Abstract
Background
The purpose of this study was to update our network meta-analysis in order to compare the efficacy of indacaterol 75 μg with that of a fixed-dose combination of formoterol and budesonide (FOR/BUD) and a fixed-dose combination salmeterol and fluticasone (SAL/FP) for the treatment of chronic obstructive pulmonary disease (COPD) based on evidence identified previously in addition to two new randomized clinical trials.
Methods
Fifteen randomized, placebo-controlled clinical trials including COPD patients were evaluated: indacaterol 75 μg once daily (n = 2 studies), indacaterol 150 μg once daily (n = 5), indacaterol 300 μg once daily (n = 4), FOR/BUD 9/160 μg twice daily (n = 2), FOR/BUD 9/320 μg twice daily (n = 2), SAL/FP 50/500 μg twice daily (n = 4), and SAL/FP 50/250 μg twice daily (n = 1). All trials were analyzed simultaneously using a Bayesian network meta-analysis and relative treatment effects between all regimens were obtained. Treatment-by-covariate interactions were included where possible to improve the similarity of the trials. Outcomes of interest were trough forced expiratory volume in 1 second (FEV1) and transitional dyspnea index at 12 weeks.
Results
Based on the results without adjustment for covariates, indacaterol 75 μg resulted in a greater improvement in FEV1 at 12 weeks compared with FOR/BUD 9/160 μg (difference in change from baseline 0.09 L [95% credible interval 0.04–0.13]) and FOR/BUD 9/320 μg (0.07 L [0.03–0.11]) and was comparable with SAL/FP 50/250 μg (0.00 L [−0.07–0.07]) and SAL/FP 50/500 μg (0.01 L [−0.04–0.05]). For transitional dyspnea index, data was available only for indacaterol 75 μg versus SAL/FP 50/500 μg (−0.49 points [−1.87–0.89]).
Conclusion
Based on results of a network meta-analysis with and without covariates, indacaterol 75 μg is expected to be at least as efficacious as FOR/BUD (9/320 μg and 9/160 μg) and comparable with SAL/FP (50/250 μg and 50/500 μg) in terms of lung function. In terms of breathlessness (transitional dyspnea index) at 12 weeks, the results are inconclusive given the limited data.
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive disorder characterized by airway obstruction and reduced lung function. Symptoms include deteriorating health status and breathlessness, and treatments aim to prevent and control symptoms, reduce exacerbations, improve health status, and increase exercise tolerance.Citation1
It has been found that a significant number of patients receive fixed-dose combinations as a first-line treatmentCitation2–Citation5 despite recommendations by the Global Initiative for Chronic Obstructive Lung Disease to use a fixed-dose combination of a long-acting beta-agonist plus an inhaled steroid only for patients with a greater degree of airway obstruction or for patients who experience repeated exacerbations.Citation1 This evidence and the absence of head-to-head, randomized, controlled trials between indacaterol and fixed-dose combinations led to an indirect comparison of indacaterol 150/300 μg with fixed-dose combinations in a previously published systematic review and network meta-analysis by Cope et al in 2011.Citation6
Indacaterol 75 μg, a novel once-daily inhaled long-acting beta-agonist recently approved in the US,Citation7 is indicated for long-term, once-daily maintenance bronchodilator treatment of airflow obstruction in patients with COPD, including chronic bronchitis and/or emphysema. The objective of the current study is to compare the efficacy of indacaterol 75 μg with that of fixed-dose formoterol and budesonide twice daily (FOR/BUD) and fixed-dose salmeterol and fluticasone twice daily (SAL/FP) for the treatment of COPD patients using the same approach as Cope et al in 2011.Citation6 The evidence base in the current analysis is consistent with the previous publicationCitation6 but includes two additional indacaterol 75 μg randomized clinical trials.Citation8 The outcomes were lung function as measured by trough forced expiratory volume in 1 second (FEV1) and breathlessness as assessed by transition dyspnea index total score at 12 weeks. At the 12-week time point, there were insufficient data available across the included randomized clinical trials to assess St George’s Respiratory Questionnaire total score.
Materials and methods
Because patients were permitted to use stable inhaled corticosteroids during the indacaterol studies, only data for patients not using inhaled corticosteroids were included in the analyses for all treatment arms in order to ensure the placebo patients in the indacaterol trials were sufficiently similar to those in the fixed-dose combination studies (where inhaled corticosteroids were not permitted).
The systematic literature review published by Cope et al identified 11 randomized clinical trialsCitation9–Citation19 based on a search of Medline® and Embase®, including two Novartis studies of indacaterol by Dahl et alCitation9 (B2334Citation20) and Feldman et alCitation10 (B2346Citation21). This review also included four randomized clinical trials from the indacaterol clinical trial program (Novartis studies B2335SCitation22 published by Donohue et al,Citation23 B2336Citation24 published by Kornmann et al,Citation25 B1302Citation26 published by Kinoshita et al,Citation27 and B2333Citation28). The current analyses was based on the same evidence base, except that two studies evaluating indacaterol 75 μg versus placebo (Novartis studies B2354 and B2355)Citation8 were added to the network and two studies by Calverley et al in 2003Citation13 and 2007Citation14 were excluded because no data were available for the current outcomes of interest. The updated network of evidence is presented in .
Figure 1 Updated network of evidence.
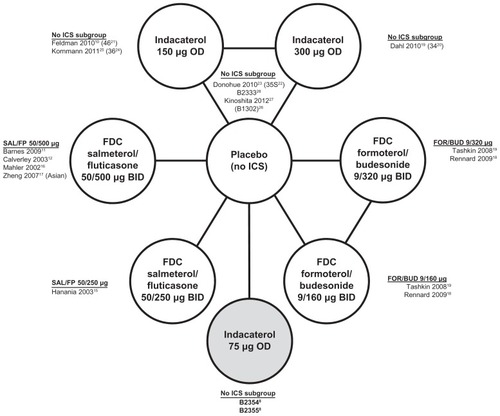
All studies were multicenter, randomized, placebo-controlled trials with a parallel design and included adult patients with COPD. The studies included patients 40 years of age or older with FEV1/forced vital capacity ≤ 0.70 and FEV1 percent predicted <80%, while the indacaterol trials required patients to have a predicted FEV1 ≥ 30%. Most studies included patients who were current or exsmokers with a smoking history of at least 10 years, although some studies included patients with a smoking history of at least 20 years (Hanania et al,Citation15 Mahler et al,Citation16 B2334,Citation20 B2346,Citation21 B2335S,Citation22 and B2336Citation24). Three studies included predominantly Asian patients (Zheng et al,Citation17 and studies B1302Citation26 and B2333Citation28), whereas the remaining studies included mostly Caucasian patients or reported study centers in Europe and the US. For additional detail regarding the study and patient characteristics, please see Cope et al.Citation6
For the two additional randomized clinical studies of indacaterol 75 μg (B2354 and B2355),Citation8 details were extracted on study design, population characteristics, and interventions. For the subgroup of patients included in the analysis who did not receive concomitant inhaled corticosteroids, data on file were provided by Novartis for the average results per treatment subgroup. The difference and associated standard error (SE) in trough FEV1 change from baseline at 12 weeks between indacaterol 75 μg and placebo were extracted for B2354 (difference 0.14 L, SE 0.025 L) and B2355 (difference 0.18 L, SE 0.026 L), as well as for transitional dyspnea index at 12 weeks from both studies (B2354, difference 1.44 points, SE 0.46 points; B2355, difference 0.49 points, SE 0.41 points).
Bayesian network meta-analysis was performed to synthesize the results of the included studies simultaneously regarding change from baseline in FEV1 and the transitional dyspnea index total score at 12 weeks to obtain relative efficacy estimates for indacaterol 75 μg versus FOR/BUD, SAL/FP, and placebo.Citation29–Citation31
A Bayesian network meta-analysis includes data, a likelihood distribution, a model with parameters, and prior distributions.Citation31 The model links the data from the individual studies to basic parameters, which represent the (pooled) relative treatment effect of each treatment versus placebo. The relative efficacy between each of the competing interventions was estimated as a function of the basic parameters. A regression model with a normal likelihood distribution was usedCitation30,Citation31 and both fixed and random effect models were tested. The residual deviance was used to select a fixed or random effects model.Citation32
Since randomization only holds within a trial and not across trials in a network meta-analysis, there is the risk that patients assessed in different comparisons are not similar, which leads to consistency violations. Therefore treatment-by-covariate interactions were incorporated in the models to minimize confounding bias.Citation33 Covariates potentially causing bias were selected based on the most influential covariates in the previous analyses, which were included simultaneously, ie, the proportion of patients who are current smokers (as opposed to ex-smokers), and the proportion of patients with severe or very severe COPD (as opposed to mild or moderate COPD). The results of the network meta-analysis provide relative treatment effects of each treatment versus a competing intervention. Noninformative prior distributions were used to avoid prior beliefs influencing the results of the model, consistent with previous analyses.Citation6
WinBUGS 1.4.1 software was used for the statistical analysis.Citation34 Summary statistics are presented for the relative treatment effects (ie, differences in transitional dyspnea index or the differences in the change from baseline for FEV1) and the 95% credible intervals, which reflects the range of true underlying effects with 95% probability. Since the posterior distribution can be directly interpreted in terms of probabilities, it was also possible to calculate the probability that indacaterol 75 μg is better than a certain regimen, which is one advantage of the Bayesian framework over the frequentist approach. Results are presented with and without adjustment for covariates for the change from baseline in FEV1 and transitional dyspnea index total score at 12 weeks.
Results
For trough FEV1 at 12 weeks, all treatments were more efficacious than placebo for the analyses without covariates, and results for indacaterol 150 μg versus placebo (difference in change from baseline 0.18 L [95% credible interval, 0.15–0.20]) and indacaterol 300 μg versus placebo (difference in change from baseline 0.17L [95% credible interval, 0.14–0.20]) were consistent with previous results. Based on the results without adjustment for covariates (), indacaterol 75 μg resulted in a greater change from baseline in FEV1 at 12 weeks compared with FOR/BUD 9/160 μg (0.09 L [0.04–0.13]) and FOR/BUD 9/320 μg (0.07 L [0.03–0.11]), and a comparable change from baseline for SAL/FP 50/250 μg (0.00 L [−0.07–0.07]) and SAL/FP 50/500 μg (0.01 L [−0.04–0.05]). Adjusting for differences in the proportion of current smokers and patients with severe or very severe COPD only had a minor impact on the point estimates for FEV1 at 12 weeks for indacaterol 75 μg versus the alternatives, although credible intervals were wider.
Table 1 Results of network meta-analysis for FEV1 and TDI at 12 weeks: indacaterol 75 μg versus alternatives without and with covariates
As with previous analyses of transitional dyspnea index at 6 months, SAL/FP 50/500 μg was more efficacious than placebo in terms of transitional dyspnea index at 12 weeks. Indacaterol 75 μg was at least as efficacious as placebo, with higher point estimates in the analyses without covariates (difference 0.90 [−0.01–1.81]) and with covariates (difference 0.81 [−0.37–2.00]). Comparative estimates versus FOR/BUD and SAL/FP 50/250 were not possible given the lack of data at 12 weeks. Indacaterol 75 μg had numerically lower transitional dyspnea index scores compared with SAL/FP 50/500 μg (difference −0.49 [−1.87–0.89]), but the credible interval included zero (). When results were adjusted for covariates, results were less favorable for indacaterol, reducing the point estimate to −1.80 versus SAL/FP 50/500 μg. A strong interpretation is not possible due to the large amount of uncertainty in these estimates, suggesting the results are inconclusive.
Discussion
The objective of this study was to update a previously published network meta-analysis by Cope et alCitation6 in order to compare the efficacy of indacaterol 75 μg once a day versus fixed-dose combinations of FOR/BUD and SAL/FP twice daily for COPD in terms of trough FEV1 and transitional dyspnea index total score. In the US, SAL/FP 50/250 μg twice daily and FOR/BUD 4.5/160 μg × two inhalations (ie, 9/320 μg) twice daily are the approved doses for COPD. Indacaterol 75 μg was at least as efficacious as FOR/BUD (9/160 μg and 9/320 μg) in terms of FEV1, and comparable with SAL/FP (50/250 μg and 50/500 μg). In terms of transitional dyspnea index total score at 12 weeks, results for indacaterol 75 μg versus SAL/FP 50/500 μg do not permit a strong interpretation given the uncertainty in the estimates. Moreover, there was no transitional dyspnea index data available at 12 weeks in order to compare indacaterol 75 μg with the approved fixed-dose combinations in the US. Indacaterol 150 μg and 300 μg estimates were consistent with the previous analysis for FEV1 at 12 weeks, suggesting that these doses are expected to be at least as good as FOR/BUD (9/320 μg and 9/160 μg) and comparable with SAL/FP (50/250 μg and 50/500 μg). There were some differences in the results for transitional dyspnea index at 12 weeks as compared with transitional dyspnea index at 6 months in the previous analyses, although indacaterol 150 μg and 300 μg are still expected to provide comparable improvements to those of SAL/FP 50/500 μg.
Randomized clinical trials form the basis of the network and allow for indirect comparisons in the absence of head-to-head comparisons. However, to yield meaningful results, the trials must be sufficiently similar. If there are systematic differences in study and patient characteristics across the different direct comparisons, and these differences are modifiers of the relative treatment effects, then the estimate of the indirect and mixed comparisons is biased.Citation30 In the indacaterol studies, patients were allowed to continue receiving concurrent inhaled corticosteroids, which was not the case in the FOR/BUD and SAL/FP studies. To avoid biased estimates of indacaterol versus FOR/BUD and SAL/FP, only a subgroup of patients who did not receive concurrent inhaled corticosteroids in the indacaterol studies were evaluated in the network meta-analysis. Meta-regression models were used to adjust for possible differences across studies in terms of the proportion of current smokers and the proportion of patients with severe or very severe COPD. Differences between adjusted and unadjusted models were not greater than the amount of uncertainty in the estimates and therefore lead to consistent interpretation. However, it was not possible to assess the similarity of the studies in terms of all patient characteristics. For example, limited information was presented with respect to the ethnicity of patients across the trials, although previous analyses suggest ethnicity was not an important factor. Similarly, there were insufficient data presented to evaluate the comorbidities of patients across the trials. Therefore, it has to be accepted that with aggregate level data there is the risk of residual confounding bias.
The current analysis focuses on the efficacy of indacaterol 75 μg in terms of FEV1 and transitional dyspnea index at 12 weeks. However, decision-makers should also consider additional patient-relevant endpoints. It was not feasible to perform a network meta-analysis for St George’s Respiratory Questionnaire (as was performed previously at 6 months) or for rescue medication use given the data available for the current evidence base, although indacaterol 75 μg was associated with significant improvements in comparison to placebo at 12 weeks for both of these outcomes. Given the existing trials for indacaterol 75 μg are 12 weeks long, it is not possible to evaluate efficacy beyond this time point. Finally, treatments should also be assessed in terms of their safety, which was not evaluated in the current study.
In conclusion, based on results of a network meta-analysis with and without covariates, indacaterol 75 μg is expected to be at least as efficacious as FOR/BUD (9/320 μg and 9/160 μg) and comparable with SAL/FP (50/250 μg and 50/500 μg) in terms of lung function (trough FEV1). In terms of breathlessness (transitional dyspnea index) at 12 weeks, results are inconclusive given the limited data.
Acknowledgment
The authors acknowledge the contribution of Rupert Gale and Jose Jardim to the original study.
Disclosure
The study was funded by Novartis Pharma AG. MK, GC-N, and JZ are employed by Novartis and SC and JPJ received funding from Novartis for this study. An abstract of this work was accepted for the American Thoracic Society conference in May 2012.
References
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of COPD2010 Available at: http://www.goldcopd.com/Guidelineitem.asp?l1=2&l2=1&intId=989Accessed June 1, 2011
- KatzPMPegoraroVL’utilizzo dei corticosteroidi nei pazienti con la broncopneumopatia cronica ostruttiva: aspetti epidemiologici ed economiciFarmeconomia e percorsi terapeutici2009104139148
- FitchKIwasakiKPyensonBPlauschinatCZhangJVariation in adherence with Global Initiative for Chronic Obstructive Lung Disease (GOLD) drug therapy guidelines: a retrospective actuarial claims data analysisCurr Med Res Opin20112771425142921599554
- JochmannANeubauerFMiedingerDGeneral practitioners’ adherence to the COPD GOLD guidelines: baseline data from the Swiss COPD cohortSwiss Med Wkly4212010 [Epub ahead of print.]
- JooMJLeeTABartleBvan de GraaffWBWeissKBPatterns of healthcare utilization by COPD severity: a pilot studyLung2008186530731218463921
- CopeSCapkun-NiggliGGaleRJardimJRJansenJPComparative efficacy of indacaterol 150 μg and 300 μg versus fixed-dose combinations of formoterol + budesonide or salmeterol + fluticasone for the treatment of chronic obstructive pulmonary disease – a network meta-analysisInt J Chron Obstruct Pulmon Dis2011632934421697997
- US Drug and Food AdministrationNews release Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm261649.htmAccessed May 3, 2012
- KerwinEMGotfriedMHLawrenceDLassenCKramerBEfficacy and tolerability of indacaterol 75 μg once daily in patients aged ≥ 40 years with chronic obstructive pulmonary disease: results from 2 double-blind, placebo-controlled 12-week studiesClin Ther201133121974198422177371
- DahlRChungKFBuhlREfficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPDThorax201065647347920522841
- FeldmanGSilerTPrasadNEfficacy and safety of indacaterol 150 microg once-daily in COPD: a double-blind, randomised, 12-week studyBMC Pulm Med201010111920051135
- BarnesNQiuYPavordIAnti-inflammatory effects of salmeterol/fluticasone propionate in chronic obstructive lung diseaseAm J Respir Crit Care Med2006173773674316424444
- CalverleyPPauwelsRVestboJrCombined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trialLancet2003361935644945612583942
- CalverleyPMBoonsawatWCsekeZZhongNPetersonSOlssonHMaintenance therapy with budesonide and formoterol in chronic obstructive pulmonary diseaseEur Respir J200322691291914680078
- CalverleyPAndersonJCelliBSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med2007356877578917314337
- HananiaNDarkenPHorstmanDThe efficacy and safety of fluticasone propionate (250 mug)/salmeterol (50 mug) combined in the Diskus inhaler for the treatment of COPDChest2003124383484312970006
- MahlerDWirePHorstmanDEffectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200216681084109112379552
- ZhengJYangLWuYThe efficacy and safety of combination salmeterol (50 mug)/fluticasone propionate (500 mug) inhalation twice daily via accuhaler in Chinese patients with COPDChest200713261756176317951625
- RennardSITashkinDMcElhattanJEfficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trialDrugs200969554956519368417
- TashkinDPRennardSIMartinPEfficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6-month randomized clinical trialDrugs200868141975200018778120
- JackDBleasdalePBerhaneIHigginsMFull Clinical Study Report for study number CQAB149B2334: a 52-week treatment, multi-center, randomized, doubleblind, double dummy, placebo-controlled, parallel-group study to assess the efficacy, safety and tolerability of indacaterol (300 and 600 μg OD) in patients with chronic obstructive pulmonary disease, using formoterol (12 μg BID) as an active controlData on file: 2008
- PrasadNPiggottSHigginsMYuTFull Clinical Study Report for study number CQAB149B2346: a 12-week treatment, multi-center, randomized, doubleblind, placebo-controlled, parallel-group study to assess the efficacy and safety of indacaterol (150 μg OD) in patients with chronic obstructive pulmonary diseaseData on file: 2008
- IqbalALeanHLawrenceDHigginsMFull Clinical Study Report for study number CQAB149B2335S: a 26-week treatment, multi-center, randomized, doubleblind, double dummy, placebo-controlled, adaptive, seamless, parallel-group study to assess the efficacy, safety and tolerability of two doses of indacaterol (selected from 75, 150, 300 and 600 μg OD) in patients with chronic obstructive pulmonary disease using blinded formoterol (12 μg BID) and open label tiotropium (18 μg OD) as active controlsData on file: 2008
- DonohueJFFogartyCLotvallJOnce-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropiumAm J Respir Crit Care Med2010182215516220463178
- LuthraAKramerBSwalesJHenleyMLassenCFull Clinical Study Report for study number CQAB149B2336: a 26-week treatment, multi-center, randomized, doubleblind, double dummy, placebo controlled, parallel-group study to assess the efficacy and safety of indacaterol (150 μg OD) in patients with chronic obstructive pulmonary disease, using salmeterol (50 μg BID) as an active controlData on file: 2009
- KornmannODahlRCentanniSOnce-daily indacaterol vs twice-daily salmeterol for COPD: a placebo-controlled comparisonEur Respir J201137227327920693243
- HosoeMOkinoNMaruyamaYFull Clinical Study Report for study number CQAB149B1302: a 12-week treatment, multi-center, randomized, doubleblind, placebo-controlled, parallel-group study to assess the efficacy, safety and tolerability of indacaterol (150 and 300 ìg OD) in patients with chronic obstructive pulmonary disease (COPD)Data on file: 2010
- KinoshitaMLeeSHHangLWEfficacy and safety of indacaterol 150 and 300 μg in chronic obstructive pulmonary disease (COPD) patients from six Asian areas including Japan: A 12-week, placebo controlled studyRespirology201217237938922122202
- FirthRHenleyMKramerBLassenCYangWOwenRFull Clinical Study Report for study number QAB149B2333: a phase III, 26-week multi-center randomized doubleblind, placebo-controlled, parallel-group study to assess the efficacy, safety and tolerability of indacaterol (150 and 300 ìg OD) in patients with chronic obstructive pulmonary diseaseData on file: 2010
- CaldwellDMAdesAEHigginsJPSimultaneous comparison of multiple treatments: combining direct and indirect evidenceBMJ2005331752189790016223826
- LuGAdesAECombination of direct and indirect evidence in mixed treatment comparisonsStat Med200423203105312415449338
- JansenJCrawfordBBergmanGStamWBayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisonsValue Health2008115966964
- DempsterAPThe direct use of likelihood for significance testingStat Comput199774247252
- CooperNJSuttonAJMorrisDAdesAEWeltonNJAddressing between-study heterogeneity and inconsistency in mixed treatment comparisons: application to stroke prevention treatments in individuals with non-rheumatic atrial fibrillationStat Med200928141861188119399825
- LunnDJThomasABestNSpiegelhalterDWinBUGS – a Bayesian modelling framework: concepts, structure, and extensibilityStat Comput200010325337
