Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality and its treatment is critical to improve quality of life, reduce symptoms, and diminish the frequency of COPD exacerbations. Due to the harmful environmental effects of pressurized metered-dose inhalers (pMDIs) containing chlorofluorocarbons (CFCs), newer systems for delivering respiratory medications have been developed.
Methods
A search of the literature in the PubMed database was undertaken using the keywords “COPD,” “albuterol,” “ipratropium bromide,” and “Respimat® Soft Mist Inhaler™”; pertinent references within the identified citations were included. The environmental effect of CFC-pMDIs, the invention of the Respimat® Soft Mist Inhaler™ (SMI) (Boehringer Ingelheim, Ingelheim, Germany), and its use to deliver the combination of albuterol and ipratropium bromide for the treatment of COPD were reviewed.
Results
The adverse environmental effects of CFC-pMDIs stimulated the invention of novel delivery systems including the Respimat SMI. This review presents its development, internal mechanism, and use to deliver the combination of albuterol and ipratropium bromide.
Conclusion
CFC-pMDIs contributed to the depletion of the ozone layer and the surge in disorders caused by harmful ultraviolet B radiation. The banning of CFCs spurred the development of novel delivery systems for respiratory medications. The Respimat SMI is an innovative device that produces a vapor of inhalable droplets with reduced velocity and prolonged aerosol duration that enhance deposition within the lower airway and is associated with improved patient satisfaction. Clinical trials have demonstrated that the Respimat SMI can achieve effects equivalent to pMDIs but with lower medication doses. The long-term safety and efficacy remain to be determined. The Respimat SMI delivery device is a novel, efficient, and well-received system for the delivery of aerosolized albuterol and ipratropium bromide to patients with COPD; however, the presence of longer-acting, less frequently dosed respiratory medications provide patients and providers with other therapeutic options.
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic, progressive lung disorder distinguished by non-normalizing airflow limitation.Citation1 Although pulmonary derangements are the primary diagnostic and clinical manifestations, COPD is increasingly being recognized as a multisystem disorder with protean features.Citation2–Citation6
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines state that the primary goals of COPD management are to reduce the clinical manifestations of COPD by relieving symptoms, improving exercise tolerance and health status, and diminishing risk by reducing mortality, preventing disease progression, and decreasing exacerbations.Citation1 The pharmacologic armamentarium for the treatment of COPD includes methylxanthines; corticosteroids; supplemental oxygen; and inhalable short- and long-acting beta agonists, anticholinergic bronchodilators, and aerosolized corticosteroids, alone or in various combinations. Most of the original inhalers utilized chlorofluorocarbon (CFC) propellants that have been shown to be environmentally deleterious. Recent innovations have developed novel devices for the delivery of respiratory medications. This review will examine the reasons for the development of new respiratory delivery systems, the design of the Respimat® Soft Mist Inhaler™ (SMI) (Boehringer Ingelheim, Ingelheim, Germany), and its use to administer the combination of albuterol (AB) and ipratropium bromide (IB) to patients with COPD. (“Albuterol” is the US term for salbutamol and the two terms are used interchangeably throughout this review.)
Review methodology
A search of the literature in the PubMed database using the keywords “COPD,” “albuterol,” “ipratropium bromide,” and “Respimat® Soft Mist Inhaler™” was undertaken. Identified citations and pertinent references within the citations were also included in the review. References were reviewed and the most clinically relevant sources are cited. Studies were not graded by criteria defined a priori.
History of chlorofluorocarbons and their environmental impact
In 1928, Thomas Midgley, Albert Henne, and Robert McNary invented Freon, a CFC, to replace the toxic hazardous chemicals such as ammonia, methyl chloride, and sulfur dioxide that were being used as refrigerants at that time.Citation7 Perhaps the first public demonstration of CFC inhalation occurred in 1928 at the American Chemical Society, when Thomas Midgley inhaled a full breath of Freon and blew it onto a candle, extinguishing the flame, to demonstrate that Freon was both safe and nonflammable.Citation8 By the 1950s, CFCs were being used as home refrigerants, cleaning and industrial solvents, foam-blowing agents, and aerosol propellants.Citation9 In 1956, Riker Laboratories manufactured the first CFC-pressurized metered-dose inhaler (pMDI) for delivering aerosolized medications.
However, in 1971, Lovelock found traces of CFC-11 in the atmosphere.Citation10 Studies of CFCs released into the environment showed that they rise from the troposphere (lower atmosphere) to the stratosphere (upper atmosphere). Due to strong bonds between carbon, chlorine, and fluorine, CFCs are resistant to degradation by environmental physical and biological systems and may remain in the stratosphere for over half a century.Citation11,Citation12 Contemporaneously, observational studies revealed an enlarging hole in the ozone layer above Antarctica ().Citation13,Citation14 These seemingly disparate observations were linked in 1974 when Molina and RowlandCitation16 theorized that chlorine radicals generated by the photolytic degradation of CFCs could cause the catalytic degradation of stratospheric ozone. Further investigations confirmed that CFCs are eventually degraded by solar radiation and release chlorine radicals that react with atmospheric ozone.Citation17 One chlorine radical is estimated to eliminate up to 100,000 ozone molecules.Citation18 Molina and Rowland received the 1995 Nobel Prize for attributing the shrinking ozone layer to the accumulation of CFCs in the stratosphere.
Figure 1 Maps of the hole in the Antarctic ozone layer demonstrating its enlargement over time.
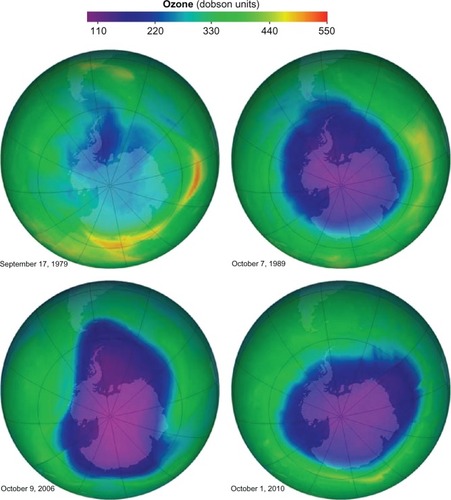
Stratospheric ozone protects humans by absorbing ultraviolet B radiation. Excess ultraviolet B may increase the risk of sunburn, skin cancer, photokeratitis, cataracts, and cause immune suppression as well as harm phytoplankton and plants.Citation19–Citation21 A 1% reduction in stratospheric ozone is estimated to cause a 3% increase in the incidence of nonmelanoma skin cancer.Citation22
By 1978, the Environmental Protection Agency, in collaboration with the US Food and Drug Administration, prohibited CFC propellants in all self-pressurized containers except pMDIs. By 1987, 27 countries including the USA had signed the Montreal Protocol on Substances that Deplete the Ozone Layer (hereafter the Montreal Protocol) and agreed to a 50% reduction in the use of ozone-depleting substances (ODSs) by 1998. The number of countries signing the Montreal Protocol subsequently increased to 188 and it was amended to call for developed countries to cease use of ODSs by 1996. Through inventive engineering, legislation, and international funding, the Montreal Protocol has led to the reduction and almost complete elimination of ODSs through the collaboration of governments, industry, scientists, and environmentalists.Citation23 Another benefit of the Montreal Protocol is the mitigation of CFCs’ effect on climate change.Citation24,Citation25 Despite the realization of the harmful effects of CFCs and their expeditious elimination through international cooperation, it is projected that the stratospheric ozone layer will not recover until at least 2060.Citation23
Alternatives to CFCs
In preparation for the elimination of CFCs from pMDIs, pharmaceutical companies formed a coalition in 1989 to develop CFC replacements and identified hydrofluoroalkanes (HFAs) (HFA-134a and HFA-227a) as propellants that do not deplete ozone. These compounds were prepared in two formulations: HFA suspension and HFA solution aerosols.Citation26 HFAs are composed of carbon, fluorine, and hydrogen atoms and do not contain chlorine. Two issues developed during the transition from CFC to HFA propellants: (1) seal leaks occurred because the seal materials used in CFC-pMDIs were not compatible with HFAs and (2) differences in thermodynamic properties of CFCs and HFAs led to solubility problems. In CFC-pMDIs, surfactants (oleic acid, lecithin, and sorbitan trioleate) were used to enhance the stability of suspensions of micronized particles but surfactants were insoluble in HFAs, causing particles to adhere to the valve and container surfaces and clog the egress aperture. These effects worsened with longer storage time, affected dose delivery, and required changes in co-solvents, gas flow, aperture of the exit, volume within the expansion chamber, and size and velocity of the drug particle-propellant droplets.Citation17,Citation27,Citation28 Once these issues were resolved, a reformulated AB with HFA propellant was approved in 1996. By 2005, more than one brand of HFA-AB was available and the US Food and Drug Administration ruled that CFC-containing AB pMDIs were no longer essential and would be prohibited in the USA after 2008. On December 31, 2008, CFC-containing pMDIs were banned in the USA and replaced by HFA-based inhalers that cost as much as threefold more;Citation29 for other drugs, replacement HFAs were launched at the same price.
Alternatives to pMDIs
In addition to HFA-based inhalers to replace CFC-containing pMDIs, other alternative delivery systems for respiratory medications have been developed.Citation30 These replacements include dry powder inhalers and small aerosol-generating devices.Citation31 Potential techniques to create aerosols have included piezoelectric vibration, extrusion through small apertures, electrohydrodynamic effect, and collision of solution jets.Citation31 The remainder of this review will concentrate on the development and use of this last technique to deliver a combination of AB and IB.
Respimat SMI design, development, and operation
The Respimat SMI is an alternative to pMDIs that forces a drug solution through a series of channels leading to two nozzles that focus two fluid jets into a precisely calculated convergence producing a vapor of inhalable droplets.Citation31,Citation32 The initial prototype for this device was constructed from a syringe containing a solution and a metal pump body (). By pushing a lever, a measured amount of fluid was withdrawn from the syringe and a spring compressed to store mechanical energy. This energy was realized when the spring was released, pushing a piston that forced the solution through small channels forming two fluid jets that collided and produced a vapor with a mean particle mass size between 1 and 5 micrometers. Although this prototype design was effective in producing an aerosol with inhalable particles sized to be deposited in the lower airway, the fabrication of nozzles by puncture of a steel disc did not reliably create the precise angle of convergence required to produce a respirable vapor. Therefore, a novel nozzle configuration was designed from silicon-glass material with a series of channels leading to two apertures that aimed the two jets into convergent paths at a reliably reproducible angle to produce the desired aerosol. This silicon-glass configuration was constructed using dependable fabrication methods developed by the microelectronics industry. Using photolithographic techniques, a precise fluid path is etched into a silicon substrate that is then enclosed by two glass plates and sealed by anodic bonding to create the “uniblock” device that produces the aerosolized vaporCitation31 ().
Figure 2 Configuration of the prototype for the Respimat® Soft Mist Inhaler™. (Boehringer Ingelheim, Ingelheim, Germany).
Note: Dalby RN, Eicher J, Zierenberg B. Development of Respimat(®) Soft Mist™ Inhaler and its clinical utility in respiratory disorders. Med Devices (Auckl). 2011;4:145–155.Citation29
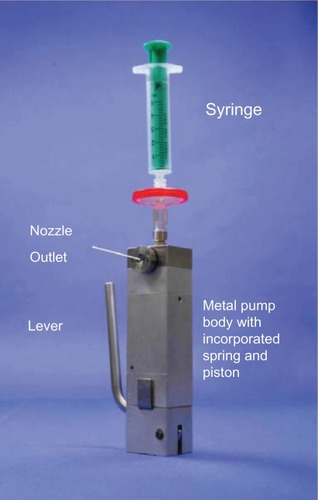
Figure 3 Internal configuration of the uniblock showing the linear flow channels that direct the medication solution to two symmetric exit ports that focus the solution jets into a precisely configured convergence to produce aerosolized droplets that exit the nozzle outlet.
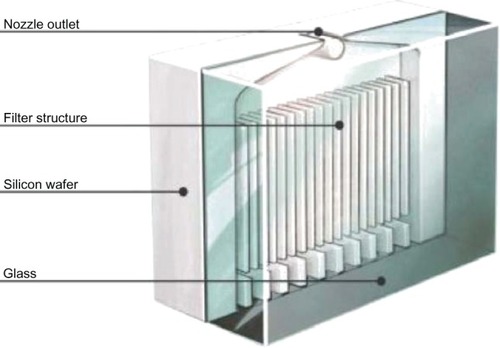
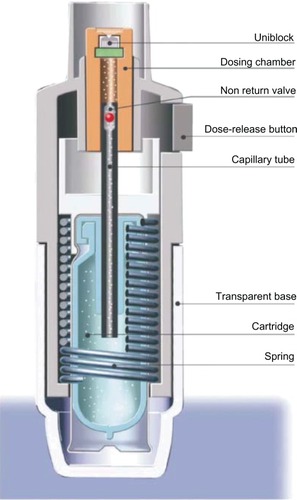
Figure 4 Internal mechanism of the Respimat® Soft Mist Inhaler™. (Boehringer Ingelheim, Ingelheim, Germany).
Subsequent modifications of the delivery device included reducing the energy required to activate the piston to a level that could be easily produced by an individual and storing the solution within a double-walled, plastic, collapsible bag within an aluminum canister. Further engineering design and manufacturing innovations were required to scale manufacture of these devices from prototypes to mass production.Citation31
The optimal aerodynamic diameter for deposition in the lower airway is between 1 and 5 micrometers; particles < 1 micrometers tend to be inhaled and exhaled without deposition and particles > 5 micrometers tend to be deposited in the upper airway. Approximately 75% of the aerosolized particles produced by the Respimat SMI have a mean aerodynamic diameter ≤ 5.8 micrometers and ≤3%–5% of the particles have a diameter < 1 micrometers.Citation32,Citation33
The efficiency of aerosolized medication delivery to the lower airway is highly affected by the deposition of medication in the inhaler and posterior pharynx that occurs due to spray momentum including aerosol ballistic effects and turbulent dispersion.Citation34–Citation38 Coordination of device activation and inhalation is theoretically more critical for devices that produce particles with greater velocity and for shorter duration. It is believed that improved lower airway drug delivery is achieved when smaller particles are generated with less momentum and longer aerosol duration.Citation39 Hochrainer et alCitation40 measured the spray velocity 10 cm from the device nozzle for ipratropium/fenoterol delivered by CFC-pMDI, HFA-pMDI, and Respimat SMI devices to be 5.6, 2.4, and 0.8 m/s, respectively. They also determined the spray duration by video recording to be 0.15, 0.21, and 1.45 s, respectively.Citation40 Thus, the Respimat SMI device produces a slower moving spray that remains aerosolized for 7–10 fold longer than pMDI’s. The slower velocity of the particles that emerge from the Respimat SMI should theoretically improve entrainment of the particles during inhalation, increase delivery to the lower airway, and reduce upper airway deposition. In addition, the longer duration of the particle mist generated by the Respimat SMI may increase the window for successful medication inhalation. Scintigraphic studies demonstrate greater lung and less oropharyngeal deposition with Respimat® Soft Mist Inhaler™ compared with dry powder inhalers and pMDIs with and without spacers.Citation41–Citation45 In studies of healthy volunteers, Newman et alCitation42,Citation43 showed that the proportion of either flunisolide or fenoterol deposited in the lungs by Respimat® Soft Mist Inhaler™ ranged from 39.2–44.6% whereas only 26.2–39.9% was deposited in the oropharynx. In contrast, lung deposition with a pMDI alone ranged from 11.0–15.3% and with a pMDI with a spacer from 9.9–28.0%.Citation42–Citation43 In patients with asthma, the Respimat® Soft Mist Inhaler™ deposited 51.6% of inhaled budesonide in the lungs and 19.3% in the oropharynx compared with 28.5% and 49.3%, respectively, with the Turbohaler® and a fast inhalation flow rate.Citation44 Lung deposition for fenoterol hydrobromide/ipratropium bromide delivered by Respimat® Soft Mist Inhaler™ in patients with COPD was 37% before training and 53% after training.Citation45 In contrast, for the same patients, lung deposition was only 21% when the same medication was delivered by HFA-pMDI before or after training.Citation45
To use the Respimat SMI, the transparent base is removed, the medication-containing cartridge is inserted, and the base replaced. After loading, the device must be activated and primed to remove air from the solution flow path prior to use. Four activations are required to insure priming of a new device. If the device is not used for more than 3 days, it must be activated once to re-prime it; if it is not used for 21 days, it must be primed as if it were a new device.Citation31 To activate the device, the base is twisted half a turn–180°–until it clicks. This motion turns a helical cam gear that compresses and loads the spring and moves a capillary tube with a one-way valve into the solution cartridge, pulling fluid into the dosing chamber (). By pressing the dose-release button, the patient releases the spring, which pushes the capillary tube toward the uniblock, closing the one-way valve and turning the capillary tube into a piston that forces the solution through the uniblock. The solution flows through the microchannels and diverges into two mirror-image exit nozzles to form two symmetric jets that converge at a precise angle of impact, producing an inhalable particle vapor that exits the device.
The Respimat SMI has a dose counter that shows the approximate number of remaining activations and turns red when approximately 1 week of medication remains in the canister. A locking mechanism inactivates the device after the maximal number of activations has been reached to prevent continued use of the device after the medication solution has been depleted or reached a level of incomplete filling of the dosing chamber. The canisters are colored for specific medications and the base is clear so that the canister color is easily visualized. The dose indicator and canister can be configured to provide 60 or 120 activations, depending on the medication and its dosing regimen.Citation31
In comparison with HFA-pMDIs, the Respimat SMI requires “cocking” to load the chamber and compressing of the spring prior to activation by pressing the dose-release button, whereas pMDIs simply require pressing the drug canister into the mouthpiece for activation and drug delivery. Although most guidelines recommend the use of a spacer device with pMDIs, the use of a spacer with the Respimat SMI is not required.Citation46
Clinical trials
The efficacy and safety of IB/AB (20/100 mcg) Respimat (Combivent Respimat®), IB/AB (36 mcg/206 mcg) metered-dose inhaler (MDI) (Combivent® MDI), or IB (20 mcg) Respimat (Ipratropium Respimat®) were compared in an international, multicenter, double-blind double-dummy, 12-week, parallel-group, active-controlled study of 1480 patients with moderate to severe COPD.Citation47 Comparing the forced expiratory volume in 1 second (FEV1) area under the curve (AUC) at baseline to day 85, this study showed:
equivalent efficacy (non-inferiority) in the change in FEV1 AUC 0–6 hours for Combivent Respimat versus Combivent MDI, −3 mL (95% confidence interval [CI], −22, 15) (the change in FEV1 AUC for the periods 0–4 and 4–6 hours were also the same)
equivalent efficacy (non-inferiority) in the change in FEV1 AUC 4–6 hours for Combivent Respimat versus Ipratropium Respimat, −17 mL (95% CI, −39, 5) (the change in FEV1 AUC for the period 0–6 hours was also equivalent)
greater change in FEV1 AUC 0–4 hours for Combivent Respimat versus Ipratropium Respimat, 47 mL (95% CI, 28, 66).
The median time to response onset and time of maximal response were also equivalent for the three treatments. The peak FEV1, maximal change in FEV1, and peak forced vital capacity (FVC) were equivalent for Combivent Respimat and Combivent MDI and greater than Ipratropium Respimat at all time points. There were no significant differences in adverse events or vital signs in the three groups. The frequency of COPD exacerbations, 10.4%–14.8%, was also equivalent.
Kilfeather et alCitation48 compared IB and fenoterol hydrobromide (FEN) in varying combinations and delivered by either Respimat or pMDI in 892 subjects with moderate to severe COPD during a 12-week trial. Patients were randomized to IB 10 mcg/FEN 25 mcg (Respimat SMI 10/25, one actuation four times per day [qid]), IB 20 mcg/FEN 50 mcg (Respimat SMI 20/50, one actuation qid), Respimat SMI placebo (Respimat SMI placebo, one actuation qid), IB 20 mcg/FEN 50 mcg (MDI 40/100, two actuations qid), and MDI placebo (MDI placebo, two actuations qid). The primary endpoint was the change in FEV1 AUC 0–1 hour prior to dosing compared with the first 60 minutes after dosing on day 85. Respimat SMI 20/50 but not Respimat 10/25 was not inferior to MDI 40/100 and both Respimat SMI doses produced significantly greater increases than Respimat SMI placebo. Interestingly, the rate of COPD exacerbations among the subjects with serious adverse events was greater in the treatment groups than in the placebo groups: Respimat SMI 10/25 (2.3%), Respimat SMI 20/50 (4.9%), Respimat SMI placebo (0.9%), MDI 40/100 (2.7%), and MDI placebo (0.9%). The overall rates of COPD exacerbations were: Respimat SMI 10/25 (20.3%), Respimat SMI 20/50 (26.8%), Respimat SMI placebo (19.3%), MDI 40/100 (20.9%), and MDI placebo (17.9%). Other reported adverse events occurred at similar rates across all groups. This study concluded that the use of the Respimat SMI delivery system produced the same bronchodilator effect as twice the dose of IB and FEN delivered by a CFC-pMDI system with similar side effects.
Iacono et alCitation49 studied the efficacy and safety of cumulative doses of IB delivered by Respimat SMI (10 or 20 mcg per actuation) or pMDI (20 mcg per actuation) in a three-period crossover trial. The primary endpoint was the increase from baseline in the average absolute FEV1 between 45 and 245 minutes after the initial inhalation. Respimat SMI 10 or 20 had similar effects on the primary endpoint that were greater than those produced by pMDI 20. The change in FEV1 (in liters) 1 hour and thereafter following the last inhalation were equivalent. Adverse events and changes in vital signs were equivalent across all the groups. This study concluded that the Respimat SMI delivery system produced the same short-term bronchodilator effect as twice the cumulative dose of IB delivered by CFC-pMDI with a similar safety profile.
Ram et alCitation50 performed a systematic review of seven randomized controlled clinical trials comparing Respimat SMI with other devices delivering aerosolized medications in patients with COPD, published before September 2010, and concluded that the Respimat inhaler does not provide any significant clinical benefit when compared with other handheld delivery devices. They found no differences in trough FEV1, trough FVC, peak FEV1, peak FVC, morning peak expiratory flow rate (PEFR), evening PEFR, exacerbations, or adverse events. The trials were of varying duration, used different endpoints, and studied various medications or combinations of medications at different concentrations including fenoterol plus ipratropium, ipratropium alone, tiotropium, ipratropium plus fenoterol, and ipratropium plus AB.
Patient response and perceptions
Besides physiologic efficacy, another critical element in determining the clinical utility of bronchodilator therapy is patients’ adherence to treatment; a highly effective medication that is not used has no clinical utility.Citation51 The factors that influence adherence to bronchodilator management are poorly understood but include patients’ attitudes and perceptions about bronchodilators and their delivery devices and patients’ experiences and reactions to their use.Citation52 Further, despite the evidence for differences in physiologic efficacy and deposition patterns, there is no overwhelming evidence to suggest that the type of inhaler device alters clinical outcomes in patients with COPD.Citation53–Citation55 Two assessments of inhaler satisfaction and preference have been developed, the Patient Device Experience Assessment and the Patient Satisfaction and Preference Questionnaire (PASAPQ).Citation56–Citation58 Hodder and Price reviewed studies of patients’ experiences with the Respimat SMI and found that the device is well accepted and patients preferred its performance to other respiratory medication delivery devices.Citation59 In a randomized, open-label, controlled, two-period, crossover study comparing IB/fenoterol delivered by Respimat SMI 20/50 mcg or HFA-pMDI 40/100 mcg in 245 patients with COPD, asthma, or mixed disease and a primary endpoint of inhaler preference, 162/201 (81%) subjects preferred Respimat SMI and 39/201 (19%) preferred HFA-pMDI.Citation60 At the end of the study, more patients were willing to continue using Respimat SMI (85) than HFA-pMDI (50). The overall satisfaction score was greater for Respimat SMI than for HFA-pMDI and appeared to be due to the subjects’ perceptions of performance rather than convenience. Clinical efficacy measured by evening PEFR, rescue inhaler use, and symptom scores was equivalent for the two treatments. In a randomized, parallel-group, double-dummy study comparing budesonide delivered by Respimat SMI (200 or 400 mcg twice daily [bid]) and Turbuhaler® (AstraZeneca, London, UK) dry powder inhaler (400 mcg bid) in 153 adults with moderate or severe asthma, subjects’ preferences were measured with the PASAPQ.Citation52 Total PASAPQ scores were 85.5 and 76.9 (P < 0.001) for Respimat SMI and Turbuhaler, respectively, and 112 (74%) subjects preferred Respimat SMI, 26 (17%) preferred Turbuhaler, and 14 (9%) had no preference. Performance scores but not convenience scores were different between the devices. Efficacy and safety data were not included in this report. It remains unknown whether the apparent preference of patients for Respimat SMI translates to increased adherence and better clinical outcomes.
Safety
Although the currently reported clinical trials evaluating the AB/IB Respimat SMI have reported no significant differences in adverse events, recent evaluations of tiotropium delivered by Respimat SMI suggest an increased risk of mortality.Citation61–Citation63 Singh et alCitation61 performed a meta-analysis of placebo-controlled, parallel-group, randomized trials of tiotropium delivered by Respimat SMI in patients with COPD and found an increased risk of mortality, with a relative risk (RR) of 1.52 (95% CI, 1.06, 2.15). The RR appeared dose dependent and was 2.15 (95% CI, 1.03, 4.51) and 1.46 (95% CI, 1.01, 2.10) for tiotropium 10 mg and 5 mg, respectively. At the 5 mg dose, one treatment-related death was estimated to occur for every 124 (95% CI, 52, 5682) COPD patients treated with tiotropium Respimat SMI. Two subsequent reviews found increased odds ratios (ORs) of all-cause mortality with tiotropium Respimat SMI of 1.47 (95% CI, 1.04, 2.08) and 1.51 (95% CI, 1.06, 2.19).Citation62,Citation63 Based on this evidence, Jenkins and BeasleyCitation64 concluded that “a recommendation can be made that the 5 and 10 mg preparations of tiotropium Respimat® should not be prescribed in the treatment of COPD.”
Several studies have suggested that IB is associated with an increased risk of cardiovascular morbidity and mortality in individuals with COPD.Citation65–Citation68 In a study of over 80,000 veterans, treatment with an anticholinergic within the previous 6 months (>99% IB and in a fixed-dose combination with AB in 38%) was associated with an increased risk of cardiovascular events.Citation68 In a large cohort of Canadians with COPD, the rate of cardiac dysrhythmias was increased significantly with initial treatment with short-acting (RR, 1.27; 95% CI, 1.03, 1.57) and long-acting beta agonists (RR, 1.47; 95% CI, 1.01, 2.15) and insignificantly with IB (RR, 1.23; 95% CI, 0.95, 1.57).Citation69 In a Taiwanese population-based, nested, case-control study of individuals with recently diagnosed COPD, ipratropium was associated with an increased risk of stroke (adjusted OR, 2.02; 95% CI, 1.71, 2.41) and the risk was further increased with concomitant treatment with a short-acting beta agonist (adjusted OR, 2.18; 95% CI, 1.81, 2.62).Citation70 However, none of these studies examined the Respimat SMI delivery system and most did not analyze fixed combination therapy with AB and IB, so further safety monitoring of combined AB and IB delivered by Respimat SMI is warranted to determine whether this delivery system may alter the safety profile of combined AB and IB treatment in individuals with COPD.
Conclusion
Although the combination of IB and AB has been repackaged in an environmentally friendly, innovative device that is more efficient, requiring half the dose for an equivalent physiologic effect with no apparent significant change in adverse events, the mixture of IB and AB itself is the oldest combination of aerosolized respiratory medications. Current recommendations for the use of AB in patients with COPD are to use short-acting beta agonists on an as-needed basis for symptomatic relief rather than on a fixed dosing schedule. In contrast, it is recommended that IB be used four times daily on a regular basis. These conflicting dosing schedules for AB and IB are at variance with the fixed dosing schedule for the combination of AB and ipratropium in Combivent MDI or Combivent Respimat. The Respimat SMI delivery device is an efficient, well-tolerated system for the delivery of aerosolized AB and IB to patients with COPD that produces equivalent physiologic effects at approximately half the dose delivered by pMDIs. However, the potential advantages of this novel system to deliver a fixed combination of two short-acting medications with different dosing schedules should be balanced by alternative therapeutic options that provide longer-acting, less frequently dosed respiratory medications.Citation71
Disclosure
The author declares no conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD): Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease2013 Available from: http://www.goldcopd.orgAccessed Dec 2012
- BarnesPJCelliBRSystemic manifestations and comorbidities of COPDEur Respir J20093351165118519407051
- DecramerMRennardSTroostersTCOPD as a lung disease with systemic consequences – clinical impact, mechanisms, and potential for early interventionCOPD20085423525618671149
- AgustiASorianoJBCOPD as a systemic diseaseCOPD20085213313818415812
- FabbriLMLuppiFBeghéBRabeKFComplex chronic comorbidities of COPDEur Respir J200831120421218166598
- van EedenSFSinDDChronic obstructive pulmonary disease: a chronic systemic inflammatory diseaseRespiration200875222423818042978
- MidgleyTJrHeeneALOrganic fluorides as refrigerantsInd Eng Chem1937225542545
- ZhongWHistory of CFCs as refrigerants Accessed http://zwhudson.myweb.uga.edu/chem8290/replacement%20of%20CFCs%20as%20refrigerants.htmAccessed March 11, 2013
- TsaiWTAn overview of environmental hazards and exposure risk of hydrofluorocarbons (HFCs)Chemosphere200561111539154715936055
- LovelockJEAtmospheric fluorine compounds as indicators of air movementsNature1971230379
- LeachCLThe CFC to HFA Transition and its impact on pulmonary drug developmentRespir Care20055091201120616122403
- RowlandFSStratospheric ozone depletionPhilos Trans R Soc Lond B Biol Sci2006361146976979016627294
- FarmanJSGardinerBGShanklinJDLarge losses of total ozone in Antarctic reveal seasonal ClOx/NOx interactionNature1985315207210
- KerrRAAnother deep Antarctic ozone holeScience1990250497937017793009
- Ozone hole through the years [web page on the Internet]Greenbelt, MDEarth Observatory2011 Available from: http://earthobservatory.nasa.gov/IOTD/view.php?id=49040Accessed Dec 2012
- MolinaMJRowlandFSStratospheric sink for chlorofluoromethanes: chlorine atom-catalysed destruction of ozoneNature1974249810814
- ButtATKamatDPansareMFinal count down to hfa albuterol inhalers: are we ready as yet?Clin Pediatr (Phila)200948213113419015282
- GribbenJThe Hole in the Sky: Man’s Threat to the Ozone LayerLondonCorgi1987
- RogersHWWeinstockMAHarrisARIncidence estimate of nonmelanoma skin cancer in the United States, 2006Arch Dermatol2010146328328720231499
- KohHKGellerACMillerDRGrossbartTALewRAPrevention and early detection strategies for melanoma and skin cancer. Current statusArch Dermatol199613244364428629848
- ReedKBBrewerJDLohseCMBringeKEPruittCNGibsonLEIncreasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, MinnesotaMayo Clin Proc201287432833422469345
- van der LeunJCTakizawaYLongstrethJDHuman healthUnited Nations Environment ProgrammeEnvironmental Effects Panel ReportNairobiUnited Nations Environment Programme19891124 Available from: http://www.ciesin.org/docs/001-538/001-538.htmlAccessed Dec 2012
- WoodcockAThe Montreal Protocol: getting over the finishing line?Lancet2009373966570570619249618
- VeldersGJAndersenSODanielJSFaheyDWMcFarlandMThe importance of the Montreal Protocol in protecting climateProc Natl Acad Sci USA2007104124814481917360370
- AndersonSOSarmaKMTaddonioKNBackground of the ozone and climate agreementsTechnology Transfer for the Ozone Layer: Lessons for Climate ChangeLondonEarthscan20072343
- ZeidlerMCorrenJHydrofluoroalkane formulations of inhaled corticosteroids for the treatment of asthmaTreat Respir Med200431354415174892
- DockhornRJWagnerDEBurgessGLProventil HFA provides protection from exercise-induced bronchoconstriction comparable to proventil and ventolinAnn Allergy Asthma Immunol199779185889236507
- ClarkAThe physics of aerosol formation by MDIs: limitations of the current approachJ Biopharm Sci1992316976
- HarrisonEChange in the air. Banning CFC-driven inhalers could levy a toll on asthma sufferersSci Am200829922022
- NewmanSPPrinciples of metered-dose inhaler designRespir Care20055091177119016122401
- DalbyRNEicherJZierenbergBDevelopment of Respimat(®) Soft Mist™ Inhaler and its clinical utility in respiratory disordersMed Devices (Auckl)2011414515522915941
- DalbyRSpallekMVoshaarTA review of the development of Respimat Soft Mist InhalerInt J Pharm20042831–21915363496
- van NoordJASmeetsJJCreemersJPGreefhorstLPDewberryHCornelissenPJDelivery of fenoterol via Respimat, a novel ‘soft mist’ inhaler. A randomised, double-blind (within device), placebo-controlled, cross-over, dose-ranging study in asthmatic patientsRespiration200067667267811124651
- LongestPWHindleMChoudhuriSDByronPRNumerical simulations of capillary aerosol generation: CFD model development and comparisons with experimental dataAerosol Sci Technol20074110952973
- LongestPWHindleMChoudhuriSDXiJComparison of ambient and spray aerosol deposition in a standard induction port and more realistic mouth–throat geometryJ Aerosol Sci2008397572591
- NewmanSPPaviaDMorénFSheahanNFClarkeSWDeposition of pressurised aerosols in the human respiratory tractThorax198136152557292382
- NewmanSPAerosol deposition considerations in inhalation therapyChest198588Suppl 2152S160S3893925
- SteinSWGabrioBJUnderstanding Throat Deposition During Cascade Impactor TestingRNDalbyPRByronSJFarrJPeartRespiratory Drug Delivery VIISerentecRaleigh, NC2000573576
- LongestPWHindleMQuantitative analysis and design of a spray aerosol inhaler. Part 1: effects of dilution air inlets and flow pathsJ Aerosol Med Pulm Drug Deliv200922327128319466904
- HochrainerDHölzHKreherCScaffidiLSpallekMWachtelHComparison of the aerosol velocity and spray duration of Respimat Soft Mist inhaler and pressurized metered dose inhalersJ Aerosol Med200518327328216181002
- KhachikianDVA Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist ExecutivesIpratropium/Albuterol (Combivent® Respimat®) Abbreviated Review2012 Available from: http://www.pbm.va.gov/ClinicalGuidance/AbbreviatedReviews/Ipratropium-albuterol(CombiventRespimat).docAccessed February 12, 2013
- NewmanSPSteedKPReaderSJHooperGZierenbergBEfficient delivery to the lungs of flunisolide aerosol from a new portable hand-held multidose nebulizerJ Pharm Sci19968599609648877887
- NewmanSPBrownJSteedKPReaderSJKladdersHLung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines: comparison of RESPIMAT with conventional metered-dose inhalers with and without spacer devicesChest199811349579639554631
- PitcairnGReaderSPaviaDNewmanSDeposition of corticosteroid aerosol in the human lung by Respimat Soft Mist inhaler compared to deposition by metered dose inhaler or by Turbuhaler dry powder inhalerJ Aerosol Med200518326427216181001
- BrandPHedererBAustenGDewberryHMeyerTHigher lung deposition with Respimat Soft Mist inhaler than HFA-MDI in COPD patients with poor techniqueInt J Chron Obstruct Pulmon Dis20083476377019281091
- Boehringer IngelheimFrequently asked questions about COMVIVENT RESPIMAT [web page on the Internet]IngelheimBoehringer Ingelheim Available from: http://www.combivent.com/respimat/faq.htmlAccessed Jan 2013
- ZuwallackRDe SalvoMCKaelinTCombivent Respimat Inhaler Study GroupEfficacy and safety of ipratropium bromide/albuterol delivered via Respimat inhaler versus MDIRespir Med201010481179118820172704
- KilfeatherSAPonitzHHBeckEImproved delivery of ipratropium bromide/fenoterol from Respimat Soft Mist Inhaler in patients with COPDRespir Med200498538739715139567
- IaconoPVelicitatPGuemasELeclercVThébaultJJImproved delivery of ipratropium bromide using Respimat (a new soft mist inhaler) compared with a conventional metered dose inhaler: cumulative dose response study in patients with COPDRespir Med200094549049510868713
- RamFSCarvallhoCRWhiteJClinical effectiveness of the Respimat inhaler device in managing chronic obstructive pulmonary disease: evidence when compared with other handheld inhaler devicesInt J Chron Obstruct Pulmon Dis2011612913921468167
- OsterbergLBlaschkeTAdherence to medicationN Engl J Med2005353548749716079372
- HodderRReesePRSlatonTAsthma patients prefer Respimat Soft Mist Inhaler to TurbuhalerInt J Chron Obstruct Pulmon Dis2009422523219554196
- RamFSBrocklebankDMMuersMWrightJJonesPWPressurised metered-dose inhalers versus all other hand-held inhalers devices to deliver bronchodilators for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20021CD00217011869627
- BrocklebankDRamFWrightJComparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literatureHealth Technol Assess2001526114911701099
- DolovichMBAhrensRCHessDRAmerican College of Chest PhysiciansAmerican College of Asthma, Allergy, and ImmunologyDevice selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and ImmunologyChest2005127133537115654001
- WelchMJNelsonHSShapiroGComparison of patient preference and ease of teaching inhaler technique for Pulmicort Turbuhaler versus pressurized metered-dose inhalersJ Aerosol Med200417212913915294063
- KozmaCMSlatonTLMonzBUHodderRReesePRDevelopment and validation of a patient satisfaction and preference questionnaire for inhalation devicesTreat Respir Med200541415215725049
- MonzBKozmaCReesePSlatonTHodderRPatient Satisfaction and Preference Questionnaire (PASAPQ) User ManualBoehringer Ingelheim International GmbH2005 [email protected]
- HodderRPriceDPatient preferences for inhaler devices in chronic obstructive pulmonary disease: experience with Respimat Soft Mist inhalerInt J Chron Obstruct Pulmon Dis2009438139019888356
- SchürmannWSchmidtmannSMoroniPMasseyDQidanMRespimat Soft Mist inhaler versus hydrofluoroalkane metered dose inhaler: patient preference and satisfactionTreat Respir Med200541536115725050
- SinghSLokeYKEnrightPFurbergCDPro-arrhythmic and pro-ischaemic effects of inhaled anticholinergic medicationsThorax201368111411622764216
- KarnerCChongJPoolePTiotropium versus placebo for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20127CD00928522786525
- DongYHLinHHShauWYChangCHLaiMSComparative safety of inhaled medications in patients with chronic obstructive pulmonary disease: systematic review and mixed treatment comparison meta-analysis of randomised controlled trialsThorax2013681485623042705
- JenkinsCRBeasleyRTiotropium Respimat increases the risk of mortalityThorax20136815723229813
- AnthonisenNRConnettJEEnrightPLManfredaJLung Health Study Research GroupHospitalizations and mortality in the Lung Health StudyAm J Respir Crit Care Med2002166333333912153966
- MacieCWooldrageKManfredaJAnthonisenNCardiovascular morbidity and the use of inhaled bronchodilatorsInt J Chron Obstruct Pulmon Dis20083116316918488440
- SinghSLokeYKFurbergCDInhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysisJAMA2008300121439145018812535
- OgaleSSLeeTAAuDHBoudreauDMSullivanSDCardiovascular events associated with ipratropium bromide in COPDChest20101371131919363211
- WilcheskyMErnstPBrophyJMPlattRWSuissaSBronchodilator use and the risk of arrhythmia in COPD: part 2: reassessment in the larger Quebec cohortChest2012142230531122871756
- WangMTTsaiCLLoYWLiouJTLeeWJLaiICRisk of stroke associated with inhaled ipratropium bromide in chronic obstructive pulmonary disease: a population-based nested case-control studyInt J Cardiol2012158227928422386700
- GordonJPanosRJInhaled albuterol/salbutamol and ipratropium bromide and their combination in the treatment of chronic obstructive pulmonary diseaseExpert Opin Drug Metab Toxicol20106338139220163324
