Abstract
Introduction
Chronic obstructive pulmonary disease (COPD) constitutes a major health challenge in Central and Eastern European (CEE) countries. However, clinical phenotypes, symptom load, and treatment habits of patients with COPD in CEE countries remain largely unknown. This paper provides a rationale for phenotyping COPD and describes the methodology of a large study in CEE.
Methods/design
The POPE study is an international, multicenter, observational cross-sectional survey of patients with COPD in CEE. Participation in the study is offered to all consecutive outpatients with stable COPD in 84 centers across the CEE region if they fulfill the following criteria: age >40 years, smoking history ≥10 pack-years, a confirmed diagnosis of COPD with postbronchodilator FEV1/FVC <0.7, and absence of COPD exacerbation ≥4 weeks. Medical history, risk factors for COPD, comorbidities, lung function parameters, symptoms, and pharmaceutical and nonpharmaceutical treatment are recorded. The POPE project is registered in ClinicalTrials.gov with the identifier NCT2119494.
Outcomes
The primary aim of the POPE study was to phenotype patients with COPD in a real-life setting within CEE countries using predefined classifications. Secondary aims of the study included analysis of differences in symptoms, and diagnostic and therapeutic behavior in participating CEE countries.
Conclusion
There is increasing acceptance toward a phenotype-driven therapeutic approach in COPD. The POPE study may contribute to reveal important information regarding phenotypes and therapy in real-life CEE.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of death worldwide and represents an important public health challenge.Citation1 On the basis of multiple studies that have been published since the 1970s, the estimate of COPD prevalence ranges between 5% and 10%.Citation2,Citation3 According to the World Health Organization estimates, COPD is predicted to become the third leading cause of death by 2030, and the burden of COPD is projected to further increase in coming decades due to continued exposure to COPD risk factors and aging of the population.Citation4–Citation6 While the major risk factor is tobacco smoking, other risk factors include age, a previous history of bronchial asthma, genetic predisposition, and respiratory infections.Citation7–Citation12 In addition to these factors, environmental and occupational exposure to gases and particles and indoor biomass inhalation may also substantially contribute to the development of COPD in affected populations.Citation13,Citation14
Although numerous studies and clinical trials regarding clinical presentation, diagnosis, and management of COPD have been recently published, very few of these studies have specifically focused on Central and Eastern Europe (CEE).Citation15–Citation23 However, patients with COPD in CEE might present with different features of the disease due to differences in environmental and nonenvironmental risk factors, age of onset of disease, comorbidities, health care access, and the level of reimbursement for COPD treatment. Thus, the objectives of the “Phenotypes of COPD in Central and Eastern Europe Study” (POPE study) are to gain a better understanding of these patient characteristics and treatment patterns of patients diagnosed with COPD among different CEE countries. This is the first CEE, multicenter, investigator-initiated, collaborative project of its kind. The purposes of this paper are to provide an introduction to the study methodology and to raise awareness toward a current hot topic in COPD research, namely, the issue of COPD phenotyping.
Methods/design
Study design
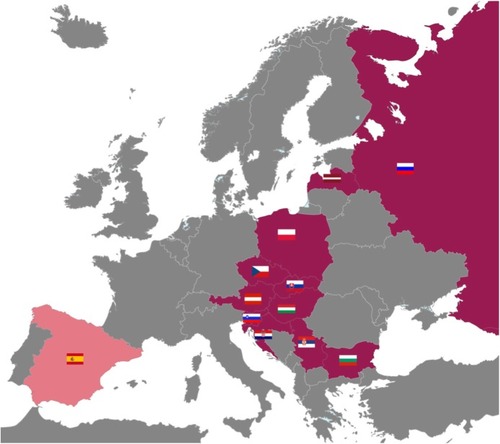
The POPE study is an international, multicenter, observational cross-sectional survey in patients with COPD in CEE. Eleven CEE countries participated in the study: Austria, Bulgaria, Croatia, Czech Republic, Hungary, Latvia, Poland, Russia, Serbia, Slovakia, and Slovenia (). The complete list of participating centers is listed in . A Steering Committee consisting of eight physicians is responsible for the scientific integrity of the study (). Each participating country is represented by one national leading expert, who coordinates the study at the national level (). Within each participating country, investigators selected by the national expert are appointed and are responsible for local data collection and organization of care. The first patient (FPI) in the database was documented in April 2014. The expected end of patient enrollment in all countries was July 2015.
Table 1 POPE study – participating centers
Table 2 POPE study – Steering Committee
The objectives
The primary aim of this study was to assess the prevalence of COPD phenotypes according to predefined criteria in an unselected group of consecutively examined patients with stable COPD in the CEE region in a real-life setting (). Secondary aims of the study included analysis of differences in symptom load, and diagnostic and therapeutic behavior in patients classified into different phenotypes. As the POPE study will actively recruit patients with COPD due to environmental risk factors other than smoking, separate analysis will be conducted to ascertain differences with a matched cohort of “smokers-related” COPD. The long-term aims of the POPE study are to educate and raise awareness for COPD phenotypes among both physicians and patients to support an individualized patient treatment approach in clinical practice.
Participants
All consecutive patients with COPD examined at office-based physician and outpatient clinics from different institutions were enrolled in this study if they fulfill the following inclusion criteria: age more than 40 years, confirmed diagnosis of COPD with postbronchodilator forced expired volume in 1 second/forced vital capacity (FEV1/FVC) <0.7, and absence of exacerbation for at least 4 weeks. The rationale for inclusion criteria imply the following points. The presence of postbronchodilator airflow limitation among persons over age 40 years is the common definition of COPD cases used worldwide. Younger subjects with bronchial obstruction represent rather a rarity. Moreover, airflow limitation in people below 40 years of age may be due to other causes (asthma, bronchiolitis, primary ciliary dyskinesia, etc). Acute exacerbation of COPD has multiple negative effects on lung functions and respiratory symptoms (important parameters of our research). Therefore, we have used 4-week exacerbation-free interval as an elimination factor against bias (in term of symptoms and pulmonary functions). POPE study patients were divided into Group A if they have a smoking history equal and/or more than 10 pack-years and Group B if they were nonsmokers or smokers of less than 10 pack-years with evidence of inhalation exposure to other risk factors. Other risk factors were also counted: workplace environment, frequent exposure to outdoor pollution, frequent exposure to indoor pollution, and cooking without ventilation. COPD is clearly defined as an enhanced chronic inflammatory response to inhaled noxious particles and/or gases. Accordingly, non-/low-smoking patients without the aforementioned predefined risk factors were excluded from POPE study. Patient enrollment started in April 2014 and continued through July 2015; thus, a relevant seasonal bias of recruitment was prevented.
Study protocol
The study protocol was conceived to capture all data routinely available for clinical phenotyping during one visit. The parameters selected were identified by the Steering Committee () together with a panel of national experts. An electronic case report form (eCRF) was used for local data collection.
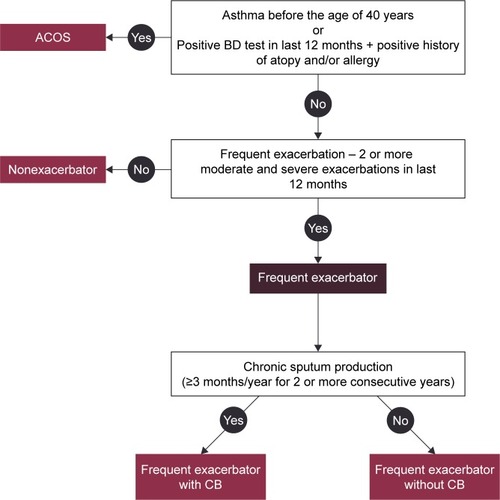
For each patient, an in-depth history was obtained, including information on allergy and atopy, COPD symptoms (dyspnoea at rest/during exercise, fatigue, cough, chronic sputum production, purulent expectoration, and hemoptysis), smoking status and other respiratory risk factors, history of acute respiratory events, including the number of COPD exacerbations with or without hospitalization, concomitant respiratory and nonrespiratory diseases, and assessment of the body composition (weight and height were routinely measured before spirometry, and self-reported weight loss or weight gain [absolute, relative rate] were registered as well). Comorbidities were scored using the Charlson comorbidity index.Citation24 Physical examination was performed on each patient. Pulmonary function data were obtained using standard equipment according to the ATS/ERS consensus guidelines.Citation25 The European Community of Coal and Steel reference equations were used in the POPE study. Postbronchodilator spirometry values for assessing COPD disease severity were reported in all patients (mandatory data). Furthermore, additional information regarding results obtained from bronchodilator reversibility testing, body plethysmography, diffusion capacity, fractional exhaled nitric oxide (FeNO), thoracic computed tomography, echocardiography, blood/sputum eosinophil assessment, serum immunoglobulin (IgE) measurement, arterial blood gases (ABG), and hematocrit (HTC) were recorded, if available, and performed within the last 12 months. Because this is a noninterventional study, obtaining the aforementioned additional information was considered optional. Thus, the information provided in this context represents the true level of diagnostic investigations for COPD in CEE countries. Patients included were classified into the Global Initiative for chronic Obstructive Lung Disease (GOLD) risk classification category on the basis of postbronchodilator FEV1, history of COPD exacerbations, respiratory symptoms using the modified Medical Research Council (mMRC) dyspnea scale, and the COPD Assessment Test (CAT).Citation1,Citation26,Citation27 With regard to CAT, total CAT score and all CAT subitems were separately noted. Any pharmaceutical treatment prescribed for COPD for at least 1 month was recorded together with medications for typical comorbidities. Nonpharmaceutical therapeutic options, including long-term oxygen therapy (LTOT), use of noninvasive ventilation, bronchoscopic or surgical volume reduction procedures, and/or relevant vaccinations for individual patients were recorded as well. An overview of the collected data is listed in . Patients were stratified according to predefined phenotypes. The phenotypes proposed by the Steering Committee consensus were consistent with a recent recommendation from Spain proposing four clinically defined groups ().Citation28 The following simple algorithm was used to determine the phenotype: 1) patients with a previous diagnosis of asthma were considered a mixed COPD–asthma phenotype (asthma–COPD overlap syndrome, ACOS), 2) patients with less than two exacerbations in the previous year were classified as nonexacerbators, 3) exacerbators with self-reported chronic cough and expectoration for more than 3 months of the year over 2 consecutive years were described as exacerbators with chronic bronchitis, and 4) the remaining exacerbators were classified as exacerbators without chronic bronchitis (predominantly with emphysema).Citation29
Table 3 POPE study – captured parameters
Analytical methods
Categorical variables were described by absolute and relative values. Median supplemented by the 5th–95th percentile range was used for continuous variables; a valid N was reported in the case of missing values in continuous variables. Mean supplemented by standard deviation or 95% confidence interval was adopted for continuous variables when normality of the data was proven. Statistical significance of differences in continuous variables between/among groups of patients was analyzed using the Mann–Whitney U-test and Kruskal Wallis test, and Student’s t-test for two groups or analysis of variance (ANOVA) followed by Tukey post hoc test. Paired t-test and/or the Wilcoxon paired test was used to analyze the statistical significance of differences of continuous variables between study time points; the McNemar test was used for the same purpose for categorical variables. Factors influencing binary end points without time to event and censoring (1 year mortality, etc) were analyzed using logistic regression. α=0.05 was used as a level of statistical significance. Analyses were performed using SPSS 22.0.0 (IBM Corporation, Armonk, NY, USA, 2013).
Sample size calculation
The background information from the available literature regarding the proportion of patients in different GOLD categories and occurrence of COPD phenotypes was utilized in the power analysis prior to the study.Citation9,Citation10,Citation30 The aim of the power analysis was to determine the sample size required to detect statistically significant differences in the prevalence of COPD phenotypes and other classification groups of interest, such as GOLD (1–4) and GOLD (A–D) measured as relative risk (RR) between participating countries within POPE study. Power analysis revealed that the optimal number of patients from the CEE region should be 3,500. This total number enables the observation of differences between various countries or groups of countries within the entire CEE region (sufficient precision guaranteed: approximately ±4% or ±2% within each participating country with categories/phenotypes of 20% or 5% prevalence, respectively; detectable RR of categories/phenotypes of 20% prevalence at least 1.5; detectable RR of categories of 5% prevalence nearly 2.0). Finally, we estimated a prevalence of nonsmoking subjects in approximately 5%–10% of the CEE COPD population.Citation31,Citation32
Organization of the study
The POPE study was an investigator-initiated study by a group of COPD researchers predominantly from CEE countries who recently formed a research forum called the “COPD Platform”. This study was managed and supervised by the Steering Committee, which was responsible for the design and scientific integrity of the study (). The project management and statistical background was provided by the Institute of Biostatistics and Analyses, Masaryk University (Brno, Czech Republic). Data in the POPE study were entered into a database system, which was originally based on a modified version of the TrialDB system.Citation33–Citation35 The TrialDB system is an easy and accessible tool for parametric data collection, validation, statistical processing, and online data management in compliance with respective legislation. A similar design was used in the multicenter, observational, cross-sectional PUMA study performed in Argentina, Colombia, Uruguay, and Venezuela.Citation36 The online application is accessible to users via the Internet browser. The security of individual records within the registry is ensured via deidentified data collection. An encryption protocol is used for data transfer between the user and central database to prevent tapping the communication between the client and server. For this reason, any communication between the client and server is achieved via the secure protocol HyperText Transfer Protocol Secure, using Secure Socket Layer encryption. The security of individual records within the registry is ensured via deidentified data collection.
The POPE study was registered in ClinicalTrials.gov with the identifier NCT2119494. More information can be obtained at http://www.copdplatform.com/. The sponsor of the study is the Ludwig Boltzmann Institute for COPD and Respiratory Epidemiology, Vienna, Austria. The research institute received an unrestricted research grant from Boehringer Ingelheim RCV GmbH & Co. KG, which provided partial support for this study but had no influence on the rationale, methodology, or analysis.
Ethics
This study was performed in accordance with the European Union laws and the respective laws of participating countries. The study, protocol, informed consent, and patient information were submitted to ethic committees in the respective countries and to regulatory agencies, where required. The rights, safety, and well-being of clinical investigation subjects were protected according to the ethical principles of the Declaration of Helsinki. All patients (except Poland, where Ethic Committee approval was not required) were requested to provide their informed consent.
Discussion
Phenotyping patients with COPD has received increasing awareness in recent years.Citation37–Citation41 A phenotype is defined as “a single or combination of disease attributes that describe differences between individuals with COPD as they relate to clinically meaningful outcomes”.Citation37 A phenotypic approach to classify COPD has been adopted by a number of national and international societies.Citation9,Citation29,Citation42–Citation44 It is actively used by the Czech and Spanish COPD guidelines to promote treatment tailored to disease presentation, beyond singular treatment of airflow obstruction.Citation42,Citation43 However, there is no general consensus on the number of phenotypes and the precise definition. Furthermore, we may need to acknowledge that individual patients may qualify for more than one phenotype.Citation42 A recent Spanish guideline proposed a classification of patients with COPD according to phenotypes similar to those used in the POPE study: infrequent exacerbators, frequent exacerbators with emphysema, frequent exacerbators with chronic bronchitis predominance, and the ACOS.Citation29,Citation43 The definition of ACOS remains controversial; however, it may include the presence of COPD with either allergic rhinitis, bronchial hyperresponsiveness, and/or a previous diagnosis of asthma with reversible airflow obstruction.Citation45 The four (aforementioned) elementary COPD phenotypes used in the POPE study were based on routine clinical practice as they have some treatment consequences. Undoubtedly, wide scope of gathered parameters allows to evaluate the presence of COPD subjects with other disease “phenotypes”, for example, COPD with pulmonary cachexia, COPD with high burden of comorbidities. Using these patient profiles in a recently published, observational, multicenter study of 3,125 patients with COPD, Miravitlles et alCitation28 observed a distribution of 60% nonexacerbators, 18% patients with ACOS, 19% exacerbators with chronic bronchitis, and 4% exacerbators without chronic bronchitis. While ACOS patients were more frequently females with better lung function, exacerbators presented with the most severe disease, with little difference between those with and without chronic bronchitis.
What is the clinical relevance of phenotyping patients with COPD?
First, there is evidence of differences in outcomes between different phenotypes. Burgel et alCitation46 observed significant differences in mortality when stratifying patients into phenotypes on the basis of airflow obstruction, evidence of emphysema, body mass index, and comorbidities. Using a very comprehensive and in-depth assessment of 342 patients with COPD, including symptoms, quality of life, exercise capacity, nutritional status, biomarkers of systemic and bronchial inflammation, sputum microbiology, computed tomography of the thorax, and echocardiography in addition lung function, Garcia-Aymerich et alCitation47 similarly demonstrated substantial differences in hospitalization rates and all-cause mortality between patient clusters. Second, there is increasing recognition and clinical acceptance to treat patients according to their phenotypic predominance. Infrequent exacerbators, defined as patients experiencing <2 exacerbations per year, may be treated with bronchodilation alone, and withdrawal of inhaled glucocorticoids may be safe in this particular population, according to data from recent studies.Citation48,Citation49 Patients with COPD and a diagnosis of asthma may in turn have a survival benefit when treated with inhaled corticosteroids.Citation50 Similarly, augmented anti-inflammatory treatment, such as Roflumilast, may only improve excarbation rates in patients with chronic bronchitis and frequent exacerbations, whereas in patients with emphysema, there is no therapeutic benefit.Citation51 The POPE study furthermore investigated whether patients received nonpharmacological treatments in the past, such as long-term oxygen therapy, noninvasive ventilation, or lung volume reduction procedures (surgical or endoscopic).
Why performing a study of COPD phenotypes in CEE?
Many previous studies have attempted to identify and quantify the prevalence of different phenotypes of COPD using populations of various sources, severities, and particularities. The health care system, however, may substantially differ in CEE compared with other systems around the globe. Differences in environmental pollution, smoking prevalence, and comorbidities may substantially contribute to differences in the level of burden of COPD across the CEE region.Citation52,Citation53 The POPE study specifically investigated symptom load, comorbidities, lung function, and exacerbation rates in both smoking and never-smoking patients with COPD in CEE and compared the results between these two groups. In fact, the prevalence of COPD in lifelong nonsmoking subjects in Poland was found to be 12%, whereas the prevalence of COPD in the nonsmoking population from Western countries usually ranged between 2% and 4%.Citation31,Citation32 These differences may potentially be due to differences in mean fine particulate matter (PM2.5) concentrations in CEE compared with Western Europe.Citation54 Moreover, different risks could lead to different clinical presentation of COPD syndrome. COPD of nonsmoking females due to biomass smoke exposure for instance is characterized by less emphysema but more air trapping than COPD due to tobacco smoke exposure.Citation55 On the other hand, access to modern therapeutic modalities due to differences in copayment may be different between Western and Eastern European countries, thus affecting prescribing behavior.Citation56 The POPE study shed new light onto the therapeutic relevance of phenotypes in a real-life setting in CEE. The multinational and multicenter approach in the POPE study was chosen not only to describe the status of patient care across the CEE region but also within the individual participating countries. Finally, in contrast to many Western European countries where patients with COPD are mostly under the long-term supervising care of general practitioners, in CEE countries, these patients are rather taken care of by respiratory specialists ().Citation30,Citation42,Citation57–Citation59
Table 4 POPE study – distribution of COPD patients between general practitioners and pulmonologists in CEE
Limitations
The POPE study design has a number of limitations that need to be acknowledged. First, it is a purely cross-sectional study aimed at assessing the prevalence of predefined phenotypes, without being able to validate these phenotypes prospectively on the basis of outcomes. Nevertheless, eligible patients underwent pre- and postbronchodilator spirometry, and completed a standardized questionnaire on demographics, environmental risk factors, symptoms, comorbidities, management, and use of health care resources.Citation36 The information provided through this comprehensive assessment is novel for the CEE region. Second, the POPE study was performed in multiple centers with different levels of health care access and differences in diagnostic and therapeutic approaches. Lung function assessment was performed in accordance with international guidelines, but without further standardizations or core laboratory evaluations. Thus, we cannot rule out differences in quality measures of performing these and other tests that might impact the comparability between sites and between countries. Nevertheless, the information provided in this context might also be considered the strength of the POPE study, as it provides real-life data regarding important information about the diagnostic approach and treatment modalities of patients with COPD in CEE.
Conclusion
The POPE provides new data regarding symptoms, clinical presentation, and treatment modalities of patients with COPD observed in daily clinical practice in the CEE region. This study may further prompt future research collaborations within participating countries with the intention to answer a number of other important unaddressed questions, such as the natural course of phenotypes, real-life prescription behavior in treatment-naive patients, and/or regional differences in treatment adherence. The long-term aims of the POPE study, however, are to educate and raise awareness for phenotypes of COPD and its potential implications regarding treatment and outcomes among both physicians and patients.
Author contributions
All authors contributed to this manuscript: ZZ and VK wrote the manuscript; MM, AV, RT, AB, NT, AS, and KZ provided valuable reviews and comment. The study design was prepared by the Steering Committee: AV, VK, RT, NT, AS, KZ, AB, and MM. MM carried out the phenotype-based view of study design and coordinated the entire project proposal. AV and VK carried out the CRF and study protocol. ZZ participated in the electronic CRF design and performed statistical analysis plan and sample size calculation. RT, AB, and ZZ carried out the study validation. NT, AS, and KZ participated in the design of the study. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank the staff of all of the study centers for their cooperation in collecting the study data. The POPE study was supported by an unrestricted scientific grant by Boehringer Ingelheim, RCV. Special thanks to the participating countries and their national experts, who were not a part of the Steering Committee, but who greatly contributed to the enrollment of patients. National experts are part of the author collective of the main manuscript describing results of the project. The eCRF design and statistical analysis for sample size calculation was per formed by J Jarkovsky, J Svancara, and M Uher, from the Institute of Biostatistics and Analyses.
Disclosure
Zuzana Zbozinkova is an employee of Institute of Biostatistics and Analyses, Masaryk University. Institute of Biostatistics and Analyses has received research grants from (in alphabetical order) AstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, and Roche.
Adam Barczyk gave presentations at symposia sponsored and received fees for advisory board participation and travel grants from Boehringer Ingelheim, Chiesi, Novartis, Pfizer, Takeda, and Teva.
Ruzena Tkacova has received speaker and consulting fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis.
Arschang Valipour received honoraria for consultancy services during advisory board meetings and/or lecture fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Novartis. He received research grants from Boehringer Ingelheim.
Neven Tudoric has received reimbursement for attending scientific conferences, and/or fees for speaking and/or consulting from AstraZeneca, Boehringer Ingelheim, Cipla, Chiesi, GlaxoSmithKline, Novartis, Pliva-Teva, Sandoz, and Takeda. He was reimbursed for participation on the advisory boards for AstraZeneca, Boehringer Ingelheim, and Novartis.
Kirill Zykov is an investigator for, has received research support from, and is a consultant and/or speaker for AstraZeneca, Bayer, Boehringer Ingelheim, KrKa, Novartis, Takeda, and Thermo Fisher Scientific.
Atilla Somfay has received speaker fees from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Orion Pharma and has received consulting fees from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Orion Pharma.
Marc Miravitlles has received speaker fees from Almirall, AstraZeneca, Boehringer Ingelheim, Esteve, Chiesi, GlaxoSmithKline, Grifols, Menarini, Novartis, and Pfizer and has received consulting fees from Almirall, Boehringer Ingelheim, CSL Behring, Gebro Pharma, GlaxoSmithKline, Grifols, MedImmune, Novartis, Pfizer, and Takeda.
Vladimir Koblizek gave presentations at symposia and sponsored and received fees for advisory board participation and travel grants from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, GlaxoSmithKline, Medicom, Mundipharma, Novartis, and Takeda. He received research grants from AstraZeneca, Boehringer Ingelheim, and Novartis. The authors report no other conflicts of interest in this work.
References
- Goldcopd.com [homepage on the Internet]Global Initiative for Chronic Obstructive Lung Disease; Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Diseasec2013 [updated January 2014; cited November 8, 2014]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2014_Jun11.pdfAccessed November 8, 2014
- Erswhitebook.org [homepage on the Internet]European Lung White Book; European Respiratory Society2013 [updated 2015; cited August 6, 2015]. Available from: http://www.erswhitebook.org/chapters/chronic-obstructive-pulmonary-disease/Accessed August 6, 2015
- BuistASMcBurnieMAVollmerWMInternational variation in the prevalence of COPD (the BOLD Study): a population-based prevalence studyLancet2007370958974175017765523
- Whoint [homepage on the Internet]Chronic obstructive pulmonary disease (COPD)World Health Organisation2007 [updated January 2015; cited August 6, 2015]. Available from: http://www.who.int/mediacentre/factsheets/fs315/en/Accessed August 6, 2015
- LopezADShibuyaKRaoCChronic obstructive pulmonary disease: current burden and future projectionsEur Respir J200627239741216452599
- QaseemAWiltTJWeinbergerSEDiagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory SocietyAnn Intern Med2011155317919121810710
- De MarcoRAccordiniSAntòJMLong-term outcomes in mild/moderate chronic obstructive pulmonary disease in the European Community Respiratory Health SurveyAm J Respir Crit Care Med20091801095696319696441
- GouldNSMinEGauthierSAging adversely affects the cigarette smoke–induced glutathione adaptive response in the lungAm J Respir Crit Care Med201018291114112220622027
- BarrechegurenMEsquinasCMiravitllesMThe asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challengesCurr Opin Pulm Med2015211747925405671
- Soler-CataluñaJJCosíoBIzquierdoJLConsensus document on the overlap phenotype COPD-asthma in COPDArch Bronconeumol201248933133722341911
- WeissSTWhat genes tell us about the pathogenesis of asthma and chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2010181111170117320133923
- MatkovicZTudoricNMiravitllesMImpact of chronic bronchial infection in the lung and beyondEur Respir Mon2013604657
- HnizdoESullivanPABangKMWagnerGAssociation between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: a study of data from the Third National Health and Nutrition Examination SurveyAm J Epidemiol2002156873874612370162
- Torres-DuqueCMaldonadoDPérez-PadillaRBiomass fuels and respiratory diseasesProc Am Thorac Soc20085557759018625750
- LeuppiJDSchuetzPBingisserRShort-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trialJAMA2013309212223223123695200
- QaseemAHopkinsRKuttyKhomepage on the InternetManagement of Chronic Obstructive Pulmonary Disease: Review of the Performance Measures by the Performance Measurement Committee of the American College of PhysiciansAmerican College of Physicians2015 [updated 2015; cited August 6, 2015]. Available from: http://www.acponline.org/clinical_information/performance_measurement/measures/pmc_copd_review.pdfAccessed August 6, 2015
- TashkinDPRennardSIUryniakTEffect Of Budesonide/Formoterol (BUD/FM) Versus FM Alone On The Rate Of Exacerbations (Either Including Or Excluding The Use Of Antibiotics As Part Of Exacerbation Definition), Lung Function, And Rescue Medication Use In Patients With Moderate To Very Severe Chronic Obstructive Pulmonary Disease (COPD). B39 COPD exacerbations: precipitating factors, prevention, and outcome [homepage on the Internet]American Thoracic Society2014A2882A2882 c1998–2015 [updated 2015; cited August 6, 2015]. Available from: http://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2014.189.1_MeetingAbstracts.A2882Accessed August 6, 2015
- MontuschiPMalerbaMSantiniGMiravitllesMPharmacological treatment of chronic obstructive pulmonary disease: from evidence-based medicine to phenotypingDrug Discov Today201419121928193525182512
- DongYHLinHHShauWYComparative safety of inhaled medications in patients with chronic obstructive pulmonary disease: systematic review and mixed treatment comparison meta-analysis of randomised controlled trialsThorax2013681485623042705
- WedzichaJADecramerMFickerJHAnalysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group studyLancet Respir Med20131319920924429126
- BarnesNCalverleyPMKaplanARabeKFChronic obstructive pulmonary disease and exacerbations: patient insights from the global Hidden Depths of COPD surveyBMC Pulm Med2013135423971625
- EmermanCLEffectiveness of inhaled steroids in the management of chronic obstructive pulmonary diseaseCurr Emerg Hosp Med Rep201314189192
- JayaramLWongCMcAuleySCombined therapy with tiotropium and formoterol in chronic obstructive pulmonary disease: effect on the 6-minute walk testCOPD201310446647223875741
- CharlsonMEPompeiPAlesKLMacKenzieCRA new method of classifying prognostic comorbidity in longitudinal studies: development and validationJ Chronic Dis19874053733833558716
- Thoracic.org [homepage on the Internet]ATS documents: statements, guidelines & reports. American Thoracic Society1998–2015 [updated 2015; cited February 12, 2015]. Available from: http://www.thoracic.org/statements/Accessed February 12, 2015
- BestallJCPaulEAGarrodRUsefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary diseaseThorax199954758158610377201
- JonesPWHardingGBerryPDevelopment and first validation of the COPD assessment testEur Respir J200934364865419720809
- MiravitllesMBarrechegurenMRoman-RodriguezMFrequency and characteristics of different clinical phenotypes of COPDInt J Tuberc Lung Dis201519899299826162367
- MiravitllesMSoler-CataluñaJJCalleMSorianoJBTreatment of COPD by clinical phenotypes: putting old evidence into clinical practiceEur Respir J20134161252125623060631
- HigginsVPriceDBakerCLReal-world characterization and differentiation of the Global Initiative for Chronic Obstructive Lung Disease strategy classificationInt J Chron Obstruct Pulmon Dis2014955156124920893
- BangKMChronic obstructive pulmonary disease in nonsmokers by occupation and exposure: a brief reviewCurr Opin Pulm Med201521214915425590955
- ZielinskiJBednarekMGóreckaDIncreasing COPD awarenessEur Respir J200627483385216585092
- NadkarniPMBrandtCMFrawleySManaging attribute – value clinical trials data using the ACT/DB client – server database systemJ Am Med Inform Assoc1998521391519524347
- NadkarniPMBrandtCMMarencoLWebEAV: automatic metadata-driven generation of web interfaces to entity-attribute-value databasesJ Am Med Inform Assoc20007434335610887163
- NadkarniPMMarencoLEasing the transition between attribute-value databases and conventional databases for scientific dataProc AMIA Symp200148348711825235
- SchiaviEStirbulovRHernández VecinoRCOPD screening in primary care in four Latin American countries: methodology of the PUMA studyArch Bronconeumol2014501146947424816036
- HanMKAgustiACalverleyPMChronic obstructive pulmonary disease phenotypesAm J Respir Crit Care Med2010182559860420522794
- LeeYMChronic obstructive pulmonary disease: respiratory review of 2014Tuberc Respir Dis (Seoul)201477415516025368660
- BlasiFChalmersJDAlibertiSCOPD and bronchiectasis: phenotype, endotype or co-morbidityCOPD201411660360425384083
- NovotnaBKoblizekVZatloukalJCzech multicenter research database of severe COPDInt J Chron Obstruct Pulmon Dis201491265127425419124
- Allen-RameyFCGuptaSDiBonaventuraMDPatient characteristics, treatment patterns, and health outcomes among COPD phenotypesInt J Chron Obstruct Pulmon Dis2012777978723226014
- KoblizekVChlumskyJZindrVChronic Obstructive Pulmonary Disease: official diagnosis and treatment guidelines of the Czech Pneumological and Phthisiological Society; a novel phenotypic approach to COPD with patient-oriented careBiomed Pap Med Fac Univ Palacky Olomouc Czech Repub2013157218920123733084
- MiravitllesMSoler-CataluñaJJCalleMSpanish guideline for COPD (GesEPOC). Update 2014Arch Bronconeumol201450Suppl 111624507959
- LouieSZekiAASchivoMThe asthma-chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerationsExpert Rev Clin Pharmacol20136219721923473596
- MiravitllesMSoler-CataluñaJJCaleMA new approach to grading and treating COPD based on clinical phenotypes: summary of the Spanish COPD guidelines (GesEPOC)Prim Care Respir J201322111712123443227
- BurgelPRPaillasseurJLPeeneBTwo distinct chronic obstructive pulmonary disease (COPD) phenotypes are associated with high risk of mortalityPLoS One2012712e5104823236428
- Garcia-AymerichJGómezFPBenetMIdentification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypesThorax201166543043721177668
- MagnussenHDisseBRodriguez-RoisinRWithdrawal of inhaled glucocorticoids and exacerbations of COPDN Engl J Med2014371141285129425196117
- RossiAGuerrieroMCorradoAWithdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO)Respir Res2014157725005873
- GershonASCampitelliMACroxfordRCombination long-acting β-agonists and inhaled corticosteroids compared with long-acting β-agonists alone in older adults with chronic obstructive pulmonary diseaseJAMA2014312111114112125226477
- RennardSICalverleyPMGoehringUMReduction of exacerbations by the PDE4 inhibitor roflumilast-the importance of defining different subsets of patients with COPDRespir Res2011121821272339
- Nizankowska-MogilnickaEMejzaFBuistASPrevalence of COPD and tobacco smoking in Malopolska region-results from the BOLD study in PolandPol Arch Med Wewn2007117940241018062562
- GolecMSkórskaCMackiewiczBRelationship between COPD and lower socioeconomic status in farmers from South-Eastern Poland (Lublin region)Rural Remote Health201414253124588301
- Eea.europa.eu [homepage on the Internet]European environment agency; Air quality in Europe – 2014 report352015 [updated July 30, 2015; cited August 6, 2015]. Available from: http://www.eea.europa.eu/publications/air-quality-in-europe-2014Accessed August 6, 2015
- CampPGRamirez-VenegasASansoresRHCOPD phenotypes in biomass smoke-versus tobacco smoke-exposed Mexican womenEur Respir J201443372573424114962
- DavidovaJPraznovcovaLLundborgCSPricing and reimbursement of pharmaceuticals in the Czech Republic and SwedenPharm World Sci2008301576417588212
- CelliBRMacNeeWAgustiAStandards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paperEur Respir J200423693294615219010
- BellamyDBouchardJHenrichsenSInternational Primary Care Respiratory Group (IPCRG) guidelines: management of chronic obstructive pulmonary disease (COPD)Prim Care Respir J2006151485716701758
- JonesPWBrusselleGDal NegroRWPatient-centred assessment of COPD in primary care: experience from a cross-sectional study of health- related quality of life in EuropePrim Care Respir J201221332933622885563