Abstract
Alpha-1 Antitrypsin Deficiency (AATD) is a rare genetic condition that predisposes patients to lung and liver disease and is often underdiagnosed due to incomplete diagnosis of chronic obstructive pulmonary disease (COPD) and asthma. Improvements in physician awareness have been made, but better strategies for both diagnosis and management are still required. The only current disease-modifying therapy for AATD is the infusion of the missing Alpha-1 Antitrypsin (AAT) protein, which can slow progression of emphysema. However, AAT treatment can impact patient freedom and quality of life due to the need for weekly intravenous infusions. A symposium was held to discuss patient-centric aspects of care that have impact on the lives of patients with AATD, including exacerbations of their lung disease, self-administration of intravenous AAT therapy and pulmonary rehabilitation. Intravenous self-infusion of drugs is an established treatment strategy for patients with a variety of conditions and can improve patient quality of life, freedom and mental well-being. Experience from these areas show that patients typically manage their treatment well and without complications. When applied to AATD, training patients to self-infuse therapy can be successful, but formal guidelines would be beneficial. In addition to pharmacological intervention, individualized pulmonary rehabilitation, exercise and educational programs can encourage health-enhancing patient behavior and further improve patient quality of life. However, differences in skeletal muscle adaptations to pulmonary rehabilitation exercise regimens have been observed between patients with AATD and non-AATD COPD, highlighting the need to develop training programs specifically designed for patients with AATD.
Video abstract

Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Alpha-1 Antitrypsin (AAT) is a 52 kDa serine proteinase inhibitor produced primarily in the liver. One of its main functions is to protect the lungs from proteinase-mediated tissue destruction.Citation1 The normal and most common form of AAT in the general population is the M-type variant, and those homozygous for the M allele (PI*MM) have AAT plasma levels >20 µmol.Citation1 However, approximately 130 mutations that result in AAT deficiency (AATD) have been identified,Citation2–Citation4 and some are associated with AAT levels of approximately 5–6 µmol.Citation1,Citation5 In patients with severe AATD, low levels of AAT can contribute to destruction of lung tissue by uninhibited proteinases, especially neutrophil elastase,Citation6 which can eventually progress to emphysema. Although patients with AATD usually present with symptoms similar to non-AATD chronic obstructive pulmonary disease (COPD), AATD is commonly associated with longer disease exacerbations, which has been shown to worsen patient health status based upon respiratory questionnaires and changes in lung function.Citation7
AATD is greatly underdiagnosed due to a lack of disease awareness and difficulties in confirming cases of AATD, which are often incompletely diagnosed as non-AATD COPD or asthma,Citation4,Citation8,Citation9 leading to long delays in obtaining a confirmed diagnosis and appropriate disease management.Citation10,Citation11 Although there have been recent improvements in raising awareness of AATD and its management, through educational initiatives and availability of testing,Citation12 efforts should be maintained to increase detection rates, which are still below 10% in most countries.Citation13 Current initiatives also aim to advance personalization of AATD management, enhancing the use of effective non-medical and medical treatments, and decreasing the burden on patient quality of life (QoL).
Currently, the only disease-modifying treatment for AATD is weekly intravenous (IV) AAT therapy, which is efficacious for slowing emphysema progression.Citation14,Citation15 However, the need for weekly visits to healthcare providers can be troublesome for patients. Emerging treatment options therefore aim to improve patient convenience with extended interval dosing and self-administration strategies. Self-administration is already practiced successfully by patients with severe hemophilia and by those with hereditary angioedema (HAE).Citation16,Citation17 In HAE, IV self-administration decreases frequency of attacks, improves patient QoL, and reduces hospitalization and burden on healthcare resources.Citation18–Citation20 However, unlike HAE, where rapid administration is essential to protect patients from life-threatening events, in AATD, self-administration primarily improves patient convenience. Improvements in patient education on type and severity of disease exacerbations should be made to ensure rapid and appropriate treatment. Tailor-made rehabilitation programs for patients with AATD are also specifically being developed to optimize and maintain response to AAT therapy.
These topics formed the basis of a symposium at the 2019 European Respiratory Society International Congress in Madrid, Spain, which had a specific focus on these areas that can help empower patients and healthcare professionals. The symposium welcomed delegates, who were predominantly clinicians, from 21 countries. This review summarizes key principles from the symposium, with the aim of supporting diagnosis and optimal management of patients with AATD.
Modifiers of Quality of Life in Patients with AATD: Focus on Exacerbations
Exacerbations of COPD are important patient-reported events, defined as a sustained worsening of symptoms beyond normal variations that may require additional treatment.Citation21 An increase in length and number of exacerbations negatively impacts patient QoL, morbidity, and mortality in AATD ()Citation7 and non-AATD COPD.Citation22,Citation23 The number of exacerbations experienced by patients with AATD is similar to that associated with non-AATD COPD.Citation7,Citation23,Citation24 However, patients with AATD typically show evidence of increased neutrophil elastase activity in the sputum at exacerbation onset; a characteristic that is greater in AATD than non-AATD COPD. In non-AATD COPD, sputum elastase activity resolves upon antibiotic treatment, whereas sputum elastase activity decreases with antibiotic treatment but still remains elevated in AATD,Citation25 highlighting a clear pathological difference between AATD and non-AATD COPD.
Figure 1 Exacerbations have a negative impact on QoL and lung function in patients with AATD. (A) Box and whisker plot showing SGRQ total scores at 12 months in AATD patients with no, infrequent and frequent AATD exacerbations.Citation7 (B) The relationship between change in vital capacity and exacerbation frequency in patients with AATD.Citation26 (C) Rationale for early intervention with antibiotic therapy: Total exacerbation length is related to the length of time in delaying treatment.
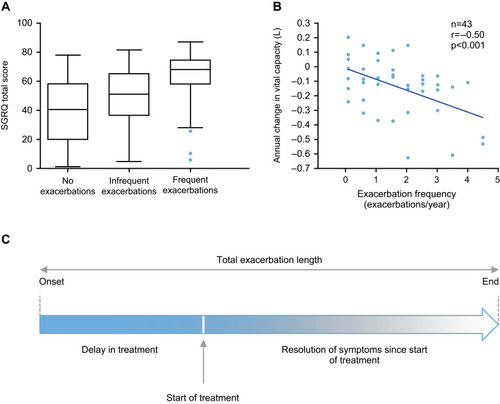
In AATD, disease progression is greatly impacted by exacerbation frequency; more frequent exacerbations are significantly associated with a decline in lung vital capacity ().Citation26 Furthermore, delay in antibiotic treatment is associated with an increase in total exacerbation length (),Citation27 highlighting the need for prompt intervention by physicians with empirical antibiotics. Exacerbations are characterized by increased dyspnea, increased sputum volume and new or increased sputum purulence. Of note, while patients with AATD more frequently seek treatment for dyspnea and sputum volume during disease exacerbations,Citation28 patients are less worried about any changes in sputum color. However, sputum color is a more reliable indicator of bacterial infection requiring antibiotic treatment and the associated increase in sputum elastase activity.Citation29 Therefore, patients experiencing a change in sputum color () should be advised to seek medical treatment and should be prescribed antibiotic therapy as early as possible to reduce exacerbation length, elastase activity, and protect lung tissues,Citation28 which can ultimately affect disease progression.
Figure 2 Sputum color chart used at Queen Elizabeth Hospital, Birmingham, UK. In symptom-based diary cards, sputum color is graded 1–5. Samples graded 3–5 indicate increasing neutrophilic infiltration and concomitant elastase activity.

IV AAT therapy reduces plasma levels of Aα-Val360,a neutrophil elastase-specific fibrinogen cleavage product and biomarker of neutrophil elastase activity, within the lungs in a dose-related manner. Given the elevated levels of elastase activity seen in patients with AATD and during disease exacerbations, biomarkers of elastase activity could be utilized to measure an effect of AAT therapy. Findings from the EXACTLE trial, where computed tomography and exacerbations were used to assess the therapeutic effect of IV AAT therapy, suggest that AAT therapy reduces exacerbation severity rather than exacerbation frequency, which was unaltered.Citation30 Furthermore, although an effect of AAT therapy on exacerbations was not seen in the RAPID trial,Citation14,Citation15 the trial was not specifically designed to address this possibility, and was therefore underpowered to assess exacerbations as an endpoint. Further research in this area is required. However, the RAPID trial did show a decrease in the rate of loss of lung tissue in treated subjects compared with controls.Citation14,Citation15
Improving the Convenience of IV AAT Therapy Through Self-Administration
The need for weekly travel to a physician/hospital or requirement for nurse visits for IV AAT therapy can have a negative effect on patient QoL and their ability to fulfill family and work-related tasks. Self-administration of IV therapy by patients at home on the other hand, may be beneficial by allowing patients to take an active role in their disease management. Self-administration of IV antibiotics has been used successfully since the 1980s for a variety of conditions and demonstrated to be well tolerated, effective and cost-efficient, whilst also improving patient well-being.Citation31 Self-administration has also been utilized and demonstrated QoL benefits in other therapy areas such as Fabry disease,Citation32 primary immune deficiency,Citation33 hemophilia,Citation34 and HAE.Citation35
The benefits of self-administration are exemplified by its implementation in HAE, a disease that has several parallels with AATD: both are genetic disorders associated with genes in the SERPIN superfamily of serine proteases and are treated with IV plasma products to replace deficient proteins. HAE is a rare autosomal dominant disorder characterized by severe attacks of swelling in the limbs, face, intestinal tract and airways, which can be fatal.Citation36 Patients with severe HAE attacks have reported improvements in QoL after IV self-administration at home in comparison to administration at the clinic.Citation35 In addition, self-administration has been shown to reduce hospital administration for acute HAE attacks and related healthcare costs.Citation37 Together, these benefits from self-administration in HAE are transferable to AATD for the perspective of patient convenience and likely to be transferable in terms of healthcare system cost.
Experience with Self-Administration in AATD from the Patient and Physician Perspective
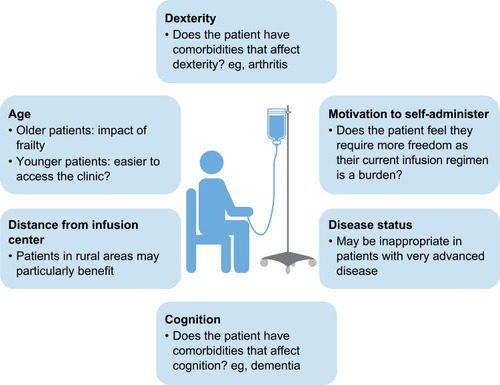
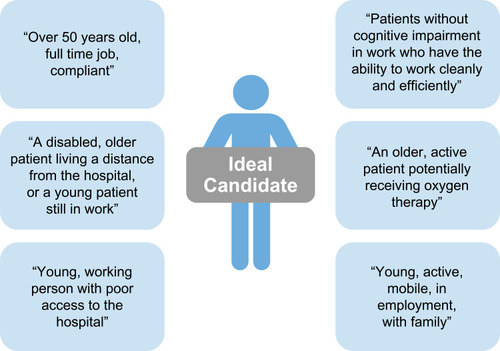
Self-administration of IV therapy is currently uncommon for AATD, particularly in Europe. However, author experience suggests that it can be easily learned and safely performed by patients at home. Self-administration with IV AAT can improve patient independence, allow patients with AATD to gain control of their lives and provide greater freedom, particularly regarding travel. Nevertheless, careful patient selection is an important aspect of the success of AATD self-administration strategies (). In a survey of 15 clinicians treating AATD in 13 European countries, AATD experts outlined their ideal candidate for AAT self-administration and suggested that a number of patient groups would benefit from self-administration ().Citation8 However, physicians may be cautious about introducing self-administration; the same survey also suggested that safety concerns surrounding IV administration was the greatest concern that physicians had regarding self-administration, as fewer resources would be available to patients at home in the event of an emergency than if the same emergency happened in clinic.Citation8 The most important advantage for self-administration was considered as giving patients greater independence.Citation8
Figure 4 Selecting the right patient for self-administration – expert pulmonologist perspectives on the ideal candidate.
Despite these reservations, AlphaNet, a not-for-profit organization providing customized patient care and innovative disease management for patients with AATD, conducted a survey of 44 patients with AATD that were actively self-administering AAT therapy in the United States. Results from this AlphaNet patient survey suggest that self-administration can be safely performed by patients with AATD.Citation38 In addition, the majority of patients surveyed were either very satisfied (95.4%), or satisfied (4.6%) with self-administration and most required minimal training, supporting the use of self-administration for AATD.Citation38 Infusion durations during this study were generally in-line with licensing recommendations and only 11% of patients reported challenges, with reports of difficulty determining injection site, problems finding a vein, port blockage, or IV stick injury.Citation38 The majority of patients (83.7%) experienced no difficulties with self-administration.Citation38 The potential success of self-administration for AATD is highlighted by the finding that 46% of patients had been self-administering IV AAT therapy for 1–5 years and 18% had been self-administering for over 10 years.Citation38
Moving Forward with Self-Administration in AATD
There is currently only one IV AAT product available in Europe that is licensed for self-administration to slow the progression of emphysema in patients with severe AATD (Respreeza®, CSL Behring).Citation39 However, initiatives are required to inform physicians regarding potential benefits of self-administration in the right patients and to encourage wider adoption of this strategy in Europe. This could lead to the implementation of case–control studies of IV AAT self-administration, to ensure that a self-administration strategy is beneficial for patients. In addition, ensuring compliance to self-administration is an important consideration given the expense of the therapy, and should ideally be monitored, eg, with mechanisms such as returning used vials to the pharmacy. A case–control study could help to establish best practice in terms of monitoring compliance of self-administration. Once further data and experience are available, the aim should be to establish formal guidelines for AAT self-administration, in addition to formal training and educational programs for both patients with AATD and their families. Guidelines for AAT self-administration can draw upon examples of guidelines developed for HAE, where all patients are considered for self-administration training.Citation40,Citation41 With AATD, some patients are unaware that self-administration is an option for treatment, and some prefer IV AAT therapy to be performed in the clinic. In either case, providing formal training and patient support would greatly improve patient confidence and their ability to self-administer independently.
Going a Step Further: The Value of Pulmonary Rehabilitation in AATD Treatment
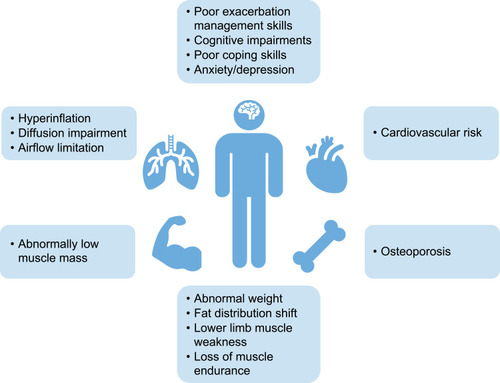
Pulmonary rehabilitation is a comprehensive exercise and educational intervention program that is based upon thorough patient assessment to achieve patient-tailored lung therapy and promote long-term adherence to health-enhancing behaviors. In patients with non-AATD COPD, pulmonary rehabilitation has been shown to improve symptoms and QoL.Citation42,Citation43 The individual program and aims of pulmonary rehabilitation need to consider comorbidities, such as cardiovascular conditions and cachexia, as well as metabolic and psychological conditions ().Citation44 Understanding individual patient needs and limitations is necessary to enable customization of rehabilitation programs to optimize patient outcome. However, it is important to note that rehabilitation only increases potential capacity of patients and does not necessarily lead to increased daily activity, emphasizing the importance of encouraging patients to translate their increased exercise capacity into adaptive behavioral changes in daily life.Citation42 One goal of pulmonary rehabilitation is to combine exercise training with optimal bronchodilation with inhaled medications. Selection of the right inhaler and training of inhalation technique increases the ability to perform high-intensity physical training; bronchodilator therapy prolongs endurance time and reduces breathlessness,Citation45,Citation46 further highlighting the importance of medication adherence in rehabilitation for COPD and AATD.
Figure 5 Treatable traits typical to patients with AATD that may affect approach to pulmonary rehabilitation. Data from Schols et al.Citation51
Although there is limited evidence for the use of pulmonary rehabilitation in patients with AATD, success has been demonstrated in a small number of studies. Short-term comprehensive pulmonary rehabilitation has been shown to improve exercise capacity and health-related QoL in patients awaiting lung transplantation due to end-stage lung disease for AATD and non-AATD COPD, independent of the underlying lung disease.Citation47 Post-pulmonary rehabilitation, improvement in patients’ physical ability was similar between lung transplant candidates with AATD and non-AATD COPD, as measured by the 6-minute walking distance assessment.Citation47 Furthermore, a significant improvement in the mental health component summary of the SF36 QoL questionnaire was also seen in patients with AATD waiting for lung transplantation.Citation47
When comparable pulmonary rehabilitation programs were carried out on patients with AATD and non-AATD COPD, differences in skeletal muscle adaptations were noted. An increase in the proportion of oxidative type I myofibers, which are more efficient over long periods of time, was observed only in patients with non-AATD COPD.Citation48 In contrast, positive adaptations in type IIA myofibers, which are more efficient for short bursts of energy, were only observed in patients with AATD.Citation48 Moreover, COPD patients with the pathogenic AATD genotype (PI*ZZ) but not the normal COPD genotype (PI*MM) showed positive adaptation in capillarization in response to exercise training.Citation48 Together, this data suggests that patients with AATD require different training regimens to patients with non-AATD COPD, but recommendations of which components and at which intensity are most efficient are lacking in clinical practice. Further studies are required to advise pulmonary rehabilitation guidelines in patients with AATD, and currently, a study aimed at determining a training program for improving the effects of pulmonary rehabilitation in patients with AATD is ongoing.Citation49 It is theorized that this study will demonstrate that high-intensity training programs, which require short bursts of energy, will benefit patients with AATD more than moderate-intensity training due to type IIA myofiber adaptations observed in these patients.Citation48 By comparison, non-AATD COPD patients show increases in type I myofibers in response to training,Citation48 and patients may, therefore, benefit more from moderate-intensity training programs that require longer bursts of energy.Citation48 Defining a training program specifically for patients with AATD may help demonstrate QoL benefits. Pulmonary rehabilitation is recommended by COPD guidelines in general, but in the more specific guidelines for patients with AATD, currently, only the Canadian Thoracic Society statement specifically mentions this treatment option,Citation50 and so there is potential to expand these recommendations into international guidelines/statements on AATD.
Conclusion
Despite general improvements in the management of patients with AATD, there are several specific considerations that may improve patient QoL and impact disease outcome. Early antibiotic therapy shortly after the onset of an exacerbation with purulent sputum can reduce total exacerbation length and can decrease progression of irreversible lung damage. Self-infusion of IV AAT therapy can be easily learned and safely performed, allowing patients with AATD to regain control of their lives, provide greater independence and improve well-being. Increased awareness of the option to self-infuse AAT therapy is needed among both physicians and patients. However, careful patient selection is crucial to the success of self-administration for AATD. In addition, formal guidelines and educational programs are required for self-administration in patients with AATD. Standardization of training recommendations may encourage more physicians to consider self-administration practices for AATD, in the right patient. Pulmonary rehabilitation, which is widely underused in patients with AATD and non-AATD COPD alike, is equally beneficial for both, improving patient QoL and decreasing breathlessness, and therefore should be encouraged along with self-administration of IV AAT therapy. However, training regimens specifically designed for patients with AATD may further improve outcome and overcome differences in muscle adaptations compared to patients with COPD.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr Robert A Sandhaus and Dr Charlie Strange are medical directors of AlphaNet, a not-for profit disease management company for AATD. Dr Robert A Sandhaus reports participation in advisory boards for Grifols, CSL Behring, AstraZeneca, Mereo BioPharma, and Inhibrx for which he receives reimbursement of travel expenses. Dr Charlie Strange consults on AATD and/or asthma and COPD with AstraZeneca, CSL Behring, Dicerna, Grifols, and Vertex. He has grants paid to MUSC from Adverum, CSA Medical, CSL Behring, Grifols, MatRx, Novartis, Nuvaira, Pulmonx, Takeda, and Vertex. Dr Andrea Zanichelli has received meeting sponsorship from and performed clinical trial research funded by BioCryst, CSL Behring, Shire, Pharming Dyax Corporation, and acts as a consultant for CSL Behring and Shire. Karen Skålvoll reports personal fees from CSL Behring which was paid to Team Alpha-1 Athlete. Dr A Rembert Koczulla reports personal fees from CSL Behring, outside the submitted work. Dr Robert A Stockley serves on the advisory boards for several pharmaceutical companies with an interest in AATD, including CSL Behring, Mereo BioPharma, Vertex, Z Factor and Kamada, and has received non-commercial funding from CSL Behring, Grifols, Takeda and Mereo BioPharma. The authors report no other conflicts of interest in this work.
Acknowledgments
Medical writing assistance for this publication was provided by Meridian HealthComms, funded by CSL Behring.
References
- American Thoracic Society/European Respiratory Society. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168(7):818–900.14522813
- DeMeoDL, SilvermanEK. Alpha1-antitrypsin deficiency. 2: genetic aspects of alpha(1) antitrypsin deficiency: phenotypes and genetic modifiers of emphysema risk. Thorax. 2004;59(3):259–264. doi:10.1136/thx.2003.00650214985567
- SilvaD, OliveiraMJ, GuimarãesM, et al. Alpha-1-antitrypsin (SERPINA1) mutation spectrum: three novel variants and haplotype characterization of rare deficiency alleles identified in Portugal. Respir Med. 2016;116:8–18. doi:10.1016/j.rmed.2016.05.00227296815
- StollerJK, LacbawanFL, AboussouanLS Alpha-1 antitrypsin deficiency. GeneReviews® [Internet]; 2006 Available from https://www.ncbi.nlm.nih.gov/books/NBK1519/. Accessed 120, 2020.
- CrystalRG. Alpha 1-antitrypsin deficiency, emphysema, and liver disease. Genetic basis and strategies for therapy. J Clin Invest. 1990;85(5):1343–1352. doi:10.1172/JCI1145782185272
- McCarthyC, ReevesEP, McElvaneyNG. The role of neutrophils in alpha-1 antitrypsin deficiency. Ann Am Thorac Soc. 2016;13(Supplement 4):S297–S304. doi:10.1513/AnnalsATS.201509-634KV27564664
- NeedhamM, StockleyRA. Exacerbations in alpha-1 antitrypsin deficiency. Eur Respir J. 2005;25(6):992–1000. doi:10.1183/09031936.05.0007470415929953
- HorváthI, CanotilhoM, ChlumskýJ, et al. Diagnosis and management of alpha-1 antitrypsin deficiency in Europe: an expert survey. ERJ Open Res. 2019;5(1):1–11. doi:10.1183/23120541.00171-2018
- Torres-DuránM, Lopez-CamposJL, BarrechegurenM, et al. Alpha-1 antitrypsin deficiency: outstanding questions and future directions. Orphanet J Rare Dis. 2018;13(1):114.29996870
- EsquinasC, BarrechegurenM, SucenaM, et al. Practice and knowledge about diagnosis and treatment of alpha-1 antitrypsin deficiency in Spain and Portugal. BMC Pulm Med. 2016;16:64. doi:10.1186/s12890-016-0222-4
- GreulichT, OttavianiS, BalsR, et al. Alpha1-antitrypsin deficiency – diagnostic testing and disease awareness in Germany and Italy. Respir Med. 2013;107(9):1400–1408. doi:10.1016/j.rmed.2013.04.02323786890
- GreulichT, VogelmeierCF. Alpha-1 antitrypsin deficiency: increasing awareness and improving diagnosis. Ther Adv Respir Dis. 2016;10(1):72–84. doi:10.1177/175346581560216226341117
- AboussouanLS, StollerJK. Detection of alpha-1 antitrypsin deficiency: a review. Respir Med. 2009;103(3):335–341. doi:10.1016/j.rmed.2008.10.00619013782
- ChapmanKR, BurdonJGW, PiitulainenE, et al. Intravenous augmentation treatment and lung density in severe alpha-1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9991):360–368. doi:10.1016/S0140-6736(15)60860-126026936
- McElvaneyNG, BurdonJ, HolmesM, et al. Long-term efficacy and safety of alpha-1 proteinase inhibitor treatment for emphysema caused by severe alpha-1 antitrypsin deficiency: an open-label extension trial (RAPID-OLE). Lancet Respir Med. 2017;5(1):51–60. doi:10.1016/S2213-2600(16)30430-127916480
- LonghurstHJ, CarrS, KhairK. C1-inhibitor concentrate home therapy for hereditary angioedema: a viable, effective treatment option. Clin Exp Immunol. 2007;147(1):11–17.17177958
- SchrijversLH, Beijlevelt-van der ZandeM, PetersM, SchuurmansMJ, FischerK. Learning intravenous infusion in haemophilia: experience from the Netherlands. Haemophilia. 2012;18(4):516–520. doi:10.1111/j.1365-2516.2012.02752.x22292416
- LeviM, ChoiG, PicavetC, et al. Self-administration of C1-inhibitor concentrate in patients with hereditary or acquired angioedema caused by C1-inhibitor deficiency. J Allergy Clin Immunol. 2006;117(4):904–908. doi:10.1016/j.jaci.2006.01.00216630950
- Martinez-SaguerI, RusickeE, Aygören-PürsünE, et al. Individual replacement therapy with a pasteurized C1-inhibitor concentrate compared to prophylaxis with danazol in patients with hereditary angioedema - a prospective study. J Allergy Clin Immunol. 2006;117(2):S180. doi:10.1016/j.jaci.2005.12.717
- RusickeE, Martinez-SaguerI, Aygören-PürsünE, et al. Home treatment in patients with hereditary angiodema. J Allergy Clin Immunol. 2006;117(2):S180
- BurgeS, WedzichaJA. COPD exacerbations: definitions and classifications. Eur Respir J. 2003;21(Supplement 41):46s–53s. doi:10.1183/09031936.03.00078002
- AnzuetoA. Impact of exacerbations on COPD. Eur Respir Rev. 2010;19(116):113. doi:10.1183/09059180.0000261020956179
- SeemungalTAR, HurstJR, WedzichaJA. Exacerbation rate, health status and mortality in COPD - a review of potential interventions. Int J Chron Obstruct Pulmon Dis. 2009;4:203–223. doi:10.2147/COPD.S338519554195
- SeemungalTAR, DonaldsonGC, PaulEA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5):1418–1422. doi:10.1164/ajrccm.157.5.97090329603117
- HillAT, CampbellEJ, BayleyDL, et al. Evidence for excessive bronchial inflammation during an acute exacerbation of chronic obstructive pulmonary disease in patients with α 1 -antitrypsin deficiency (PiZ). Am J Respir Crit Care Med. 1999;160(6):1968–1975. doi:10.1164/ajrccm.160.6.990409710588615
- DowsonLJ, GuestPJ, StockleyRA. Longitudinal changes in physiological, radiological, and health status measurements in alpha-1 antitrypsin deficiency and factors associated with decline. Am J Respir Crit Care Med. 2001;164(10):1805–1809. doi:10.1164/ajrccm.164.10.210603611734427
- VijayasarathaK, StockleyRA. Reported and unreported exacerbations of COPD: analysis by diary cards. Chest. 2008;133(1):34–41. doi:10.1378/chest.07-169217989153
- EjioforSI, StolkJ, FernandezP, et al. Patterns and characterization of COPD exacerbations using real-time data collection. Int J Chron Obstruct Pulmon Dis. 2017;12:427–434.28182151
- StockleyRA, O’BrienC, PyeA, et al. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117(6):1638–1645. doi:10.1378/chest.117.6.163810858396
- DirksenA, PiitulainenE, ParrDG, et al. Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha-1 antitrypsin deficiency. Eur Respir J. 2009;33(6):1345–1353. doi:10.1183/09031936.0015940819196813
- StiverHG, TroskySK, CoteDD, et al. Self-administration of intravenous antibiotics: an efficient, cost-effective home care program. Can Med Assoc J. 1982;127(3):207–211.6809305
- ConcolinoD, AmicoL, CappelliniMD, et al. Home infusion program with enzyme replacement therapy for Fabry disease: the experience of a large Italian collaborative group. Mol Genet Metab Rep. 2017;12:85–91. doi:10.1016/j.ymgmr.2017.06.00528702361
- GardulfA, NicolayU. Replacement IgG therapy and self-therapy at home improve the health-related quality of life in patients with primary antibody deficiencies. Curr Opin Allergy Clin Immunol. 2006;6(6):434–442. doi:10.1097/01.all.0000246619.49494.4117088648
- KhairK, MeerabeauL, GibsonF. Self-management and skills acquisition in boys with haemophilia. Health Expect. 2015;18(5):1105–1113. doi:10.1111/hex.1208323711015
- BygumA, AndersenKE, MikkelsenCS. Self-administration of intravenous C1-inhibitor therapy for hereditary angioedema and associated quality of life benefits. Eur J Dermatol. 2009;19(2):147–151.19264579
- SchmaierAH. The hereditary angioedema syndromes. J Clin Invest. 2019;129(1):66–68. doi:10.1172/JCI12537830530986
- ZanichelliA, AzinGM, CristinaF, et al. Safety, effectiveness, and impact on quality of life of self-administration with plasma-derived nanofiltered C1 inhibitor (Berinert®) in patients with hereditary angioedema: the SABHA study. Orphanet J Rare Dis. 2018;13(1):51. doi:10.1186/s13023-018-0797-329631595
- SandhausRA, BoydBS. Alpha-1 antitrypsin therapy: a satisfaction survey of individuals self-administering. Am J Respir Crit Care Med. 2018;A1758–A1758.
- Electronic Medicines Compendium. Respreeza SmPC; 2015 Available from https://www.medicines.org.uk/emc/product/7026/smpc. Accessed 120, 2020.
- BorkK, Aygören-PürsünE, BasM, et al. Guideline: hereditary angioedema due to C1 inhibitor deficiency. Allergo J Int. 2019;28(1):16–29. doi:10.1007/s40629-018-0088-5
- ShapiroRS, ZacekL. Training hereditary angioedema patients to self-administer intravenous C1 esterase inhibitor concentrate. J Infus Nurs. 2014;37(4):284–290. doi:10.1097/NAN.000000000000004924983261
- SpruitMA, PittaF, McAuleyE, et al. Pulmonary rehabilitation and physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(8):924–933. doi:10.1164/rccm.201505-0929CI26161676
- SpruitMA, SinghSJ, GarveyC, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–e64. doi:10.1164/rccm.201309-1634ST24127811
- VanfleterenLEGW, SpruitMA, GroenenM, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–735. doi:10.1164/rccm.201209-1665OC23392440
- BeierJ, KirstenA-M, MrózR, et al. Efficacy and safety of aclidinium bromide compared with placebo and tiotropium in patients with moderate-to-severe chronic obstructive pulmonary disease: results from a 6-week, randomized, controlled phase IIIb study. COPD. 2013;10(4):511–522. doi:10.3109/15412555.2013.81462623819698
- CasaburiR, HamiltonAL, MerrillDD, et al. Influence of spirometric impairment on improvement in constant work rate cycling endurance in COPD patients: differences in response to bronchodilator therapy and exercise training. Am Thorac Soc. 2019;A4275–A4275.
- KennK, GloecklR, SoennichsenA, et al. Predictors of success for pulmonary rehabilitation in patients awaiting lung transplantation. Transplantation. 2015;99(5):1072–1077. doi:10.1097/TP.000000000000047225393161
- JaroschI, GehlertS, JackoD, et al. Different training-induced skeletal muscle adaptations in COPD patients with and without alpha-1 antitrypsin deficiency. Respiration. 2016;92(5):339–347. doi:10.1159/00044950927686000
- ClinicalTrials.gov. Effects of different exercise training modalities in alpha-1 antitrypsin deficiency patients. Available from https://clinicaltrials.gov/ct2/show/NCT3802357. Accessed 1120, 2019.
- MarciniukDD, HernandezP, BalterM, et al. Alpha-1 antitrypsin deficiency targeted testing and augmentation therapy: a Canadian Thoracic Society clinical practice guideline. Can Respir J. 2012;19(2):109–116. doi:10.1155/2012/92091822536580
- ScholsAM, FerreiraIM, FranssenFM, et al. Nutritional assessment and therapy in COPD: a European respiratory society statement. Eur Respir J. 2014;44(6):1504. doi:10.1183/09031936.0007091425234804