Abstract
Background
Inpatient admissions for chronic obstructive pulmonary disease (COPD) represent a significant economic burden, accounting for over half of direct medical costs. Reducing 30-day readmissions could save health care resources while improving patient care. Recently, the Patient Protection and Affordable Care Act authorized reduced Medicare payments to hospitals with excess readmissions for acute myocardial infarction, heart failure, and pneumonia. Starting in October 2014, hospitals will also be penalized for excess COPD readmissions. This retrospective database study investigated whether use of arformoterol, a nebulized long-acting beta agonist, during an inpatient admission, had different 30-day all-cause readmission rates compared with treatment using nebulized short-acting beta agonists (SABAs, albuterol, or levalbuterol).
Methods
A US nationally representative hospital database was used to study adults aged ≥40 years, discharged between January, 2006 and March, 2010, and with a diagnosis of COPD. Patients receiving arformoterol on ≥80% of days following treatment initiation were compared with patients receiving a nebulized SABA during hospitalization. Arformoterol and nebulized SABA patients were matched (1:2) for age, sex, severity of inpatient admission, and primary/secondary COPD diagnosis. Logistic regression compared the odds of readmission while adjusting for age, sex, race, admission type, severity, primary/secondary diagnosis, other respiratory medication use, respiratory therapy use, oxygen use, hospital size, and teaching status.
Results
This retrospective study compared 812 arformoterol patients and 1,651 nebulized SABA patients who were discharged from their initial COPD hospital admission. An intensive care unit stay was more common among arformoterol patients (32.1% versus 18.4%, P<0.001), suggesting more severe symptoms during the initial admission. The observed readmission rate was significantly lower for arformoterol patients than for nebulized SABA patients (8.7% versus 11.9%, P=0.017), as were the adjusted odds of readmission (odds ratio 0.69, 95% confidence interval 0.51–0.92).
Conclusion
All-cause 30-day readmission rates were significantly lower for arformoterol patients than nebulized SABA patients, both before and after adjusting for patient and hospital characteristics.
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality. The worldwide prevalence of COPD, estimated at 7.6%, increases with age to 9.9% in those aged 40 years or older and 14.2% in those aged 65 years or older.Citation1 In high-income countries, the World Health Organization has identified COPD as the seventh leading cause of disability-adjusted life years and the fourth global leading cause of death.Citation2 In the US, chronic lower respiratory tract diseases, such as COPD, have been identified as the third leading cause of death.Citation3 However, the mortality associated with COPD may be underestimated because the cause of death for individuals with COPD is often attributed to one of the common associated comorbidities.Citation4
COPD imposes a substantial economic burden on the health care system. In 2008, the US spent $53.7 billion on direct health care costs for treating asthma and COPD.Citation3 Average annual health care costs for COPD patients varied dramatically from $2,003 to $43,471 depending on the types of medical services utilized, with hospitalized patients requiring treatment in the intensive care unit incurring the greatest expense.Citation5 Across all COPD patients, hospital admissions account for the majority (>50%) of direct health care costs.Citation5–Citation7
The course of COPD is characterized by a slow, progressive deterioration in airflow that is punctuated by exacerbations and eventually may lead to severe disability or death. Many of the exacerbations can be treated on an outpatient basis, but more severe exacerbations may require hospitalization. Lung function generally declines after each exacerbation, particularly for those requiring admission to hospital.Citation8 A recent large-scale naturalistic study of COPD found that the risk of readmission or death increased threefold after the first hospital readmission, and grew progressively to a 25-fold increase following the tenth readmission.Citation9 The risk for readmission was particularly elevated during the 3 months following discharge from hospital.Citation9 Reducing readmissions through more effective treatment and disease management could potentially reduce treatment costs and slow disease progression in COPD.
In the US, Medicare is a federal insurance program funded through the Social Security system that provides health care coverage for individuals who are aged 65 years and older or who are permanently disabled. The program is administered by the Centers for Medicare & Medicaid Services (CMS), a component of the Department of Health and Human Services. Among US Medicare beneficiaries who were hospitalized for COPD, nearly one in four (22.6%) were readmitted within 30 days.Citation10 Due to high rates of 30-day readmissions for US Medicare beneficiaries, the Patient Protection and Affordable Care Act of 2010 authorized the CMS to reduce payments to hospitals with higher than expected 30-day readmission rates for select conditions.Citation11,Citation12 Currently, heart failure, acute myocardial infarction, and pneumonia are targeted,Citation13 but beginning in the fall of 2014, COPD will be added to the list of conditions.Citation11,Citation12
The interest in 30-day readmission rates is growing. The Journal of the American Medical Association recently published a special issue on this topic, including research that reviewed the diagnoses, timing, and demographics associated with the currently targeted 30-day readmissions,Citation14 and the outcomes from a CMS-sponsored quality improvement program that showed a modest reduction of 0.56 readmissions per 1,000 beneficiaries per quarter in the intervention communities (where hospitals implemented care transition interventions, medication management improvements, and standardization of the discharge planning process) relative to the control communities.Citation15
The Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines recommend maintenance therapy using long-acting bronchodilators for all patients who require continuous bronchodilation because of frequent daily symptoms.Citation16 However, despite these treatment recommendations, short-acting bronchodilators are used alone in usual clinical care for 20% of patients with moderate COPD.Citation17 Short-acting bronchodilators, on the other hand, are recommended for use as rescue medications or to treat the intermittent symptoms for patients with early-stage COPD.Citation16 Because of the advantages of using long-acting bronchodilators in patients with more advanced disease, the use of the these agents in patients hospitalized with an exacerbation of COPD may represent a potential approach for reducing 30-day readmission rates and, consequently, overall readmission rates.Citation18
The purpose of this retrospective study was to examine 30-day readmission rates for patients treated with either nebulized arformoterol or a short-acting β2 agonist (SABA) bronchodilator during a hospitalization for COPD. In general, long-acting β2 agonists (LABAs) can be delivered via a metered-dose inhaler, dry powder inhaler, or nebulizer. Nebulized administration may have several advantages. For example, it may yield a more consistent medication dose due to fewer user errors, especially in older patients with COPD, and many patients prefer to use nebulizers over metered-dose inhalers and dry-powder inhalers.Citation19,Citation20 In view of these potential advantages, we hypothesized that arformoterol, a LABA medication delivered via a nebulizer, would result in fewer hospital readmissions within 30 days than a nebulized SABA, when used in similar hospitalized patients with COPD who were subsequently discharged. The objective of this retrospective study was to compare all-cause 30-day readmission rates, subsequent to an initial inpatient stay, among matched COPD patients who were treated with nebulized arformoterol or a nebulized SABA (at least 80% of the time spent in hospital, in order to simulate use once the patient was admitted) during the initial hospital stay.
Materials and methods
Study design
This retrospective administrative database study used a matched case-control design. The administrative hospital data were from the database maintained by Premier, Inc. (Charlotte, NC, USA), the largest hospital-based, service-level database in the US. The database contained detailed service information from over 500 hospitals and more than 50 million inpatient discharges since 2000. It was nationally representative of hospital coverage by number of beds, US census region, urban setting, and teaching status. The database did not include any identifiable protected health information and, pursuant to the Health Insurance Portability and Accountability Act of 1996, the study did not require institutional review board waiver or approval.Citation21
Inclusion and exclusion criteria
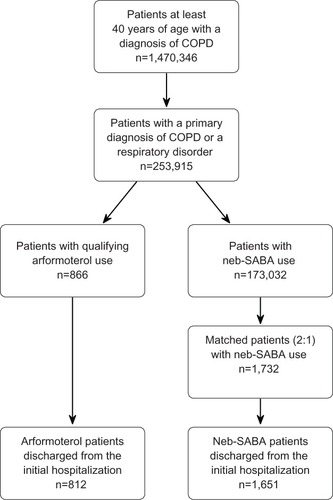
This retrospective study included patients who were at least 40 years of age when they were initially admitted to the hospital between January 1, 2006 and March 31, 2010. All patients were required to have either a primary discharge diagnosis for COPD (ICD-9-CM codes 491.xx, 492.xx, or 496.xx) or a secondary diagnosis for COPD along with a primary discharge diagnosis for another respiratory condition (ICD-9-CM codes 460.xx–519.xx). Patients in the primary treatment cohort were required to initiate arformoterol treatment and receive arformoterol for at least 80% of the inpatient days following initiation. The comparison cohort was comprised of patients who were treated with a nebulized SABA (either albuterol or levalbuterol) on each day during admission and who did not receive any nebulized LABA treatment. The arformoterol patients could have been treated with a SABA prior to and after initiating arformoterol. Patients were excluded if they died during the initial admission. shows the patient flow through the inclusion and exclusion criteria.
Outcomes measure
The primary outcome variable was 30-day all-cause readmission. It was operationally defined as readmission to the same hospital within 30 days of the discharge date.
Matching procedure
Retrospective studies that effectively control for selection bias have a high degree of validity. Selection bias could have occurred in this study if the patients treated with arformoterol systematically differed from those treated with a nebulized SABA on background characteristics that were associated with the risk of 30-day readmission. All background characteristics were identified from information collected during the initial hospitalization, prior to assessing 30-day all-cause readmission.
Demographic characteristics that were controlled for in the analysis included age, sex, and race, given that the risk of hospital admissions has been associated with age and other demographic characteristics.Citation10,Citation22 The analysis also controlled for characteristics of the hospital, including geographic location, urban or rural setting, teaching status, and total number of beds, because the likelihood of readmission has been shown to vary by hospital and hospital characteristics such as geographic location and size.Citation10
Clinical characteristics from the initial hospitalization that were controlled for included primary versus secondary COPD diagnosis, admission type (emergency room versus other), length of stay, and severity. Disease progression and severity have been closely linked to the risk of readmission.Citation9 Severity was assessed using the 3M™ All Patient Refined™ Diagnosis-Related Groups (APR-DRG) severity of illness classes.Citation23 These severity of illness classes range from 1 (mild) to 4 (extreme) and are based on age, primary and secondary diagnoses, and certain procedures performed during hospitalization.Citation23 Finally, the analysis controlled for other treatments that were administered during the initial admission, including oxygen therapy, respiratory therapy, anticholinergic treatment, corticosteroid treatment, antibiotic treatment, and other COPD medications. The concomitant treatments could have directly affected readmission, but could also reflect important differences in case complexity.
In part, because there were more nebulized SABA patients in the data, and to develop more similar cohorts for comparison, each patient treated with arformoterol was matched to two patients treated with a nebulized SABA. Exact matches were made on age, sex, APR-DRG severity of illness, and primary versus secondary COPD diagnosis. Matches were made prior to excluding patients who died during the baseline admission. All of the other background characteristics described above were statistically controlled in the data analyses.
Statistical methods
Descriptive statistics included means and standard deviations for continuous measures and number and percentage of patients for categorical measures. Unadjusted differences between patients treated with arformoterol or a nebulized SABA during the initial admission were assessed using t-tests for continuous measures and chi-square tests for categorical variables.
The adjusted odds of being readmitted in 30 days were assessed using multivariate logistic regression. The regression analysis compared this primary outcome between the arformoterol patients and nebulized SABA patients while statistically adjusting for the previously described set of variables. The two-tailed alpha level was set to 0.05 and all analyses were completed using SAS version 9.2 (SAS Inc, Cary, NC, USA) and WinSQL (Synametrics, Plainsboro, NJ, USA).
Results
Sample description
During the index hospital stay, a total of 866 patients initiated arformoterol and met all of the inclusion criteria. These patients were matched (1:2) on age, sex, APR-DRG severity of illness class, and primary COPD diagnosis with 1,732 patients treated with a nebulized SABA (). During the index hospital stay, 135 patients died (54 [6.2%] arformoterol and 81 [4.7%] nebulized SABA patients) and could not be included in the readmission analysis, leaving a final sample of 812 arformoterol and 1,651 nebulized SABA patients. Among patients who died during the initial hospital admission, arformoterol patients were significantly more likely to have intensive care admissions compared with nebulized SABA patients (85% versus 51%), which was a significant predictor of mortality (data not shown). Almost all patients (99.2%) with intensive care admissions in the arformoterol cohort had this event prior to or concurrent with their treatment initiation with arformoterol.
Age, sex, APR-DRG severity of illness classes, and primary COPD diagnosis were nearly identical between the cohorts because the patients were matched on these characteristics. However, significant differences between patients treated with arformoterol and those treated with a nebulized SABA were observed on other demographic, hospital, clinical, and initial admission treatment characteristics (). Notably, arformoterol appeared to be selected for patients with a more severe symptom profile based on greater intensive care (32.1% versus 18.4%, P<0.001), oxygen therapy (78.0% versus 71.3%, P<0.001), and corticosteroid treatment (87.2% versus 77.1%, P<0.001).
Table 1 Characteristics of arformoterol and nebulized SABA patients during initial hospitalization
Readmissions
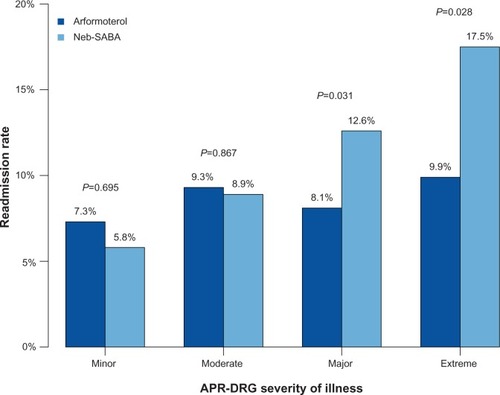
Seventy-one (8.7%) of the arformoterol patients and 197 (11.9%) of the nebulized SABA patients were readmitted to the same hospital within 30 days of their index discharge (P=0.016). The unadjusted 30-day readmission rates for the two cohorts varied by APR-DRG severity class (), with significantly fewer readmissions among patients treated with arformoterol in the major (8.1% versus 12.6%, P=0.031) and extreme (9.9% versus 17.5%, P=0.028) severity categories.
Figure 2 Unadjusted all-cause 30-day readmission rates for arformoterol-treated and nebulized SABA-treated patients by APR-DRG severity.
Abbreviations: APR-DRG, 3M™ All Patient Refined™-Diagnosis Related Groups; neb-SABA, nebulized short-acting β2 agonist.
A multivariate logistic regression was used to estimate the odds of 30-day readmission between the arformoterol and nebulized SABA groups, after adjusting for covariates of sex, age, race, hospital characteristics, diagnoses, admission, treatment, length of stay, and severity of illness. After adjusting for initial admission differences, the odds for readmission were 31% lower (odds ratio 0.69; 95% confidence interval 0.51–0.92) for patients treated with arformoterol than for patients treated only with a nebulized SABA. Patients with APR-DRG major (odds ratio 1.89, P=0.042) and extreme (odds ratio 2.49, P=0.007) severity classes during the index hospital stay were significantly more likely to be readmitted to the hospital within 30 days ().
Table 2 Logistic regression model
Discussion
In this retrospective observational study of usual clinical care, patients treated with nebulized arformoterol, had significantly lower (8.7% versus 11.9%) 30-day readmissions compared with patients receiving nebulized SABA treatment. Despite baseline differences in intensive care stays and oxygen use suggesting that the arformoterol patients were more severe, patients treated with arformoterol had significantly lower adjusted odds (odds ratio 0.69) of being readmitted within 30 days of discharge than matched patients treated with a nebulized SABA. The reduced 30-day readmission rates for arformoterol-treated patients, despite poor baseline status, suggests that those treated with arformoterol may have better stabilization status at discharge. Further investigations into discharge status and consequent outcomes post-discharge are needed. Future research using prospectively collected data, rather than retrospective data, can also examine any additional hospital factors, such as discharge planning, device training, and patient education, which may contribute to the differences in 30-day readmission observed in this study.
The 30-day readmission rates observed in this study were lower compared with the recent literature.Citation10 This difference may be explained by the younger patient mix in this study; a third of patients were below 65 years of age, whereas the other study focused solely on Medicare beneficiaries. Consistent with the literature findings, patients with higher APR-DRG severity were more likely to be readmitted than patients with lower severity in our study. Interestingly, the unadjusted difference in readmission rates between the arformoterol and nebulized SABA cohorts was most pronounced among the patients with major or extreme APR-DRG severity scores. While the sample size was too small to conduct inferential analysis, additional studies comparing outcomes between arformoterol and use of other agents may shed light on the role of arformoterol in severely compromised exacerbating COPD patients.
A previous meta-analysis of randomized controlled trials in COPD found that LABA treatment reduced exacerbations relative to placebo by 21%;Citation24 however, there is a paucity of randomized controlled trials comparing the rates of exacerbations or hospitalizations between LABA and SABA treatments.Citation25 Two previously published, retrospective outpatient studies had similar findings. A retrospective study of usual care in Scotland found that outpatients treated with LABAs had reduced rates of hospitalization relative to outpatients treated with only SABAs or antimuscarinic agents.Citation18 Using electronic health records, another study comparing patients treated with LABA and inhaled corticosteroids with patients treated with SABA and inhaled corticosteroid recipients also found reduced readmissions for the LABA-treated and inhaled corticosteroid-treated cohort.Citation26 The findings of this study are consistent with prior research and treatment guidelines recommending the use of long-acting rather than short-acting bronchodilators for maintenance treatment in patients with moderate to severe COPD.Citation16 Our finding that initiating hospitalized patients (once admitted) with COPD on arformoterol rather than a nebulized SABA have lower 30-day readmissions appears to be an important and unique contribution to the literature.
Reducing 30-day readmissions among patients with COPD has important implications for hospitals since the Patient Protection and Affordability Care Act has authorized the CMS to reduce hospital payments for hospitals with excessive rates of 30-day readmissions.Citation11,Citation12 Debate about this pay for performance program is ongoing, including questions regarding the incentive size and structure,Citation27 the focus on 30-day readmissions rather than broader clinical outcomes,Citation28 and the potential for sharing risks and reward among all entities in the community responsible for improving care.Citation29 The program will begin to target COPD patients at high risk for readmission within 30 daysCitation10 beginning in the fall of 2014.Citation11 These study results suggest that hospitals with excess 30-day readmission rates for COPD may want to consider quality improvement initiatives that encourage the use of LABA instead of SABA when appropriate,Citation16 and may also want to consider including such agents on their formularies that add intrinsic value through an impact on hospital readmissions.
An examination of the average annual costs of treating COPD in the US in 2006 revealed that the average patient incurred $3,943 in direct expenses, but those who were hospitalized incurred $15,093, and those requiring treatment in the intensive care unit during hospitalization incurred $43,471.Citation5 Although most COPD patients are not hospitalized in a given year, inpatient costs are the largest driver of direct health care costs.Citation7 In the current analysis, the difference in readmission rates between arformoterol and nebulized SABA was most pronounced for patients with a more severe illness profile. Initiating patients on a nebulized LABA, such as arformoterol, instead of using nebulized SABAs alone during an inpatient admission for COPD could reduce health care costs by preventing expensive readmissions. Decreasing readmission rates may also lead to better patient outcomes by potentially preventing a decline in lung functionCitation8 and reduction in quality of life.Citation30
Limitations
The administrative hospital data used in this study were originally collected for clinical and economic benchmarking rather than research purposes. Therefore, the information may not be as precise as prospectively collected clinical research data. Patients with COPD discharge diagnoses in the hospital were assumed to have met formal COPD diagnostic criteria, but no spirometry results were available to confirm the diagnoses. Although the analyses controlled for a large number of background characteristics, the data were limited in scope. Some important measures of severity in COPD, such as measures of lung function, smoking status, and medication treatment outside of the hospital, were not available and may have differed between patients treated with arformoterol or a nebulized SABA. However, the arformoterol group had greater intensive care unit use during the baseline admission, suggesting that the selection bias for this cohort was to have more severe patients who were more likely, rather than less likely, to be readmitted. Readmissions to hospitals, other than the one with the index admission, were not captured in the data, potentially resulting in underestimation of the 30-day readmission rates. Cohorts were not matched on hospital, so hospital represents an uncontrolled variable. Finally, variations in treatment and discharge protocol between hospital admissions could not be controlled for in this study due to the absence of such information and small sample sizes precluded the use of institution as a matching variable. Prospective, interventional research that compares the rates of hospital readmission for patients with COPD randomized to arformoterol or a nebulized SABA may be warranted to confirm these findings.
Conclusion
This retrospective comparative study of the nebulized LABA, arformoterol, versus nebulized SABAs found that patients treated with arformoterol had significantly reduced odds of all-cause 30-day hospital readmissions relative to matched patients treated with SABAs. Initiating hospitalized COPD patients with a LABA instead of a SABA may help prevent all-cause readmissions, reduce health care costs, and prevent greater deterioration in lung function, particularly among patients with a more severe illness profile.
Acknowledgments
The authors would like to acknowledge Bernadette Johnson of Premier Healthcare solutions, Inc. for her analytical support, and Michael D Stensland of Agile Outcomes Research, Inc. for his technical writing support.
Disclosure
VB, JK, and KR are all full-time employees of Sunovion Pharmaceuticals, Inc., the study sponsor. SBR and FRE are employees of Premier Healthcare solutions, Inc., which contracted with Sunovion Pharmaceuticals, Inc. to conduct this research. SSB reports no financial interests in this work.
References
- HalbertRJNatoliJLGanoABadamgaravEBuistASManninoDMGlobal burden of COPD: systematic review and meta-analysisEur Respir J200628352353216611654
- MathersCBoermaTFatDMThe Global Burden of Disease: 2004 UpdateGeneva, SwitzerlandWorld Health Organization2008
- National Heart Lung and Blood InstituteMorbidity and Mortaility: 2012 Chart Bood on Cardiovascular, Lung, and Blood Diseases2012 Available from: http://www.nhlbi.nih.gov/resources/docs/cht-book.htmAccessed August 1, 2013
- CamilliAERobbinsDRLebowitzMDDeath certificate reporting of confirmed airways obstructive diseaseAm J Epidemiol199113387958002021146
- DalalAAChristensenLLiuFRiedelAADirect costs of chronic obstructive pulmonary disease among managed care patientsInt J Chron Obstruct Pulmon Dis2010534134921037958
- HillemanDEDewanNMaleskerMFriedmanMPharmacoeconomic evaluation of COPD. 2000Chest2009136Suppl 5e3020162783
- StrasselsSASmithDHSullivanSDMahajanPSThe costs of treating COPD in the United StatesChest2001119234435211171708
- DonaldsonGCSeemungalTARBhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax2002571084785212324669
- SuissaSDell’anielloSErnstPLong-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortalityThorax2012671195796322684094
- JencksSFWilliamsMVColemanEARehospitalizations among patients in the Medicare fee-for-service programN Engl J Med2009360141418142819339721
- Medicare Payment Advisory CommissionReport to Congress: Promoting Greater Efficiency in Medicare2007 Available from: http://www.medpac.gov/documents/jun07_entirereport.pdfAccessed August 1, 2013
- United States CongressPatient Protection and Affordable Care Act2010 Available from: http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdfAccessed August 1, 2013
- WilliamsMVA requirement to reduce readmissions: take care of the patient, not just the diseaseJAMA2013309439439623340642
- DharmarajanKHsiehAFLinZDiagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumoniaJAMA2013309435536323340637
- BrockJMitchellJIrbyKAssociation between quality improvement for care transitions in communities and rehospitalizations among Medicare beneficiariesJAMA2013309438139123340640
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for the Diagnosis, Management, and Prevention of COPD (Updated 2013)2013 Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.htmlAccessed August 1, 2013
- FitchKIwasakiKPyensonBPlauschinatCZhangJVariation in adherence with Global Initiative for Chronic Obstructive Lung Disease (GOLD) drug therapy guidelines: a retrospective actuarial claims data analysisCurr Med Res Opin20112771425142921599554
- ShortPMLipworthSIWElderDHJSchembriSLipworthBJEffect of beta blockers in treatment of chronic obstructive pulmonary disease: a retrospective cohort studyBMJ2011342d254921558357
- GellerDEComparing clinical features of the nebulizer, metered-dose inhaler, and dry powder inhalerRespir Care200550101313132116185367
- DhandRDolovichMChippsBMyersTRRestrepoRFarrarJRThe role of nebulized therapy in the management of COPD: evidence and recommendationsCOPD201291587222292598
- United States CongressHealth Insurance Portability and Accountability Act of 19961996 Available from: http://www.gpo.gov/fdsys/pkg/PLAW-104publ191/html/PLAW-104publ191.htmAccessed September 19, 2012
- KirbySEDennisSMJayasingheUWHarrisMFPatient related factors in frequent readmissions: the influence of condition, access to services and patient choiceBMC Health Serv Res20101021620663141
- AverillRFGoldfieldNHughesJSAll Patient Refined Diagnosis Related Groups (APR-DRGs), Version 20.0: Methodology Overview2003 Available from: http://www.hcup-us.ahrq.govAccessed June 20, 2012
- RodrigoGJNanniniLJRodríguez-RoisinRSafety of long-acting beta-agonists in stable COPD: a systematic reviewChest200813351079108718460518
- BolluVKKarafilidisJColosiaABennettLHananiaNComparison of efficacy and safety outcomes in randomized trials of long-acting and short-acting β2-agonists for chronic obstructive pulmonary disease: a reviewJ Pulmon Resp Med201331137
- KiriVABettoncelliGTestiRViegiGInhaled corticosteroids are more effective in COPD patients when used with LABA than with SABARespir Med20059991115112415921904
- JhaAKTime to get serious about pay for performanceJAMA2013309434734823340633
- VaduganathanMBonowROGheorghiadeMThirty-day readmissions: the clock is tickingJAMA2013309434534623340632
- McCarthyDJohnsonMBAudetA-MRecasting readmissions by placing the hospital role in community contextJAMA2013309435135223340635
- LlorCMolinaJNaberanKCotsJMRosFMiravitllesMExacerbations worsen the quality of life of chronic obstructive pulmonary disease patients in primary health careInt J Clin Pract200862458559218266710