Abstract
Background
The role of the antioxidant N-acetylcysteine (NAC) in the treatment of chronic obstructive pulmonary disease (COPD) has not been clarified as yet. In early studies, we found that the proportion of smokers with COPD having extremely slow/slow microsomal epoxide hydrolase (EPHX1) enzyme activity is significantly higher than that in healthy smokers. The purpose of this study was to evaluate whether different EPHX1 enzyme activity is related to differential therapeutic effects of treatment with NAC in COPD.
Methods
A total of 219 patients with COPD were randomly allocated to an extremely slow/slow EPHX1 enzyme activity group (n=157) or a fast/normal EPHX1 enzyme activity group (n=62) according to their EPHX1 enzyme activity. Both groups were treated with NAC 600 mg twice daily for one year. The main study parameters, including forced expiratory volume in one second (FEV1), St George’s Respiratory Questionnaire (SGRQ), and yearly exacerbation rate, were measured at baseline and at 6-month intervals for one year.
Results
Both FEV1 and SGRQ symptom scores were improved after treatment with NAC in the slow activity group when compared with the fast activity group. Further, changes in FEV1 and SGRQ symptom score in patients with mild-to-moderate COPD were more significant than those in patients with severe-to-very severe COPD. The yearly exacerbation rates were reduced in both groups, but the reduction in the slow activity group was significantly lower than in the fast activity group.
Conclusion
NAC treatment in COPD patients with extremely slow/slow EPHX1 enzyme activity improves FEV1 and the SGRQ symptom score, especially in those with mild-to-moderate COPD, and polymorphism in the EPHX1 gene may have a significant role in differential responses to treatment with NAC in patients with COPD.
Introduction
It is well known that chronic obstructive pulmonary disease (COPD) is strongly associated with genetic factors and that susceptibility to COPD depends in part on the genetic phenotypes and gene polymorphism of a variety of factors involved in the pathogenesis of COPD, such as inflammatory cytokines, proteases, antiproteases, oxidoreductases, and detoxifying enzymes. There is good evidence to suggest that increasing oxidative stress is a key factor in the pathogenesis of COPD.Citation1–Citation3 The body has a perfect enzymatic and nonenzymatic antioxidation system to cope with oxidative stress and protect the body from attack by oxidants. The main known oxidation inhibition enzymes in the body including glutathione-S-transferase, microsomal epoxide hydrolase (EPHX1), and heme oxygenase, hydrolyze and inactivate oxygen metabolites, thus fighting against or neutralizing the oxidative damage caused by oxidative stress, and eventually maintaining the dynamic balance of oxidation/antioxidation in the body. When the production of oxidation inhibitors is decreased or their activity is diminished as a result of genetic variation, the dynamic balance of oxidation/antioxidation is lost, leading to oxidative damage.
In our early oxidation inhibition enzyme and antiprotease gene polymorphism studies, we found that there was no significant correlation between GSTP1 I105V polymorphism and COPD, and we did not find any association between polymorphisms in the serine protein inhibitor E2 gene and COPD in the Han population of southwest China.Citation4,Citation5 However, we did find that the exon 3 heterozygous genotype of EPHX1 (Tyr113/His113) in smokers with COPD was significantly higher than in otherwise healthy smokers, and so was the proportion of subjects with extremely slow/slow EPHX1 enzyme activity.Citation6,Citation7
Being a representative antioxidant and mucus-modifying drug,Citation8–Citation10 N-acetylcysteine (NAC) is the focus of a good deal of pharmaceutical research at present. Numerous researchers have demonstrated that NAC can reduce the number of acute exacerbations of COPD,Citation11–Citation14 but the evidence for whether NAC can improve lung function or not remains equivocal. Stav and Raz conducted a double-blind, randomized, placebo-controlled study and found that treating COPD patients with NAC had a beneficial effect on physical performance, probably due to a reduction in air trapping.Citation15 The results of a large multicenter study conducted in Europe show that although NAC could improve symptoms in patients with COPD and reduce their average medical expenditure, the decline in forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) was not significantly different between the NAC group and the placebo group.Citation16 Another multicenter study followed 523 patients with COPD for 3 years and found that there was no difference in lung function or in prevention of exacerbations between NAC and placebo.Citation17
What types of patients with COPD may benefit more from NAC treatment? Our hypothesis is that the equivocal effect of NAC as antioxidant therapy in patients with COPD may be associated with genetic phenotypes and gene polymorphism in oxidation inhibiting enzymes. This study was conducted to explore whether there was a difference in the curative effect when COPD patients with different EPFX1 genotypes were given the same NAC treatment. To our knowledge, no relevant reports have been published to date.
Materials and methods
Subjects
A total of 219 unselected smokers with stable COPD (diagnostic criteria in accordance with the 2011 Global Initiative for Chronic Obstructive Lung Disease guideline) were recruited from the Department of Respiratory Critical Care Medicine, First Affiliated Hospital of Kunming Medical University, Kunming, People’s Republic of China, between June 2012 and December 2013. Two hundred and twenty-three otherwise healthy smokers without COPD and matched for age and sex were recruited as controls from the medical center at the First Affiliated Hospital of Kunming Medical University. All subjects were of Han nationality and from southwest China. Eligible patients with stable COPD and post-bronchodilator spirometry FEV1/FVC <70%, aged 60–80 years, and with the duration of COPD of 5 years or more were included. Patients were excluded if they had asthma or other chronic bronchial lung or allergic disease, had a history of upper respiratory tract infection in the 6 weeks before enrolment or upper respiratory tract infection history in the first 2 weeks of the screening period, or had a history of lung surgery, malignancy, or other severe organ dysfunction. Patients with severe hypoxemia who failed to cooperate with lung function testing or had poor reliability or compliance were also excluded.
None of the patients needed to change their current medication (including inhaled long-acting beta-agonists and corticosteroid). The study was approved by the ethics committee of Kunming Medical University. All subjects provided their written informed consent.
DNA genotyping
Genomic DNA was extracted from peripheral blood leukocytes in the 219 COPD patients and 223 healthy smokers by a phenol-chloroform method. We identified the EPHX1 polymorphisms using direct sequencing for EPHX1 exon 3 and polymerase chain reaction-restriction fragment length polymorphism for EPHX1 exon 4. Based on their EPHX1 exon 3 and exon 4 polymorphism status, according to the classification of Smith and Harrison,Citation18 the patients could be classified into four groups according to putative EPHX1 phenotype for enzyme activity, ie, normal, fast, slow, and extremely slow, as we have described previously.Citation6
Study design
Eligible patients were allocated to either an extremely slow/slow EPHX1 enzyme activity group or a fast/normal EPHX1 enzyme activity group based on their level of enzyme activity. All subjects were given NAC 600 mg twice daily (Zambon Pharma, Bresso, Italy) for one year. Study parameters, including age, sex, smoking status, body mass index, current medication, exacerbation rate during the previous year, lung function, SGRQ score, and 6MWD, were recorded at baseline before the beginning of the trial. Symptoms, changes in current medication, and patient compliances with NAC therapy were recorded at each follow-up visit day (every 3 months). Lung function, SGRQ score, and 6MWD were measured at 6 and 12 months. An exacerbation episode was defined as an increase in cough, sputum, or dyspnea, making it necessary to use antibiotics and/or oral corticosteroids or to hospitalize the patient during the study. Adverse events were also recorded throughout the study.
Lung function tests
Spirometry was performed by a trained specialist technologist using a Pony FX portable spirometer (Cosmed, Rome, Italy) according to the American Thoracic Society standards for lung function tests.Citation19 Spirometric data (FEV1, FVC, FEV1/FVC ratio, FEV1% predicted) were measured at 0, 6, and 12 months during the trial.
Statistical analysis
The sample size was estimated so that the genetic association would be adequately powered to detect in excess of 200 subjects using Quanto (assuming 90% power, Kp 0.1, RG 2.6). Statistical analyses were performed using Statistical Package for the Social Sciences version 21.0 (IBM Corporation, Armonk, NY, USA). All hypothesis tests were two-sided, and P<0.05 was defined as being statistically significant. Data are expressed as the mean ± standard deviation unless indicated otherwise.
The frequencies of the EPHX1 genotypes between patients with COPD and controls were compared using the two-tailed chi-squared test. Odds ratios (ORs) and 95% confidence intervals (CIs) were also calculated to assess the relative risk of COPD. The differences in enumeration data between the two groups at baseline (such as age, smoking index, FEV1% predicted, FEV1/FVC) were compared using the unpaired t-test, and measurement data (such as sex, composition, and frequency of EPHX1 genotype distribution) were compared using the chi-squared test. Changes in lung function parameters and other variables in the two groups were analyzed before and after treatment with NAC using the paired t-test.
Results
We recruited 240 patients with COPD and 223 healthy smokers from June 2012 to December 2013, but 21 patients with COPD had dropped out by the end of the study. Six patients with COPD withdrew their consent, eight were lost to follow-up, four showed poor compliance, and three died during the study, leaving 219 patients who completed the study.
shows the baseline characteristics of the 219 patients with COPD and the 223 healthy smokers; shows the distribution of EPHX1 genotypes between these two groups. The proportion of heterozygous (Tyr113/His113) individuals was significantly higher in the COPD group than in the control group (63.9% versus 38.1%, respectively, P<0.001; OR 1.6, 95% CI 1.4–1.9). Conversely, the frequency of homozygous wild-type (Tyr113/Tyr113) subjects was significantly lower in the COPD group than in the control group (18.7% versus 41.3%, respectively, P<0.001; OR 0.4, 95% CI 0.3–0.6). However, there was no significant difference in distribution of exon 4 genotype polymorphism between the COPD group and control group. shows the distribution of EPHX1 phenotypes in the COPD and healthy smokers. The frequency of the slow activity EPHX1 phenotype was significantly higher in the COPD group than in the control group (54.3% versus 32.3%, P<0.001, OR 1.4, 95% CI 1.2–1.6), and the frequency of the fast activity EPHX1 phenotype was lower in the COPD group than in the control group (4.1% versus 15.7%, P<0.001, OR 0.2, 95% CI 0.1–0.4).
Table 1 Baseline characteristics of COPD patients and healthy smokers
Table 2 Distribution of EPHX1 genotypes in COPD patients and healthy smokers
Table 3 Distribution of EPHX1 phenotypes in COPD patients and healthy smokers
All eligible patients with COPD were allocated to either a slow activity group (n=157) or fast activity group (n=62) based on EPHX1 enzyme activity. Baseline characteristics and lung function parameters for patients with COPD did not differ between the slow and fast activity groups ().
Table 4 Baseline characteristics of patients with COPD in different EPHX1 enzyme activity groups
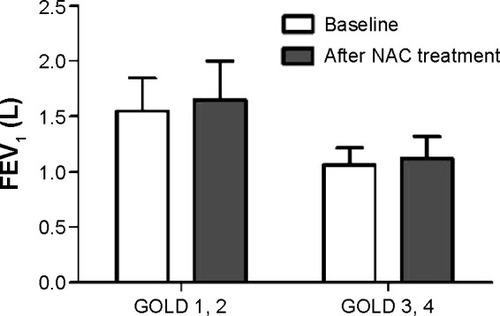
There were significant improvements in FEV1 (from 1.37±0.35 L to 1.46±0.39 L, P=0.041) and FEV1% predicted (from 59.1±16.5 to 63.2±17.8, P=0.038) in the slow activity group after treatment with NAC for one year, but there were no significant differences with regard to changes in FEV1 and FEV1% predicted in the fast activity group after treatment with NAC for one year ( and ). When the patients in the slow activity group were classified as having either mild to moderate COPD (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage 1 and 2) or severe to very severe COPD (GOLD 3 and 4) based on disease severity, we noted that the changes in FEV1 (1.55±0.30 L versus 1.65±0.35 L, P=0.030) and FEV1% predicted (68.4±12.4 versus 73.1±13.2, P=0.01) were statistically significant in the mild to moderate subgroup (). There were also improvements in FEV1 (1.06±0.16 L versus 1.12±0.20 L, P=0.075) and FEV1% predicted (42.4±7.2 versus 45.2±8.5, P=0.064) in the severe to extremely severe subgroup, but these did not reach statistical significance.
Table 5 Characteristics of subjects with COPD in different EPHX1 enzyme activity groups before and after treatment with NAC for 12 months
Figure 1 FEV1 versus NAC treatment in the two groups.
Abbreviations: FEV1, forced expiratory volume in one second; NAC, N- acetylcysteine.
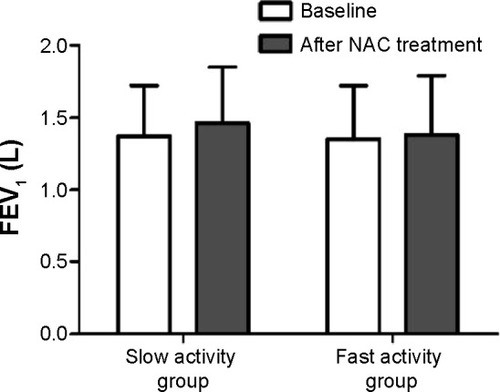
Figure 2 FEV1 versus NAC treatment in the two subgroups of the slow activity group.
Exacerbation rates in both the slow activity and fast activity groups were significantly lower than at baseline after treatment with NAC for one year. Moreover, the mean exacerbation rate was lower in the slow activity group than in the fast activity group (0.88±0.59 versus 1.21±0.60, respectively, P<0.001).
There was a significant difference in SGRQ symptom score in the slow activity group after one year of treatment with NAC when compared with baseline (40.9±12.3 versus 37.8±13.1, P=0.033), but there was no significant difference in the fast activity group. Further, the change in SGRQ symptom score after treatment with NAC was statistically significant compared with baseline in the mild to moderate subgroup (34.3±8.9 versus 31.2±9.8, P=0.018), but there was no statistically significant change after treatment with NAC in the severe to extremely severe subgroup (52.7±7.8 versus 49.8±9.2, P=0.071). There were no statistically significant differences in SGRQ total score or any other domains in the two groups between baseline and after treatment with NAC. Changes from baseline in body mass index and 6MWD did not differ in the two groups after one year of treatment with NAC.
Except for mild gastrointestinal complaints, no major adverse events occurred in each of the groups. Three patients died during the trial, one in the slow activity group (pneumonia) and two in the fast activity group (acute exacerbation of COPD and acute heart failure). None of these deaths were thought to be related to NAC.
Discussion
Our study demonstrated significant improvements in FEV1, FEV1% predicted, and SGRQ symptom score in COPD patients with extremely slow/slow activity of EPHX1 who were treated with NAC 600 mg twice daily for one year. Unlike in previous studies, this study of NAC treatment was carried out in COPD patients with different EPHX1 phenotypes for enzyme activity, and that may be one of the reasons why findings such as ours have not been reported previously. In an earlier study of 256 COPD patients and in this study, we found that the extremely slow/slow activity EPHX1 phenotype exhibited decreased enzyme activity and thus had an increased risk of COPD.Citation6 Other studies have also revealed a significant association between the extremely slow/slow activity EPHX1 phenotype and an increased risk of COPD.Citation18,Citation20–Citation23 The EPHX1 enzyme detoxifies the harmful epoxides produced by smoking and hydrolyzes various exogenous pro-oxidants to form water and soluble dihydrodiol compounds, so has a protective effect on the lung.Citation24 The extremely slow/slow activity phenotype has a decrease in EPHX1 enzyme activity of more than 50%.Citation25 This decreased EPHX1 enzyme activity may result in an oxidant/antioxidant imbalance within the lung. The body’s antioxidant capacity may be enhanced by antioxidant drugs such as NAC in COPD patients with decreased EPHX1 enzyme activity. This may be the reason why NAC was more effective in the group with the extremely slow/slow EPHX1 activity phenotype than in the group with fast/normal EPHX1 activity phenotype.
Numerous studies have been performed over the last two decades to assess whether NAC has potential therapeutic effects in patients with COPD. No previous studies have shown a significant increase in FEV1 with either high-dose or low-dose NAC, although some studies reported improvements in some parameters of lung function after treatment with NAC. For example, Stav and Raz found that inspiratory capacity and FVC were higher, in particular, post exercise, after treatment with NAC 600 mg twice daily compared with placebo,Citation15 and Tse et al found that high-dose NAC resulted in significantly improved small airway function.Citation13 Some of the interindividual differences in effect associated with NAC treatment might be explained by genetic polymorphism of the antioxidase.
Another important finding of this study is that the changes in FEV1, FEV1% predicted, and SGRQ symptom score were statistically significant in the mild to moderate subgroup after treatment with NAC when stratified by disease severity. Although there was also a small improvement in the severe to very severe subgroup, the change was not statistically significant. Our analysis of the treatment effects in COPD stratified by severity showed a greater improvement in FEV1 in less advanced COPD. This suggests that NAC might have a more crucial preventive effect in patients in the early stages of COPD. The effect of early intervention with NAC in COPD patients might be better with regard to controlling symptoms, slowing disease progression, and improving the quality of life and prognosis.
There was also a significant reduction in exacerbation rate in the slow activity group and in the fast activity group after treatment with NAC, but there the exacerbation rate was lower in the slow activity group than in the fast activity group (0.88±0.59 exacerbations per patient-year versus 1.21±0.60 exacerbations per patient-year, respectively, P<0.001). There is increasing evidence that use of NAC can decrease the risk of exacerbation in patients with COPD,Citation26–Citation30 and our findings is also in agreement with recent studies.Citation13,Citation14
Except for SGRQ symptom score, there were no significant differences in BMI, 6MWD, or SGRQ total score or other domains in the two groups between baseline and after treatment with NAC. The reason for this might be two-fold. Firstly, COPD is a complex polygenic disease, and previous studies have shown that the hereditary susceptibility to COPD may depend on the coincidence of several gene polymorphisms acting together,Citation31,Citation32 but our study only observed the effect of NAC treatment in COPD patients stratified by EPHX1. Will the effect of NAC treatment be better in COPD patients with deficiencies in several antioxidant enzyme genes? Secondly, some studies have shown that it takes more than 6 months for the antioxidant drug to go into effect,Citation14,Citation33 and suggested that the therapeutic effect of the antioxidant drug was slow but progressive, so that longer treatment times might be required to improve BMI and exercise capacity in patients with COPD.
In conclusion, we have found preliminary evidence that FEV1, SGRQ symptom score, and risk of exacerbation can be improved by NAC in COPD patients with extremely slow/slow EPHX1 enzyme activity, which means that polymorphism in the EPHX1 gene may have a significant role in the different responses to NAC seen in patients with COPD.
Our findings have crucial clinical implications with regard to our understanding of the hereditary factors determining responsiveness to antioxidant treatment with NAC in patients with COPD. More and larger prospective studies in groups of COPD patients with different ethnic origins and various antioxidant enzyme gene polymorphisms will help to define which patients may benefit more from treatment with NAC.
Acknowledgments
We are grateful to Shen Ling from the School of Public Health, Kunming Medical University, for help with the statistical analysis and to Gan Ping, Kunming Institute of Zoology, for providing technical assistance. This study was supported by a grant from the National Natural Science Foundation of China (81160006).
Disclosure
The authors report no conflicts of interest in this work.
References
- MacNeeWPulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary diseaseProc Am Thorac Soc200521506016113469
- SpruitMASinghSJGarveyCAn official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitationAm J Respir Crit Care Med20131888e13e6424127811
- VestboJHurdSSAgustiAGGlobal Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- ZhongLZhangYPFuWPDaiLMSunCWangYQThe relationship between GSTP1 I105V polymorphism and COPD: a reappraisalAm J Respir Crit Care Med2010181776376520335387
- ZhongLFuWPSunCDaiLMZhangYPAbsence of association between SERPINE2 genetic polymorphisms and chronic obstructive pulmonary disease in Han Chinese: a case-control cohort studyBMC Med Genet2009106619604412
- FuWPSunCDaiLMYangLFZhangYPRelationship between COPD and polymorphisms of HOX-1 and mEPH in a Chinese populationOncol Rep200717248348817203192
- LiHFuWPHongZHMicrosomal epoxide hydrolase gene polymorphisms and risk of chronic obstructive pulmonary disease: a comprehensive meta-analysisOncol Lett2013531022103023426996
- DekhuijzenPNAntioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary diseaseEur Respir J200423462963615083766
- UenoTYamadaMIgarashiYOgawaTN-acetyl cysteine protects osteoblastic function from oxidative stressJ Biomed Mater Res A201199452353121913320
- van OverveldFJDemkowUGoreckaDde BackerWAZielinskiJNew developments in the treatment of COPD: comparing the effects of inhaled corticosteroids and N-acetylcysteineJ Physiol Pharmacol200556Suppl 413514216204787
- GerritsCMHeringsRMLeufkensHGLammersJWN-acetylcysteine reduces the risk of re-hospitalisation among patients with chronic obstructive pulmonary diseaseEur Respir J200321579579812765423
- PelaRCalcagniAMSubiacoSIsidoriPTubaldiASanguinettiCMN-acetylcysteine reduces the exacerbation rate in patients with moderate to severe COPDRespiration199966649550010575333
- TseHNRaiteriLWongKYHigh-dose N-acetylcysteine in stable COPD: the 1-year, double-blind, randomized, placebo-controlled HIACE studyChest2013144110611823348146
- ZhengJPWenFQBaiCXTwice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trialLancet Respir Med20142318719424621680
- StavDRazMEffect of N-acetylcysteine on air trapping in COPD: a randomized placebo-controlled studyChest2009136238138619447919
- SchermerTChavannesNDekhuijzenRFluticasone and N-acetylcysteine in primary care patients with COPD or chronic bronchitisRespir Med2009103454255119138505
- DecramerMRutten-van MolkenMDekhuijzenPNEffects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trialLancet200536594701552156015866309
- SmithCAHarrisonDJAssociation between polymorphism in gene for microsomal epoxide hydrolase and susceptibility to emphysemaLancet199735090786306339288046
- American Thoracic SocietyStandardization of Spirometry, 1994 UpdateAm J Respir Crit Care Med19951523110711367663792
- ParkJYChenLWadhwaNTockmanMSPolymorphisms for microsomal epoxide hydrolase and genetic susceptibility to COPDInt J Mol Med200515344344815702235
- RodriguezFJardiRCostaXDetection of polymorphisms at exons 3 (Tyr113 – >His) and 4 (His139 – >Arg) of the microsomal epoxide hydrolase gene using fluorescence PCR method combined with melting curves analysisAnal Biochem2002308112012612234472
- SandfordAJChaganiTWeirTDConnettJEAnthonisenNRParePDSusceptibility genes for rapid decline of lung function in the lung health studyAm J Respir Crit Care Med2001163246947311179124
- YoshikawaMHiyamaKIshiokaSMaedaHMaedaAYamakidoMMicrosomal epoxide hydrolase genotypes and chronic obstructive pulmonary disease in JapaneseInt J Mol Med200051495310601573
- MaekawaKItodaMHaniokaNNon-synonymous single nucleotide alterations in the microsomal epoxide hydrolase gene and their functional effectsXenobiotica200333327728712637245
- HassettCAicherLSidhuJSOmiecinskiCJHuman microsomal epoxide hydrolase: genetic polymorphism and functional expression in vitro of amino acid variantsHum Mol Genet1994334214287516776
- De BackerJVosWVan HolsbekeCEffect of high-dose N-acetylcysteine on airway geometry, inflammation, and oxidative stress in COPD patientsInt J Chron Obstruct Pulmon Dis2013856957924293993
- ShenYCaiWLeiSZhangZEffect of high/low dose N-acetylcysteine on chronic obstructive pulmonary disease: a systematic review and meta-analysisCOPD201411335135824378052
- SteyCSteurerJBachmannSMediciTCTramerMRThe effect of oral N-acetylcysteine in chronic bronchitis: a quantitative systematic reviewEur Respir J200016225326210968500
- SutherlandERCrapoJDBowlerRPN-acetylcysteine and exacerbations of chronic obstructive pulmonary diseaseCOPD20063419520217361500
- ZuinRPalamideseANegrinRCatozzoLScardaABalbinotMHigh-dose N-acetylcysteine in patients with exacerbations of chronic obstructive pulmonary diseaseClin Drug Invest2005256401408
- LomasDASilvermanEKThe genetics of chronic obstructive pulmonary diseaseRespir Res200121202611686861
- SampsonasFKarkouliasKKaparianosASpiropoulosKGenetics of chronic obstructive pulmonary disease, beyond a1-antitrypsin deficiencyCurr Med Chem200613242857287317073633
- ZhengJPKangJHuangSGEffect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebo-controlled studyLancet200837196292013201818555912

