Abstract
At present there is no cure for chronic obstructive pulmonary disease (COPD). However, some nonpharmacologic treatments, such as rehabilitation and lung volume reduction surgery, as well as pharmacologic intervention, can relieve some of the patient’s symptoms and improve quality of life, while also reducing the rate of exacerbations and hospitalizations. There needs to be a paradigm shift away from the unjustified nihilistic approach to COPD towards considering it a preventable and treatable disease. After patients quit smoking and start to lead healthier lifestyles, long-acting bronchodilators, such as long-acting beta-adrenergic agents (LABA) and long-acting antimuscarinic agents (LAMA), are recommended as the cornerstone of treatment for COPD, either as monotherapy or in combination. COPD is characterized by a reduced maximum expiratory flow and slow forced emptying of the lungs, which progress over time and are not completely reversible. In this condition, gas gets trapped in the lungs and pulmonary hyperinflation occurs. LABA and LAMA improve airway patency and deflate the lungs. Indacaterol is the first once-daily LABA approved for treatment of COPD, and is administered by inhalation through the Breezhaler® device. The speed of bronchodilation is similar to that with salbutamol (ie, about five minutes) and longer (ie, 24 hours) than that with traditional LABA, with the same 12-hour effect as salmeterol and formoterol, both of which require twice-daily administration. This is why indacaterol has been called the “ultra-LABA”. On the one hand, the fast onset of action provides immediate relief of symptoms, and on the other, its constant 24-hour bronchodilation provides “pharmacologic stenting” which facilitates lung emptying, thereby decreasing trapped gas and pulmonary hyperinflation. Once-daily administration of a fast and long-acting bronchodilator can improve patient adherence with therapy, which is known to be a major problem for many medical treatments. Dose-finding trials have shown that 75 μg is the minimum dose needed to achieve clinically important improvement. However, indacaterol 150 μg and 300 μg achieve an even greater improvement in lung function and patient-oriented outcomes. Further, these two doses of indacaterol significantly reduce pulmonary hyperinflation, thereby improving exercise tolerance and ability to perform day-to-day activities. It is more effective on lung volumes at the 300 μg dose than formoterol, and better than salmeterol and tiotropium at the 150 μg dose, at least in the acute setting. It is noteworthy that few studies document these results in patients with COPD and moderate airflow obstruction. These are exactly the kind of patients our research should be concentrating on, in view of the accelerated decay in forced expiratory volume in one second at this stage of the disease. Finally, all the relevant studies show that indacaterol is consistently well tolerated by patients with COPD at every stage, and that it has a high safety profile.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major medical problem as well as a burden on global public health services. It causes tremendous difficulties for individuals and societies due to it being the cause of severe lifelong disability and even premature death. COPD is one of the leading causes of morbidity and mortality worldwide.Citation1 Cigarette smoking is the principal cause of COPD, and quitting is the most effective measure to combat progression of the disease.Citation2,Citation3 At present there is no cure for COPD. However, some nonpharmacologic treatments, such as rehabilitationCitation4,Citation5 and lung volume reduction surgery,Citation6,Citation7 as well as pharmacologic treatment, can relieve symptoms and improve quality of life while also reducing the rate and severity of exacerbations as well as the number of hospital visits and admissions.Citation8,Citation9 In other words, COPD cannot be cured, but substantial benefits can be obtained for patients and society by abandoning the conventional approach,Citation10 and taking the view, once and for all, that COPD is a preventable and treatable disease.Citation11
COPD has been defined as a “disorder”,Citation12 a “disease state”,Citation13 or a “pathologic condition”Citation14 resulting from chronic bronchitis, pulmonary emphysema, and small airway disease.Citation15–Citation18 It has also been suggested that bronchial asthma might be considered among the phenotypes of COPD.Citation19 Asthma can, in fact, cause poorly reversible airflow obstruction.Citation20 However, we share the view that COPD and asthma are two different disease entities,Citation21 each with their own pathway of pharmacologic treatment. In asthma, inhaled corticosteroids (ICS) are the cornerstone of pharmacotherapy for the disease, whereas monotherapy with bronchodilators is strongly discouraged.Citation22 Long-acting beta-adrenergic agents (LABA) can be added to ICS to improve asthma control.Citation22 In contrast, long-acting bronchodilators, such as LABACitation23,Citation24 and/or long-acting antimuscarinic agents (LAMA),Citation25 are recommended as the foundation of treatment for COPD, either as monotherapy or used in combination.Citation26–Citation28 It has been suggested that ICS can be added to LABACitation29 and LAMACitation30 in patients with severe airflow obstruction (forced expiratory volume in one second [FEV1] <50% or <60% of predicted values), who remain symptomatic despite regular treatment with long-acting bronchodilators and have frequent exacerbations.Citation31,Citation32 FEV1 < 50% predicted is the threshold suggested by the 2011 Global Initiative for Chronic Obstructive Lung Disease document,Citation9 and FEV1 < 60% predicted is the threshold indicated by the European Medicines Agency.
Many clinical studies have demonstrated the efficacy of LABA and LAMA in reducing the symptoms of COPD and the incidence of exacerbations.Citation33 Understanding of the mechanisms via which long-acting bronchodilators provide benefits for patients with COPD requires a brief description of the underlying pathophysiology.
Pathophysiologic background
COPD is characterized by reduced maximum expiratory flow and slow forced emptying of the lungs, which progresses over time and is not completely reversible. These pathophysiologic abnormalities are determined by a varying combination of airway disease and lung parenchymal destruction. The latter causes not only a reduction in lung elastic recoil pressure, but also loss of alveolar attachments supporting the small airways.Citation34 Unsupported small airways are compressed during expiration by the positive intrathoracic pressure, such that expiratory flow limitation develops. Under these circumstances, gas gets trapped in the lungs and pulmonary hyperinflation occurs.Citation35
After the seminal work by Fletcher and Peto,Citation36 an accelerated decline in FEV1 has been universally accepted as the paradigm for the natural history of COPD. However, air trapping causes vital capacity and forced vital capacity to decrease, while residual volume increases due to both loss of elastic recoil and small airway obstruction/closure.Citation37 As the disease progresses, the residual volume expands, vital capacity falls, along with a fall in FEV1, and functional residual capacity and total lung capacity increase. Hence, a new paradigm should be adopted to understand the natural history of COPD, ie, modification of lung volumes with evolution of the disease. This would emphasize the decline in FEV1 secondary to the modification in lung volume determined by air trapping, as shown in . This concept is important because it brings the focus of therapy onto the escape of trapped gas rather than on the conventional issue of dilation of the large airways.
Figure 1 Schematic illustration showing that the natural history of chronic obstructive pulmonary disease is characterized by a progressive increase in gas trapping measured as a progressive increase in residual volume.
Abbreviations: FEV1, forced expiratory volume in one second; FRC, functional residual capacity; IC, inspiratory capacity; TLC, total lung capacity; VC, vital capacity; RV, residual volume; L, liters.
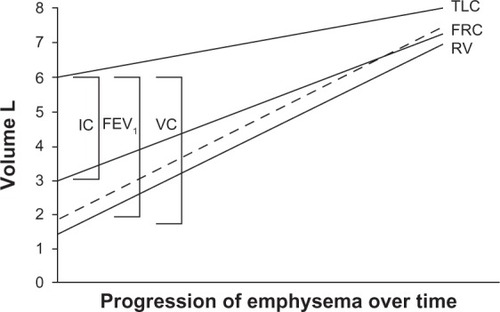
The rise in residual volume, functional residual capacity, and total lung capacity above predicted values is termed pulmonary hyperinflation. With the act of breathing, abnormal airway resistance and expiratory flow limitation may prevent full expiration, such that the end-expiratory lung volume is set above the relaxed functional residual capacity. This condition is defined as “dynamic pulmonary hyperinflation”. A slight degree of dynamic pulmonary hyperinflation is present even at rest in patients with severe COPD.Citation38,Citation39 Dynamic pulmonary hyperinflation magnifies during acute exacerbationCitation40,Citation41 and exerciseCitation42 even in mild COPD.Citation43 Dynamic pulmonary hyperinflation increases the elastic work of breathing while decreasing the pressure-generating capacity of the respiratory muscles due to its unfavorable geometric arrangement. This combination of events causes poor exercise tolerance and dyspnea,Citation44 as well as cardiovascular complications.Citation45
A simple measurement of dynamic hyperinflation is the change in inspiratory capacity, which mirrors the concomitant variation in functional residual capacity. Both resting inspiratory capacity and changes in inspiratory capacity are better correlated with dyspnea than FEV1.Citation46
By relaxing smooth muscle in the airways, LABA and LAMA improve the patency of both the large and small airways, thereby decreasing air trapping.Citation47,Citation48 With sustained bronchodilation, less dynamic hyperinflation occurs during exercise or exacerbations, and breathing becomes less uncomfortable because of ventilation at a lower lung volume. It has been suggested that these complex effects of bronchodilation on pulmonary mechanics and the dynamics of breathing might be the major mechanism via which long-acting bronchodilators decrease the frequency and severity of exacerbations.Citation49
Indacaterol: an “ultra-LABA”
Indacaterol is the first once-daily LABACitation50 approved for treatment of COPD, and is administered by inhalation through the Breezhaler device (Novartis Pharma AG, Basel, Switzerland). This paper reviews and discusses some of the recent data on the effects of indacaterol in patients with COPD.
Extensive preclinical studies on indacaterol have documented its rapid onset and long duration of action due to its biochemical structure.Citation51–Citation53 The speed of bronchodilation is similar to that of salbutamol (ie, both of five minutes) and longer than that of traditional LABA (ie, 24 hours) such as salmeterol and formoterol, which require twice-daily administration. This is why indacaterol has been called an “ultra-LABA”.Citation54 This rapid onset of bronchodilation is not affected by time of administration, ie, in the morning or in the evening.Citation55 However, due to the circadian rhythm of bronchomotor tone, with a peak of airflow resistance in the morning, administration in the morning might be preferable for rapid relief of the patient’s symptoms.Citation56
The duration of bronchodilation is also important. On the one hand, it has been suggested that once-daily administration might improve patient adherence with treatment,Citation57 and on the other, persistent 24-hour airway patency provides a type of “pharmacologic stenting” which facilitates lung emptying and thereby decreases trapped gas and pulmonary hyperinflation.Citation58 As mentioned earlier, this action should be regarded as key to the effectiveness of bronchodilating therapy in COPD.
Dose-finding studies are an essential step in drug development. Studies using different doses have been performed with indacaterol.Citation59–Citation61 Indacaterol 150 μg was found to be the lowest effective dose for global targeted improvement in FEV1, but with the 300 μg dose being selected for the second step. No safety signal was observed with any dose of indacaterol.Citation33,Citation62 Some recent studies have focused on indacaterol 75 μg and documented that it improves lung function and symptoms, compared with placebo, in patients with COPD and moderate-to-severe airflow obstruction, suggesting that it can be used as a regular therapy for COPD.Citation63,Citation64 This dose has been selected in the US,Citation65 whereas the 150 μg and 300 μg doses are marketed in other countries.
Analysis of the relationship between improvement in lung function, most commonly FEV1, and patient-related outcomes, such as dyspnea and quality of life, is important. Indeed, it is a common belief that spirometry may not fully capture the impact of COPD on patients’ health status. However, in a pooled analysis of three clinical studies on indacaterol, involving more than 3000 patients with COPD and moderate-to-severe airflow obstruction, Jones et alCitation66 showed significant improvements in patient-related outcomes such as dyspnea (assessed by the transitional dyspnea index)Citation67 and quality of life (assessed by the Saint George Respiratory Questionnaire),Citation68 which were associated with greater improvements in FEV1.Citation66 These authors concluded that interventions which significantly improve FEV1 are also likely to produce better clinical and patient-related outcomes. A recently reported study investigated the effectiveness of indacaterol in patients with COPD on regular treatment with ICS as well as in ICS-naive patients.Citation69 Decramer et al report that the positive action of indacaterol on lung function and symptoms was not affected by ICS treatment.Citation69 They also found that, whereas indacaterol 150 μg was effective in patients with COPD and moderate airflow obstruction, higher doses of 300 μg might be needed in patients with more severe functional impairment. However, a recent study of the acute effects of indacaterol by Cazzola et al concluded that a subgroup of patients with COPD can gain some benefits in lung function from an increase of the dose from 150 μg to 300 μg, although that improvement may be associated with a mild transient decrease in pulse-oximetry.Citation70
Safety profile
The safety message by Chapman et alCitation33,Citation62 was reinforced by analysis of a data base containing data on more than 4000 patients with COPD and moderate-to-severe airflow obstruction, enrolled in studies lasting more than six months, by Worth et al.Citation71 The overall cerebrocardiovascular profile of indacaterol was similar to that of placebo and comparable with that of other long-acting bronchodilators. Further, Donohue et al pooled data from 11 clinical studies investigating indacaterol 75 μg, 150 μg, 300 μg, and 600 μg, formoterol 12 μg twice daily, salmeterol 50 μg twice daily, tiotropium 18 μg, and placebo.Citation72 Overall, almost 10,000 data/patients were analyzed to investigate whether any dose of indacaterol might be associated with adverse events compared to placebo; in particular, serious adverse events, such as plasma potassium, blood glucose, QTc intervals and vital signs. The risk of acute respiratory adverse events was not significantly increased with any of the active treatments compared with placebo. However, some patients reported mild cough, but with attenuation over time. This can occur within seconds of inhalation and with rapid resolution, and has not been associated with bronchospasm.Citation33 In summary, indacaterol has a good safety and tolerability profile, and is appropriate for maintenance treatment of patients with COPD and moderate-to-severe airflow obstruction. This may be relevant in particular for elderly patients with COPD and multiple comorbidity.
Efficacy versus established therapies
A recent review addressed the comparative efficacy of indacaterol in COPD.Citation33 In particular, indacaterol at different doses was compared with formoterolCitation73–Citation75 and salmeterol,Citation76,Citation77 which are well established LABA used as regular pharmacotherapy for patients with COPD. Indacaterol 150 μg and 300 μg achieved greater bronchodilation and better patient-related outcomes than formoterol 12 μg or salmeterol 50 μg twice daily in patients with moderate-to-severe airflow obstruction.
Compared with tiotropium, the published data indicated that indacaterol was at least as effective and safe as tiotropium in an “open-label” six-month study.Citation78 Hence, two effective and safe long-acting bronchodilators provide patients and physicians with more flexibility for treating patients with COPD.Citation78 However, to circumvent the limitations related to the open-label design, Vogelmeier et al performed a blinded noninferiority comparison of indacaterol 150 μg and 300 μg, tiotropium 18 μg, and placebo. Both indacaterol doses had a faster onset of action on day 1, providing clinically relevant treatment-placebo differences of 120–130 mL in FEV1 five minutes post-dosing.Citation79 At this time point, treatment with both indacaterol doses resulted in a statistically superior FEV1 (about 80 mL) compared with tiotropium. In a blinded parallel-group comparison by Buhl et al, indacaterol 150 μg and tiotropium 18 μg had similar effects on trough FEV1.Citation80 However, patients treated with indacaterol showed greater improvements in terms of dyspnea and quality of life. In this regard, it is worth mentioning the study by Chapman et al, which reported that patients with COPD may show a preference for one inhaler (Breezhaler) over another (HandiHaler®, Boeheringer-Ingelheim, Ingelheim, Germany).Citation81 This may be an important factor for optimum dose delivery and successful management of COPD.
Regarding concerns about the scientific credibility of open-label protocols versus full blinded designs, Beeh et al, on analyzing the published studies, reported that there was no difference between the two types of design in terms of objective measurements such as lung function, whereas some slight bias might be introduced for subjective measurements, such as dyspnea and quality of life.Citation82 The authors recommend transparency concerning study design and consideration of any potential source of bias, suggesting that, under such circumstances, data from open-label studies can provide valuable and credible evidence of the effects of therapy.
Rodrigo and Neffen undertook a systematic comparison between indacaterol, tiotropium, and twice-daily LABA from five trials representing almost 6000 patients.Citation83 This systematic review suggests that patients with COPD and moderate-to-severe airflow obstruction treated with indacaterol had better clinical outcomes with regard to dyspnea and health status than those treated with tiotropium or twice-daily LABA. Cope et al investigated the efficacy of indacaterol relative to alternative bronchodilators by means of a patient-level mixed-treatment comparison, involving a combination of four randomized controlled trials.Citation84 They found that indacaterol 150 μg provided better FEV1 than formoterol or salmeterol, and a greater improvement in quality of life than tiotropium, while the 300 μg dose demonstrated the greatest response overall. A multiple comparison study investigated indacaterol 75 μg, tiotropium 18 μg, salmeterol 50 μg twice daily, and formoterol 12 μg twice daily at 12 weeks in a network meta-analysis from 21 clinical trials.Citation85 They concluded that indacaterol 75 μg provided levels of improvement in health-related quality of life and lung function comparable with those of tiotropium, salmeterol, and formoterol. However, although 75 μg can be accepted as the minimum effective dose of indacaterol, it should be noted that the 150 μg and 300 μg doses provide better bronchodilation without significant side effects. In fact, these two doses are marketed in almost all countries, while the US Food and Drug Administration allows only the minimum 75 μg dose.
Indacaterol versus LABA-ICS combinations
A number of studies have addressed the issue of indacaterol versus fixed-dose combination of salmeterol-fluticasone. Balint et al compared the onset of action of indacaterol 150 μg and 300 μg with that of salbutamol 200 μg and salmeterol-fluticasone 50/500 μg in a randomized, double-blind, crossover trial using FEV1 at five minutes post-dosing as the primary variable.Citation86 Indacaterol was faster-acting than salmeterol-fluticasone, and as fast as salbutamol. Two other studies investigating indacaterol versus a LABA-ICS combination were performed as a network meta-analysis. Cope et al compared indacaterol 150 μg and 300 μg with two doses of formoterol-budesonide (9/320 μg and 9/160 μg) and salmeterol-fluticasone (50/250 μg and 50/500 μg).Citation87 They found that indacaterol monotherapy at both doses was as good as both LABA-ICS combinations in terms of lung function, health status, and breathlessness. A similar study was performed with indacaterol 75 μg, which was shown to be an effective monotherapy in terms of lung function, although the study was inconclusive in terms of breathlessness and health status.Citation88 At present, there are still no appropriately designed prospective studies comparing indacaterol directly with LABA-ICS combinations. This is an important issue, given that some publications have shown that the LABA-ICS combination is widely used in patients with COPD and moderate airflow obstruction,Citation32,Citation89 despite no guidelines recommending treatment with ICS in the absence of frequent exacerbations. In this regard, it should be remembered that, in the COPD population with moderate airflow obstruction, the prevalence of “frequent exacerbations” averages about 22%, ie, occurs in a minority of the overall “moderate” patient population.Citation32
Maintenance-naive patients with moderate COPD
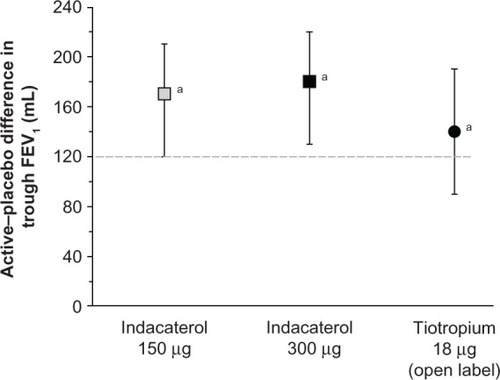
Decramer et al have addressed the issue of regular pharmacotherapy using indacaterol 150 μg and 300 μg in patients with COPD not receiving other maintenance treatment.Citation90 The data were pooled from three randomized, placebo-controlled studies, and the results from an open-label tiotropium treatment arm in one study were also available for comparison. Both doses of indacaterol led to clinically relevant and statistically significant improvements over placebo at week 26. Tiotropium also improved trough FEV1, and there was no statistically significant difference between the treatments, as shown in . However, indacaterol was slightly better than tiotropium in improving patient-related outcomes, such as dyspnea, bad days, and quality of life. All treatment regimens reduced the exacerbation rate compared with placebo, namely by about 0.70 in the treated groups versus 0.91 in the placebo group, with no difference between treatments. The overall incidence of adverse events was not significantly different between the groups, and none of these events were serious. A potential limitation of this study is the fact that the subgroup analyses were not preplanned and no statistical power calculation was done. In addition, the use of open-label tiotropium may have introduced bias. However, its value relies on the investigation of patients with COPD naive to regular pharmacotherapy.
Figure 2 Effect of active treatments (differences compared with placebo) on trough FEV1 at week 26.
Abbreviation: FEV1, forced expiratory volume in one second.
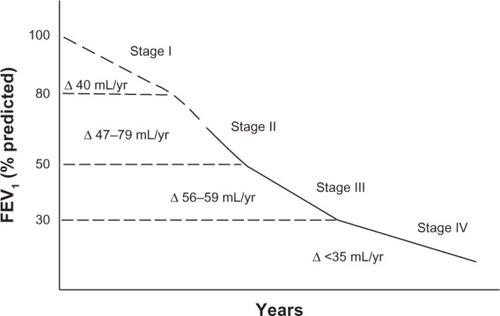
The effect of tiotropium in patients naive to maintenance therapy was reported by Troosters et al in a subgroup of patients from the UPLIFT population.Citation91–Citation93 They found that regular treatment with tiotropium was associated with significant benefits and slower disease progression in patients with COPD who were not on maintenance therapy. The annual rate of decline of FEV1 was about 10 mL less, on average, in the tiotropium group compared with the placebo arm.Citation91 This was not the case in the whole UPLIFT population, where a difference amounting to an average of 7 mL was observed in patients with moderate obstruction in the active arm compared with conventional treatment.Citation92,Citation93 Data from recent clinical investigations indicate that clinicians continue to be confronted with patients newly detected as having COPD.Citation94,Citation95 In the work by Decramer et al, the majority of patients naive to treatment (62%–70%) had mild-to-moderate airflow obstruction.Citation90 Therefore, research on the impact of pharmacotherapy in patients naive to maintenance treatment is important. In particular, greater attention should be paid to patients with COPD and moderate airflow obstruction. Their symptoms might well be underestimated due both to a lower impact on daily activity and to the fact that the exacerbation rate is lower than in more severe COPD.Citation32 Further, a recent study by Tantucci and Modina reviewed spirometric data for patients with COPD recruited in the placebo arms of 14 recent clinical trials, and analyzed the decline in FEV1 according to separation in the classic four-stage Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification.Citation96,Citation97 They found that the loss of lung function, assessed as reduction in expiratory airflow, was more rapid in the early stages of COPD than in the later stages, as shown in .
Figure 3 Range of average rate of decline of FEV1 in patients with chronic obstructive pulmonary disease according to severity of airflow obstruction.
Abbreviation: FEV1, forced expiratory volume in one second.
In conclusion, the studies by Decramer et al and Troosters et al show that long-acting bronchodilators, such as indacaterol at 150 μg and 300 μg and tiotropium 18 μg, are effective in gaining benefits for patients with COPD naive to other maintenance pharmacologic therapies.Citation90,Citation91 On the other hand, long-acting bronchodilation seems to slow the FEV1 decline in patients with moderate COPD, where the rate of loss of lung function is accelerated.Citation93,Citation96 These conclusions are meaningful in terms of planning studies specifically designed to address the effects of long-acting bronchodilators in treatment-naive patients in the early stages of the disease, for whom regular pharmacotherapy with long acting bronchodilators might reduce the faster progression of the disease.Citation3
Combination of indacaterol and tiotropium
It has been well known since the 1980s that a combination of bronchodilators can be more effective than any single agent used alone. In the COMBIVENT study, a short-term adrenergic agonist (albuterol) was combined with a short-term anticholinergic agent (ipratropium) to obtain greater bronchodilation than possible with either drug used alone.Citation98 LABA and LAMA are also powerful bronchodilators with different mechanisms of action. They can be associated either by separate administration, as was done for indacaterol with tiotropium, or in a single device, as was done for indacaterol with glycopyrronium.Citation27,Citation28 The latter is a new LAMA with a fast onset similar to indacaterol, and a long duration of action similar to that of tiotropium.Citation99 Van Noord et al have shown that combination of indacaterol and glycopyrronium in the same device allows rapid and sustained bronchodilation with significant improvements when compared with indacaterol 150 μg and 300 μg in patients with COPD.Citation28 No significant cardiovascular adverse event was observed.Citation100 Recently, Mahler et al reported on data from two studies comparing indacaterol 150 μg and placebo in patients already taking tiotropium 18 μg.Citation27 They found greater bronchodilation and lung deflation (increase in inspiratory capacity) with indacaterol and tiotropium compared with tiotropium monotherapy. Adverse effects were similar in the two groups and mild. Therefore, combination of long-acting bronchodilators with different mechanisms of action may be useful in the management of patients with COPD for whom a greater degree of bronchodilation and lung deflation is needed. In this regard, a recent clinical trial reported by Vogelmeier et al compared a once-daily indacaterol-glycopyrronium combination with a twice-daily salmeterol-fluticasone combination.Citation101 They found better data in terms of bronchodilation and patient-related outcomes with the former combination than with the latter in patients with COPD and moderate-to-severe airflow obstruction but without exacerbations in the previous year.
Indacaterol, hyperinflation, and exercise tolerance
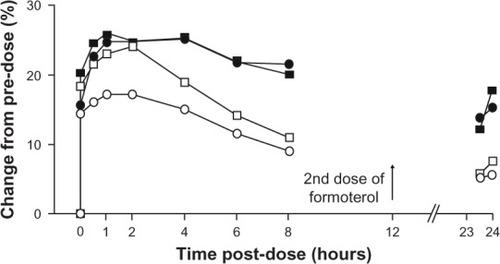
It is known that pulmonary hyperinflation causes poor exercise tolerance in patients with COPD, as discussed earlier. It has been shown that bronchodilators, both short-actingCitation47,Citation48,Citation102 and long-acting,Citation103,Citation104 can improve exercise tolerance by decreasing lung hyperinflation. Some recent studies have found that indacaterol is also effective at doing this. Beier et al reported a greater increase in inspiratory capacity after indacaterol 300 μg than after formoterol twice daily, although the effect on FEV1 was essentially equivalent, as shown in .Citation74
Figure 4 Comparison of effects of indacaterol and formoterol on FEV1 and inspiratory capacity as percent change in unadjusted mean values from predose.
Abbreviation: FEV1, forced expiratory volume in one second.
Rossi et al noted a significant improvement in peak inspiratory capacity in a crossover protocol comparing the acute effect of indacaterol 150 μg versus open-label tiotropium 18 μg and placebo in patients with COPD and moderate airflow obstruction.Citation105 Spirometry and lung volumes were both measured. The data show that indacaterol 150 μg has a significant bronchodilating action, similar to the effect of the recommended dose of tiotropium on spirometric variables (FEV1 and forced vital capacity), but slightly superior at improving inspiratory capacity and reducing lung hyperinflation, as seen in .
Figure 5 Changes in FRC, TLC, and RV at hour 4 after administration.
Abbreviations: FRC, functional residual capacity; TLC, total lung capacity; RV, residual volume.
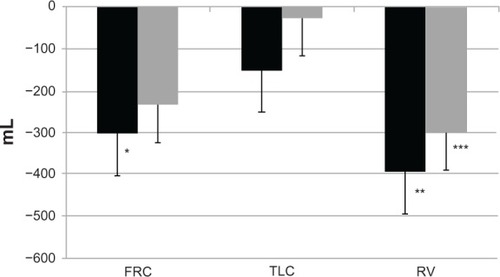
Hence, these data and those of Beier et al show that indacaterol, at two different doses, is very effective at reducing lung hyperinflation, which remains a key target in the management of patients with COPD and moderate to severe airflow obstruction.Citation74 Beeh et al investigated the effect of indacaterol 300 μg on peak inspiratory capacity in patients with COPD and a significant degree of resting pulmonary hyperinflation (functional residual capacity > 120% predicted) during exercise in a crossover study with evaluation after two weeks of treatment.Citation106 They found that peak inspiratory capacity was significantly greater after indacaterol than after placebo. Resting inspiratory capacity, trough FEV1, dyspnea indices, and endurance time were also improved by indacaterol 300 μg. Similar results were obtained by O’Donnell et al, who studied the impact of the lower dose of indacaterol, ie, 150 μg, on exercise tolerance in patients with COPD and moderate to severe airflow obstruction.Citation107 They found that indacaterol 150 μg was effective in improving endurance time from the first day of treatment onwards. Taken together, the data from these studies show that indacaterol, at both doses, ie, 300 μg and 150 μg, is successful in reducing pulmonary hyperinflation, which in turn decreases dyspnea and increases exercise tolerance.Citation74,Citation105–Citation107 In this regard, the studies by Ofir et al and O’Donnell et al in patients with COPD and mild airflow obstruction should also be borne in mind.Citation43,Citation102
One could speculate that greater exercise tolerance would encourage patients with COPD to pursue a more active lifestyle with significant implications for their quality of life and perhaps even survival. In fact, a recent study by Hataji et al documents that indacaterol can improve daily activity in patients with COPD.Citation108 It is noteworthy that such a great body of literature suggests that daily exercise, even mild and pleasant, can be a powerful tool to reducing mortality from all causes.Citation109
Conclusion
COPD is no longer considered an irreversible disease and can now be treated effectively.Citation110 Long-acting bronchodilators are not simply symptomatic drugs. Their complex effects on the underlying pathophysiology of COPD and benefits in terms of patient-related outcomes could encourage a paradigm shift from symptomatic drugs to “disease modifiers”. Indacaterol is a new long-acting bronchodilator approved as regular therapy for patients with COPD and moderate to severe airflow obstruction. The available literature shows that significant bronchodilation can be obtained following inhalation of indacaterol from the Breezhaler device at the three marketed doses, ie, 75 μg in the US, and 150 μg and 300 μg in other countries worldwide.
Indacaterol has a rapid onset of action, as fast as that of salbutamol and formoterol, and faster than salmeterol, tiotropium, and the fluticasone-salmeterol combination. Indacaterol also has a longer-lasting effect, ie, 24 hours, which is longer than for formoterol and salmeterol and similar to that of tiotropium. At present, indacaterol is the only bronchodilator available for COPD therapy which combines rapid onset with a 24-hour effect. Hence its definition as an “ultra-LABA”.
The data show that the significant bronchodilation achieved by indacaterol translates into perceivable benefits for the patient, including relief of symptoms, better quality of life, and reduction of exacerbations. Indacaterol decreases pulmonary hyperinflation, thereby improving exercise tolerance and everyday activities. Its key effect on lung volumes appears superior to that of formoterol for the 300 μg dose, and better than salmeterol and tiotropium for the 150 μg dose, at least in acute settings. It is noteworthy that some studies document these results in patients with COPD and moderate airflow obstruction. These are exactly the type of patients our research should be concentrating on, in view of the accelerated decrease in FEV1 seen at this stage of the disease. Further, all the studies have shown that indacaterol is consistently well tolerated by patients with COPD at every stage and that it has a good safety profile.
Therefore, we can conclude that the “ultra-LABA” indacaterol is effective and safe in the treatment of COPD and it will eventually replace the traditional LABA. Just as importantly, indacaterol can be successfully and safely combined with a LAMA, to maximize bronchodilation and deflate the lungs efficiently and continuously in patients with stable COPD.
Acknowledgments
The authors thank Chris Botterill for copy editing the manuscript and Pamela Micheletti for technical and editing assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
- HananiaNAMarciniukDDA unified front against COPD: clinical practice guidelines from the American College of Physicians, the American College of Chest Physicians, the American Thoracic Society, and the European SocietyChest2011140356556621896511
- AnthonisenNRConnettJEMurrayRPSmoking and lung function of Lung Health Study participants after 11 yearsAm J Respir Crit Care Med2002166567567912204864
- MaltaisFDennisNChanCKRationale for earlier treatment in COPD: a systematic review of published literature in mild-to-moderate COPDCOPD20131017910323272663
- LacasseYGoldsteinRLassersonTJMartinSPulmonary rehabilitation for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20064CD00379317054186
- EganCDeeringBMBlakeCShort term and long term effects of pulmonary rehabilitation on physical activity in COPDRespir Med2012106121671167923063203
- National Emphysema Treatment Trial Research GroupPatients at high risk of death after lung-volume-reduction surgeryN Engl J Med2001345151075108311596586
- SciurbaFCErnstAHerthFJA randomized study of endobronchial valves for advanced emphysemaN Engl J Med2010363131233124420860505
- National Clinical Guideline CentreChronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary careLondon, UKNational Clinical Guideline Centre Available from: http://guidance.nice.org.uk/CG101/Guidance/pdf/EnglishAccessed April 12, 2013
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease Revised 2011. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011_Feb21.pdfAccessed April 12, 2013
- Van der PalenJMonninkhofEvan der ValkPVisserAManaging COPD: no more nihilism!Patient Educ Couns200452322122314998589
- CelliBRMacNeeWATS/ERS Task ForceStandards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paperEur Respir J200423693294615219010
- SiafakasNMVermeirePPrideNBOptimal assessment and management of chronic obstructive pulmonary disease (COPD)Eur Respir J199588139814207489808
- ATS StatementStandards for the diagnosis and care of patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20051525S78S121
- StämpfliMRAndersonGPHow cigarette smoke skews immune responses to promote infection, lung disease and cancerNat Rev Immunol20099537738419330016
- HoggJCMacklemPTThurlbeckWMSite and nature of airway obstruction in chronic obstructive lung diseaseN Engl J Med196827825135513605650164
- CosioMGhezzoHHoggJCThe relations between structural changes in small airways and pulmonary-function testsN Engl J Med19782982312771281651978
- HoggJCChuFUtokaparchSThe nature of small-airway obstruction in chronic obstructive pulmonary diseaseN Engl J Med2004350262645265315215480
- McDonoughJEYuanRSuzukiMSmall-airway obstruction and emphysema in chronic obstructive pulmonary diseaseN Engl J Med2011365171567157522029978
- KraftMAsthma and chronic obstructive pulmonary disease exhibit common origins in any country!Am J Respir Crit Care Med2006174323824416864716
- FabbriLMRomagnoliMCorbettaLDifferences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2003167341842412426229
- BarnesPJAgainst the Dutch hypothesis: asthma and chronic obstructive pulmonary disease are distinct diseasesAm J Respir Crit Care Med2006174324024316864717
- Pocket Guide for Asthma Management and PreventionA pocket guide for physician and nurses Updated 2011Global Initiative for Asthma2011 Available from: http://www.ginasthma.org/Accessed April 12, 2013
- RossiAKhiraniSCazzolaMLong-acting β2-agonists (LABA) in chronic obstructive pulmonary disease: efficacy and safetyInt J Chron Obstruct Pulmon Dis2008341918488425
- TashkinDPFabbriLMLong-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agentsRespir Res201011114921034447
- RodrigoGJNanniniLJTiotropium for the treatment of stable chronic obstructive pulmonary disease: a systematic review with meta-analysisPulm Pharmacol Ther200720549550216621638
- SethiSCoteCBronchodilator combination therapy for the treatment of chronic obstructive pulmonary diseaseCurr Clin Pharmacol201161486121235463
- MahlerDAD’UrzoABatemanEDConcurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomized, double-blind comparisonThorax201267978178822544891
- van NoordJABuhlRLaForceCQVA149 demonstrates superior bronchodilation compared with indacaterol or placebo in patients with chronic obstructive pulmonary diseaseThorax201065121086109120978028
- CalverleyPMAndersonJACelliBTORCH InvestigatorsSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med2007356877578917314337
- AaronSDVandemheenKLFergussonDTiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease. A randomized trialAnn Intern Med2007146854555517310045
- QaseemAWiltTJWeibergerSEDiagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the ACP, ACCP, ATS and ERSAnn Intern Med2011155317919121810710
- HurstJRVestboJAnzuetoAEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Susceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- RibeiroMChapmanKRComparative efficacy of indacaterol in chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis20127314515222419862
- SaettaMGhezzoHKimWDLoss of alveolar attachments in smokers. A morphometric correlate of lung function impairmentAm Rev Respir Dis198513248949004051324
- CooperCBThe connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and functionAm J Med200611910S1213117113394
- FletcherCPetoRTinkerCThe Natural History of Chronic Bronchitis and Emphysema An Eight-Year Study of Early Chronic Obstructive Lung Disease in Working Men in LondonOxford, UKOxford University Press1976
- MacklemPTTherapeutic implications of the pathophysiology of COPDEur Respir J201035367668020190332
- Dal VecchioLPoleseGPoggiRRossiA“Intrinsic” positive end-expiratory pressure in stable patients with chronic obstructive pulmonary diseaseEur Respir J19903174802178961
- KhiraniSPoleseGAlivertiAOn-line monitoring of lung mechanics during spontaneous breathing: a physiologic studyRespir Med2010104346347120096552
- O’ DonnellDEParkerCMCOPD exacerbations – 3: pathophysiologyThorax200661435436116565268
- RossiAKhiraniSPEEPi and the air-bag effectRespiration200977325625819346761
- O’DonnellDERevillSMWebbKADynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2001164577077711549531
- OfirDLavenezianaPWebbKALamYMO’DonnellDEMechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2008177662262918006885
- CalverleyPMKoulourisNGFlow limitation and dynamic hyperinflation: key concepts in modern respiratory physiologyEur Respir J200525118619915640341
- TzaniPAielloMEliaDDynamic hyperinflation is associated with a poor cardiovascular response to exercise in COPD patientsRespir Res20111215022074289
- DiazOVillafrancaCGhezzoHRole of inspiratory capacity on exercise tolerance in COPD patients with and without tidal expiratory flow limitation at restEur Respir J200016226927510968502
- TantucciCDuguetASimilowskiTZelterMDerenneJPMilic-EmiliJEffect of salbutamol on dynamic hyperinflation in chronic obstructive pulmonary diseaseEur Respir J19981247998049817148
- DellacàRLPompilioPPWalkerPPDuffyNPedottiACalverleyPMEffect of bronchodilation on expiratory flow limitation and resting lung mechanics in COPDEur Respir J20093361329133719164347
- WedzichaJADecramerMSeemugalTAThe role of bronchodilator treatment in the prevention of exacerbations of COPDEur Respir J20124061545155422835613
- SlatonRMCruthirdsDLIndacaterol (Arcapta Neohaler) for chronic obstructive pulmonary diseaseP T2012372869822605898
- CazzolaMPageCPCalzettaLMateraMGPharmacology and therapeutics of bronchodilatorsPharmacol Rev201264345050422611179
- YorganciogluAIndacaterol in chronic obstructive pulmonary disease: an update for cliniciansTher Adv Chronic Dis201231253623251766
- JonesPWBarnesNVogelmeierCLawrenceDKramerBEfficacy of indacaterol in the treatment of patients with COPDPrim Care Respir J201120438038821785813
- CazzolaMMateraMGLötvallJUltra-long acting β2 agonists in development for asthma and chronic obstructive pulmonary diseaseExpert Opin Investig Drugs2005147775783
- MagnussenHVerkindreCJackDIndacaterol once-daily is equally effective dosed in the evening or morning in COPDRespir Med2010104121869187620850959
- CalverleyPMLeeATowseLvan NoordJWitekTJKelsenSEffect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary diseaseThorax2003581085586014514937
- ToyELBaulieuNUMcHaleJLTreatment of COPD: relationship between daily dosing frequency, adherence, resource use, and costsRespir Med2011105343544120880687
- BeehKMBeierJThe short, the long, and the “ultra-long”: why duration of bronchodilator action matters in chronic obstructive pulmonary diseaseAdv Ther201027315015920411368
- RennardSBantjeTCentanniSA dose-ranging study of indacaterol in obstructive airways disease, with a tiotropium comparisonRespir Med200810271033104418479895
- BarnesPJPocockSJMagnussenHIntegrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless designPulm Pharmacol Ther201023316517120080201
- RenardDLoobyMKramerBLawrenceDMorrisDStanskiDRCharacterization of the bronchodilatory dose response to indacaterol in patients with chronic obstructive pulmonary disease using model-based approachesRespir Res2011125421518459
- ChapmanKRRennardSIDograAOwenRLassenCKramerBLong-term safety and efficacy of indacaterol, a novel long-acting β2 agonist in subjects with COPD: a randomized, placebo-controlled studyChest20111401687521349928
- KerwinEMGotfriedMHLawrenceDLassenCKramerBEfficacy and tolerability of indacaterol 75 μg once daily in patients aged ≥ 40 years with chronic obstructive pulmonary disease: results from 2 double-blind, placebo-controlled 12-week studyClin Ther201133121974198422177371
- GotfriedMHKerwinEMLawrenceDLassenCKramerBEfficacy of indacaterol 75 μg once daily on dyspnea and health status: results of two double-blind, placebo-controlled 12 week studiesCOPD20129662963623020650
- ChowdhuryBASeymourSMMicheleTMDurmowiczAGLiuDRosebraughCJThe risks and benefits of indacaterol – the FDA’s reviewN Engl J Med2011365242247224922168640
- JonesPWDonohueJFNedelmanJPascoeSPinaultGLassenCCorrelating changes in lung function with patient outcomes in chronic obstructive pulmonary disease: a pooled analysisRespir Res20111216122206353
- MahlerDAWeinbergDHWellsCKFeinsteinARThe measurements of dyspnea* Contents, interobserver agreement, and physiologic correlates of two new clinical indexesChest1984857517586723384
- JonesPWQuality of life measurements for patients with diseases of the airwaysThorax1992466766821835178
- DecramerMDahlRKornmannOKornSLawrenceDMcBryanDEffects of long-acting bronchodilators in COPD patients according to COPD severity and ICS useRespir Med2013107222323223219347
- CazzolaMSegretiAStirpeEEffect of an additional dose of indacaterol in COPD patients under regular treatment with indacaterolRespir Med2012107110711123083839
- WorthHChungKFFelserJMHuHRueeggPCardio- and cerebrovascular safety of indacaterol vs formoterol, salmeterol, tiotropium and placebo in COPDRespir Med2011105457157921227674
- DonohueJFSinghDKornmannOLawrenceDLassenCKramerBSafety of indacaterol in the treatment of patients with COPDInt J Chron Obstruct Pulmon Dis2011647749222003293
- BauwensONinaneVVan de MaeleB24-hour bronchodilator efficacy of single doses of indacaterol in subjects with COPD: comparison with placebo and formoterolCurr Med Res Opin200925246347019192991
- BeierJBeehKMBrookmanLPeacheyGHmissiAPascoeSBronchodilator effects of indacaterol and formoterol in patients with COPDPulm Pharmacol Ther200922649249619465142
- DahlRChungKFBuhlREfficacy of a new once-daily long-acting inhaled β2-agonist indacaterol versus twice-daily formoterol in COPDThorax201065647347920522841
- KornSKerwinEAtisSIndacaterol once-daily provides superior efficacy to salmeterol twice-daily in COPD: a 12-week studyRespir Med2011510571972621367594
- KornmannODahlRCentanniSOnce-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparisonEur Respir J201137227327920693243
- DonohueJFFogartyCLötvallJOnce-daily bronchodilators for chronic obstructive pulmonary disease. Indacaterol versus tiotropiumAm J Respir Crit Care Med2010182215516220463178
- VogelmeierCRamos-BarbonDJackDIndacaterol provides 24-hour bronchodilation in COPD: a placebo-controlled blinded comparison with tiotropiumRespir Res20101113520920365
- BuhlRDunnLJDisdierCBlinded 12-week comparison of once-daily indacaterol and tiotropium in COPDEur Respir J201138479780321622587
- ChapmanKRFogartyCMPeckittCDelivery characteristics and patients’ handling of two single-dose dry-powder inhalers used in COPDInt J Chron Obstruct Pulmon Dis2011635336321760722
- BeehKMBeierJDonohueJFClinical trial design in chronic obstructive pulmonary disease: current perspectives and considerations with regard to blinding of tiotropiumRespir Res2012135222726538
- RodrigoGJNeffenHComparison of indacaterol with tiotropium or twice-daily long-acting beta-agonists for stable COPD: a systematic reviewChest201214251104111022383666
- CopeSCapkun-NiggliGGaleREfficacy of once-daily indacaterol relative to alternative bronchodilators value in COPD patients: a patient-level mixed treatment comparisonValue Health201215352453322583463
- CopeSZhangJWilliamsJJansenJPEfficacy of once-daily indacaterol 75 μg relative to alternative bronchodilators in COPD: a study level and a patient level network meta-analysisBMC Pulm Med2012122922732017
- BalintBWatzHAmosCOwenRHigginsMKramerBOnset of action of indacaterol in patients with COPD: comparison with salbutamol and salmeterol-fluticasoneInt J Chron Obstruct Pulmon Dis2010531131820856830
- CopeSCapkun-NiggliGGaleRJardimJRJansenJPComparative efficacy of indacaterol 150 μg and 300 μg versus fixed-dose combinations of formoterol + budesonide or salmeterol + fluticasone for the treatment of chronic obstructive pulmonary disease – a network meta-analysisInt J Chron Obstruct Pulmon Dis2011632934421697997
- CopeSKraemerMZhangJCapkun-NiggliGJansenJPEfficacy of indacaterol 75 μg versus fixed-dose combinations of formoterol-budesonide or salmeterol-fluticasone for COPD: a network meta-analysisInt J Chronic Obstruct Pulmon Dis20127415420
- CorradoARossiAHow far is real life from COPD therapy guidelines. An Italian Observational StudyRespir Med2012106798999722483189
- DecramerMRossiALawrenceDMcBryanDIndacaterol therapy in patients with COPD not receiving other maintenance treatmentRespir Med2012106121706171423031496
- TroostersTCelliBLystigTTiotropium as a first maintenance drug in COPD: secondary analysis of the UPLIFT trialEur Respir J2010361657320185426
- TashkinDPCelliBSennSA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med2008359151543155418836213
- DecramerMCelliBKestenSLystigTMehraSTashkinDPEffect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trialLancet200937496961171117819716598
- PelkonenMNotkolaILNissinenATukiainenHKostelaHThirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural menChest200613041129113717035447
- van DurmeYMVerhammeKMStijnenTPrevalence, incidence, and lifetime risk for the development of COPD in the elderly: the Rotterdam studyChest2009135236837719201711
- TantucciCModinaDLung function decline in COPDInt J Chron Obstruc Pulmon Dis201279599
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease Update 2010. Available from: http://www.goldcopd.com
- [No authors listed]In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. An 85-day multicenter trial. COMBIVENT Inhalation Aerosol Study GroupChest19941055141114198181328
- BuhlRBanerjiDProfile of glycopyrronium for once-daily treatment of moderate-to-severe COPDInt J Chron Obstruct Pulmon Dis2012772974123118536
- Van de MaeleBFabbriLMMartinCHortonRDolkerMOverendTCardiovascular safety of QVA149, a combination of indacaterol and NVA237, in COPD patientsCOPD20107641842721166630
- VogelmeierCFBatemanEDPallanteJEfficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group studyLancet Respir Med2013115160
- O’DonnellDELavenezianaPOraJWebbKALamYAOfirDEvaluation of acute bronchodilator reversibility in patients with symptoms of GOLD stage I COPDThorax200964321622319052054
- O’DonnellDEVoducNFitzpatrickMWebbKAEffect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary diseaseEur Respir J2004241869415293609
- O’DonnellDEFlϋgeTGerkenFEffects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPDEur Respir J200423683284015218994
- RossiACentanniSCerveriIAcute effects of indacaterol on lung hyperinflation in moderate COPD: a comparison with tiotropiumRespir Med20121061849022035851
- BeehKMWagnerFKhindriSDrollmannAFEffect of indacaterol on dynamic lung hyperinflation and breathlessness in hyperinflated patients with COPDCOPD20118534021793716
- O’DonnellDECasaburiRVinckenWINABLE 1 study groupEffect of indacaterol on exercise endurance and lung hyperinflation in COPDRespir Med201110571030103621498063
- HatajiONaitoMItoKWatanabeFGabazzaECTaguchiOIndacaterol improves daily physical activity in patients with chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis201381523293514
- ChangYHChenRCWahlqvistMLLeeMSFrequent shopping by men and women increases survival in the older Taiwanese populationJ Epidemiol Commun Health2012667e20
- RussellRAnzuetoAWeismanIOptimizing management of chronic obstructive pulmonary disease in the upcoming decadeInt J Chron Obstruct Pulmon Dis20116476121311693
