Abstract
Background
Coadministration of mometasone furoate (MF) and formoterol fumarate (F) produces additive effects for improving symptoms and lung function and reduces exacerbations in patients with asthma and chronic obstructive pulmonary disease (COPD). The present study assessed the relative systemic exposure to MF and characterized the pharmacokinetics of MF and formoterol in patients with COPD.
Methods
This was a single-center, randomized, open-label, multiple-dose, three-period, three-treatment crossover study. The following three treatments were self-administered by patients (n = 14) with moderate-to-severe COPD: MF 400 μg/F 10 μg via a metered-dose inhaler (MF/F MDI; DULERA®/ZENHALE®) without a spacer device, MF/F MDI with a spacer, or MF 400 μg via a dry-powder inhaler (DPI; ASMANEX® TWISTHALER®) twice daily for 5 days. Plasma samples for MF and formoterol assay were obtained predose and at prespecified time points after the last (morning) dose on day 5 of each period of the crossover. The geometric mean ratio (GMR) as a percent and the corresponding 90% confidence intervals (CI) were calculated for treatment comparisons.
Results
Systemic MF exposure was lower (GMR 77%; 90% CI 58, 102) following administration by MF/F MDI compared to MF DPI. Additionally, least squares geometric mean systemic exposures of MF and formoterol were lower (GMR 72%; 90% CI 61, 84) and (GMR 62%; 90% CI 52, 74), respectively, following administration by MF/F MDI in conjunction with a spacer compared to MF/F MDI without a spacer. MF/F MDI had a similar adverse experience profile as that seen with MF DPI. All adverse experiences were either mild or moderate in severity; no serious adverse experience was reported.
Conclusion
Systemic MF exposures were lower following administration by MF/F MDI compared with MF DPI. Additionally, systemic MF and formoterol exposures were lower following administration by MF/F MDI with a spacer versus without a spacer. The magnitude of these differences with respect to systemic exposure was not clinically relevant.
Introduction
Current treatment guidelines for the long-term management of chronic obstructive pulmonary disease (COPD) and asthma recommend, for certain degrees of severity, combination therapy with an inhaled corticosteroid (ICS) and a long-acting β2-agonist.Citation1–Citation5 Clinically, coadministration of an ICS and long-acting β2-agonist has been shown to have additive effects for improving symptoms and lung function and reducing the frequency of disease exacerbations.Citation1–Citation5
Mometasone furoate (MF) is a potent ICS with relatively low potential to cause significant systemic side effects typically associated with oral corticosteroids, such as hypothalamic-pituitary-adrenal axis suppression.Citation6–Citation8 MF has been shown to produce clinical benefit for treating asthma and COPD by reducing symptoms and exacerbations, and improving lung function, with no significant safety risks.Citation6–Citation10 Formoterol fumarate (F) is a potent, selective, long-acting β2-agonist that exerts a preferential effect on β2-adrenergic receptors of bronchial smooth muscle.Citation11 Bronchodilator activity observed in patients with asthma after F inhalation is characterized by a rapid onset (within 3 minutes of inhalation) and long duration (at least 12 hours) of action.Citation11 F is approved for maintenance treatment in patients with asthma and COPD. Merck Sharp & Dohme Corp (Whitehouse Station, NJ, USA) and Novartis (East Hanover, NJ, USA) have jointly developed a fixed-dose combination product combining MF and F in a metered-dose inhaler device (MF/F MDI; marketed as DULERA® in the United States and ZENHALE® in Canada and elsewhere; Merck & Co. Inc., Whitehouse Station, NJ, USA) for the treatment of asthma. MF/F MDI is also in late-stage clinical development for the treatment of patients with COPD. In addition to producing additive beneficial effects on symptoms and lung function, an MF/F combination product is expected to be more convenient for patients with asthma or COPD.
Due to the effects of decreases in lung function on exposure to inhaled products in patients with COPD, the systemic exposure and pharmacokinetics of MF and formoterol in these patients were expected to differ from healthy volunteers, as has been reported for other ICSs.Citation12,Citation13 A previous pharmacokinetic study showed lower mean (area under the curve [AUC]; 25%; geometric mean ratio [GMR]: 75%; 90% confidence interval [CI]: 61%–91%; mean maximum concentration [Cmax] 39% [GMR: 61%; 90% CI: 49%–75%]) systemic exposure of MF after steady-state dosing from the MDI compared to the dry-powder inhaler (DPI) ASMANEX® TWISTHALER® ([MF] Merck Sharp & Dohme Corp) in healthy patients (data on file, Merck Sharp & Dohme Corp, 2010). Similar differences in systemic drug exposure with MDIs and DPIs have been reported for other ICSs.Citation14,Citation15
As part of the clinical development program for MF/F MDI for the treatment of patients with moderate to severe COPD, the present study was conducted primarily to define the systemic exposure of MF when administered using a new combination product containing MF and F in an MDI device (MF/F MDI) versus the approved DPI monotherapy product (MF DPI) for which there is extensive clinical use and safety experience.Citation16 As a secondary objective, this study examined the potential effect of using a spacer device in conjunction with the MF/F MDI on MF and formoterol exposure (versus use of MDI device without a spacer). In addition, this study provided descriptive multiple-dose pharmacokinetic data for MF and formoterol in patients with COPD.
Methods
Patient selection
This study enrolled men and women between the ages of 40 and 75 years with the following inclusion criteria: moderate to severe COPD (as defined by post-bronchodilator forced expiratory volume in 1 second [FEV1] ≥30% and <80%) within 1 week prior to the baseline visit (day 1); current smoker or ex-smoker with at least 10 pack-years of smoking history; and receiving only albuterol/salbutamol for relief of symptoms for at least 2 weeks prior to randomization. Subjects were excluded from participation in this study based on the following criteria: increase in absolute volume of FEV1 of ≥400 mL within 30 minutes after administration of four inhalations of albuterol/salbutamol (total dose of 360 to 400 μg), or nebulized 2.5 mg albuterol/salbutamol; inability to use the MF/F MDI device or the MF DPI device; female patients who were pregnant, intended to become pregnant (within 3 months of ending the study), or were breastfeeding; history of any infectious disease within 4 weeks prior to drug administration; or tested positive for hepatitis B surface antigen, hepatitis C antibodies, or human immunodeficiency virus.
Study design
This was a randomized, open-label, multiple-dose, three-period, three-treatment crossover study conducted at a single study center. Subjects were screened within 21 days prior to dosing.
All patients were trained in the use of the devices and proper inhalation techniques using placebo, MDI, and DPI. If necessary, patients could be retrained in the proper use of these inhaler devices prior to the start of each period. Subjects were instructed by the investigator regarding when and how to take the daily treatment.
Subjects were admitted to the study center on day 1 to confirm continued eligibility and for baseline assessments. The investigator or designee reviewed the inclusion/exclusion criteria and recorded adverse events (AEs) and medications taken within the previous 14 days. A repeat drug and pregnancy screen, laboratory safety tests (hematology, blood chemistry, urinalysis, and electrocardiography), and vital signs also were performed on day 1. On day 1 of the first treatment period, after a 10-hour overnight fast, each patient was randomized to a crossover treatment sequence according to a computer-generated randomization schedule provided by the sponsor, and then received the first dose. The following three treatments were self-administered by patients: treatment A, MF 400 μg/F 10 μg twice a day (BID) via MDI oral inhalation (two puffs × 200 μg/5 μg MF/F per burst combination product); treatment B, MF 400 μg/F 10 μg BID via MDI oral inhalation and in conjunction with a spacer device (two puffs × 200 μg/5 μg MF/F per burst combination product); and treatment C, MF 400 μg BID via DPI oral inhalation (two puffs × 200 μg MF per oral inhalation from the MF DPI). Subjects self-administered the treatments under observation of the site staff for 4 days every 12 hours between approximately 8 am and 9 am and again between approximately 8 pm and 9 pm, and a single morning dose on day 5. After taking their treatment, subjects were instructed to rinse their mouth with water and then spit it out (not swallow it). Based on a previous pharmacokinetic study where the effective half-life (based on accumulation) of MF after administration from an MDI was approximately 25 hours, and since dosing for five half-lives is typically required to attain steady state conditions, a dosing period of 5 days was chosen for this study. Subjects were confined to the study center for the duration of treatment in each period of the crossover. After a 1-week washout between dosing, patients returned to start confinement for the next treatment period.
MF/F MDIs and placebo MDI devices (for the training of the inhalation technique) were manufactured by 3M Health Care Ltd (Loughborough, Leicestershire, UK, for Schering-Plough Corp [now Merck Sharp & Dohme Corp], Kenilworth, NJ, USA). The spacer device (AeroChamber Plus® Valved Holding Chamber; Monaghan Medical Corp, Plattsburgh, NY, USA) and MF DPI were obtained commercially by the site. Placebo DPI to match the MF DPI was manufactured and supplied by Schering-Plough Corp (now Merck Sharp & Dohme Corp).
The study population was identified from the surrounding urban and suburban communities of Madisonville, KY, USA. Subjects were recruited from a pool of volunteers obtained from the database of Commonwealth Biomedical Research, LLC and by word-of-mouth advertisement. The protocol and informed consent were approved by Independent Investigational Review Board, Plantation, FL, USA, as required by the US Code of Federal Regulations and the internal Standard Operating Procedures of the sponsor. The study was conducted in accordance with good clinical practice and was approved by the appropriate institutional review boards and regulatory agencies. Written consent was obtained from all patients prior to the conduct of any study related procedures.
Pharmacokinetic assessments
Pharmacokinetic parameters were calculated via noncompartmental analysis. Day 5 plasma concentrations and actual sampling times were used to calculate the following pharmacokinetic parameters of MF and formoterol: area under the plasma concentration–time curve from 0 to 12 hours (AUC0–12 hr), the maximum plasma concentration (Cmax), trough plasma concentration (Ctrough), and time to maximum plasma concentration (Tmax). Pharmacokinetic parameters were calculated using WinNonlin® software (v 5.0.1; Pharsight Corporation, Mountain View, CA, USA). AUC0–12 hr was calculated using the linear trapezoidal method for ascending concentrations and log trapezoidal method for descending concentrations. Values for Cmax, Ctrough, and Tmax were obtained by visual inspection of the blood concentration data.
For the determination of MF plasma concentration, whole blood was collected in K3-ethylenediaminetetraacetic acid-containing tubes at predose (0 hours) and at 0.5, 1, 2, 4, 8, and 12 hours after the morning dose on day 5 and centrifuged for 15 minutes at 1500 × g. Plasma was removed and stored in a freezer at −20°C. A 1 mL sample aliquot was fortified with 50 μL of internal standard (mometasone furoate-d3). Analytes were isolated through liquid–liquid extraction with 5.0 mL of 15:85 ethyl acetate/hexane, v/v. The extracts were further purified by solid phase extraction with Bond Elut® LRC NH2 cartridges (Agilent Technologies, Strathaven, Scotland). Analytes were eluted with 6.0 mL of 65:35 ethyl acetate/hexane, v/v. The solvent was evaporated under a nitrogen stream at approximately 50°C, and the remaining residue was reconstituted with 125 μL of methanol and 75 μL of 20 μM sodium acetate. The final extract was analyzed using a Sciex API 5000 triple quadrupole liquid chromatography with tandem mass spectrometry system equipped with an electrospray ionization source (AB SCIEX, Framingham, MA, USA) and having a lower limit of quantitation (LLOQ) of 0.250 pg/mL. The LC system employed a Luna (Phenomenex, Torrance, CA, USA) C18 3 × 150 mm (3 μm particle size) column and gradient elution. The retention time for both MF and the internal standard was approximately 14 minutes. The range of the standard curve using a 1.00 mL sample of human plasma was 0.250 to 25.0 pg/mL. At the LLOQ (0.250 pg/mL) for the MF assay, the between-day mean (standard deviation) was 0.253 (0.023) pg/mL, mean % bias was 1.10%, and the mean coefficient of variation was 8.95%.
For the determination of the plasma concentration of formoterol, whole blood was collected in tubes containing lithium heparin with eserine (physostigmine) hemisulfate as a preservative at predose (0 hours) and at 0.167 (10 minutes), 0.25, 0.5, 1, 2, 4, 8, and 12 hours after the morning dose on day 5 and centrifuged for 15 minutes at 1500 × g. Plasma was removed and stored in a freezer at −20°C. A 500 μL plasma sample aliquot was fortified with 20 μL of internal standard (formoterol-d6) and 200 μL of 2% ammonium hydroxide. Extraction solvent was added, and the tubes were vortexed and centrifuged. The aqueous layer was frozen and the organic layer was decanted to a clean tube containing keeper solution. The organic solution was evaporated and the remaining residue was reconstituted with 200 μL of reconstitution solution. A 40-μL volume of the final extract was injected and analyzed using a Sciex API 5000 triple quadrupole liquid chromatography with tandem mass spectrometry system equipped with a turbo ion spray source (AB SCIEX) and having an LLOQ of 1.45 pmol/L. The LC system employed a 10 × 3 mm (5 μm particle size) Thermo BETASIL Silica-100 loading column (Thermo Fisher Scientific, Waltham, MA, USA) and a 50 × 3 mm (5 μm particle size) Thermo BETASIL Silica-100 analytical column. Formoterol and the internal standard were separated from the other plasma components using a mobile phase A consisting of 0.1% formic acid in 10 mM ammonium formate and a mobile phase B consisting of a 95:5 acetonitrile:mobile Phase A. The retention time for both formoterol and the internal standard were approximately 2.5 minutes. The range of the standard curve using a 500 μL sample of human plasma was 1.45 pmol/L to 727 pmol/L. At the LLOQ (1.45 pmol/L) for the formoterol assay, the between-day mean (standard deviation) was 1.44 (0.135) pmol/L, mean % bias was −1.23%, and the mean % coefficient of variation was 9.37%.
Safety assessments
Clinical laboratory tests, vital signs, electrocardiography, and physical examinations were assessed at screening and clinical laboratory tests and vital signs at prespecified times during the study. Subjects were continually monitored for possible occurrence of AEs. At study conclusion (day 6 of period 3), vital signs, clinical laboratory tests, and physical examinations were repeated.
Statistical analysis
Summary statistics including means and coefficients of variation were provided for MF concentration data at each time point and the derived pharmacokinetic parameters. The primary objective was to compare the MF AUC0–12 hr and Cmax values for the MF/F MDI combination with those for MF DPI monotherapy (ie, treatment A versus treatment C, respectively). The AUC0–12 hr and Cmax values were log-transformed and analyzed using an appropriate analysis of variance (ANOVA) model for a three-period crossover design extracting sources of variation due to treatment, patient, sequence, and period. As a secondary objective, the MF AUC0–12 hr and Cmax values for the MF/F MDI combination administered without and with a spacer were compared (ie, treatment A versus treatment B). The geometric means of treatment A to treatment C or treatment A to treatment B were expressed as a ratio (GMR), and the corresponding 90% CIs were computed. In addition, the log-transformed AUC0–12 hr and Cmax values for formoterol were analyzed similarly for treatments A and B, and comparisons between treatment A and treatment B were performed using a ratio and the corresponding 90% CI.
Assuming an intrapatient variability of 28% (based on the variability observed in a similarly designed crossover study in healthy adults), this study with a targeted sample size of 12 patients should have been able to detect a 30% difference in MF pharmacokinetics between treatments B or C versus A with 80% power and a 90% CI.
No inferential analysis of safety data was planned. The number of patients reporting any AEs, the occurrence of specific AEs, and discontinuation due to AEs were tabulated.
Results
Demographic and baseline characteristics
A total of 14 patients (five men and nine women) aged 45 to 72 years (mean, 62.7 years) were treated. Of these, 13 (93%) were white and one (7%) was black/African-American. Subjects had a mean (range) 50 (20–100) pack-year smoking history and a mean (range) predicted post-bronchodilator FEV1 of 58% (42%–73%). All 14 patients completed the study.
Pharmacokinetic results
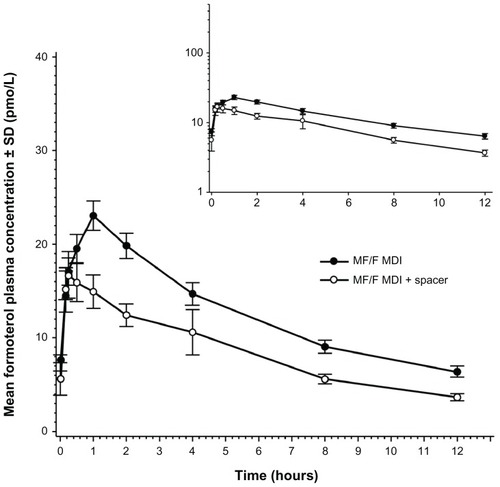
Mean plasma concentration-time profiles showed prolonged absorption of MF following administration of MF by MDI (). Median MF Tmax values were 3.00, 2.00, and 1.00 hours for treatments A, B, and C, respectively (). Median Tmax values of formoterol were 1.02 and 0.52 hours for Treatments A and B, respectively ().
Table 1 MF and F pharmacokinetic parameters on day 5 following twice-daily administration of MF/F MDI with or without a spacer or MF DPI in patients with chronic obstructive pulmonary disease
Figure 1 Mean (SD) MF plasma concentration-time profiles at day 5 in patients with chronic obstructive pulmonary disorder.
Abbreviations: DPI, dry-powder inhaler; F, formoterol fumarate; MDI, metered-dose inhaler; MF, mometasone furoate; SD, standard deviation.
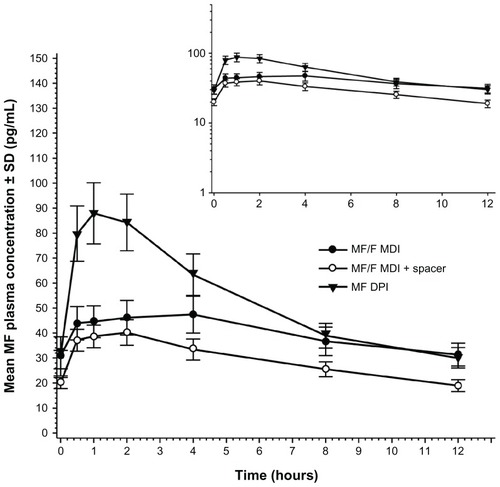
For the comparison of the MF exposure following inhalation of the DPI and MDI (primary objective), the ANOVA model included all treatments. Intrapatient variabilities for MF AUC0–12 hr and Cmax of 44% and 47%, respectively, were obtained from the model (). Despite this large observed variability, MF Cmax values were significantly different between treatments, with the mean Cmax for the MDI being 44% lower than that for DPI (). Mean MF AUC0–12 hr following inhalation by MDI alone was 23% lower than the mean value following administration of MF by DPI. The large CI reflects the small sample size and the larger than expected intrapatient variability (expected variability was approximately 28%; observed was 44%).
Table 2 Day 5 exposures to MF following twice-daily administration of MF/F MDI with or without a spacer or MF DPI in patients with chronic obstructive pulmonary disorder
A secondary objective of the study was to compare MF and formoterol exposure following inhalation using the MDI with and without a spacer. Following inhalation by MDI with the spacer, MF exposures based on AUC0–12 hr were lower than for MDI alone (). In the initial analysis with all treatments (), the ratio estimates for MF AUC0–12 hr and Cmax included 100%. However, because the larger intrapatient variability was related to the DPI treatment group, a reanalysis without treatment C showed that the AUC0–12 hr and Cmax values were 28% and 18% lower, respectively, for MDI with a spacer compared with MDI alone (). Intrapatient variability for MF AUC0–12 hr and Cmax values in the original three-treatment ANOVA ranged from 44% and 47%, respectively, to 23% and 24%, when comparing only the MDI data.
Table 3 Day 5 exposures to MF and formoterol following twice-daily administration of MF/F MDI with or without a spacer in patients with chronic obstructive pulmonary disorder
Mean plots of formoterol plasma concentrations showed rapid absorption of F (). For formoterol, AUC0–12 hr and Cmax values were 38% and 20% lower, respectively, for MDI with the spacer compared to MDI alone ().
Figure 2 Mean (SD) formoterol plasma concentration-time profiles at day 5 after twice-daily oral inhalation of MF/F MDI (MF 400 μg/F 10 μg) with (treatment B) and without (treatment A) a spacer device in patients with chronic obstructive pulmonary disorder.
Abbreviations: F, formoterol fumarate; MDI, metered-dose inhaler; MF, mometasone furoate; SD, standard deviation.
Safety
A total of ten (71.4%) patients reported at least one AE during the study: seven (50%) during treatment A (MF 400 μg + F 10 μg via MDI), three (21.4%) during treatment B (MF 400 μg + F 10 μg via MDI with spacer), and four (28.6%) during treatment C (MF 400 μg via DPI). The most common AEs were headache and dyspepsia, occurring in eight (57.1%) and two (14.3%) patients, respectively. All reported AEs were either mild or moderate in severity. No death or serious AEs occurred during the study.
There were no clinically significant changes in blood chemistry or hematologic parameters, vital signs, or electrocardiography in any of the treatment groups.
Discussion
Rapid and sustained relief from bronchoconstriction and improvement in lung function are critical for the long-term management of patients with persistent symptoms and exacerbation of COPD. Coadministration of MF and F has been shown to produce additive effects in rapidly improving symptoms and lung function and reducing the frequency of exacerbation of asthma and COPD.Citation9,Citation10,Citation17–Citation19 The decreases in lung function seen in patients with COPD may affect systemic exposure to drugs, such as MF/F MDI, which are administered via inhalation, and thereby may alter the pharmacokinetics of MF and formoterol. This has previously been described for ICS fluticasone propionate in patients with COPD compared to healthy controls.Citation12,Citation13 Therefore, the current study was conducted in patients with moderate-to-severe COPD to assess the pharmacokinetics of MF and formoterol in the intended target population. The rationale for including the MF DPI comparison arm in this study was to assess the relative systemic exposure of MF as administered by the MF/F MDI to the MF DPI, for which there is extensive clinical use and safety experience.Citation16 A previous pharmacokinetic study in healthy subjects showed lower systemic exposure to MF after steady state dosing from the MDI compared to the DPI device (data on file, Merck Sharp & Dohme Corp, 2006). Similar differences in the systemic exposure of ICS between MDI and DPI devices have been reported for other ICSs. For example, for SYMBICORT® (AztraZeneca, London, UK), an approved fixed-dose combination product containing budesonide and formoterol, the systemic exposure of budesonide was approximately 30% lower in both pediatric and adult patients with asthma after administration from an MDI device compared to the same dose delivered from a DPI device.Citation14,Citation15
The current study demonstrated that mean systemic exposures to MF were 23% lower following administration by MF/F MDI compared to MF DPI (primary objective). Additionally, mean systemic exposures of MF and formoterol were 28% lower and 38% lower, respectively, following administration by MF/F MDI in conjunction with a spacer (AeroChamber Plus® Valved Holding Chamber) compared to MF/F MDI without a spacer (secondary objective). The high intrapatient variability in MF exposures observed in this study may in part be attributed to differences in lung function over time (eg, reduced lung inflammation over time with observed dosing) and/or day-to-day variations in patient inhalation technique. In order to control for variations in inhalation technique, patients were extensively trained on the proper use of inhalation devices at baseline and, if necessary, prior to the start of each treatment period. However, even after allowing for differences in the inhalation techniques between the MDI and DPI for the observed intrapatient variability, MF and formoterol exposure following MDI treatment were still lower than those following DPI administration. These observed differences were not due to differences between the two formulations/devices in the oral deposition and subsequent gastrointestinal absorption of MF, since patients were instructed to rinse their mouths with water and spit it out after treatment administration. Furthermore, after oral administration as a solution, MF has been shown to have very low systemic bioavailability due to extensive first pass metabolism (unpublished data). The magnitude of the observed differences in systemic exposure between the MDI and DPI are probably due to formulation differences, which may result in differences in regional lung deposition and clearance from the lungs. The high intrapatient variability was related to inclusion of data from MF DPI treatment group (ie, treatment C) in the ANOVA model. A reanalysis of the results excluding treatment C showed lower overall mean exposures when a spacer was used with the MF/F MDI compared to when the MF MDI was used alone.
The present results are in agreement with previous studies showing that spacer devices reduce the systemic absorption of ICS in healthy volunteers.Citation20,Citation21 In those studies, a major factor that contributed to the lower dosage delivery using a spacer was the static charge of the spacer, which attracted medication particles. The authors also reported that multiple actuations and delayed inhalation of the drug after actuation may also cause reductions in dose delivery through a spacer. Application of antistatic material or washing the spacer was useful to reduce the static effect.Citation21 It should be noted, however, that the demonstrated differences in systemic exposure seen in this study in the presence/absence of a spacer device were observed in COPD patients who were trained for good, reproducible inhalation techniques with an MDI device and therefore would be unlikely to benefit from the use of a spacer in clinical practice. Spacer devices are indicated for patients with poor coordination and poor inhalation technique in order to improve drug delivery to the lungs. In clinical practice, pharmacotherapy with an inhalation product, such as MF/F MDI, is individualized, and each patient is titrated to a desired therapeutic response. Therefore, considering that patients who require a spacer device will be dosed with, and if necessary, titrated with a spacer, the use of a spacer device is not expected to have an efficacy implication in the target population.
In this study, MF/F MDI was shown to have a similar AE profile to MF DPI. All reported AEs were either mild or moderate in intensity and no serious AE was reported in this study. These safety findings are in agreement with those of previous studies conducted in asthma patients, which also reported that treatment with MF/F was generally well tolerated.Citation22,Citation23 Nevertheless, the current short term, multiple-dose pharmacokinetic study does not address the long-term safety and tolerability profile of chronic MF/F MDI therapy in patients with COPD. Two recently published articles demonstrated treatment with MF/F MDI was well tolerated in patients with moderate-to-severe COPD over 52 weeks.Citation9,Citation10
Considering that this study demonstrated a lower systemic exposure to the MDI product compared with the DPI in patients with COPD, there may be some concern regarding the appropriate interchangeability of the DPI monotherapy for the MDI combination therapy with regard to the comparability of the delivery of MF dose in each device. While a comparison of the systemic exposure of inhaled drugs is an acceptable way to evaluate the relative risk of ICS with regard to their systemic safety, there is considerable debate whether similar systemic exposure reflects comparable localized drug concentrations in the lung. Therefore, the clinical development program for the MF/F fixed-dose combination product has focused on demonstrating the clinical efficacy and safety of the MF/F device compared with the MF MDI device and placebo rather than the DPI reference products.Citation9,Citation10
In this study, the observed mean difference in systemic availability between MDI and DPI formulations was 23%. However, the difference between formulations in actual lung deposition may be smaller than those noted in systemic exposure and may be due to, at least in part, differences in lung retention. This hypothesis is supported by the apparently longer MF effective half-life (25 hours) after administration from the MDI versus the DPI (effective 13 hours; unpublished data). In addition, the majority of dose response studies conducted with inhaled corticosteroids have failed to demonstrate clinically meaningful differences even between doubling of doses,Citation24,Citation25 let alone a 25% difference. This point is illustrated by formoterol/budesonide FDC (SYMBICORT®) where the same delivered doses are approved for both the MDI (aerosol inhaler) and DPI (Turbuhaler®; AstraZeneca) formulations despite the ~30% lower systemic budesonide exposure from the MDI.Citation14,Citation15 Consistent with the relatively flat dose-response relationship of ICS, the current European Medicines Agency therapeutic equivalence guideline for orally inhaled products (CPMP/EWP/4151/00) has expanded equivalence acceptance criteria margins of 0.67 and 1.5,Citation26 which assume a mean difference between treatments of up to 1.5-fold. In view of the aforementioned considerations, the 23% lower systemic exposure to MF from the MDI relative to the DPI formulation is not considered clinically important.
Conclusion
This multiple-dose pharmacokinetic study demonstrated that systemic exposure to MF was lower following administration by MF/F MDI compared to MF DPI in patients with mild-to-moderate COPD. Additionally, systemic exposures of MF and formoterol were lower following administration by MF/F MDI with an AeroChamber Plus® Valved Holding Chamber spacer compared to MF/F MDI without this spacer. The magnitude of the differences in systemic exposure to MF seen with MF/F MDI versus MF DPI as well as MF/F MDI administered with a spacer versus MF/F MDI without a spacer were not clinically relevant. There also was no clinically relevant difference in systemic exposure to formoterol seen with MF/F MDI administered in the presence and absence of a spacer. Finally, MF/F delivered twice daily by MDI was generally well tolerated among patients with moderate-to-severe COPD in this short-term study.
Acknowledgments
The authors wish to thank Amy O Johnson-Levonas (Merck Sharp & Dohme Corp.) for writing and editing sections of the manuscript as well as Kathleen Newcomb and Jennifer Pawlowski (Merck Sharp & Dohme Corp.) for assistance with the preparation of this manuscript for submission and publication.
Disclosure
This study was financially supported by Merck Sharp & Dohme Corp, a subsidiary of Merck and Co, Inc (Whitehouse Station, NJ, USA). T Kosoglou, J Hubbell, F Xuan, AG Meehan, BS Kantesaria, and DL Cutler all declare that they are full-time employees of Merck Sharp & Dohme Corp. BA Wittmer received research grant support from Schering-Plough Corporation (now Merck Sharp & Dohme Corp). The authors report no other conflicts of interest in this work.
References
- American Thoracic Society/European Respiratory Society Task ForceStandards for the Diagnosis and Management of Patients with COPD 2004New York, NYAmerican Thoracic Society/European Respiratory Society Task Force2005
- National Collaborating Centre for Chronic ConditionsChronic obstructive pulmonary disease: national clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary careThorax200459 Suppl 11232
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary DiseaseGlobal Initiative for Chronic Obstructive Lung Disease, Inc2011 Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011_Feb21.pdfAccessed August 7, 2011
- GINA – the Global Initiative for Asthma [webpage on the Internet]Global strategy for asthma management and preventionNational Heart, Lung, and Blood Institute. Global Initiative for Asthma (GINA)2010 Available from: http://www.ginasthma.org/guideline-report-2010.htmlAccessed August 7, 2011
- NHLBI, Diagnosis and Management of Asthma [webpage on the Internet]Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthmaNational Heart Lung and Blood Institute updated 2012. Available from: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htmAccessed August 7, 2011
- BernsteinDIBerkowitzRBChervinskyPDose-ranging study of a new steroid for asthma: mometasone furoate dry powder inhalerRespir Med199993960361210542973
- KarpelJPNayakALumryWInhaled mometasone furoate reduces oral prednisone usage and improves lung function in severe persistent asthmaRespir Med2007101362863716875813
- NoonanMKarpelJPBenschGWComparison of once-daily to twice-daily treatment with mometasone furoate dry powder inhalerAnn Allergy Asthma Immunol2001861364311206236
- DohertyDETashkinDPKerwinEEffects of mometasone furoate/formoterol fumarate fixed-dose combination formulation on chronic obstructive pulmonary disease (COPD): results from a 52-week Phase III trial in subjects with moderate-to-very severe COPDInt J Chron Obstruct Pulmon Dis20127577122334769
- TashkinDPDohertyDEKerwinEEfficacy and safety characteristics of mometasone furoate/formoterol fumarate fixed-dose combination in subjects with moderate to very severe COPD: findings from pooled analysis of two randomized, 52-week placebo-controlled trialsInt J Chron Obstruct Pulmon Dis20127738622334770
- LaForceCPrennerBMAndrianoKLavecchiaCYegenUEfficacy and safety of formoterol delivered via a new multidose dry powder inhaler (Certihaler) in adolescents and adults with persistent asthmaJ Asthma200542210110615871441
- SinghSDWhaleCHoughtonNDaley-YatesPKirbySMWoodcockAAPharmacokinetics and systemic effects of inhaled fluticasone propionate in chronic obstructive pulmonary diseaseBr J Clin Pharmacol200355437538112680886
- EdsbäckerSWollmerPSelroosOBorgströmLOlssonBIngelfJDo airway clearance mechanisms influence the local and systemic effects of inhaled corticosteroids?Pulm Pharmacol Ther200821224725817950641
- TrondeAGillenMBorgströmLLötvallJAnkerstJPharmacokinetics of budesonide and formoterol administered via 1 pressurized metered-dose inhaler in patients with asthma and COPDJ Clin Pharmacol200848111300130818974284
- Symbicort® (budesonide/formaterol fumarate dehydrate) [package insert]Wilmington, DEAstraZeneca2010
- ZeidlerMCorrenJTashkinDPUse of mometasone furoate administered via a dry powder inhaler in the treatment of asthmaCurr Med Res Opin20102661295130520370376
- NathanRANolteHPearlmanDSfor P04334 Study InvestigatorsTwenty-six-week efficacy and safety study of mometasone furoate/formoterol 200/10 microg combination treatment in patients with persistent asthma previously receiving medium-dose inhaled corticosteroidsAllergy Asthma Proc201031426927920678306
- WeinsteinSFMurphyKRCorrenJNolteHWhiteMVEfficacy and safety of medium and high doses of mometasone furoate/formoterol (MF/F) combination treatment in subjects with severe persistent asthma [abstract]J Allergy Clin Immunol2010125AB196
- WeinsteinSFCorrenJMurphyKNolteHWhiteMStudy Investigators of P04431Twelve-week efficacy and safety study of mometasone furoate/formoterol 200/10 microg and 400/10 microg combination treatments in patients with persistent asthma previously receiving high-dose inhaled corticosteroidsAllergy Asthma Proc201031428028920687982
- WalesDMakkerHKaneJMcDowellPO’DriscollBRSystemic bioavailability and potency of high-dose inhaled corticosteroids: a comparison of four inhaler devices and three drugs in healthy adult volunteersChest199911551278128410334140
- ClarkDJLipworthBJEffect of multiple actuations, delayed inhalation and antistatic treatment on the lung bioavailability of salbutamol via a spacer deviceThorax199651109819848977596
- MasperoJCherrezINolteHLong-term safety and tolerability of two doses of mometasone furoate/formoterol (MF/F) combination, administered via a metered-dose inhaler, for the treatment of moderate-to-severe persistent asthmaJ Allergy Clin Immunol20091232S159
- MasperoJFNolteHCherrez-OjedaIP04139 Study GroupLong-term safety of mometasone furoate/formoterol combination for treatment of patients with persistent asthmaJ Asthma201047101106111520874458
- AdamsNBestallJJonesPBudesonide at different doses for chronic asthmaCochrane Database Syst Rev20014CD00327111687182
- AdamsNPBestallJCJonesPLassersonTJGriffithsBCatesCJFluticasone at different doses for chronic asthma in adults and childrenCochrane Database Syst Rev20084CD00353418843646
- Committee for Medicinal Products for Human Use (CHMP)Guideline on the Requirements for Clinical Documentation for Orally Inhaled Products (OIP) Including the Requirements for Demonstration of Therapeutic Equivalence between Two Inhaled Products for Use in the Treatment of Asthma and Chronic Obstructive Pulmonary Disease (COPD) in Adults and for Use in the Treatment of Asthma in Children and AdolescentsLondonEuropean Medicines Agency2009 Available from: http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500003504Accessed August 7, 2011
