Abstract
Three long-acting muscarinic antagonists (LAMAs) are now available in Europe, providing clinicians and patients with a choice of interventions, which is important in COPD, which is clinically a heterogeneous disease. The first LAMA, tiotropium, has been widely used over the last decade as a once-daily maintenance therapy in stable COPD to improve patients’ health-related quality of life and to reduce the risk of exacerbations. Administered via the HandiHaler® device, it is safe and well tolerated. Another new once-daily LAMA, glycopyrronium, has also been shown to improve health status and reduce exacerbations, and is well tolerated. The subject of this review is a third LAMA, aclidinium bromide, which was approved as a twice-daily maintenance bronchodilator treatment. In the pivotal Phase III clinical trials, patients receiving aclidinium achieved significantly greater improvements in lung function, reductions in breathlessness, and improvements in health status compared with placebo, for up to 24 weeks. In continuation studies, these improvements were sustained for up to 52 weeks. Pooled data showed exacerbation frequency was significantly reduced with aclidinium versus placebo. Preclinical and pharmacological studies demonstrating low systemic bioavailability and a low propensity to induce cardiac arrhythmias were translated into a favorable tolerability profile in the clinical trial program – the adverse event profile of aclidinium was similar to placebo, with a low incidence of anticholinergic and cardiac adverse events. While additional studies are needed to evaluate its full clinical potential, aclidinium is an important part of this recent expansion of LAMA therapeutic options, providing clinicians and patients with an effective and well-tolerated COPD treatment.
Introduction
Anticholinergic agents play a key role in COPD management, being recommended as a first choice, either as monotherapy or in combination with a long-acting β2-agonist (LABA).Citation1 For around a decade, only one long-acting muscarinic agonist (LAMA) – tiotropium bromide – was available, but this picture changed in 2012, with the approval of two new LAMAs – aclidinium bromide and glycopyrronium bromide. All three LAMAs are noted as potential treatment options in the recent update from the Global initiative for chronic Obstructive Lung Disease (GOLD).Citation2
Tiotropium bromide
Tiotropium has been widely used over the last decade as once-daily maintenance therapy in stable COPD. Currently, it can be delivered via the HandiHaler®, a single-dose dry powder inhaler, and the Respimat®, a soft mist device which is a propellant-free, multidose inhaler. It has been extensively studied in patients with COPD – a recent Cochrane review identified 22 studies of good methodological quality that had enrolled 23,309 participants with COPD.Citation3 This review showed that tiotropium improved patients’ health-related quality of life and reduced exacerbations and hospitalization.Citation3
In the 4-year Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT) trial using tiotropium via the HandiHaler® in moderate to severe COPD, improvements in forced expiratory volume in 1 second (FEV1) compared with placebo were maintained throughout the trial (ranging from 87 to 103 mL pre-bronchodilator). The mean number of exacerbations was reduced by 14% and mean total St George’s Respiratory Questionnaire (SGRQ) score was higher than with placebo at each time point throughout the 4-year period by 2.3 to 3.3 units. However, the rate of decline in FEV1 – the primary outcome of the trial – was not significantly reduced by the use of tiotropium.Citation4
In the UPLIFT trial, overall rates of serious cardiac adverse events (AEs) were all significantly lower in the tiotropium group than the placebo group (relative risk [RR]=0.84), although mortality was not significantly lower.Citation4 Other publications have described conflicting observations regarding tiotropium and cardiovascular risk,Citation3,Citation5–Citation9 including a new user cohort study in the UK, which identified a numerically (but not significantly) increased risk of stroke with tiotropium HandiHaler® versus LABA, but a significantly lower all-cause mortality (hazard ratio [HR]=0.70).Citation6 In recent years, there has been increasing concern that tiotropium delivered via the Respimat® may have been associated with higher mortality,Citation3,Citation5–Citation10 but the large, randomized Tiotropium Safety and Performance in Respimat® study (TioSPIR; NCT1126437), in which outcomes with tiotropium via Respimat® (2.5 and 5 μg doses) and HandiHaler® (18 μg) were compared in over 17,000 patients, showed no difference in all-cause mortality and no difference in efficacy as measured by exacerbation rate.Citation11
Glycopyrronium bromide
Glycopyrronium bromide is a synthetic quaternary ammonium compound, which has been used for many years to reduce secretions and block cardiac vagal reflexes before surgery.Citation12 Previously it was administered orally or as an injection, but a dry-powder formulation has now been developed, administered once daily (QD) via a single dose dry-powder device – the Breezhaler®. Following promising results in early preclinical and clinical studies,Citation13–Citation16 a Phase III development program called GLycopyrronium bromide in COPD airWays (GLOW) was developed and has shown that glycopyrronium 50 μg QD improved trough FEV1, breathlessness, and health status,Citation17 and reduced exacerbations.Citation18 In the GLOW 3 trial, glycopyrronium treatment was superior to placebo with respect to exercise endurance time after 3 weeks of treatment.Citation19 The program also showed that it had an acceptable safety profile and low incidence of cardiac and anticholinergic AEs.Citation17–Citation19
Aclidinium bromide
Aclidinium bromide 400 μg has been approved for the maintenance treatment of COPD.Citation20,Citation21 It has a twice-daily (BID) dosing regimen and is delivered by a multidose dry powder device named Genuair® in the EU and Pressair® in the USA.
Pharmacologic and pharmacokinetic profile
Preclinical studies have shown that aclidinium displays high affinity for all five muscarinic receptors, with kinetic selectivity for M3 receptors over M2, and a shorter duration of action and a faster onset compared with tiotropium bromide.Citation22 The drug’s preclinical cardiac safety profile is also favorable.Citation23
Pharmacokinetic studies in healthy volunteers showed that it is poorly absorbed into plasma and rapidly hydrolyzed into two major inactive metabolites, resulting in limited systemic exposure.Citation24,Citation25 Further studies in healthy individuals demonstrated that steady state was achieved within 2 days for aclidinium at all doses testedCitation25 and that there was no effect on the QT interval at doses of up to 800 μg BID.Citation26 Renal impairment does not appear to increase systemic exposure to aclidinium,Citation27 and its pharmacokinetic profile appears to be similar in younger (40–59 years of age) and more elderly (≥70 years of age) patients with COPD.Citation28
Efficacy and safety
Aclidinium has been extensively evaluated in patients with COPD ()Citation29–Citation39 and has also been the subject of a recent Cochrane systematic review.Citation40 Early studies of aclidinium examined a QD schedule,Citation37,Citation38 but while in a dose of 200 μg QD it significantly improved trough FEV1 in patients with COPD versus placebo,Citation39 the improvement (59–67 mL) was below the suggested minimum clinically important difference (MCID) of 100 mL,Citation41 although significant improvements in breathlessness and health status were seen and exacerbations were reduced.Citation39
Table 1 Overview of Phase II, Phase III, and long-term trials of aclidinium in COPD
Subsequently, studies investigating higher doses and alternative dosing regimens were conducted,Citation29,Citation30 leading to two Phase III studies: the 12-week AClidinium in Chronic Obstructive Respiratory Disease I (ACCORD COPD I) study ()Citation31 and the 24-week Aclidinium To Treat Airway obstruction In COPD PatieNts (ATTAIN) study ().Citation32 These evaluated aclidinium at doses of 200 and 400 μg BID versus placebo. Supportive studies include the 12-week ACCORD COPD II studyCitation33 and two long-term studiesCitation34,Citation35 (). Most recently, a 6-week Phase IIIb trial has compared aclidinium 400 μg BID with placebo and tiotropium QD via the HandiHaler® in patients with stable, moderate to severe COPD.Citation36
Figure 1 Study designs for the two pivotal Phase III studies of aclidinium BID: (A) ACCORD COPD I,Citation31 and (B) ATTAIN.Citation32
Impact of aclidinium on lung function
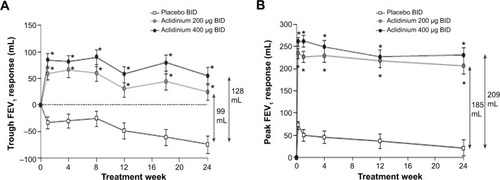
In the ACCORD COPD I study, the mean pre-bronchodilator FEV1 improved by 124 mL versus placebo () and peak FEV1 by 192 mL versus placebo after 12 weeks (). The peak FEV1 achieved with aclidinium was significantly greater than for placebo from the first dose onwards (P<0.0001).Citation31 In the 24-week ATTAIN study, the results were very similar: mean improvement in pre-bronchodilator FEV1 at Week 24 compared with placebo was 128 mL (), and mean improvement in peak FEV1 at Week 24 was 209 mL. Again, these benefits were seen from the first dose until the end of the study ().Citation32 The 12-week ACCORD COPD II study showed smaller improvements in trough FEV1, and this result is thought to be due to statistically significant imbalances between study arms in terms of baseline FEV1 and COPD severity.Citation33 Two long-term studies have shown that improvements in FEV1 from baseline with aclidinium are sustained for up to 52 weeks.Citation34,Citation35 The Cochrane meta-analysis confirmed that aclidinium therapy resulted in statistically significant improvements in both trough and peak FEV1 compared with placebo.Citation40
Figure 2 Change from baseline in (A) trough FEV1 and (B) peak FEV1 at Week 24 in ACCORD COPD I study.
Notes: *P<0.001 vs placebo; †P<0.05, ‡P<0.01 vs aclidinium 200 μg. From Kerwin EM, D’Urzo AD, Gelb AF, et al. COPD 2012;9(2):90–101. Copyright © 2012, Informa Healthcare. Reproduced with permission of Informa Healthcare.Citation31
Abbreviations: ACCORD, AClidinium in Chronic Obstructive Respiratory Disease I; FEV1, forced expiratory volume in 1 second; LS, least squares; SE, standard error.
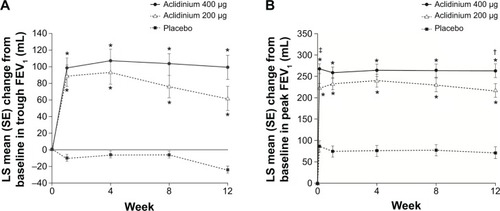
Figure 3 Change from baseline in (A) trough FEV1 and (B) peak FEV1 at Week 24 in ATTAIN study.
Abbreviations: ATTAIN, Aclidinium To Treat Airway obstruction In COPD patieNts; BID, twice daily; FEV1, forced expiratory volume in 1 second.
In a recently reported 6-week trial comparing aclidinium BID with placebo and tiotropium QD in patients with stable, moderate to severe COPD compared with placebo, improvements in the area under the curve (AUC) over 24 hours were significantly greater with aclidinium (156 mL) than with tiotropium (117 mL, P<0.05). This difference was largely driven by a significantly greater improvement in overnight AUC with aclidinium (168 mL) compared with tiotropium (100 mL, P<0.01), most likely arising due to the different pharmacokinetics associated with QD and BID dosing.Citation36
Breathlessness, health status, and COPD symptoms with aclidinium
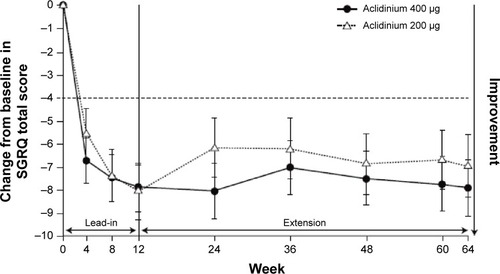
Significant improvements were seen in breathlessness, health status, and COPD symptoms in the pivotal trials. In both ACCORD COPD I and ATTAIN, by the end of the study, compared with placebo, the improvement in transition dyspnea index score reached the MCID.Citation31,Citation42,Citation43 In ATTAIN at Week 24, the improvement over placebo in SGRQ score exceeded the MCID.Citation42,Citation44 In the two 52-week studies, 45% of patients in LAS-MD-35 achieved a clinically significant improvement (≥4.0-unit improvement from baseline) in SGRQ score at Week 52;Citation34 similarly, in the ACCORD COPD I extension at 64 weeks, 64% of patients improved by more than this amount ().Citation35
Figure 4 Least squares mean (standard error) change from baseline in SGRQ total score in patients on continuous aclidinium in 1-year extension study of ACCORD COPD I.
Abbreviations: ACCORD, AClidinium in Chronic Obstructive Respiratory Disease I; SGRQ, St George’s Respiratory Questionnaire.
The clinical study data synthesis presented in the Cochrane review demonstrated significant improvements in transition dyspnea index (eight trials, 4,490 patients) and SGRQ (seven trials, 4,420 patients) with aclidinium therapy compared with placebo. Furthermore, a higher proportion of patients treated with aclidinium achieved the MCID in each of these measures, compared with placebo.Citation40
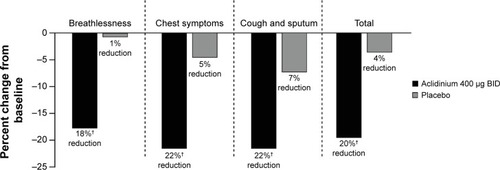
In the ACCORD COPD I study,Citation31 night-time and morning COPD symptoms were all significantly reduced among patients treated with aclidinium compared with those who received placebo (), and the impact of breathlessness on early morning activities was also significantly reduced with aclidinium versus placebo (). In the ATTAIN study,Citation32 the EXAcerbations of Chronic pulmonary disease Tool-Respiratory Systems (EXACT-RS) daily diary was used as an exploratory outcome measure. This showed that aclidinium improved the total score and the component scores (breathlessness, chest symptoms, and cough and sputum) significantly more than placebo ().
Figure 5 Percent change from baseline in frequency of night-time COPD symptoms at Week 12 in the ACCORD COPD I study.
Note: †P≤0.0023 vs placebo.
Abbreviations: ACCORD, AClidinium in Chronic Obstructive Respiratory Disease I; BID, twice daily.
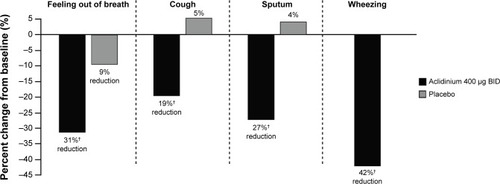
Figure 6 Percent change from baseline in severity and impact of early morning symptoms at Week 12 in the ACCORD COPD I study.
Notes: †P=0.0009, ‡P=0.0002.
Abbreviations: ACCORD, AClidinium in Chronic Obstructive Respiratory Disease I; BID, twice daily.
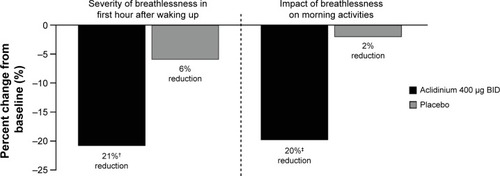
Figure 7 Percent change from baseline in daily COPD symptoms as measured by EXACT-RS scores at Week 24 in the ATTAIN study.
Abbreviations: ATTAIN, Aclidinium to Treat Airway Obstruction in COPD Patients; BID, twice daily; EXACT-RS, EXAcerbations of Chronic pulmonary disease Tool-Respiratory Systems.
Effect of aclidinium on COPD exacerbations
The impact of aclidinium BID on COPD exacerbations was examined in pooled analyses of data from ACCORD COPD I and ATTAIN.Citation45,Citation46 Two methods were used to capture COPD exacerbations – health care resource utilization, in which an exacerbation was defined as an increase in symptoms on ≥2 consecutive days requiring a change in treatment, and EXACT.Citation47 Pooled analyses of health care resource utilization assessments showed that aclidinium significantly reduced moderate to severe exacerbation rates by 29% compared with placebo.Citation46 The synthesized data in the Cochrane review from ten aclidinium clinical studies in 5,624 patients found that the reduction in moderate exacerbations requiring treatment with systemic steroids and/or antibiotics did not reach significance for aclidinium versus placebo, but that aclidinium significantly reduced the frequency of exacerbations requiring hospitalization.Citation40 However, it should be noted that these studies were not powered to investigate exacerbations, and the populations included were not enriched by recruiting patients with a history of frequent exacerbations.
Inhaler preference
In two randomized, double-blind, double-dummy, crossover studies (n=109 patients in total), more patients found the Genuair® easier to use than Aerolizer® or HandiHaler® and reported that dose preparation with Genuair® was “very easy” compared with the other two inhalers (65% vs 24% for Genuair® vs Aerolizer®, respectively; 80% vs 53% for Genuair® vs HandiHaler®, respectively).Citation48 Overall, more patients expressed a preference for Genuair® compared with Aerolizer® or HandiHaler®.Citation48 In a further study, significantly fewer patients made a critical error using Genuair® (10.5%) compared with HandiHaler® (26.7%).Citation49
Safety and tolerability of aclidinium
In the Phase III and IIIb studies, aclidinium exhibited a good tolerability profile ().Citation29–Citation36 In the 12-week ACCORD COPD I study, the overall incidence of AEs was very low and similar in aclidinium- and placebo-treated patients, with no evidence of a dose–harm relationship. In fact, COPD exacerbation was the only AE reported in >5% of patients in any treatment group (placebo, 12.4%; aclidinium 200 μg, 9.2%; aclidinium 400 μg, 7.4%).Citation31 Other AEs were headache (≤3.3%), nasopharyngitis (≤2.6%), back pain (≤2.7%), dyspnea (≤2.6%), and arthralgia (≤2.6%). A similar picture was observed in the ATTAIN trial, in which the most common AE was COPD exacerbation (placebo, 20.5%; aclidinium 200 μg, 15.9%; aclidinium 400 μg, 14.1%), followed by headache (≤12.3%), nasopharyngitis (≤11.6%), back pain (≤4.3%), and rhinitis (≤3.3%).Citation32 Cardiac AEs and anticholinergic AEs occurred in <2% of patients in any treatment group in the two studies.Citation31,Citation32
The good safety and tolerability profile of aclidinium was confirmed in the longer-term studies, LAS-MD-35 and the ACCORD COPD I extension ().Citation34,Citation35 In particular, major adverse cardiac events in pooled data were low, with no evidence of dose dependency (1.6% events with aclidinium 200 μg BID and 1.4% events with aclidinium 400 μg BID).Citation50 In the 6-week study comparing aclidinium with placebo and tiotropium, the incidence of AEs (28.0% overall) was similar between treatment groups, with few patients experiencing anticholinergic AEs (<2.0%, any group).Citation36 Further confirmation of the tolerability of aclidinium is provided by the Cochrane meta-analysis, including ten trials and 5,651 patients, which found no significant difference in the occurrence of AEs between aclidinium and placebo.Citation40
Dose selection
Several of the Phase II and III studies described in this review included two doses of aclidinium – 200 μg and 400 μg BID; however, the data have not revealed a clear dose–response relationship. The 400 μg dose provides numerically greater improvements in most study endpoints compared with aclidinium 200 μg, although there are exceptions, such as the improvements in SGRQ observed over 64 weeks (). However, these studies were not designed to directly compare the two doses, and confidence intervals often overlapped. As there are no apparent differences in the safety profile of the two doses, and the 400 μg dose consistently provided the greatest improvements, this was the dose licensed by the EMA and FDA.Citation51,Citation52
Discussion
As noted in the current GOLD guidelines, tiotropium, aclidinium, and glycopyrronium can all be considered as appropriate options for maintenance treatment in the stable COPD patient.Citation2 This review concerned aclidinium in the context of two other LAMAs. What stands out as the difference between it and them, since they seem to have similar efficacy and safety profiles?
The low systemic bio-availability of aclidinium may be an advantage, but more data are needed, as this is a class of drugs with a generally low side-effect rate. The BID dosing does not appear to be a disadvantage compared to the QD regimes of tiotropium and glycopyrronium, since it may confer better overnight bronchodilation that may be particularly beneficial for patients with significant night and morning symptoms.
The three LAMAs also provide patients with a choice, as each is delivered by a different device, and some patients may prefer one over another. For example, the multidose Genuair®/Pressair® was preferred by more patients than the HandiHaler®.Citation48 More importantly, the critical error rate (errors of use that potentially result in poor lung deposition of the drug) was lower with the aclidinium device than the HandiHaler®.Citation49 The device plays a crucially important role in determining the reliability of inhaled therapy, since poor technique is associated with increased health care resource use.Citation53
In conclusion, when considering new inhaled drugs, it is important to look beyond the chemical entity and its pharmacology. Dosing regimens and inhaler performance may be equally important in determining relative advantages of one drug over another. That may be the case with aclidinium.
Acknowledgments
Dr Alison Humphries from Complete Medical Communications provided medical writing support funded by Almirall S.A., Barcelona, Spain. Prior to peer review, Almirall S.A. was offered the opportunity to review this paper for scientific accuracy. Professor Paul Jones is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Disclosure
The author participated in the clinical development of aclidinium as an investigator for the AClidinium CLinical trial Assessing efficacy and safety In Moderate to severe COPD patients (ACCLAIM) COPD I (coordinating investigator), ACCLAIM COPD II (coordinating investigator), and ATTAIN (principal investigator) studies. The author and his institution have received consulting and lecture fees from Almirall S.A. in association with the aclidinium development program, but no fees for the writing of this paper. The author reports no other conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung Disease [webpage on the Internet]Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease [updated Jan 2014] Available from: http://www.goldcopd.com/guidelines-global-strategy-for-diagnosis-management.htmlAccessed November 12, 2014
- Global Initiative for Chronic Obstructive Lung Disease [webpage on the Internet]Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [updated 2014] Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdfAccessed December 12, 2014
- KarnerCChongJPoolePTiotropium versus placebo for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20147CD00928525046211
- TashkinDPCelliBSennSA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med2008359151543155418836213
- SinghSLokeYKFurbergCDInhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysisJAMA2008300121439145018812535
- SinghSLokeYKEnrightPLFurbergCDMortality associated with tiotropium mist inhaler in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis of randomised controlled trialsBMJ2011342d321521672999
- JaraMWentworthC3rdLanesSA new user cohort study comparing the safety of long-acting inhaled bronchodilators in COPDBMJ Open201223e000841
- ShortPMWilliamsonPAElderDHLipworthSISchembriSLipworthBJThe impact of tiotropium on mortality and exacerbations when added to inhaled corticosteroids and long-acting beta-agonist therapy in COPDChest20121411818621799028
- DongYHLinHHShauWYWuYCChangCHLaiMSComparative safety of inhaled medications in patients with chronic obstructive pulmonary disease: systematic review and mixed treatment comparison meta-analysis of randomised controlled trialsThorax2013681485623042705
- VerhammeKMAfonsoARomioSStrickerBCBrusselleGSturkenboomMUse of tiotropium Respimat® SMI vs tiotropium Handihaler® and mortality in patients with COPDEur Respir J201342360661523520322
- WiseRAAnzuetoACottonDTIOSPIR InvestigatorsTiotropium Respimat inhaler and the risk of death in COPDN Engl J Med2013369161491150123992515
- UlrikCSOnce-daily glycopyrronium bromide, a long-acting muscarinic antagonist, for chronic obstructive pulmonary disease: a systematic review of clinical benefitInt J Chron Obstruct Pulmon Dis2012767367823055716
- SechaudRRenardDZhang-AubersonLde la MotteSDrollmannAKaiserGPharmacokinetics of multiple inhaled NVA237 doses in patients with chronic obstructive pulmonary disease (COPD)Int J Clin Pharmacol Ther201250211812822257577
- FogartyCHattersleyHDi ScalaLDrollmannABronchodilatory effects of NVA237, a once daily long-acting muscarinic antagonist, in COPD patientsRespir Med2011105333734221144724
- VerkindreCFukuchiYFlémaleASustained 24-h efficacy of NVA237, a once-daily long-acting muscarinic antagonist, in COPD patientsRespir Med2010104101482148920541381
- VogelmeierCVerkindreCCheungDSafety and tolerability of NVA237, a once-daily long-acting muscarinic antagonist, in COPD patientsPulm Pharmacol Ther201023543844420416390
- D’UrzoAFergusonGTvan NoordJAEfficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trialRespir Res20111215622151296
- KerwinEHébertJGallagherNEfficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 studyEur Respir J20124051106111423060624
- BeehKMSinghDDi ScalaLDrollmannAOnce-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trialInt J Chron Obstruct Pulmon Dis2012750351322973092
- European Medicines Agency [webpage on the Internet]Eklira® Genuair® (aclidinium bromide)London, UKEuropean Medicines Agency2014 [updated Dec, 2014]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002211/human_med_001571.jsp&mid=WC0b01ac058001d124Accessed December 1, 2014
- US Food and Drug Administration [webpage on the Internet]Tudorza® Pressair® (aclidinium bromide)Rockville, MDUS Food and Drug Administration2012 [updated 2012]. Available from: http://www.access-data.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Drug-DetailsAccessed December 1, 2014
- GavaldàAMiralpeixMRamosICharacterization of aclidinium bromide, a novel inhaled muscarinic antagonist, with long duration of action and a favorable pharmacological profileJ Pharmacol Exp Ther2009331274075119710368
- GavaldàAViñalsMAubetsJGrasJEffect of formoterol alone and in combination with aclidinium on electrocardiograms in dogsEur Respir J201240Suppl 56P2116
- JansatJMLamarcaRde MiquelGSchrödterAMiletzkiBGurniakMSafety and pharmacokinetics of multiple doses of aclidinium bromide, a novel long-acting muscarinic antagonist for the treatment of chronic obstructive pulmonary disease, in healthy participantsJ Clin Pharmacol200949101239124619592595
- LasseterKCDilzerSJansatJMGarcia GilECaractaCOrtizSSafety and pharmacokinetics of multiple doses of aclidinium bromide administered twice daily in healthy volunteersPulm Pharmacol Ther20122519319922366196
- LasseterKCAubetsJChuecosFGarcia GilEAclidinium bromide, a long-acting antimuscarinic, does not affect QT interval in healthy subjectsJ Clin Pharmacol201051692393220959525
- SchmidKPascualSGarcia GilEOrtizSJansatJMPharmacokinetics and safety of aclidinium bromide, a muscarinic antagonist, in adults with normal or impaired renal function: A phase I, open-label, single-dose clinical trialClin Ther201032101798181221194604
- de la MotteSBeierJSchmidKPascualSJansatJMGarcia GilEPharmacokinetics and safety of aclidinium bromide in younger and elderly patients with chronic obstructive pulmonary diseaseInt J Clin Pharmacol Ther201250640341222541745
- FuhrRMagnussenHSaremKEfficacy of aclidinium bromide 400 μg twice daily compared with placebo and tiotropium in patients with moderate to severe COPDChest2012141374575221903737
- SinghDMagnussenHKirstenAA randomised, placebo- and active-controlled dose-finding study of aclidinium bromide administered twice a day in COPD patientsPulm Pharmacol Ther201225324825322497752
- KerwinEMD’UrzoADGelbAFACCORD I study investigators. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I)COPD2012929010122320148
- JonesPWSinghDBatemanEDEfficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN studyEur Respir J201240483083622441743
- RennardSIScanlonPDFergusonGTACCORD COPD II: a randomized clinical trial to evaluate the 12-week efficacy and safety of twice-daily aclidinium bromide in chronic obstructive pulmonary disease patientsClin Drug Investig201313312893904
- GelbAFTashkinDPMakeBJZhongXGarcia GilECaractaCLAS-MD-35 study investigatorsLong-term safety and efficacy of twice-daily aclidinium bromide in patients with COPDRespir Med2013107121957196523916502
- D’UrzoAKerwinERennardSHeTGarcia GilECaractaCOne-year extension study of ACCORD COPD I: safety and efficacy of two doses of twice-daily aclidinium bromide in patients with COPDCOPD201310450051023679347
- BeierJKirstenAMMrózREfficacy and safety of aclidinium bromide compared with placebo and tiotropium in patients with moderate-to-severe chronic obstructive pulmonary disease: results from a 6-week, randomized, controlled Phase IIIb studyCOPD201310451152223819698
- ChanezPBurgePSDahlRAclidinium bromide provides long-acting bronchodilation in patients with COPDPulm Pharmacol Ther2010231152119683590
- JoosGFSchelfhoutVJPauwelsRABronchodilatory effects of aclidinium bromide, a long-acting muscarinic antagonist, in COPD patientsRespir Med2010104686587220044242
- JonesPWRennardSIAgustiAEfficacy and safety of once-daily aclidinium in chronic obstructive pulmonary diseaseRespir Res2011125521518460
- NiHSoeZMoeSAclidinium bromide for stable chronic obstructive pulmonary diseaseCochrane Database Syst Rev20149CD01050925234126
- DonohueJFMinimal clinically important differences in COPD lung functionCOPD20052111112417136971
- AgustiAJonesPWBatemanEDImprovement in symptoms and rescue medication use with aclidinium bromide in patients with chronic obstructive pulmonary disease: results from ATTAINEur Respir J201138Suppl 55874
- MahlerDAWitekTJJrThe MCID of the transition dyspnea index is a total score of one unitCOPD2005219910317136969
- JonesPWQuirkFHBaveystockCMThe St George’s Respiratory QuestionnaireRespir Med199185Suppl B25311759018
- KerwinEJonesPD’UrzoARekedaLGarcia GilECaractaCTwice-daily aclidinium bromide in COPD patients: a pooled analysis of lung function in the ACCORD-COPD I and ATTAIN trialsEur Respir J201240Suppl 56P2892
- JonesPWSinghDKerwinELamarcaRCaractaCGarcia GilEReduced COPD exacerbations associated with aclidinium bromide versus placebo: a pooled analysis of Phase III dataThorax201267Suppl 2A146
- LeidyNKWilcoxTKJonesPWRobertsLPowersJHSethiSEXACT-PRO Study GroupStandardizing measurement of chronic obstructive pulmonary disease exacerbations: reliability and validity of a patient-reported diaryAm J Respir Crit Care Med2011183332332920813886
- FuhrRMagnussenHSinghDde MiquelGCaractaCGarcia GilEPatient assessments of ease of use of Genuair® versus Aerolizer® and HandiHaler®Eur Respir J201138Suppl 55P3979
- van der PalenJGinkoTKrokerAPreference, satisfaction and errors with two dry powder inhalers in patients with COPDExpert Opin Drug Deliv20131081023103123745954
- DonohueJRennardSCelliBRekedaLGarcia GilECaractaCSafety and tolerability of aclidinium bromide in patients with COPD: pooled results from long-term phase III studiesChest20121424688A
- European Medicines Agency Committee for Medicinal Products for Human Use (CHMP)Eklira Genuair: CHMP assessment report5242012 http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002211/WC500132663.pdfAccessed February 25, 2015
- Food and Drug AdministrationTudorza Pressair: Summary review of regulatory action7202012 http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202450Orig1s000SumR.pdfAccessed February 25, 2015
- MelaniASBonaviaMCilentiVInhaler mishandling remains common in real life and is associated with reduced disease controlRespir Med2011105693093821367593

