Abstract
Background
Randomized, controlled trials comparing long-acting muscarinic antagonist (LAMA) efficacy in COPD are limited. This network meta-analysis (NMA) assessed the relative efficacy of tiotropium 18 µg once-daily (OD) and newer agents (aclidinium 400 µg twice-daily, glycopyrronium 50 µg OD, and umeclidinium 62.5 µg OD).
Methods
A systematic literature review identified randomized, controlled trials of adult COPD patients receiving LAMAs. A NMA within a Bayesian framework examined change from baseline in trough forced expiratory volume in 1 second (FEV1), transitional dyspnea index focal score, St George’s Respiratory Questionnaire score, and rescue medication use.
Results
Twenty-four studies (n=21,311) compared LAMAs with placebo/each other. Aclidinium, glycopyrronium, tiotropium, and umeclidinium, respectively, demonstrated favorable results versus placebo, for change from baseline (95% credible interval) in 12-week trough FEV1 (primary endpoint: 101.40 mL [77.06–125.60]; 117.20 mL [104.50–129.90]; 114.10 mL [103.10–125.20]; 136.70 mL [104.20–169.20]); 24-week trough FEV1 (128.10 mL [84.10–172.00]; 135.80 mL [123.10–148.30]; 106.40 mL [95.45–117.30]; 115.00 mL [74.51–155.30]); 24-week St George’s Respiratory Questionnaire score (−4.60 [−6.76 to −2.54]; −3.14 [−3.83 to −2.45]; −2.43 [−2.92 to −1.93]; −4.69 [−7.05 to −2.31]); 24-week transitional dyspnea index score (1.00 [0.41–1.59]; 1.01 [0.79–1.22]; 0.82 [0.62–1.02]; 1.00 [0.49–1.51]); and 24-week rescue medication use (data not available; −0.41 puffs/day [−0.62 to −0.20]; −0.52 puffs/day [−0.74 to −0.30]; −0.30 puffs/day [−0.81 to 0.21]). For 12-week trough FEV1, differences in change from baseline (95% credible interval) were −12.8 mL (−39.39 to 13.93), aclidinium versus tiotropium; 3.08 mL (−7.58 to 13.69), glycopyrronium versus tiotropium; 22.58 mL (−11.58 to 56.97), umeclidinium versus tiotropium; 15.90 mL (−11.60 to 43.15), glycopyrronium versus aclidinium; 35.40 mL (−5.06 to 76.07), umeclidinium versus aclidinium; and 19.50 mL (−15.30 to 54.38), umeclidinium versus glycopyrronium. Limitations included inhaler-related factors and safety; longer-term outcomes were not considered.
Conclusion
The new LAMAs studied had at least comparable efficacy to tiotropium, the established class standard. Choice should depend on physician’s and patient’s preference.
Introduction
The overarching goals for the management of COPD include prevention of further disease progression, symptom relief, reduction in exacerbations, treatment of complications (eg, infections), and the maintenance or improvement of overall health status.Citation1 Treatment options for COPD depend on symptom burden and exacerbation risk, but bronchodilators are a cornerstone of therapy. Long-acting muscarinic antagonists (LAMAs) are recommended for patients in Global Initiative for Chronic Obstructive Lung Disease (GOLD) groups A to D.Citation1 LAMAs are associated with improved lung function, improved quality of life, and reduced exacerbations.Citation2,Citation3
Until 2012, tiotropium bromide was the only LAMA widely available for the treatment of COPD.Citation3–Citation5 Tiotropium is a once-daily (OD) treatment, and has been widely prescribed for COPD. However, several new LAMAs have since been introduced, including aclidinium bromide (twice-daily [BD] for COPD maintenance) and glycopyrronium bromide (OD for COPD maintenance), which could be used as alternatives to tiotropium.Citation6–Citation9 Umeclidinium bromide has been the most recent addition; this is a OD inhaled LAMA approved for COPD maintenance therapy in adults in the EU, USA, and several other countries. Compared with placebo, umeclidinium OD (metered dose 62.5 µg, delivered dose 55 µg) significantly improved lung function, dyspnea, and health status over 12 weeks in a randomized study of 246 patients.Citation9 With this new addition to the LAMA class, there is a need to understand the relative comparative efficacy of the available agents.
Primary comparative efficacy data from randomized controlled trials for newer LAMAs are limited. With the introduction of new agents, such as umeclidinium, it is often not feasible to conduct clinical trials to compare the new treatment against all alternative agents in clinical trials to determine relative efficacy. Accordingly, there are no published direct head-to-head comparisons on the clinical efficacy between all LAMAs. Therefore, alternative methodologies need to be employed to better inform health care practitioners of the anticipated relative efficacy for important clinical endpoints. A number of network meta-analyses (NMAs) have been published in recent years, comparing LAMAs (tiotropium and glycopyrronium) with other COPD therapies,Citation10 and aclidinium versus glycopyrronium and tiotropium.Citation11 However, since the introduction of a new treatment option (umeclidinium), further analyses are needed. A systematic literature review and Bayesian NMA was undertaken to assess the relative efficacy of aclidinium, glycopyrronium, tiotropium, and umeclidinium for the treatment of COPD.
Methods
Study objectives
The primary objective of this study (GSK study number: 201280)Citation12 was to assess the relative efficacy of all LAMAs available in the market at the licensed doses, namely: aclidinium 400 µg BD (hereafter referred to as aclidinium), glycopyrronium 50 µg OD (glycopyrronium), tiotropium 18 µg OD (tiotropium), and umeclidinium 62.5 µg OD (umeclidinium), by means of lung function (difference in change from baseline for trough forced expiratory volume in 1 second [FEV1]) at 12 weeks. The doses of each of the four LAMAs chosen for this NMA were the only approved doses for the dry powder inhaler formulations. Other formulations such as tiotropium 5 µg OD via a soft mist device, which is considered as equivalent to 18 µg via the Handihaler, or alternative BD glycopyrronium/glycopyrrolate investigational formulations have not been included. Secondary objectives were to assess the relative efficacy of the LAMA for the following endpoints: 1) difference in change from baseline for trough FEV1 (at 24 weeks); 2) difference in change from baseline in St George’s Respiratory Questionnaire (SGRQ) total score (at 12 and 24 weeks); 3) differences in the transitional dyspnea index (TDI) focal score (at 12 and 24 weeks); and 4) differences in change in rescue medication use (mean number of puffs per day) (at 12 and 24 weeks). The 12- and 24-week time points used in our study were chosen to reflect the expected data availability; these are commonly used time intervals in COPD trials.
Data sources
A systematic review including a broad range of search terms following Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines was performed.Citation13 The following databases were searched: MEDLINE (through Ovid platform); MEDLINE In-Process (Ovid); EMBASE (Ovid); The Cochrane Database of Systematic Reviews (CDSR) and Cochrane Central Register of Controlled Trials (CENTRAL); Database of Abstracts of Reviews of Effects (DARE); and Health Technology Assessment (HTA) websites, HTA database and National Institute for Health Research (NIHR). The following clinical trial registries were searched: Clinicaltrials.gov; World Health Organization International Clinical Trials Registry Platform (WHO ICTRP); Current Controlled Trials; EU Clinical Trials Register (EU-CTR); Klinische Prüfungen PharmNet.Bund; and The International Prospective Register of Systematic Reviews (PROSPERO). The searches were performed on April 14, 2014–April 16, 2014, for studies in English and German language without time restrictions. Predefined search strategies were used (available in Table S1), tailored for each database.
Inclusion criteria and study selection process for systematic literature review
The relevance of each identified citation was assessed based on the title and abstract according to predefined selection criteria (Table S2). For the abstracts that met the selection criteria, available publications were obtained and evaluated using the full-text selection criteria. Studies (randomized, controlled trials) had to include adults with COPD reporting on at least one of: umeclidinium; aclidinium; tiotropium; glycopyrronium compared with each other or placebo. The outcomes examined were trough FEV1, TDI focal score, SGRQ score, and rescue medication. The time points of interest for all outcomes were 12 and 24 weeks, while outcomes between 8 and 16 weeks or 20 and 28 weeks were reported as proxy outcomes for 12 and 24 weeks, respectively. The selection was performed by two researchers independently and any discrepancies were resolved by consensus. The final selected citations were grouped per study.
Data abstraction and quality assessment
Key data from each eligible study were extracted, including study design (treatments, duration, inclusion/exclusion criteria, and background treatment) and patient characteristics (eg, age, sex, and lung function parameters; Table S3) into a data extraction form. Data extraction was performed by one researcher and verified by another researcher. Data of interest presented in graphs were extracted using DigitizeIT version 1.5 software (DigitizeIT, Braunschweig, Germany). The methodological and reporting quality of the included trials was assessed with a checklist based on the guidance by the Institute for Quality and Efficiency in Health Care.Citation14 The risk of bias in each study was classified as “high” or “low” based on the following items: appropriate generation of a randomization sequence; adequate allocation concealment; blinding of patients, treating staff, and staff responsible for follow-up treatment; reporting of all relevant outcomes independent of results; no other aspects that could lead to bias. The results of the risk of bias assessment are presented in Table S4.
Data synthesis
The existence of a connected network of studies per outcome, as well as the study design and patient characteristics of the identified studies, was used to assess the feasibility of a valid NMA.Citation15 The identified evidence was used to perform a NMA within a Bayesian framework to simultaneously synthesize the results of the included studies and to obtain relative treatment effects.Citation16,Citation17 A generalized linear model with normal likelihood distribution was used.Citation18 Non-informative prior distributions of the relative treatment effects (normal distributions with zero mean and a variance of 10,000) were used as a widely accepted option for all outcomes of interest. The analysis was based on the difference between the least square mean at follow-up or the difference in change from baseline for the active treatment versus the comparator as well as the associated standard error (SE) of the difference. To assess the consistency of the network, the node splitting method was followed by separating and comparing direct and indirect evidence per outcome for each one of these three pairwise comparisons.Citation19
For each outcome, a fixed- and a random-effects model was evaluated. The fixed-effects model assumed that the differences in true relative treatment effects across studies in the network of evidence were only due to differences in treatment comparisons (ie, that there was no variation in relative treatment effects for a particular pairwise comparison). The random-effects model assumed that differences in observed treatment effects across the studies in the network were not only caused by the different treatment comparisons, but that there was also heterogeneity in the relative effects for a particular type of comparison caused by factors that modify the relative treatment effect. With the NMA models used, the heterogeneity was assumed to be constant for every treatment comparison. Due to the relatively low number of studies, treatment-by-covariate interactions could not be incorporated into the models; instead, scenario analyses were developed to test the impact of certain studies on the relative treatment estimates.
The goodness of fit of each model to the data was assessed using the Deviance Information Criterion.Citation20 The posterior densities for the outcomes of interest were estimated using the Markov Chain Monte Carlo simulations for each model. The results were based on 80,000 iterations on three chains, with a burn-in of 20,000 iterations. Convergence assessment was based on visual inspection of trace plots. Accuracy of the posterior estimates was assessed using the Monte Carlo error for each parameter (Monte Carlo error <5% of the posterior standard deviation [SD]). Given the dataset used, the fixed-effects model was chosen over the random-effects model unless there was enough evidence to suggest that the random-effects model was substantially different (ie, Deviance Information Criterion value was lower and Monte Carlo error was not out of proportion). WinBUGS 1.4.3 statistical software was used for the analysesCitation21 and the models were based on those defined by Dias et al (programs 7(b) and 8(a) in the Appendix of Dias et al).Citation18
The NMA provided posterior distributions of the relative treatment effects between interventions for each outcome of interest. The posterior distributions were summarized with the median to reflect the most likely value of the estimate, and the 2.5th and 97.5th percentile to capture the 95% credible interval (CrI).Citation18 The 95% CrI represents the range of true underlying effects with 95% probability. For each endpoint, the probability that each treatment was better than a certain comparator was established.
If a study only reported mean differences without a measure of uncertainty (SE, SD, or confidence interval [CI]), the following steps were executed to impute SE values: 1) SD of the difference for each study reporting sufficient data were calculated by the formula SD of difference = SE of difference × square root of N; 2) the average SD of the trials in the network was calculated; 3) the average SD of the trials in the network is imputed for the trial that did not report a SD/SE/95% CI; or 4) the SE of the difference = average SD/square root of N.
Results
Search and selection results
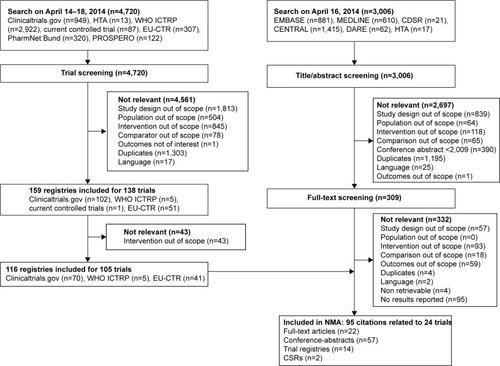
A total of 3,006 citations and 4,720 clinical trials were identified (). After screening, 95 citations (publications and trials) reporting on 24 different trials with 21,311 patients were included in the analysis.
Figure 1 Flow chart of study selection process.
Study characteristics
All studies included in the analyses were parallel-group, multicenter, randomized, controlled trials and the number of patients randomized per arm ranged from 46Citation22 to 3,006Citation3 (). All trials were double blind, with the exception of one tiotropium trial that included tiotropium as an open-label arm.Citation23 Inhaled corticosteroids were allowed in all the studies where information on inhaled corticosteroid background was reported. Long-acting β2-agonist (LABA) background treatment was allowed in five tiotropium studies (LABA use at baseline ranged from 38% to 61% of the study arms, where data were available).Citation3,Citation4,Citation24,Citation25 Information on LABA use was missing in three studies,Citation26–Citation28 and was not allowed in the remaining studies.
Table 1 Key study characteristics for all studies included (only arms of interest)
Patient characteristics
Patient populations ranged from 49% to 99% male (), but the mean age was similar across the studies (mean range 60–67 years). Spirometry measures were generally consistent at baseline, with most studies requiring a FEV1/forced vital capacity of ≤0.70. The mean FEV1 % predicted ranged between 50% and 56% for aclidinium-treated patients, 37%–56% for glycopyrronium, 35%–55% for tiotropium, and 45%–48% for umeclidinium. The proportion of patients with severe or very severe COPD was reported in seven studies,Citation3,Citation29–Citation35 and ranged from 31% to 100% (per treatment arm). Across all studies, the proportion of patients per arm who used inhaled corticosteroids at baseline ranged from 22% to 76%. All studies included patients who were current or ex-smokers and most specified a smoking history of at least 10 years; the mean number of pack-years ranged from 38.6 to 69.4 years.
Table 2 Key patient characteristics at baseline for all studies included (only arms of interest)
Network meta-analysis
Although there was some degree of variation in patient characteristics across studies, in general the studies were of good quality and homogeneous, and thus a valid NMA was feasible.Citation36 The network diagram for the randomized clinical trials included in the NMA is shown in . Studies were identified that compared aclidinium, glycopyrronium, tiotropium, and umeclidinium with placebo as the common comparator. The NMA results for trough FEV1 at 12 weeks (primary endpoint) and 24 weeks are presented, as well as secondary endpoints at 24 weeks. Supportive analyses of secondary endpoints at 12 weeks are presented in Tables S5 and S6.
Figure 2 Overall network of studies in the network meta-analysis of umeclidinium versus other LAMAs or placebo for (A) trough FEV1 at 12 weeks, (B) trough FEV1 at 24 weeks, (C) SGRQ total score at 24 weeks, (D) TDI focal score at 24 weeks, and (E) rescue medication use at 24 weeks.
Abbreviations: BD, twice-daily; FEV1, forced expiratory volume in 1 second; LAMA, long-acting muscarinic antagonist; OD, once-daily; PBO, placebo; SGRQ, St George’s Respiratory Questionnaire; TDI, transitional dyspnea index.
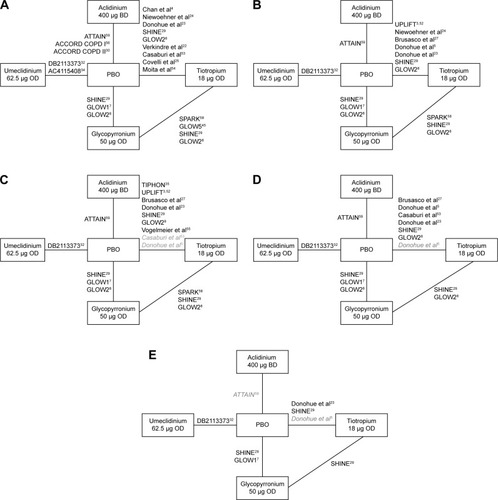
Given the geometry of each network (containing only one closed loop; ), direct and indirect evidence for all outcomes was only available for the comparative efficacy of tiotropium versus placebo, glycopyrronium versus placebo, and tiotropium versus glycopyrronium. No important deviation between direct and indirect evidence was observed when the network consistency was assessed, suggesting that the consistency assumption was valid.
Trough FEV1 at 12 weeks (primary outcome)
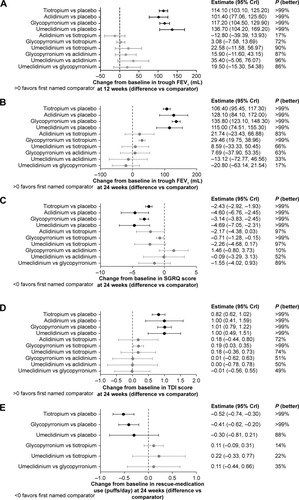
In total, 17 studies (11,935 patients) were included for the FEV1 endpoint ( and ). The minimal clinically important difference for FEV1 is 100 mL.Citation37 All LAMAs investigated were more efficacious than placebo, with a mean change from baseline greater than the minimal clinically important difference (). The mean change from baseline in trough FEV1 was highest for umeclidinium, with a difference of 136.7 mL (95% Crl: 104.20–169.20) from placebo and a >99% probability of being better than placebo. The probability of umeclidinium being a better treatment than tiotropium, aclidinium, or glycopyrronium was 90%, 96%, or 86%, respectively.
Table 3 Individual study results for trough FEV1, SGRQ total scores, TDI focal scores, and rescue medication use
Figure 3 Differences in intervention versus the comparator for change from baseline in (A) trough FEV1 (mL) at 12 weeks, (B) trough FEV1 (mL) at 24 weeks, (C) SGRQ total scores at 24 weeks, (D) TDI focal scores at 24 weeks, and (E) rescue medication use at 24 weeks (95% CrI and probability of the intervention being better than the comparator).
Trough FEV1 at 24 weeks
In total, eleven studies (15,663 patients) were included for the FEV1 endpoint at 24 weeks ( and ). Again, the mean change from baseline was greater than the minimal clinically important difference for all active agents. The highest change from baseline in trough FEV1 was found with glycopyrronium, with a difference of 135.8 mL (95% Crl: 123.10–148.30). Glycopyrronium had a >99% chance of being better than tiotropium, which had the next highest difference in change from baseline trough FEV1. The newest agent, umeclidinium, had a mean difference in change from baseline of 115.0 mL compared with placebo (95% CrI: 74.51–155.30), with >99% probability of being better than placebo (). Umeclidinium was comparable to other LAMAs for this endpoint, with only a 66%, 33%, and 17% probability of being better than tiotropium, aclidinium, and glycopyrronium, respectively.
SGRQ total score at 24 weeks
Thirteen studies (15,739 patients) were included in the examination of this endpoint ( and ). Two studies reported only the mean difference in change from baseline without any measure of uncertainty, such as SE, SD, or 95% CI.Citation5,Citation26 An imputed value was calculated based on the average SD of the difference in change from baseline of trials in the network. Imputing this value and adding the studies to the analysis did not impact the results.
The minimal clinically important difference for SGRQ score is 4 units.Citation38 Relative to placebo, only umeclidinium and aclidinium mean scores were reduced by more than 4 units, although all agents had 99% probability of being better than placebo (). The highest difference was seen with umeclidinium, which had a 97%, 52%, and 89% chance of being better than tiotropium, aclidinium, or glycopyrronium, respectively.
TDI focal score at 24 weeks
Nine studies (7,285 patients) were included ( and ). One studyCitation5 did not report any measure of uncertainty or an exact P-value; this was imputed and did not impact the results.
The minimal clinically important difference for TDI score is 1 unit.Citation39 Aclidinium, glycopyrronium, and umeclidinium had a mean difference in change from baseline in TDI score of ≥1.00 (). Only the mean change in TDI score for tiotropium did not reach the minimal clinically important difference.
Rescue medication use at 24 weeks
A total of six studies (4,502 patients) were included ( and ). Glycopyrronium, tiotropium, and umeclidinium reduced rescue medication use to comparable extents, with mean changes of −0.41 (95% CrI: −0.62 to −0.20), −0.52 (95 CrI: −0.74 to −0.30), and −0.30 puffs/day (95% CrI: −0.81 to 0.21), relative to placebo ().
Discussion
In the absence of head-to-head study data and in light of new available agents, a systematic literature review and NMA was carried out to assess the relative efficacy of LAMAs for the treatment of COPD. Overall, a large number of patients (21,311) were included in our analyses. Endpoints (change from baseline in trough FEV1, SGRQ total scores, TDI focal scores, and rescue medication use) were selected because they were consistently reported across all studies and deemed to be clinically important endpoints in those studies. Other endpoints, such as adverse events, exercise tolerance, and exacerbation rate, were not included, for several reasons. First, the definitions and methodology for reporting adverse events and exercise tolerance were variable across trials, precluding accurate comparisons. Second, exacerbations were studied in some longer-term trials, where a history of these events was required at entry, but were not key endpoints in most 3- and 6-month studies. Although exacerbations were beyond the scope of this NMA, another NMA performed without the inclusion of umeclidinium suggested that efficacy was comparable between aclidinium, glycopyrronium, and tiotropium for the prevention of COPD exacerbations; all reduced moderate-to-severe exacerbations, compared with placebo, and all were equally effective.Citation40
As expected, this NMA revealed that all the active LAMA treatments (aclidinium, glycopyrronium, tiotropium, and umeclidinium) were more efficacious than placebo, with each of the active therapies providing clinically relevant improvements in trough FEV1 (>100 mL) at 12 and 24 weeks. Improvements in other measures (SGRQ score, TDI focal score, and rescue medication use), versus placebo, were also observed. The estimates met the minimal clinically important differences for umeclidinium (SGRQ and TDI focal score), aclidinium (SGRQ and TDI focal score), and glycopyrronium (TDI focal score only) versus placebo at 24 weeks. Overall, these findings suggest that all LAMAs are effective, compared with placebo.
Aclidinium and umeclidinium had broadly similar efficacy for lung function and patient-reported outcomes, compared with the other LAMAs examined and each other. Overall, there was no evidence that a BD regimen (ie, aclidinium) was more efficacious than OD regimens. For umeclidinium, the newest agent, there were some modest numerical improvements in 12-week lung function, compared with other LAMAs; however, the CrI crossed zero in all cases. In some cases, there were indications that glycopyrronium had superior efficacy to tiotropium, with the newer agent having a >99% probability of being better in terms of 24-week FEV1 and SGRQ score than tiotropium. However, it should be acknowledged that the patients in the glycopyrronium trials had predominantly moderately severe COPD, compared with tiotropium trials, which tended to include patients with severe COPD.
Although there have been no direct comparisons of umeclidinium with other LAMAs in the literature (noting that head-to-head trials of umeclidinium versus glycopyrronium [NCT2236611],Citation41 and umeclidinium versus tiotropium [NCT2207829],Citation42 are currently ongoing), there have been recent direct comparisons of aclidinium versus tiotropium. In one randomized, controlled trial aclidinium had comparable bronchodilation and significantly improved symptom control, relative to tiotropium at 6 weeks, in line with our data.Citation43 A small, randomized crossover study also suggested some improvements for FEV1 area under the curve and COPD symptoms with aclidinium versus tiotropium.Citation44 The GLOW5 study concluded that glycopyrronium and tiotropium had similar efficacy, noting that there were non-significant improvements with glycopyrronium for TDI focal score, SGRQ total score, rescue medication use, and the rate of COPD exacer-bations.Citation45 The current analysis failed to entirely corroborate these findings, highlighting small potential efficacy differences in favor of glycopyrronium. As noted previously, this discrepancy could result from differences in baseline COPD severity between glycopyrronium and tiotropium trials.
Limitations
There are some potential limitations to this analysis. Although the endpoints selected were clinically important (and commonly reported in randomized controlled trials), they were also relatively short-term endpoints. At present, all four of the LAMAs investigated here have reported positive effects on exacerbations outcomes relative to placebo,Citation7,Citation46–Citation48 but differences in study methodology, populations, and reporting methods precluded robust comparisons of LAMAs against one another in our analysis. We also focused on mean outcomes; alternative analyses examining percentage of responders, if performed on patient level data, might highlight incremental differences between the LAMAs that were not apparent when means were used. Differences in the patient populations, particularly the approximately 20% range in mean baseline FEV1 % predicted values, and background medications may have resulted in some residual confounding influences that could not be adequately addressed with our methodology, despite attempts to select similar studies. Consequently, the findings do not carry the same weight as head-to-head randomized controlled trials; such studies are warranted to corroborate our data. Finally, the data used in the NMA were obtained from highly controlled studies with patients who have been trained in the use of different inhaler devices. Our analysis cannot account for potential handling errors or preferences for a particular device (these factors were likely to have been minimized within studies due to blinding). These inhaler-related factors highlight a need for more pragmatic COPD-effectiveness studies (less controlled) when LAMAs are compared. Such studies may allow for increased differentiation within the LAMA class driven by device choice and posology differences within the drug class. Until such head-to-head studies are available, our findings provide reassurance that umeclidinium has an efficacy profile at least on a par with the standard-of-care LAMA, tiotropium, and a profile at least as effective as other new alternative LAMAs.
Conclusion
The current data on LAMAs suggest that aclidinium, glycopyrronium, tiotropium, and umeclidinium are efficacious, relative to placebo, and the efficacy profile of newer LAMAs appears at least on a par with the standard-of-care LAMA, tiotropium. Until randomized controlled head-to-head trials can be carried out, there is little robust evidence to suggest that one is more efficacious than the others, and the choice of LAMA should depend on physician’s and patient’s preference.
Author contributions
ASI, ELH, YSP, and AK contributed to the study design and were involved in the analysis or interpretation of the data. ELH was also involved in the data acquisition. All authors drafted the manuscript.
Acknowledgments
The analysis was sponsored by GSK (GSK study number: 201280). Editorial support in the form of development of the draft outline in consultation with the authors, editorial suggestions to draft versions of this paper, assembling tables and figures, collating author comments, copyediting, fact checking, referencing, and graphic services was provided by Emma McConnell, PhD, at Gardiner-Caldwell Communications (Macclesfield, UK) and was funded by GSK.
Supplementary materials
Table S1 Search strategy for the systematic review
Table S2 Participants, interventions, comparisons, outcomes, and study design (PICOS) criteria
Table S3 Data extraction
Table S4 Risk of bias assessment for the included studies
Table S5 Individual study results for trough SGRQ total scores, TDI focal scores, and rescue medication use
Table S6 Differences in intervention versus the comparator for change for SGRQ total scores, TDI focal scores, and rescue medication use at 12 weeks (95% CrI and probability of the intervention being better than the comparator)
References
- ChanCKNMaltaisFSigouinCHaddonJMFordGTA randomized controlled trial to assess the efficacy of tiotropium in Canadian patients with chronic obstructive pulmonary diseaseCan Respir J20071446547218060091
- Spiriva Assessment of FEV1 (SAFE)The effect of inhaled tiotropium bromide (18 mcg once daily) on the change in FEV1 during long-term treatment in patients with COPD. A one-year parallel group, double-blind, randomised, placebo-controlled studyBoehringer Ingelheim Clinical Trial Register. NLM: NCT02772642005 Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/205/205.259_U05-3345.pdfAccessed October 19, 2015
- Spiriva® Assessment of FEV1 (SAFE)Boehringer Ingelheim2013 NLM identifier: NCT0277264. Available from: http://trials.boeh-ringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/205/205.259_U05-3345.pdfAccessed October 19, 2015
- Spiriva (Tiotropium Bromide) Assessment of FEV1 – (SAFE-Portugal)Boehringer Ingelheim2013 NLM identifier: NCT0239408. Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/205/205.282_U06-2124.pdfAccessed October 19, 2015
- TonnelABPerezTGrosboisJMVerkindreCBravoMLBrunMEffect of tiotropium on health-related quality of life as a primary efficacy endpoint in COPDInt J Chron Obstruct Pulmon Dis2008330131018686739
- TashkinDPCelliBSennSA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med20083591543155418836213
- Evaluation of the long-term effects of spiriva on lung function in COPD patientsBoehringer Ingelheim2005 NLM identifier: NCT0144339
- NiewoehnerDERiceKCoteCPrevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trialAnn Intern Med200514331732616144890
- BrusascoVHodderRMiravitllesMKorduckiLTowseLKestenSHealth outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPDThorax20035839940412728159
- DonohueJFvan NoordJABatemanEDA 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterolChest2002122475512114338
- CasaburiRMahlerDAJonesPWA long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J20021921722411866001
- DonohueJFFogartyCLotvallJOnce-daily bronchodilators for chronic obstructive pulmonary disease indacaterol versus tiotropiumAm J Respir Crit Care Med201018215516220463178
- BatemanEDFergusonGTBarnesNDual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE studyEur Respir J2013421484149423722616
- D’UrzoAFergusonGTvan NoordJAEfficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trialRespir Res20111215622151296
- KerwinEHebertJGallagherNEfficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 studyEur Respir J2012401106111423060624
- VerkindreCBartFAguilaniuBThe effect of tiotropium on hyperinflation and exercise capacity in chronic obstructive pulmonary diseaseRespiration20067342042716484769
- CasaburiRBriggsDDDonohueJFSerbyCWMenjogeSSWitekTJThe spirometric efficacy of once-daily dosing with tiotropium in stable COPD – a 13-week multicenter trialChest20001181294130211083677
- CovelliHBhattacharyaSCassinoCConoscentiCKestenSAbsence of electrocardiographic findings and improved function with once-daily tiotropium in patients with chronic obstructive pulmonary diseasePharmacotherapy2005251708171816305289
- GarciaRFA randomised, double-blind, placebo-controlled, 12 weeks trial to evaluate the effect of Tiotropium Inhalation Capsules on the magnitude of exercise, measured using an accelerometer, in patients with Chronic Obstructive Pulmonary Disease (COPD)Boehringer Ingelheim Trial Results2007 NLM Identifier: NCT0144326. Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/205/205.269.pdfAccessed October 19, 2015
- MoitaJBarbaraCCardosoJTiotropium improves FEV1 in patients with COPD irrespective of smoking statusPulm Pharmacol Ther20082114615117693107
- VogelmeierCKardosPHarariSGansSJMStengleinSThirlwellJFormoterol mono- and combination therapy with tiotropium in patients with COPD: a 6-month studyRespir Med20081021511152018804362
- KerwinEMD’UrzoADGelbAFLakkisHGarciaGECaractaCFEffi-cacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I)COPD201299010122320148
- RennardSIScanlonPDFergusonGTACCORD COPD II: a randomized clinical trial to evaluate the 12-week efficacy and safety of twice-daily aclidinium bromide in chronic obstructive pulmonary disease patientsClin Drug Invest201333893904
- JonesPWSinghDBatemanEDEfficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN studyEur Respir J20124083083622441743
- GlaxoSmithKlineClinical Study Report – DB21133732013
- DonohueJFMaleki-YazdiMRKilbrideSMehtaRKalbergCChurchAEfficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPDRespir Med20131071538154623830094
- GlaxoSmithKlineClinical Study Report – AC41154082013
- WedzichaJADecramerMFickerJHAnalysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group studyLancet Respir Med2013119920924429126
- ChapmanKRBeehKMBeierJA blinded evaluation of the efficacy and safety of glycopyrronium, a once-daily long-acting muscarinic antagonist, versus tiotropium, in patients with COPD: the GLOW5 studyBMC Pulm Med201414424438744
Disclosure
ASI and YSP are employees of GSK and hold stocks in GSK. ELH and AK are employees of Mapi and received payment from GSK for consultancy during the conduct of this study. The authors report no other conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) [database on the internet]Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease2015 Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Apr2.pdfAccessed May 11, 2015
- KewKMDiasSCatesCJLong-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysisCochrane Database Syst Rev2014263
- TashkinDPCelliBSennSA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med20083591543155418836213
- ChanCKNMaltaisFSigouinCHaddonJMFordGTA randomized controlled trial to assess the efficacy of tiotropium in Canadian patients with chronic obstructive pulmonary diseaseCan Respir J20071446547218060091
- DonohueJFvan NoordJABatemanEDA 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterolChest2002122475512114338
- JonesPWAgustiAChanezPA phase III study evaluating aclidinium bromide, a novel long-acting antimuscarinic, in patients with COPD: ACCLAIM/COPD IAm J Res Crit Care Med2009179A6180
- D’UrzoAFergusonGTvan NoordJAEfficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trialRespir Res20111215622151296
- KerwinEHebertJGallagherNEfficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 studyEur Respir J2012401106111423060624
- TrivediRRichardNMehtaRChurchAUmeclidinium in patients with COPD: a randomised, placebo-controlled studyEur Respir J201443728123949963
- CopeSDonohueJFJansenJPComparative efficacy of long-acting bronchodilators for COPD – a network meta-analysisRespir Res201314
- KarabisALindnerLMocarskiMHuismanEGreeningAComparative efficacy of aclidinium versus glycopyrronium and tiotropium, as maintenance treatment of moderate to severe COPD patients: a systematic review and network meta-analysisInt J COPD20138405423
- GSK Study 201280 [database on the internet] Available from: http://www.gsk-clinicalstudyregister.com/study/201280#psAccessed March 31, 2015
- PRISMA Guidelines [database on the internet] Available from: http://www.prisma-statement.org/Accessed April 1, 2015
- Allgemeine Methoden [database on the internet] Available from: https://www.iqwig.de/download/IQWiG_Methoden_Version_4-2.pdfAccessed June 25, 2015
- CopeSZhangJSaletanSSmiechowskiBJansenJPSchmidPA process for assessing the feasibility of a network meta-analysis: a case study of everolimus in combination with hormonal therapy versus chemotherapy for advanced breast cancerBMC Med2014129324898705
- SalantiGIndirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis toolRes Synth Methods201238026062083
- HoaglinDCHawkinsNJansenJPConducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: Part 2Value Health20111442943721669367
- DiasSWeltonNJSuttonAJAdesAENICEDSU Technical Support Document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials [database on the internet] Available from: http://www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%2015April2014.pdfAccessed May 11, 2015
- DiasSWeltonNJCaldwellDMAdesAEChecking consistency in mixed treatment comparison meta-analysisStat Med20102993294420213715
- SpiegelhalterDJBestNGCarlinBRvan der LindeABayesian measures of model complexity and fitJ R Stat Soc Series B Stat Methodol200264583616
- LunnDJThomasABestNSpiegelhalterDWinBUGS – a Bayesian modelling framework: concepts, structure, and extensibilityStat Comput200010325337
- VerkindreCBartFAguilaniuBThe effect of tiotropium on hyperinflation and exercise capacity in chronic obstructive pulmonary diseaseRespiration20067342042716484769
- DonohueJFFogartyCLotvallJOnce-daily bronchodilators for chronic obstructive pulmonary disease indacaterol versus tiotropiumAm J Respir Crit Care Med201018215516220463178
- NiewoehnerDERiceKCoteCPrevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trialAnn Intern Med200514331732616144890
- CovelliHBhattacharyaSCassinoCConoscentiCKestenSAbsence of electrocardiographic findings and improved function with once-daily tiotropium in patients with chronic obstructive pulmonary diseasePharmacotherapy2005251708171816305289
- CasaburiRMahlerDAJonesPWA long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J20021921722411866001
- BrusascoVHodderRMiravitllesMKorduckiLTowseLKestenSHealth outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPDThorax20035839940412728159
- GarciaRFA randomised, double-blind, placebo-controlled, 12 weeks trial to evaluate the effect of Tiotropium Inhalation Capsules on the magnitude of exercise, measured using an accelerometer, in patients with Chronic Obstructive Pulmonary Disease (COPD)Boehringer Ingelheim Trial Results NLM Identifier: NCT01443262007 Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/205/205.269.pdfAccessed October 19, 2015
- BatemanEDFergusonGTBarnesNDual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE studyEur Respir J2013421484149423722616
- RennardSIScanlonPDFergusonGTACCORD COPD II: a randomized clinical trial to evaluate the 12-week efficacy and safety of twice-daily aclidinium bromide in chronic obstructive pulmonary disease patientsClin Drug Invest201333893904
- GlaxoSmithKlineA multicenter trial comparing the efficacy and safety of GSK573719/GW642444 with GSK573719 and with tiotropium over 24 weeks in subjects with COPDClinical Study Report – DB2113374. NLM identifier: NCT13169132013 Available from: https://gsk.sylogent.com/files/sam-2588-gsk-113374-clinical-study-report-redact-v02.pdfAccessed October 19, 2015
- GlaxoSmithKlineA 24-week, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of GSK573719/GW642444 inhalation powder and the individual components delivered once-daily via a novel dry powder inhaler in subjects with chronic obstructive pulmonary diseaseClinical Study Report – DB2113373. NLM identifier: NCT13136502013 Available from: https://gsk.sylogent.com/files/sam-3623-gsk-113373-clinical-study-report-redact-v03.pdfAccessed October 19, 2015
- GlaxoSmithKline24-week trial comparing GSK573719/GW642444 with GW642444 and with tiotropium in chronic obstructive pulmonary diseaseClinical Study Report – DB2113360. NLM identifier: NCT13169002013 Available from: https://gsk.sylogent.com/files/sam-2504-gsk-113360-clinical-study-report-redact-v02.pdfAccessed October 19, 2015
- GlaxoSmithKlineA 12-week, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of GSK573719 delivered once-daily via a novel dry powder inhaler in subjects with chronic obstructive pulmonary diseaseClinical Study Report – AC4115408. NLM identifier: NCT13872302013 Available from: https://gsk.sylogent.com/files/sam-2802-gsk-115408-clinical-study-report-redact.pdfAccessed October 19, 2015
- TonnelABPerezTGrosboisJMVerkindreCBravoMLBrunMEffect of tiotropium on health-related quality of life as a primary efficacy endpoint in COPDInt J Chron Obstruct Pulmon Dis2008330131018686739
- JansenJPTrikalinosTCappelleriJCIndirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force ReportValue Health20141715717324636374
- DonohueJFMinimal clinically important differences in COPD lung functionCOPD2005211112417136971
- JonesPWSt George’s respiratory questionnaire: MCIDCOPD20052757917136966
- MahlerDAWitekTJThe MCID of the transition dyspnea index is a total score of one unitCOPD200529910317136969
- ObaYLoneNAComparative efficacy of long-acting muscarinic antagonists in preventing COPD exacerbations: a network meta-analysis and meta-regressionTher Adv Respir Dis2015931525586493
- GlaxoSmithKlineA randomized, parallel-group, open-label study to evaluate the efficacy and safety of umeclidinium (UMEC) 62.5 mcg compared with glycopyrronium 44 mcg in subjects with chronic obstructive pulmonary disease (COPD) Available from: http://www.gsk-clinicalstudyregister.com/study/201315#psAccessed June 25, 2015
- GlaxoSmithKlineA randomized, blinded, double-dummy, parallel-group study to evaluate the efficacy and safety of umeclidinium (UMEC) 62.5 mcg compared with tiotropium 18 mcg in subjects with chronic obstructive pulmonary disease (COPD) Available from: http://www.gsk-clinicalstudyregister.com/study/201316#psAccessed June 25, 2015
- BeierJKirstenAMMrozREfficacy and safety of aclidinium bromide compared with placebo and tiotropium in patients with moderate-to-severe chronic obstructive pulmonary disease: results from a 6-week, randomized, controlled phase IIIb StudyCOPD20131051152223819698
- FuhrRMagnussenHSaremKEfficacy of aclidinium bromide 400 µg twice daily compared with placebo and tiotropium in patients with moderate to severe COPDChest201214174575221903737
- ChapmanKRBeehKMBeierJA blinded evaluation of the efficacy and safety of glycopyrronium, a once-daily long-acting muscarinic antagonist, versus tiotropium, in patients with COPD: the GLOW5 studyBMC Pulm Med201414424438744
- TashkinDCelliBKestenSLystigTDecramerMEffect of tiotropium in men and women with COPD: results of the 4-year UPLIFT (R) trialRespir Med20101041495150420418083
- JonesPWRennardSIAgustiAEfficacy and safety of once-daily aclidinium in chronic obstructive pulmonary diseaseRespir Res2011125521518460
- DonohueJFNiewoehnerDBrooksJO’DellDChurchASafety and tolerability of once-daily umeclidinium/vilanterol 125/25 mcg and umeclidinium 125 mcg in patients with chronic obstructive pulmonary disease: results from a 52-week, randomized, double-blind, placebo-controlled studyRespir Res2014157825015176
- Spiriva Assessment of FEV1 (SAFE)The effect of inhaled tiotropium bromide (18 mcg once daily) on the change in FEV1 during long-term treatment in patients with COPD. A one-year parallel group, double-blind, randomised, placebo-controlled studyBoehringer Ingelheim Clinical Trial Register. NLM: NCT02772642005 Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clini-caltrial/com_EN/results/205/205.259_U05-3345.pdfAccessed October 19, 2015
- Spiriva® Assessment of FEV1 (SAFE)Boehringer Ingelheim2013 NLM identifier: NCT0277264. Available from: http://trials.boeh-ringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/205/205.259_U05-3345.pdfAccessed October 19, 2015
- Spiriva (Tiotropium Bromide) Assessment of FEV1 – (SAFE-Portugal)Boehringer Ingelheim2013 NLM identifier: NCT0239408. Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/205/205.282_U06-2124.pdfAccessed: October 19, 2015
- Evaluation of the long-term effects of Spiriva on lung function in COPD patientsBoehringer Ingelheim2005 NLM identifier: NCT0144339. Available from: https://clinicaltrials.gov/ct2/show/results/NCT0144339Accessed October 19, 2015
- CasaburiRBriggsDDDonohueJFSerbyCWMenjogeSSWitekTJThe spirometric efficacy of once-daily dosing with tiotropium in stable COPD – a 13-week multicenter trialChest20001181294130211083677
- MoitaJBarbaraCCardosoJTiotropium improves FEV1 in patients with COPD irrespective of smoking statusPulm Pharmacol Ther20082114615117693107
- VogelmeierCKardosPHarariSGansSJMStengleinSThirlwellJFormoterol mono- and combination therapy with tiotropium in patients with COPD: a 6-month studyRespir Med20081021511152018804362
- KerwinEMD’UrzoADGelbAFLakkisHGarciaGECaractaCFEfficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I)COPD201299010122320148
- DonohueJFMaleki-YazdiMRKilbrideSMehtaRKalbergCChurchAEfficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPDRespir Med20131071538154623830094
- WedzichaJADecramerMFickerJHAnalysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group studyLancet Respir Med2013119920924429126
- JonesPWSinghDBatemanEDEfficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN studyEur Respir J20124083083622441743

