Abstract
Background
Real-world prescription pathways leading to triple therapy (TT) (inhaled corticosteroid [ICS] plus long-acting β2-agonist bronchodilator [LABA] plus long-acting muscarinic antagonist) differ from Global initiative for chronic Obstructive Lung Disease [GOLD] and National Institute for Health and Care Excellence treatment recommendations. This study sets out to identify COPD patients without asthma receiving TT, and determine the pathways taken from diagnosis to the first prescription of TT.
Methods
This was a historical analysis of COPD patients without asthma from the Optimum Patient Care Research Database (387 primary-care practices across the UK) from 2002 to 2010. Patient disease severity was classified using GOLD 2013 criteria. Data were analyzed to determine prescribing of TT before, at, and after COPD diagnosis; the average time taken to receive TT; and the impact of lung function grade, modified Medical Research Council dyspnea score, and exacerbation history on the pathway to TT.
Results
During the study period, 32% of patients received TT. Of these, 19%, 28%, 37%, and 46% of patients classified as GOLD A, B, C, and D, respectively, progressed to TT after diagnosis (P<0.001). Of all patients prescribed TT, 25% were prescribed TT within 1 year of diagnosis, irrespective of GOLD classification (P=0.065). The most common prescription pathway to TT was LABA plus ICS. It was observed that exacerbation history did influence the pathway of LABA plus ICS to TT.
Conclusion
Real life UK prescription data demonstrates the inappropriate prescribing of TT and confirms that starting patients on ICS plus LABA results in the inevitable drift to overuse of TT. This study highlights the need for dissemination and implementation of COPD guidelines to physicians, ensuring that patients receive the recommended therapy.
Introduction
Chronic obstructive pulmonary disease (COPD) contributes significantly to health care costs worldwide.Citation1 While there is no cure for COPD, it is preventable and treatable by reducing and relieving the impact of symptoms, such as chronic cough, chronic sputum production, and dyspnea while also reducing the risk of exacerbations.Citation2 The current Global initiative for chronic Obstructive Lung Disease (GOLD) strategy classifies COPD patients, according to an assessment of lung function (spirometry), current symptoms, and future exacerbation risks, into one of four groups: GOLD A, B, C, and D.Citation2
The GOLD 2015 strategy document also proposes suitable first and secondary choice pharmacologic therapies for each of these groups.Citation2 Specifically, the prescribing of triple therapy (TT), designated as long-acting β2-agonist bronchodilator (LABA) with a long-acting muscarinic antagonist (LAMA) and an inhaled corticosteroid (ICS), is recommended exclusively for COPD patients in group D (forced expiratory volume in 1 second [FEV1]<50% predicted and/or a history of two or more exacerbations per year; or one or more hospitalizations for a COPD exacerbation according to the GOLD 2013 updateCitation3 and a modified Medical Research Council [mMRC] grade ≽2; or a COPD assessment test score ≽10; or a clinical COPD questionnaire >1). Similarly the National Institute for Health and Clinical Excellence (NICE) in the UK has created an algorithm for prescribing inhaled pharmacotherapies based on a multidimensional assessment of COPD patients.Citation4 Despite these guidelines, real-world studies indicate that pharmacological treatments often differ from the recommendations.Citation5–Citation8 To date, studies have indicated that there is insufficient evidence to determine whether TT is clinically superior to dual therapy optionsCitation9,Citation10 and that there is a requirement for longer-term studies to assess the value of this therapy.Citation11 NICE guidelines state that the use of TT is only cost-effective in patients with a predicted FEV1<50% and who are frequent exacerbators (two or more exacerbations in the past 12 months),Citation4 thereby the overuse of TT is probably wasteful of limited health care resources when probably only LAMA, LABA, or dual bronchodilator (LABA plus LAMA) is required.
The use of TT exposes patients to the long-term use of ICS, which is already reported to be widely used in patients with GOLD A and B where it is not clinically indicated.Citation5,Citation6,Citation12–Citation17 Early studies have shown that patients with more severe COPD and who have a history of frequent exacerbations were most likely to benefit from ICS treatment.Citation18–Citation20 However, recent studies have shown the overuse of ICS in COPD patients with mild to moderate diseaseCitation21 which is contrary to current GOLD recommendations and to the approved COPD indications for ICS in combination with LABAs. NICE guidelines also indicate that ICS, in combination with a LABA, is not cost-effective in COPD patients with a predicted FEV1>50%.Citation4 The inappropriate use of ICS results in these patients being exposed early in the disease to the known side effects associated with ICS,Citation22–Citation25 in particular pneumonia.Citation26–Citation28 Furthermore, ICS use does not necessarily result in superior efficacy when compared to other treatment optionsCitation10,Citation21,Citation29–Citation31 or decrease hospitalizations in COPD patients.Citation32
In order to suggest solutions for how alignment with recommendations could be improved, it is necessary to understand what treatment decisions are being made by general practitioners and how they evolve over time and lead to TT being prescribed. This historical, observational study sets out to understand prescribing practices by using COPD patient data from a large UK patient dataset, Optimum Patient Care Research Database (OPCRD). The aims of this study were to explore the prescribing pathways to TT; specifically, what patients were prescribed prediagnosis and at the time of diagnosis and what they changed to over time. Furthermore, the aim was to identify the most common treatment pathways to TT and evaluate what influenced these pathways to TT.
Methods
Data source
The study utilized data from the OPCRD which is a quality-controlled longitudinal primary-care database mainly containing respiratory data. At the time of data extraction for this study, the OPCRD comprised anonymous patient data from 387 primary-care practices across the UK serving a population of over 3 million patients, of which data from 318 primary-care practices were utilized for this study.Citation33
The first type of data consisted of routine clinical data where Optimum Patient Care software interfaced with primary-care practice management systems to extract information which included patient demographics, standard COPD comorbidities, exacerbation history, mMRC dyspnea score, and current therapy.
The second type of data collected was on patient-reported outcomes in a subset of patients. Eligible patients with respiratory disease (those with diagnoses and/or in receipt of prescriptions for COPD and approved for participation by their general practitioner) completed validated disease-assessment questionnaires, which contained questions to calculate mMRC scores.
All data in the OPCRD is anonymized. This database has been approved by Trent Multi Centre Research Ethics Committee for clinical research use, and the OPCRD’s independent Anonymized Data Ethics Protocols and Transparency committee verified and approved the planned analysis.Citation33 This study is registered at www.clinicaltrials.gov with identifier number NCT1786720.
Study design and patients
A historical cohort study design was utilized in this study, which contained data from 1997–2010. The analyses utilized data from 2002–2010. Data prior to 2002 was excluded to ensure the full analysis of treatment pathways including a LAMA treatment, tiotropium, which was approved in the UK in 2002.Citation34
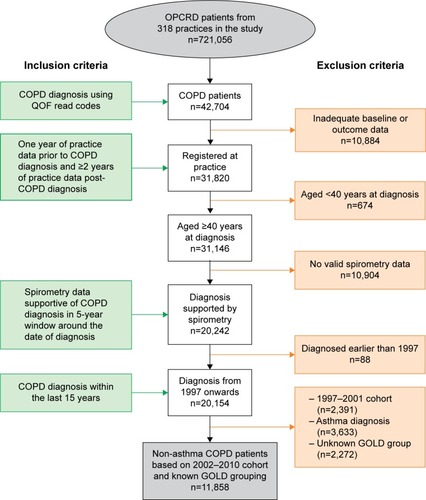
The analysis included patients ≥40 years of age at initial date of COPD diagnosis (quality outcome framework diagnostic codeCitation35), with spirometry data supportive of a COPD diagnosis (FEV1/forced vital capacity <0.7) in the 5 years prior to and inclusive of the date of diagnosis, and having data 1 year prior to and a minimum of 2 years post-initial date of diagnosis.
The pathway to TT was examined over time from patients with an initial date of COPD diagnosis from the years 2002–2010. GOLD 2013 group classification was utilized in this study and was based on COPD symptoms (mMRC), spirometric classification, and number of exacerbations.Citation3 Other exclusion criteria consisted of: patients whose initial date of COPD diagnosis was before 2002 (due to UK approval of tiotropium in 2002Citation34); patients who had an asthma diagnosis; and patients with unknown GOLD classification ().
Figure 1 Patient selection.
COPD initial therapy was defined as pharmacological therapy prescribed during 1 year prior to and at the initial date of COPD diagnosis (baseline period). Therapies were grouped into four categories: no TT prescription, TT prescribed prior to initial date of COPD diagnosis, TT prescribed at initial date of COPD diagnosis, and TT prescribed after initial date of COPD diagnosis.
Analysis of data
Summaries were produced for all characteristics at the time of diagnosis. Data were analyzed for the number of patients who were and were not prescribed TT prior to, at, and after COPD diagnosis. For patients who were prescribed TT after COPD diagnosis, the time taken for these patients to receive TT was categorized into years: up to 1 year, 1–2 years, 2–3 years, 3–4 years, 4–5 years, 5–6 years, 6–7 years, 7–8 years, 8–9 years, 9–10 years, and >10 years.
Common treatment pathways were identified based on a number of patients ≥1% of the total study population following a particular pathway. Pathways followed by <1% of patients were grouped together and presented as other non-frequent pathways. Treatment pathways were mapped from the initial therapy (defined as the sum of therapies prescribed during 1 year prior to and at the initial date of COPD diagnosis) to the first prescription of TT.
Lung function grade, mMRC, and exacerbation history were calculated during 1 year prior to and at the initial date of COPD diagnosis. The impact of lung function grade, mMRC score (dyspnea), and exacerbation history at baseline on treatment pathways and treatment pathways to TT was also described.
Statistical differences between the patients split into cohorts was calculated for those who were: 1) not prescribed TT; 2) prescribed TT prior to the initial date of COPD diagnosis; 3) prescribed TT on the date of COPD diagnosis; and 4) prescribed TT after the date of COPD diagnosis. A chi-square test was used to compare whether all groups analyzed were statistically different (the P-value applies to all groups being compared).
Results
Patient selection
Of the 42,704 patients diagnosed with COPD between 2002 and 2010, 11,858 subjects met the eligibility criteria (). Subsequent analyses were carried out on this cohort of patients.
Patient characteristics and demographics
A summary of all eligible patient characteristics at time of diagnosis is provided in . The median age of patients was 66 years with an interquartile range from 60 to 74 years. The proportion of males was 54%. Most patients were ex-smokers (50%) or current smokers (47%). In the year preceding the study, 67% of patients did not experience an exacerbation, 19% of patients experienced one exacerbation, and 14% experienced two or more exacerbations. COPD diagnosis, according to GOLD 2013 lung function grade,Citation3 classified 70% of patients as having mild-to-moderate airflow limitation (FEV1≥50%), while 25% and 5% of patients had severe and very severe airflow limitation, respectively. At the closest date to initial date of COPD diagnosis, eligible patients were classified according to GOLD 2013 as 41% (n=4,822) as GOLD A, 25% (n=2,933) as B, 17% (n=2,055) as C, and 17% as D (n=1,988) ().
Table 1 Summary of patient characteristics at time of diagnosis
TT status and progression to TT in COPD patients
Regardless of GOLD category, 32% of patients received TT during the study period (). A small proportion of GOLD A, B, and C patients received TT prior to their initial recorded COPD diagnosis (1%, 1%, and 2%, respectively) (). A similar percentage of GOLD A, B, and C patients were prescribed TT at initial diagnosis or within 1 year (1%, 1%, and 2%, respectively) (). However, after initial COPD diagnosis, many GOLD A, B, and C patients (19%, 28%, and 37%, respectively; P<0.001) were prescribed TT ().
Table 2 Triple therapy status by GOLD group (2002–2010)
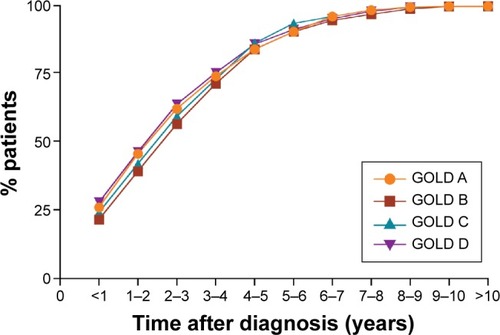
Analysis of the time to TT demonstrated that 25% of patients who progressed to TT did so within 1 year after diagnosis (GOLD A: 26%; GOLD B: 22%; GOLD C: 24%; and GOLD D: 28%; P=0.065) (). Within 2 years of initial diagnosis, >40% of patients progressed to TT (GOLD A: 46%; GOLD B: 39%; GOLD C: 42%; and GOLD D: 47%; ), with >50% of patients progressing to TT within 3 years after diagnosis (GOLD A: 62%; GOLD B: 57%; GOLD C: 60%; and GOLD D: 64%; P=0.065) (). Almost 100% of patients who progressed to TT in GOLD A, B, C, and D did so within 8 years after initial diagnosis ().
Therapy pathway to TT
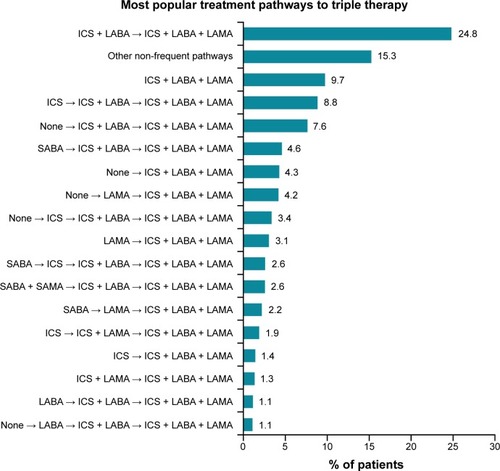
A total of 86 treatment pathways were identified in the COPD population (n=3,505). Among these, 17 frequent treatment pathways were identified (n=2,970) (). The most frequent treatment pathway was ICS plus LABA → ICS plus LABA plus LAMA (n=869 [25%]) (). The second and third most frequent treatment pathways to TT were patients who were prescribed TT as their initial therapy (ICS plus LABA plus LAMA) and ICS → ICS plus LABA → ICS plus LABA plus LAMA (n=341 [10%] and n=310 [9%], respectively). Approximately 15% (n=535) of patients were prescribed inhaled therapy according to treatment pathways with <1% frequency overall (other non-frequent pathways).
Figure 3 Distribution of different treatment pathways leading to triple therapy (ICS plus LABA plus LAMA), identified from total triple therapy population (n=3,505).
Notes: Pathways with a percent frequency of less than 1% were grouped under the category “other non-frequent pathways”. The first drug listing in the treatment pathway was considered to be the patient’s initial therapy, and the second drug listing was the prescription after the initial date of COPD diagnosis.
The impact of mMRC score (dyspnea) revealed that, in patients with a mMRC score of 4, 25% of this group followed the most common pathway to TT (ICS plus LABA → ICS plus LABA plus LAMA) (). In patients with a mMRC score of 0, 1, 2, and 3, approximately 24% took the most common treatment pathway (ICS plus LABA → ICS plus LABA plus LAMA). A similar trend was observed for the other 16 most frequent treatment pathways.
Table 3 Pathways to triple therapy split by mMRC score
The impact of the number of exacerbations at baseline on the most common treatment pathways was also analyzed (). Of patients who experienced three or more exacerbations, 43% followed the pathway of ICS plus LABA → ICS plus LABA plus LAMA. The percentage of patients with two, one, or no history of exacerbations following this pathway was 33%, 26%, and 20%, respectively. A similar trend was observed for the remaining treatment pathways.
Table 4 Pathways to triple therapy split by exacerbation history
A similar percentage of patients in each of the GOLD 2013 lung function grades followed the most common pathway, ICS plus LABA → ICS plus LABA plus LAMA (grade 1=25%, grade 2=24%, grade 3=26%, and grade 4=23%) (). A similar trend was observed for the other 16 possible treatment pathways.
Table 5 Pathways to triple therapy split by lung function grade
Discussion
In this study, an analysis of the pathways leading to TT indicated that one-third of COPD patients with no concomitant asthma diagnosis between 2002 and 2010 progressed to TT. Results demonstrated that TT was inappropriately used in GOLD A, B, and C patients. Approximately 25% of all patients prescribed TT received their first TT prescription 1 year after diagnosis, rising to 50% within 3 years post diagnosis, and eventually resulting in 100% 8 years postdiagnosis. The most common pathway to TT observed was ICS plus LABA pre-COPD diagnosis followed by TT postdiagnosis of COPD (ICS plus LABA → ICS plus LABA plus LAMA). The mMRC dyspnea score and impairment in lung function were not evaluating factors that influenced pathways leading to TT; however, the number of exacerbations was shown to influence this progression from ICS plus LABA to TT. Throughout all the common pathways to TT (>1% frequency) identified, the use dual bronchodilators (LABA plus LAMA) as a therapy option was not observed.
NICE guidelines suggest that patients with persistent breathlessness and exacerbations should progress to TT,Citation4 with GOLD indicating the use of TT in GOLD D patients.Citation2 Results from this study demonstrated that approximately one-third of all COPD patients in this study were receiving TT, which falls in the range observed in similar studies that were conducted in Japan (21%),Citation36 France (33%),Citation37 and Greece (44.8%).Citation38 Of the patients prescribed TT in the study presented here, 19%, 28%, and 37% of GOLD A, B, and C patients, respectively, were prescribed TT after their initial diagnosis of COPD. The frequency of TT being prescribed is comparable to that observed from other studies. In a French study, the percentage of GOLD A, B, C, and D patients being prescribed TT was 20%, 32%, 25%, and 48%, respectively.Citation37 A study by Miyazaki et alCitation36 also observed similar rates of TT being prescribed to GOLD B, C, and D with slightly less TT prescribed to GOLD A patients (9%). Similarly, in a Canadian study, 20% and 30% of GOLD A and GOLD B patients, respectively, were prescribed TT.Citation39 Overall, this indicates that the prescription behaviors observed in this UK study do not differ from those reported by other countries. A study by Corrado et alCitation14 suggested that some respiratory specialists prefer to use TT even in patients who do not have severe COPD. This was evident in the present study, where the percentage of TT being prescribed to GOLD A, B, and C patients pre-COPD and post-COPD diagnosis was similar. One could assume that administration of TT is optimal for patients with COPD, because they receive optimal bronchodilation plus anti-inflammatory therapy, but to date there is no conclusive evidence on the superiority of dual-bronchodilation with an ICS (TT) over other therapy options, in particular in patients at low risk of exacerbations.Citation9,Citation10
We next examined the pathways to TT, with the most common pathway being ICS plus LABA → TT. This results in the issue that once patients are started on ICS plus LABA, the next therapeutic option is inevitably TT. It is also key to note that, of all the pathways identified with a frequency above 1%, none contained dual bronchodilators (LABA plus LAMA) despite the LAMA tiotropium being available in the UK in 2002.Citation34 The recent WISDOM study evaluated the impact of stepping patients down from TT to dual bronchodilators. Results from this study demonstrated that even in severe and very severe COPD patients, the medium-term risk of moderate or severe exacerbations was similar between patients who were taken off ICS in a stepwise approach, and those who continued to receive ICS as part of TT.Citation30 This removal of ICS has also been supported in the INSTEAD study where the withdrawal of ICS therapy from patients receiving ICS plus LABA, contrary to guidelines recommendations, did not have a negative impact on lung function and symptoms when compared to patients who remained on ICS plus LABA.Citation21,Citation40 There is also real-world evidence to further confirm the ability to withdraw ICS, as demonstrated by the OPTIMO study.Citation41 These studies indicate that many patients with COPD do not need ICS therapy, which suggests that the appropriate role of ICS in COPD management has not been truly established.Citation40 Furthermore, such studies support the possibility of stepping back from ICS-containing regimes in patients who do not require them.
When the pathways to TT were further examined according to mMRC scores (dyspnea) and lung function impairment, it was observed that these had no impact on the pathways to TT, with ICS plus LABA → TT being the most common pathway to TT. A similar scenario, whereby lung function did not influence the prescription of therapies to COPD patients, has been previously reported. In the INSTEAD study, patients were receiving ICS plus LABA for 3 or more months prior to enrolling, despite having a mean FEV1 of 64% and having no history of exacerbations.Citation21,Citation40 Contrary to mMRC and lung function data, exacerbation history was observed to have an impact on the prescription pathways to TT, specifically on the pathway ICS plus LABA → TT. Overall, this reconfirms the point that starting patients with COPD on ICS plus LABA inevitably results in the drift to TT, irrespective of severity of lung function impairment and breathlessness. While it was observed that patients with a previous history of exacerbations are justified to be prescribed TT, in this study it was also relatively common for patients with no exacerbation history, or with low symptoms or protected lung function, to eventually drift to TT, without being prescribed more appropriate therapies like dual bronchodilators. This highlights the need to amend behavior and provide better education on how exacerbations in COPD can be avoided and what are the recommended therapeutic options for patients with COPD. It should be noted though that in the year prior to this study, approximately 14% of patients had frequent exacerbations compared with 67% of patients who did not experience any exacerbations. The addition of ICS would only be indicated in a subgroup of COPD patients where exacerbations required treatment with systemic corticosteroids (with or without antibiotics), not if these exacerbations were treated with antibiotics only.Citation42,Citation43 This is similar to data from a study by Lange et alCitation44 where approximately 2% and 7% of COPD patients had two or more exacerbations a year (frequent exacerbators) in COPD GOLD stages 2 and 3, respectively. It is important to highlight that, generally, there are lower frequencies of frequent exacerbators in primary-care-based COPD studies (eg, OPCRD) and population-based COPD studies (eg, Copenhagen studies and Rotterdam Study) when compared with secondary-/tertiary-care-based studies, such as the ECLIPSE study (22%, 33%, and 49% in COPD GOLD stage 2, 3, and 4, respectively).Citation45
From the observed pathways to TT, it was particularly evident that there was extensive overuse of ICS within the frequent pathways, especially in patients with no indication for ICS. NICE specifically recommends that ICS should be given to patients with a FEV1<50% and two or more exacerbations in the past 12 months.Citation4 ICS is also indicated for patients who have asthma and COPD, and for COPD patients who are frequent exacerbators.Citation46 Previous studies have also reported the inappropriate use of ICS,Citation5,Citation6,Citation12–Citation17 which can add unnecessary additional health care costsCitation47 without providing any superior efficacy.Citation10,Citation21,Citation29–Citation31 Aside from efficacy and cost concerns with the overuse of ICS, there is a safety concern, whereby the long-term exposure to ICS can result in several side effects which include pneumonia,Citation26 osteoporosis,Citation25 diabetes,Citation24 cataracts,Citation22 and tuberculosis.Citation23 With respect to pneumonia, studies have demonstrated that ICS use was associated with a 70% increase in the rate of hospitalization for pneumoniaCitation27 and a 69% increase in serious pneumonia, defined as hospitalization for or death from pneumonia.Citation28 A recent real-world study by Harries et alCitation32 has indicated that despite the increases in prescribing of LABA plus ICS combinations in the UK, the rate of COPD hospitalizations has not reduced. The high use of ICS could be due to the fact that clinicians are more confident in maintaining patients on ICS than switching to other therapies,Citation48 along with the fact that GOLD always cites ICS first (before LABA and LAMA) in the treatment recommendations, which is based on “alphabetical order” instead of the most important drug/drugs being cited first (long-acting bronchodilators). Additionally, no fixed combination dual bronchodilators were available previously. GOLD recommendations for primary-care patients are based on randomized clinical trials and observational studies usually performed in secondary and tertiary care, such as ELIPSE,Citation49 which may overestimate the occurrence of COPD exacerbations, as well as the benefits of ICS.
There are limitations to this study that have to be acknowledged. The data presented here are historical, and the quality of the data is dependent on coding used in the clinical records, although prescribing and quality outcome framework-related data were well recorded. No centralized spirometry was carried out so the quality of spirometry could impact on the data collected and classification of patients into the relevant GOLD groups. Only one-quarter of all patients met entry criteria, which could mean that the treatment received by the patients in the study was not typical of all patients. GOLD categories might not necessarily be the best way to assess prescribing practices for COPD patients, given that FEV1 is a poor guide to future exacerbations, but symptoms, lung function, and exacerbations seemed to have little impact on prescribing patterns within this study.
Prescription pathways leading to TT in COPD patients without asthma highlight the common practice of prescribing ICS-containing regimens and overuse of TT, particularly in low-risk patients. The results also highlight the relatively low prescribing of bronchodilators as first-line therapy. This reflects the persistent uncertainties of physicians in prescribing the most appropriate therapy to patients with COPD, and emphasizes that more efforts are required to improve education on and the implementation of COPD guidelines regarding COPD therapies.
Acknowledgments
The analyses reported in this study were funded by Novartis Pharma AG (Basel, Switzerland) and were conducted by Research in Real-Life Ltd (Cambridge, UK), an independent company. The authors also thank David Bergin, Danielle Corbett, and Vivek Khanna (professional medical writers; Novartis) for assistance in the preparation of this paper. Writing support was funded by Novartis Pharma AG. The present affiliation of M Baldwin is Boehringer Ingelheim, Frankfurt, Germany.
Disclosure
D Price has board membership with Aerocrine, Almirall, Amgen Inc., AstraZeneca plc, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis International AG, and Teva; consultancy with Aerocrine, Almirall, Amgen Inc., AstraZeneca plc, Boehringer Ingelheim, Chiesi, GlaxoSmith-Kline plc, Meda, Mundipharma, Napp, Novartis International AG, Pfizer, Inc., and Teva; grants and unrestricted funding for investigator-initiated studies from UK National Health Service, British Lung Foundation, Aerocrine, AKL Ltd, Almirall, AstraZeneca plc, Boehringer Ingelheim, Chiesi, Eli Lilly, GlaxoSmithKline plc, Meda, Merck & Co., Inc., Mundipharma, Napp, Novartis International AG, Orion, Pfizer, Inc., Respiratory Effectiveness Group, Takeda, Teva, and Zentiva; payments for lectures/speaking from Almirall, AstraZeneca plc, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline plc, Kyorin, Meda, Merck & Co., Inc., Mundipharma, Novartis International AG, Pfizer, Inc., SkyePharma, Takeda, and Teva; payment for manuscript preparation from Mundi pharma and Teva; patents (planned, pending, or issued) with AKL Ltd.; payment for the development of educational materials from GlaxoSmithKline plc and Novartis International AG; stock/stock options/shares in AKL Ltd which produces phytopharmaceuticals and owns 80% of Research in Real Life Limited and its subsidiary social enterprise Optimum Patient Care; received payment for travel/accommodations/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis International AG, and Teva; funding for patient enrolment or completion of research from Almirall, Chiesi, Teva, and Zentiva; and peer reviewer for grant committees with Medical Research Council (2014), Efficacy and Mechanism Evaluation programme (2012), and HTA (2014). G Brusselle has, within the last 5 years, received honoraria for lectures from AstraZeneca plc, Boehringer Ingelheim, Chiesi, GlaxoSmithKline plc, Merck & Co., Inc., Novartis International AG, Pfizer, Inc., and UCB; and he is a member of advisory boards for AstraZeneca plc, Boehringer Ingelheim, GlaxoSmithKline plc, and Novartis International AG. K Gruffydd-Jones acted as a consultant for and has spoken on behalf of AstraZeneca plc, Novartis International AG, GlaxoSmithKline plc, Teva, Chiesi, and Boehringer Ingelheim. M Miravitlles has received speaker fees from Almirall, Boehringer Ingelheim, Pfizer, Inc., AstraZeneca plc, Chiesi, GlaxoSmithKline plc, Menarini, Grifols, and Novartis International AG, and consulting fees from Almirall, Boehringer Ingelheim, Pfizer, Inc., GlaxoSmithKline plc, Gebro Pharma, CLS Behring, Cipla, MediImmune, Takeda, Novartis International AG, and Grifols. DL Keininger is an employee of Novartis International AG. R Stewart is an employee of Research in Real Life Limited. M Baldwin, at time of this project, was an employee of Novartis International AG Research and Development, Horsham, UK, and now is an employee of Boehringer Ingelheim, Ingelheim, Germany. R Jones reports personal fees from Almirall, AstraZeneca plc, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline plc, and Health Intelligence; grants, personal fees, and nonfinancial support from Novartis International AG; and personal fees and nonfinancial support from Napp/Mundipharma, Optimal Patient Care, and Respiratory Education Alliance. The authors report no other conflicts of interest in this work.
References
- ChapmanKRManninoDMSorianoJBEpidemiology and costs of chronic obstructive pulmonary diseaseEur Respir J200627118820716387952
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary diseaseGlobal Initiative for Chronic Obstructive Lung Disease2015 Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015.pdfAccessed July 2, 2015
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary diseaseGlobal Initiative for Chronic Obstructive Lung Disease2013 Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdfAccessed July 2, 2015
- National Institute for Health and Care ExcellenceChronic obstructive pulmonary disease: Management of chronic obstructive pulmonary disease in adults in primary and secondary careLondonNational Institute for Health and Care Excellence2004 Available from: https://www.nice.org.uk/guidance/cg101/evidence/cg101-chronic-obstructive-pulmonary-disease-update-full-guideline2Accessed July 2, 2015
- AscheCVLeaderSPlauschinatCAdherence to current guidelines for chronic obstructive pulmonary disease (COPD) among patients treated with combination of long-acting bronchodilators or inhaled corticosteroidsInt J Chron Obstruct Pulmon Dis2012720120922500120
- FitchKIwasakiKPyensonBPlauschinatCZhangJVariation in adherence with Global Initiative for Chronic Obstructive Lung Disease (GOLD) drug therapy guidelines: a retrospective actuarial claims data analysisCurr Med Res Opin20112771425142921599554
- MasoompourSMMohammadiAMahdaviazadHAdherence to the Global Initiative for Chronic Obstructive Lung Disease guidelines for management of COPD: a hospital-base studyClin Respir J Epub20141013
- MiravitllesMSicrasACrespoCCosts of chronic obstructive pulmonary disease in relation to compliance with guidelines: a study in the primary care settingTher Adv Respir Dis20137313915023653458
- Canadian Agency for Drugs and Technologies in Health (CADTH)Triple Therapy for Moderate-to-Severe Chronic Obstructive Pulmonary DiseaseCADTH Technol Overv201014e012922977419
- GaebelKMcIvorRAXieFTriple therapy for the management of COPD: a reviewCOPD20118320624321513437
- KarnerCCatesCJThe effect of adding inhaled corticosteroids to tiotropium and long-acting beta(2)-agonists for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20119CD00903921901729
- JochmannANeubauerFMiedingerDSchafrothSTammMLeuppiJDGeneral practitioner’s adherence to the COPD GOLD guidelines: baseline data of the Swiss COPD Cohort StudySwiss Med Wkly Epub2010421
- SuissaSBarnesPJInhaled corticosteroids in COPD: the case againstEur Respir J2009341131619567599
- CorradoARossiAHow far is real life from COPD therapy guidelines? An Italian observational studyRespir Med2012106798999722483189
- de Miguel-DíezJCarrasco-GarridoPRejas-GutierrezJInappropriate overuse of inhaled corticosteroids for COPD patients: impact on health costs and health statusLung2011189319920621499887
- FranssenFMSpruitMAWoutersEFDeterminants of polypharmacy and compliance with GOLD guidelines in patients with chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2011649350122069360
- WongGWMiravitllesMChisholmAKrishnanJARespiratory guidelines – which real world?Ann Am Thorac Soc201411Suppl 2S85S9124559026
- CalverleyPPauwelsRVestboJCombined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trialLancet2003361935644945612583942
- JonesPWWillitsLRBurgePSCalverleyPMInhaled Steroids in Obstructive Lung Disease in Europe study investigatorsDisease severity and the effect of fluticasone propionate on chronic obstructive pulmonary disease exacerbationsEur Respir J2003211687312570111
- MahlerDAWirePHorstmanDEffectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200216681084109112379552
- RossiAvan der MolenTdel OlmoRINSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPDEur Respir J20144461548155625359348
- CummingRGMitchellPLeederSRUse of inhaled corticosteroids and the risk of cataractsN Engl J Med199733718149203425
- KimJHParkJSKimKHJeongHCKimEKLeeJHInhaled corticosteroid is associated with an increased risk of TB in patients with COPDChest201314341018102423079688
- SuissaSKezouhAErnstPInhaled corticosteroids and the risks of diabetes onset and progressionAm J Med2010123111001100620870201
- WeatherallMJamesKClayJDose-response relationship for risk of non-vertebral fracture with inhaled corticosteroidsClin Exp Allergy20083891451145818537983
- DiSantostefanoRLSampsonTLeHVHindsDDavisKJBakerlyNDRisk of pneumonia with inhaled corticosteroid versus long-acting bronchodilator regimens in chronic obstructive pulmonary disease: a new-user cohort studyPloS One201495e9714924878543
- ErnstPGonzalezAVBrassardPSuissaSInhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumoniaAm J Respir Crit Care Med2007176216216617400730
- SuissaSPatenaudeVLapiFErnstPInhaled corticosteroids in COPD and the risk of serious pneumoniaThorax201368111029103624130228
- KraemerMEllisABaldwinMJansenJPCapkun-NiggliGCopeSPRS5 dual bronchodilation with indacaterol and tiotropium in combination versus triple therapy, fixed-dose combinations, and monotherapy in COPD – a network meta-analysis of FEV1Value in Health157A560
- MagnussenHDisseBRodriguez-RoisinRWithdrawal of inhaled glucocorticoids and exacerbations of COPDN Engl J Med2014371141285129425196117
- VogelmeierCFBatemanEDPallanteJEfficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group studyLancet Respir Med201311516024321804
- HarriesTHSeedPTJonesSSchofieldPWhitePChronic obstructive pulmonary disease hospital admissions and drugs – unexpected positive associations: a retrospective general practice cohort studyNPJ Prim Care Respir Med2014241400624842126
- Optimum Patient Care Research Database (OPCRD) [webpage on the Internet]Cambridge: Optimum Patient Care2014 Available from: http://www.optimumpatientcare.org/Html_Docs/OPCRD.htmlAccessed July 2, 2015
- YohannesAMConnollyMJHananiaNATen years of tiotropium: clinical impact and patient perspectivesInt J Chron Obstruct Pulmon Dis2013811712523515335
- 2014/15 General Medical Services (GMS)Contract Quality and Outcomes Framework (QOF) [webpage on the Internet]LondonBritish Medical Association2014 Available from: http://bma.org.uk/practical-support-at-work/contracts/independent-contractors/qof-guidanceAccessed July 2, 2015
- MiyazakiMNakamuraHTakahashiSThe reasons for triple therapy in stable COPD patients in Japanese clinical practiceInt J Chron Obstruct Pulmon Dis2015101053105926082629
- BurgelPRDesléeGJebrakGReal-life use of inhaled corticosteroids in COPD patients versus the GOLD proposals: a paradigm shift in GOLD 2011?Eur Respir J20144341201120324176996
- PapalaMKerenidiNGourgoulianisKIEveryday clinical practice and its relationship to 2010 and 2011 GOLD guideline recommendations for the management of COPDPrim Care Respir J201322336236423989678
- SafkaKMcIvorLMcIvorAP252 Gold Category And Optimal Management: A Canadian PerspectiveThorax201469A187A188
- CalverleyPMWhat to use INSTEAD of inhaled corticosteroids in COPD?Eur Respir J20144461391139325435522
- RossiAGuerrieroMCorradoAOPTIMO/AIPO Study GroupWithdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO)Respir Med20141577
- BrusselleGGBrackeKLahousseLTargeted therapy with inhaled corticosteroids in COPD according to blood eosinophil countsLancet Respir Med20153641641725892351
- PascoeSLocantoreNDransfieldMTBarnesNCPavordIDBlood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trialsLancet Respir Med20153643544225878028
- LangePMarottJLVestboJPrediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general populationAm J Respir Crit Care Med20121861097598122997207
- BrusselleGWhy doesn’t reducing exacerbations decrease COPD mortality?Lancet Respir Med20142968168325209987
- PriceDYawnBBrusselleGRossiARisk-to-benefit ratio of inhaled corticosteroids in patients with COPDPrim Care Respir J20132219210023135217
- WhitePThorntonHPinnockHGeorgopoulouSBoothHPOvertreatment of COPD with inhaled corticosteroids – implications for safety and costs: cross-sectional observational studyPloS One2013810e7522124194824
- SinDDManSFSteroids in COPD: still up in the air?Eur Respir J201035594995120436169
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247