysis was to assess whether a blood eosinophil count of ≥2% in patients undergoing ICS therapy was associated with a greater reduction in COPD exacerbation rate and pneumonia incidence.
Materials and methods
An electronic search was performed using the keywords “COPD”, “eosinophil”, and “clinical trial” in the PubMed and EMBASE databases to retrieve articles, up to 2017, relevant to our focus. Data were extracted, and a meta-analysis was conducted using RevMan 5 (version 5.3.5).
Results
Five studies comprising 12,496 patients with moderate-to-very severe COPD were included. At baseline, 60% of the patients had ≥2% blood eosinophils. Our meta-analysis showed a 17% reduction in exacerbation of moderate/severe COPD in patients with ≥2% blood eosinophils undergoing ICS therapy compared to the non-ICS/ICS withdrawal/placebo group. The difference between the two types of treatment was significant (risk ratio [RR], 0.816; 95% CI, 0.67–0.99; P=0.03). Furthermore, the risk of pneumonia-related events was significantly increased in the subgroup with eosinophil count ≥2% undergoing ICS-containing treatments (RR, 1.969; 95% CI, 1.369–2.833; P<0.001). There was no significant difference in the subgroup with eosinophil count <2% (RR, 1.29; 95% CI, 0.888–1.879; P<0.181).
Conclusion
The results of our meta-analysis suggest that the 2% threshold for blood eosinophils could accurately predict ICS treatment response in patients with COPD, but increased the risk of pneumonia.
Keywords:
Introduction
Inhaled corticosteroids (ICSs) can reduce acute exacerbation in patients with COPD having moderate-to-very severe lung function defects and a history of frequent exacerbation.Citation1–Citation5 However, the risks and benefits of ICS treatment are still controversial, especially the purported increase in pneumonia incidence.Citation6 Airway eosinophilia is a hallmark inflammatory response for asthma pathogenesis and is now known to be involved in the airway inflammatory process in COPD.Citation7–Citation9 Eosinophilic COPD is defined using sputum eosinophil counts of ≥3% and has been reported during acute exacerbations in up to 28% of cases.Citation10 Interestingly, it is seen in ~34%Citation11 (or 38%Citation12) of patients with COPD in stable condition. Airway eosinophilia is an important marker for treatment effectiveness of inhaled and oral corticosteroid therapies in patients with COPD.Citation12–Citation15
A diagnostic tool for the measurement and detection of airway eosinophilia is induced sputum assessment.Citation8 Sputum induction is thought to be a direct and reliable method for evaluating airway inflammation; however, it has several limitations.Citation16,Citation17 For instance, it is unsuitable for point-of-care testing, requires experience, and has a failure rate of up to 30%.Citation16,Citation17 Due to these limitations, the search for minimally invasive and easily available methods that can identify and evaluate the status of sputum eosinophilia inflammation in asthma and COPD has been intensified.Citation10,Citation11,Citation18–Citation21 The use of peripheral blood cell counts is a simple and attractive tool that has potential in clinical practice. The prediction and accuracy of blood eosinophils and sputum eosinophilia in patients with asthma have been assessed and have demonstrated promising results.Citation21–Citation24 However, limited studies have examined this issue in patients with COPD in a stable condition. One study reported a correlation between bronchial and blood eosinophil counts in 20 patients with COPD and 21 healthy controls.Citation25 Previous reports have also confirmed that blood eosinophils can serve as a good biomarker for steroid therapy in exacerbatingCitation26 and stableCitation27,Citation28 patients with COPD. Therefore, blood eosinophil count could be a predictive biomarker to indicate stable or exacerbating status and may indicate the effectiveness of ICS treatment in patients with COPD.
At present, there is still a lack of consensus about the optimal threshold of blood eosinophils for guiding ICS treatment in patients with stable COPD. Hence, the aim of this systematic review and meta-analysis was to investigate the predictive value of blood eosinophil count (cutoff point of 2%) as a biomarker of ICS efficacy in reducing the annual rate of moderate/severe exacerbations for patients with moderate-to-very severe COPD and a history of exacerbations.
Materials and methods
Search strategy and inclusion/exclusion criteria
This systematic review was performed in accordance with the guidelines on “Systematic Reviews and Meta-analysis: Understanding the Best Evidence in Primary Healthcare” and “How to Review a Meta-analysis”. A comprehensive search was conducted using the terms “COPD”, “eosinophil”, and “clinical trial” in PubMed and EMBAS to identify relevant studies published up to 2017. All articles identified in the initial database search were screened based on title, abstract, and full text to confirm eligibility and avoid overlapping data. Primary research articles were compared for the following items: studies of ICS treatment and annual rates of moderate/severe exacerbations in patients with COPD with <2% and ≥2% blood eosinophils. Studies involving patients with COPD that had acute exacerbation, preclinical studies, and conference abstracts were excluded from our meta-analysis.
Data extraction
Relevant data were extracted from the eligible publications: the name of the first author, the year of publication, trial name, the number of patients analyzed, inclusion criteria for participants, baseline patient characteristics, treatment regimens, study duration, the annual rate of moderate/severe exacerbations (including time-to-first exacerbations), and pneumonia events. Data on the therapeutic partners for ICS medications were obtained from seven studies from 1 to 3 years. The therapeutic partners included fluticasone propionate, fluticasone furoate, salmeterol+tiotropium, and ICS+long-acting β2-agonist (LABA).
Statistical analysis
To compare patients with COPD with eosinophil counts of <2% and ≥2% in terms of the risk of exacerbation, time-of-first exacerbation, and pneumonia events, the meta-analysis software, “Comprehensive Meta-Analysis” version 2.2.055 (Englewood, NJ, USA) was used. Heterogeneity across studies was assessed by the Cochran’s Q statistic test and the I2 test. I2 values ≥25%, 50%, and 75% were considered to reflect mild, moderate, and high degrees of heterogeneity, respectively. A random-effects model was used for pooled outcome measures with I2 >50%. The difference in moderate/severe exacerbation rate between both treatment arms was expressed as the risk ratio (RR). The time-to-first exacerbation was expressed as the hazard ratio (HR). The relative risk of pneumonia events was expressed as RR. Data are presented as 95% CIs.
Results
Search strategy and data extraction
From the search using the keywords “COPD”, “eosinophil”, and “clinical trial” from PubMed and EMBASE, a total of 57 primary articles that were potentially relevant were obtained to determine further eligibility. Of these, 52 articles did not fulfill our inclusion criteria and were excluded. Of the five remaining publicationsCitation27,Citation29–Citation32 published from 2015 to 2016, a total of 12,496 patients were included in this meta-analysis/systematic review. The characteristics of the eligible studies are summarized in . Overall, the total number of patients included in our study was 12,496. All participants were current or ex-smokers with a mean age of 64 years and were diagnosed with moderate-to-vey severe COPD. The asthma diagnosis was excluded in all trials. The proportion of patients with ≥2% blood eosinophils at baseline ranged from 32% to 75% in seven studies (mean 60%). In total, ICS-containing treatments included 854 patients treated with ICS monotherapy, 5,134 patients treated with ICS+LABA combination therapy; and 1,115 patients treated with ICS+LABA+long-acting muscarinic antagonist (LAMA) triple therapy. Non-ICS/ICS withdrawal/placebo included 4,269 patients treated with non-ICS regimens/placebo and 1,124 patients in withdrawal from ICS+LABA+LAMA triple therapy.
Table 1 Summary of five articles from PubMed and EMBASE that were determined eligible using the keywords “COPD”, “eosinophil”, and “clinical trial” based on systematic review criteria
Primary outcome: risk of moderate/severe exacerbation
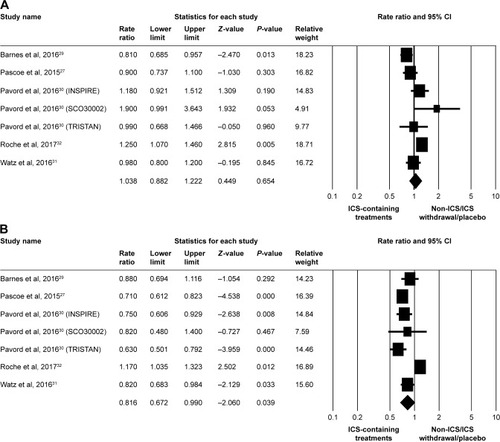
In the meta-analysis of primary outcomes, seven studiesCitation27,Citation29–Citation32 were pooled for moderate/severe exacerbation RR analysis. Overall, there was no significant difference between ICS-containing treatments and non-ICS/ICS withdrawal/placebo treatments at eosinophil counts of <2% (RR, 1.038; 95% CI, 0.882–1.222; P=0.654), as shown in . ICS-containing treatments in the subgroup with eosinophil counts of ≥2% had a significantly reduced RR of moderate/severe exacerbations than did non-ICS/ICS withdrawal/placebo treatment groups (RR, 0.816; 95% CI, 0.672–0.990; P=0.039), as shown in . The publication bias of pooled risk for moderate/severe exacerbations was minimized by selecting high-quality articles and well-compared systematic review items, whereby either eosinophil counts of <2% or ≥2% had a low risk difference ().
Figure 1 Forest plots of studies comparing the pooled risk ratio for moderate/severe exacerbation in patients with COPD receiving ICS-containing treatment or non-ICS/ICS withdrawal/placebo treatments by subgroup.
Note: (A) Eosinophil counts <2% and (B) eosinophil counts ≥2%.
Abbreviation: ICS, inhaled corticosteroid.
Secondary outcomes: risk of time-to-first moderate/severe exacerbation and pneumonia
ICS-continuing treatment in patients with COPD with baseline blood eosinophil levels ≥2% was associated with reduced risk of moderate/severe exacerbation. The secondary outcomes of the meta-analysis (pooled HRs for time-to-first moderate/severe exacerbation and pooled relative risk of pneumonia events) were significantly higher in the subgroup with eosinophil counts ≥2% with ICS-containing treatments.
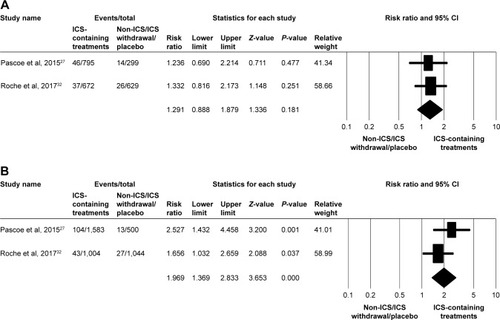
Two populations of five studiesCitation29,Citation32 were pooled to analyze the HR for time-to-first moderate/severe exacerbation between ICS-containing treatments and non-ICS/ICS withdrawal/placebo treatments in the subgroup with eosinophil counts <2%, but no significant association was observed (HR, 1.148; 95% CI, 0.743–1.775; P=0.534), as shown in . In the subgroup with eosinophil counts ≥2%, ICS-containing treatments showed a significantly higher association with moderate/severe exacerbation than did non-ICS/ICS withdrawal/placebo treatments (HR, 1.173; 95% CI, 1.043–1.319; P=0.008), as shown in . Although numerous trials have confirmed the efficacy of ICS for the treatment of COPD, ICS may also increase the risk of pneumonia in patients with COPD. Analysis of two populations of five studiesCitation27,Citation32 revealed that the risk of pneumonia events was significantly increased in the subgroup with eosinophil counts ≥2% with ICS-containing treatments (RR, 1.969; 95% CI, 1.369–2.833; P<0.001), as shown in . There was no significant difference in the subgroup with eosinophil counts <2% (RR, 1.291; 95% CI, 0.888–1.879; P=0.181), as shown in .
Figure 3 Forest plots of studies comparing the pooled hazard ratio for time-to-first moderate/severe exacerbation in patients with COPD receiving ICS-containing treatment or non-ICS/ICS withdrawal/placebo treatments by subgroup.
Note: (A) Eosinophil counts <2% and (B) eosinophil counts ≥2%.
Abbreviation: ICS, inhaled corticosteroid.
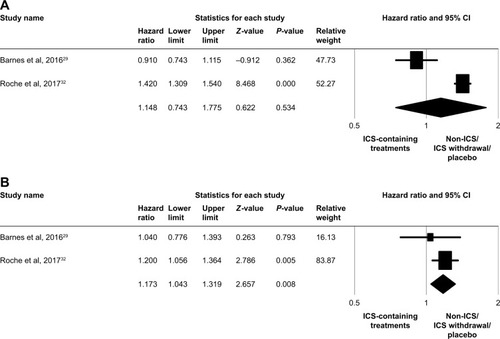
Figure 4 Forest plots of studies comparing the pooled relative risk of pneumonia events in patients with COPD receiving ICS-containing treatment or non-ICS/ICS withdrawal/placebo treatments by subgroup.
Abbreviation: ICS, inhaled corticosteroid.
Discussion
Summary of main results
We conducted a systematic review and meta-analysis to investigate the predictive role of baseline blood eosinophil counts as a biomarker of response to ICS in patients with moderate-to-very severe COPD. A total of five studies with 12,496 patients were used for analysis. The cutoff of 2% blood eosinophil counts was utilized to categorize patients in this study, and 60% of patients had ≥2% blood eosinophils at baseline. For patients with ≥2% blood eosinophils, the five studies provided inconsistent results when comparing the efficacy of ICS-containing regimens and non-ICS regimens/ICS withdrawal/placebo treatments at reducing exacerbation risk. In the meanwhile, we excluded the patients treated with other anti-inflammatory agents such as oral steroid or phosphodiesterase type 4 inhibitors at the same time. Our meta-analysis showed a significantly decreased acute exacerbation risk in favor of ICS-containing treatments vs non-ICS/ICS withdrawal/placebo treatments for reducing COPD exacerbations in patients with ≥2% blood eosinophils (RR, 0.81; 95% CI, 0.67–0.99; P=0.03).
The characteristics of COPD include chronic inflammation and structural changes in the respiratory tract, which are mediated by inflammatory cells and a complex cytokine network.Citation33,Citation34 Neutrophils are the predominant cell type involved in airway inflammation in patients with COPD and account for >70% of sputum cells.Citation35,Citation36 However, ~20%–40% of patients with stable COPD show elevated eosinophil levels (>3%) in sputum.Citation7 Furthermore, blood eosinophilia (≥2%) has been reported in 50%–70% of patients with COPD.Citation37 Eosinophils are multifunctional leukocytes characterized by the storage of cytotoxic basic proteins in secondary granules as well as the production of reactive oxygen species, lipid mediators, and cytokines.Citation38,Citation39 The release of cytotoxic basic proteins, reactive oxygen species, and lipid mediators is associated with damage to airway epithelial cells.Citation7,Citation39 During a COPD exacerbation, both absolute and relative eosinophil counts in sputum are significantly increased compared with those during the stable phase.Citation36 Therefore, eosinophils may contribute to increased airway inflammation during acute exacerbations of COPD.Citation36 Additionally, blood eosinophilia is associated with a higher risk of COPD exacerbation,Citation40,Citation41 which may lead to a decline in lung functionCitation7 and increased mortality.Citation42
The molecular mechanisms for the anti-inflammatory activity of corticosteroids include repression of activated inflammatory genes, activation of anti-inflammatory genes, and posttranscriptional effects.Citation43,Citation44 At the cellular level, corticosteroids can inhibit the survival of eosinophils in the airways.Citation43,Citation45 Leigh et al reported that 4-week treatment with inhaled budesonide (1,600 µg/day) normalized the sputum eosinophil counts in patients with COPD with sputum eosinophilia.Citation12 In patients without sputum eosinophilia, the sputum eosinophil counts stayed within the normal range after budesonide treatment.Citation12 Budesonide treatment did not significantly affect the sputum neutrophil counts of all enrolled patients.Citation12 Similar results were observed in patients with COPD treated with a combination therapy of inhaled fluticasone (200 µg/day) and salmeterol (100 µg/day) for 2 months.Citation46 In patients with sputum eosinophilia, the percentage of eosinophils in sputum significantly decreased from 8.9% to 1.6% (P=0.003) after treatment with inhaled fluticasone–salmeterol.Citation46 In contrast, sputum neutrophils remained unchanged after fluticasone–salmeterol combination therapy.Citation46 Based on currently available data, ICS can significantly suppress eosinophilic airway inflammation, but has less of an effect on neutrophilic airway inflammation, which predominates in COPD.Citation12,Citation46 There is some evidence suggesting that maintenance treatment with ICS is more effective in patients with COPD with higher eosinophil counts, with regards to reducing exacerbation frequency and improving lung function.Citation27–Citation31,Citation47,Citation48
Although asthma and COPD are two different chronic respiratory diseases, a significant proportion of patients aged >40 years present with overlapping conditions.Citation49 These patients are categorized as having asthma–COPD overlap syndrome (ACOS).Citation49 A joint project of the Global Initiative for Asthma and the Global initiative for chronic Obstructive Lung Disease (GOLD) provides the clinical description for ACOS:
ACOS is characterized by persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD. ACOS is therefore identified in clinical practice by the features that it shares with both asthma and COPD.Citation49
However, there is no universally accepted definition of ACOS at present. Previous large-scale studies have revealed that the prevalence rates of ACOS in patients with COPD are between 5.6% and 55%, depending on the diagnostic criteria applied.Citation50–Citation57 The mixed COPD–asthma phenotype is associated with more respiratory symptoms, increased risk of exacerbation and hospitalization, and worse health-related quality of life compared with the pure COPD phenotype.Citation53,Citation54,Citation56,Citation58,Citation59 Regarding eosinophils, patients with ACOS show significantly higher sputum and blood eosinophil counts than those with pure COPD.Citation60 A blood eosinophil count >5% was used as a minor criterion for defining ACOS in a cohort of Spanish patients with COPD.Citation51
Several studies suggested that ACOS patients were more likely to benefit from ICS treatment because of sputum eosinophilia.Citation52,Citation60,Citation61 Kitaguchi et al reported that patients with ACOS showed a significant increase in forced expiratory volume 1 (FEV1; 32.4%) than did those with pure COPD (12.8%) after being treated with inhaled fluticasone propionate (400 µg/day) for 2–3 months.Citation60 Moreover, a significant correlation was observed between the increase in FEV1 and sputum eosinophil counts.Citation60 In a study conducted by Lee et al, the improvement in FEV1 was significantly greater in patients with ACOS (240.2±33.5 mL) than that in patients with pure COPD (124.6±19.8 mL) after 3 months of treatment with ICS+LABA (salmeterol 50 µg/fluticasone 500 µg or formoterol 9 µg/budesonide 320 µg twice daily).Citation61 A Canadian longitudinal cohort study revealed that new use of ICS+LABA was associated with a modestly reduced risk of death or COPD hospitalization in patients with COPD compared with new use of LABA alone (HR, 0.92; 95% CI, 0.88–0.96).Citation52 A more pronounced reduction in risk was observed in the ACOS subgroup (HR, 0.84; 95% CI, 0.77–0.91).Citation52
Based on clinical evidence, the GOLD 2017 guidelines recommend ICS+LABA as the first-line treatment in patients with high blood eosinophil counts and/or ACOS.Citation62 In a joint project by Global Initiative for Asthma and GOLD, ICS was considered as the initial treatment for ACOS patients, and add-on treatment with LABA and/or LAMA is also usually necessary.Citation49
It is still debated whether high eosinophil levels in either sputum or blood are associated with a severe COPD allergic phenotype, including greater exacerbation frequency, and whether blood eosinophils are predictive of sputum eosinophils. Hastie et al reported that high concentrations of sputum eosinophils were a better biomarker than high concentrations of blood eosinophils to identify a patient subgroup with more severe disease, more frequent exacerbations, and increased emphysema by quantitative computer tomography.Citation63 Further studies should be performed to demonstrate the roles of sputum or blood eosinophil counts in COPD.
The most commonly used cutoff point for blood eosinophil counts in previous studies was 2%Citation27,Citation29–Citation32 because this level showed high sensitivity for identifying sputum eosinophilia of ≥3%.Citation10 However, our meta-analysis indicated that the 2% threshold may be too low to accurately identify the group of patients most likely to benefit from ICS treatment. In several studies, the choice of a cutoff point showing the best predictive ability of blood eosinophils was based on the analyses of treatment outcomes, such as COPD exacerbation rate, stratified by a number of possible blood eosinophil levels.Citation28,Citation31,Citation47,Citation48 In the NCT1009463 and NCT1017952 trials, there was a linear relationship between blood eosinophil count and the RR of annual exacerbation rate for fluticasone furoate–vilanterol vs vilanterol, and a blood eosinophil cutoff of 2.4% was used in the cluster analysis (.2.4% blood eosinophils: RR, 0.68; 95% CI, 0.58–0.79; blood eosinophil count ≤2.4%: RR, 0.94; 95% CI, 0.80–1.11).Citation47 Results of the WISDOM trial suggested that blood eosinophil counts of ≥4% or ≥300 cells/µL may identify patients with COPD requiring continuation treatment with ICS (triple therapy consisting of fluticasone propionate, salmeterol, and tiotropium).Citation31 According to the post hoc analysis of the FORWARD trial, the greatest differences in COPD exacerbation rate (reduction: 46%; P≤0.001) and improvements in FEV1 (difference: 0.102; P=0.001) and St George’s Respiratory Questionnaire for COPD score (difference: −5.9; P≤0.001) between dipropionate–formoterol fumarate and formoterol fumarate were observed in patents with blood eosinophil counts of ≥279.8 cells/µL.Citation28 A pooled analysis of the LANTERN and ILLUMINATE trials demonstrated that fluticasone–salmeterol was more effective than indacaterol–glycopyrronium at reducing exacerbation risk in patients with COPD with blood eosinophil counts >300 cells/µL (RR, 0.59).Citation48
The optimal cutoff points of blood eosinophil counts (2.4%–4% and 279.8–300 cells/µL) obtained from the studies mentioned aboveCitation28,Citation31,Citation47,Citation48 might not represent the true predictive ability because of the implicit multiple comparisons with different possible cutoff points. Further randomized studies with a biomarker-stratified design should be performed in order to find the most appropriate cutoff point for blood eosinophils.
Since COPD is a highly heterogeneous disease and several molecular mechanisms are associated with corticosteroid resistance,Citation43 the combination of blood eosinophil counts with other markers may achieve the best predictive accuracy in predicting treatment response to ICS.Citation17 Cigarette smoking is not only the primary cause of COPD but also associated with a poor response to ICS.Citation64 Various mechanisms may be involved in the development of resistance to ICS, in particular, HDAC2 dysfunction.Citation64–Citation66 Peroxynitrite produced by the reaction of nitric oxide and superoxide anion from cigarette smoke may cause reduced HDAC2 activity and expression, which decreases the efficacy of ICS.Citation65,Citation66
A cluster analysis of the patients who may benefit more from fluticasone furoate–vilanterol vs vilanterol identified three separate clusters in 3,255 patients with smoking-related COPD from the NCT1009463 and NCT1017952 trials.Citation47 Cluster 1 included patients with >2.4% blood eosinophils (n=1,777, 54.6%), cluster 2 included patients with ≤2.4% blood eosinophils and a smoking history of ≤46 pack-years (n=891, 27.4%), and cluster 3 included patients with ≤2.4% blood eosinophils and a smoking history of >46 pack-years (n=587, 18.0%). The RRs of annual exacerbation rates for fluticasone furoate–vilanterol vs vilanterol revealed that the greatest treatment effect of fluticasone furoate–vilanterol was found in cluster 1 (RR, 0.68; 95% CI, 0.58–0.79). Patients in cluster 2 may also benefit from fluticasone furoate–vilanterol treatment (RR, 0.78; 95% CI, 0.63–0.96); however, those in cluster 3 were considered to be non-responders to fluticasone furoate–vilanterol (RR, 1.22; 95% CI, 0.94–1.58). Blood eosinophil count was the most important factor affecting the treatment response to fluticasone furoate–vilanterol, followed by smoking history. The impact of smoking history on ICS treatment in patients with COPD also warrants further investigation.
Limitations of this study
Our meta-analysis was limited to only five studies on the post hoc analyses of eight randomized trials. None of these trials were originally designed to assess the usefulness of blood eosinophils as a biomarker for predicting the response to ICS in patients with COPD. Heterogeneity in study design and patient populations was observed across the eight trials. Randomized withdrawal study design was only utilized in the WISDOM trial.Citation31 The experimental and active comparator arms were somewhat heterogeneous among trials. The combination therapy of ICS+LABA or ICS+LABA+LAMA was studied in seven trials,Citation27,Citation30–Citation32 and the results suggest that the therapeutic response to ICS may be influenced by LABA or LABA+LAMA to some degree. The FLAME trial recruited patients with moderate-to-very severe airflow limitation.Citation32 Five trials involved patients with moderate-to-severe COPD;Citation27,Citation29,Citation30 however, two trials involved patients with more severe disease (severe-to-very severe COPD).Citation30,Citation31 Patients without a history of exacerbations could only be enrolled in the SCO30002 trial,Citation30 while patients who had never smoked were excluded from all trials.Citation27,Citation29,Citation31,Citation32,Citation67–Citation69 Therefore, our results may not be generalizable to real-world patients suitable for ICS therapy as recommended by the GOLD guidelines.Citation62 The withdrawal rates in the ICS-containing treatment arms and active comparator/placebo arms were 18.3%–34.5% and 16.6%–41.7%, respectively.Citation2,Citation32,Citation67–Citation71 The highest withdrawal rates (34.5% in the fluticasone propionate–salmeterol group and 41.7% in the tiotropium group) were observed in the INSPIRE trial, which included patients with severe and very severe COPD.Citation69 In the INSPIRE,Citation69 ISOLDE,Citation70 and TRISTAN 66 trials, significantly higher proportions of patients in the active comparator or placebo arms failed to complete the study than did those in the ICS-containing arms. High withdrawal rates could lead to bias in the estimates of treatment effect because patients who completed the study may be healthier than the population at study entry.
A comprehensive assessment of the relationship between blood eosinophil counts and the treatment effects of ICS regimens is necessary. However, the meta-analysis of other efficacy parameters, such as time to first exacerbation, FEV1, St George’s Respiratory Questionnaire for COPD total score, and mortality was not performed in this study because some of these data stratified by blood eosinophil counts were not presented in the five studies.
Conclusion
Our meta-analysis suggested a modest benefit from ICS-containing treatments vs non-ICS/ICS withdrawal/placebo treatments in reducing the annual rate of moderate/severe exacerbations in patients with COPD with ≥2% blood eosinophils at baseline. However, the incidence of pneumonia risk was also increased in patients with COPD with higher eosinophil counts who used ICS treatment. To be useful in clinical practice, further prospectively designed studies with prespecified criteria for blood eosinophil subgroups of patients with COPD are required to identify the best cutoff point for blood eosinophil count.
Disclosure
The author reports no conflicts of interest in this work.
References
- FergusonGTAnzuetoAFeiREmmettAKnobilKKalbergCEffect of fluticasone propionate/salmeterol (250/25 microg) or salmeterol (50 microg) on COPD exacerbationsRespir Med200810281099110818614347
- DransfieldMTBourbeauJJonesPWOnce-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trialsLancet Respir Med20131321022324429127
- SzafranskiWCukierARamirezAEfficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary diseaseEur Respir J2003211748112570112
- SharafkhanehASouthardJGGoldmanMUryniakTMartinUJEffect of budesonide/formoterol pMDI on COPD exacerbations: a double-blind, randomized studyRespir Med2012106225726822033040
- AgarwalRAggarwalANGuptaDJindalSKInhaled corticosteroids vs placebo for preventing COPD exacerbations: a systematic review and metaregression of randomized controlled trialsChest2010137231832519783669
- SuissaSNumber needed to treat in COPD: exacerbations versus pneumoniasThorax201368654054323125170
- SahaSBrightlingCEEosinophilic airway inflammation in COPDInt J Chron Obstruct Pulmon Dis200611394718046901
- BrightlingCEClinical applications of induced sputumChest200612951344134816685028
- SinghDKolsumUBrightlingCEEosinophilic inflammation in COPD: prevalence and clinical characteristicsEur Respir J20144461697170025323230
- BafadhelMMcKennaSTerrySAcute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkersAm J Respir Crit Care Med2011184666267121680942
- McDonaldVMHigginsIWoodLGGibsonPGMultidimensional assessment and tailored interventions for COPD: respiratory utopia or common sense?Thorax201368769169423503624
- LeighRPizzichiniMMMorrisMMMaltaisFHargreaveFEPizzichiniEStable COPD: predicting benefit from high-dose inhaled corticosteroid treatmentEur Respir J200627596497116446316
- BrightlingCEMonteiroWWardRSputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trialLancet200035692401480148511081531
- BrightlingCEMcKennaSHargadonBSputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary diseaseThorax200560319319815741434
- PizzichiniEPizzichiniMMGibsonPSputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitisAm J Respir Crit Care Med19981585 Pt 1151115179817701
- PavordIDBafadhelMExhaled nitric oxide and blood eosinophilia: independent markers of preventable riskJ Allergy Clin Immunol2013132482882924001802
- BainesKJPavordIDGibsonPGThe role of biomarkers in the management of airways diseaseInt J Tuberc Lung Dis201418111264126825299856
- PavordIDGibsonPGInflammometry: the current state of playThorax201267319119222344396
- YapEChuaWMJayaramLZengIVandalACGarrettJCan we predict sputum eosinophilia from clinical assessment in patients referred to an adult asthma clinic?Intern Med J2013431465221790924
- KorevaarDAWesterhofGAWangJDiagnostic accuracy of minimally invasive markers for detection of airway eosinophilia in asthma: a systematic review and meta-analysisLancet Respir Med20153429030025801413
- WesterhofGAKorevaarDAAmelinkMBiomarkers to identify sputum eosinophilia in different adult asthma phenotypesEur Respir J201546368869626113672
- ZhangXYSimpsonJLPowellHFull blood count parameters for the detection of asthma inflammatory phenotypesClin Exp Allergy20144491137114524849076
- FowlerSJTavernierGNivenRHigh blood eosinophil counts predict sputum eosinophilia in patients with severe asthmaJ Allergy Clin Immunol2015135382282425445828
- WagenerAHde NijsSBLutterRExternal validation of blood eosinophils in asthmaThorax201570211512025422384
- EltboliOMistryVBarkerBBrightlingCERelationship between blood and bronchial submucosal eosinophilia and reticular basement membrane thickening in chronic obstructive pulmonary diseaseRespirology201520466767025645275
- BafadhelMMcKennaSTerrySBlood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trialAm J Respir Crit Care Med20121861485522447964
- PascoeSLocantoreNDransfieldMTBarnesNCPavordIDBlood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trialsLancet Respir Med20153643544225878028
- SiddiquiSHGuasconiAVestboJBlood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2015192452352526051430
- BarnesNCSharmaRLettisSCalverleyPMBlood eosinophils as a marker of response to inhaled corticosteroids in COPDEur Respir J20164751374138226917606
- PavordIDLettisSLocantoreNBlood eosinophils and inhaled corticosteroid/long-acting β-2 agonist efficacy in COPDThorax201671211812526585525
- WatzHTetzlaffKWoutersEFBlood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trialLancet Respir Med20164539039827066739
- RocheNChapmanKRVogelmeierCFBlood eosinophils and response to maintenance chronic obstructive pulmonary disease treatment. Data from the FLAME trialAm J Respir Crit Care Med201719591189119728278391
- BarnesPJThe cytokine network in chronic obstructive pulmonary diseaseAm J Respir Cell Mol Biol200941663163819717810
- BarnesPJImmunology of asthma and chronic obstructive pulmonary diseaseNat Rev Immunol20088318319218274560
- BathoornELieskerJJPostmaDSChange in inflammation in out-patient COPD patients from stable phase to a subsequent exacerbationInt J Chron Obstruct Pulmon Dis2009410110919436694
- FujimotoKYasuoMUrushibataKHanaokaMKoizumiTKuboKAirway inflammation during stable and acutely exacerbated chronic obstructive pulmonary diseaseEur Respir J200525464064615802337
- IyerASDransfieldMTSerum eosinophils as a COPD biomarker: ready for prime time?Lancet Respir Med20164534134327066738
- GeorgeLBrightlingCEEosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary diseaseTher Adv Chronic Dis201671345126770668
- NixonJNewboldPMustelinTAndersonGPKolbeckRMonoclonal antibody therapy for the treatment of asthma and chronic obstructive pulmonary disease with eosinophilic inflammationPharmacol Ther2017169577727773786
- HasegawaKCamargoCAPrevalence of blood eosinophilia in hospitalized patients with acute exacerbation of COPDRespirology201621476176426699685
- PriceDRigazioAPostmaDBlood eosinophilia and the number of exacerbations in COPD patientsEur Respir J201444Suppl 584416
- HospersJJSchoutenJPWeissSTPostmaDSRijckenBEosinophilia is associated with increased all-cause mortality after a follow-up of 30 years in a general population sampleEpidemiology200011326126810784241
- BarnesPJGlucocorticosteroids: current and future directionsBr J Pharmacol20111631294321198556
- BarnesPJInhaled corticosteroidsPharmaceuticals20103351454027713266
- MeagherLCCousinJMSecklJRHaslettCOpposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytesJ Immunol199615611442244288666816
- PerngDWWuCCSuKCLeeYCPerngRPTaoCWInhaled fluticasone and salmeterol suppress eosinophilic airway inflammation in chronic obstructive pulmonary disease: relations with lung function and bronchodilator reversibilityLung2006184421722217006748
- HindsDRDisantostefanoRLLeHVPascoeSIdentification of responders to inhaled corticosteroids in a chronic obstructive pulmonary disease population using cluster analysisBMJ Open201666e010099
- WedzichaJAPriceDMezziKFogelRBanerjiDQVA149 compared with salmeterol/fluticasone (SFC) on exacerbations and its correlation with baseline blood eosinophils: a pooled analysis of LANTERN and ILLUMINATEEur Respir J201546Suppl 59PA1005
- Global Initiative for Asthma (GINA) and Global Initiative for Chronic Obstructive Lung Disease (GOLD) [webpage on the Internet]Diagnosis of diseases of chronic airway limitation: asthma, COD and asthma–COPD overlap syndrome (ACOS) [updated 2015] Available from: http://goldcopd.org/asthma-copd-asthma-copd-overlap-syndrome/Accessed April 27, 2017
- BaarnesCBKjeldgaardPNielsenMMiravitllesMUlrikCSIdentifying possible asthma–COPD overlap syndrome in patients with a new diagnosis of COPD in primary careNPJ Prim Care Respir Med2017271608428055002
- CosioBGSorianoJBLópez-CamposJLDefining the asthma–COPD overlap syndrome in a COPD cohortChest20161491455226291753
- GershonASCampitelliMACroxfordRCombination long-acting β-agonists and inhaled corticosteroids compared with long-acting β-agonists alone in older adults with chronic obstructive pulmonary diseaseJAMA2014312111114112125226477
- HardinMChoMMcDonaldMLThe clinical and genetic features of COPD–asthma overlap syndromeEur Respir J201444234135024876173
- HardinMSilvermanEKBarrRGThe clinical features of the overlap between COPD and asthmaRespir Res20111212721951550
- MarshSETraversJWeatherallMProportional classifications of COPD phenotypesThorax200863976176718728201
- MenezesAMBMontes de OcaMPérez-PadillaRIncreased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD–asthmaChest2014145229730424114498
- RheeCKYoonHKYooKHMedical utilization and cost in patients with overlap syndrome of chronic obstructive pulmonary disease and asthmaCOPD201411216317024111662
- KauppiPKupiainenHLindqvistAOverlap syndrome of asthma and COPD predicts low quality of lifeJ Asthma201148327928521323613
- MiravitllesMSorianoJBAncocheaJCharacterisation of the overlap COPD–asthma phenotype. Focus on physical activity and health statusRespir Med201310771053106023597591
- KitaguchiYKomatsuYFujimotoKHanaokaMKuboKSputum eosinophilia can predict responsiveness to inhaled corticosteroid treatment in patients with overlap syndrome of COPD and asthmaInt J Chron Obstruct Pulmon Dis2012728328922589579
- LeeSYParkHYKimEKCombination therapy of inhaled steroids and long-acting beta2-agonists in asthma–COPD overlap syndromeInt J Chron Obstruct Pulmon Dis2016112797280327877033
- VogelmeierCFCrinerGJMartinezFJGlobal strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summaryAm J Respir Crit Care Med2017195555758228128970
- HastieATMartinezFJCurtisJLAssociation of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohortLancet Respir Med201751295696729146301
- ItoKLimSCaramoriGChungKFBarnesPJAdcockIMCigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophagesFASEB J20011561110111211292684
- BarnesPJItoKAdcockIMCorticosteroid resistance in chronic obstructive pulmonary disease: inactivation of histone deacetylaseLancet2004363941073173315001333
- TamimiASerdarevicDHananiaNAThe effects of cigarette smoke on airway inflammation in asthma and COPD: therapeutic implicationsRespir Med2012106331932822196881
- CalverleyPPauwelsRVestboJFor TRial of Inhaled STeroids ANd long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trialLancet2003361935644945612583942
- GlaxoSmithKlineClinical study register. SFCT 01/SCO30002 Available from: http://www.gsk-clinicalstudyregister.com/files/pdf/23674.pdfAccessed April 6, 2017
- WedzichaJACalverleyPMSeemungalTAThe prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromideAm J Respir Crit Care Med20081771192617916806
- BurgePSCalverleyPMJonesPWSpencerSAndersonJAMaslenTKRandomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trialBMJ200032072451297130310807619
- MagnussenHDisseBRodriguez-RoisinRWithdrawal of inhaled glucocorticoids and exacerbations of COPDN Engl J Med2014371141285129425196117