Abstract
Current guidelines for the management of chronic obstructive pulmonary disease (COPD) recommend limiting the use of inhaled corticosteroids (ICS) to patients with more severe disease and/or increased exacerbation risk. However, there are discrepancies between guidelines and real-life practice, as ICS are being overprescribed. In light of the increasing concerns about the clinical benefit and long-term risks associated with ICS use, therapy needs to be carefully weighed on a case-by-case basis, including in patients already on ICS. Several studies sought out to determine the effects of withdrawing ICS in patients with COPD. Early studies have deterred clinicians from reducing ICS in patients with COPD as they reported that an abrupt withdrawal of ICS precipitates exacerbations, and results in a deterioration in lung function and symptoms. However, these studies were fraught with numerous methodological limitations. Recently, two randomized controlled trials and a real-life prospective study revealed that ICS can be safely withdrawn in certain patients. Of these, the WISDOM (Withdrawal of Inhaled Steroids During Optimized Bronchodilator Management) trial was the largest and first to examine stepwise withdrawal of ICS in patients with COPD receiving maintenance therapy of long-acting bronchodilators (ie, tiotropium and salmeterol). Even with therapy being in line with the current guidelines, the findings of the WISDOM trial indicate that not all patients benefit from including ICS in their treatment regimen. Indeed, only certain COPD phenotypes seem to benefit from ICS therapy, and validated markers that predict ICS response are urgently warranted in clinical practice. Furthermore, we are now better equipped with a larger armamentarium of novel and more effective long-acting β2-agonist/long-acting muscarinic antagonist combinations that can be considered by clinicians to optimize bronchodilation and allow for safer ICS withdrawal. In addition to providing a review of the aforementioned, this perspective article proposes an algorithm for the stepwise withdrawal of ICS in real-life clinical practice.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
While inhaled corticosteroid (ICS)-containing regimens benefit certain patients with chronic obstructive pulmonary disease (COPD), concerns about the inappropriate overuse of ICS in real-life practice, their clinical benefit and long-term risks, and considerable waste of health care resources that could be better used on other more appropriate management strategies prompted studies to evaluate whether certain patients already on ICS therapy fare better without it.Citation1 This perspective paper explores the evidence available on ICS withdrawal in patients with COPD and the potential phenotypes that can be withdrawn from ICS therapy. Furthermore, an algorithm for clinical practice is proposed to address the following critical questions: 1) In which patients is ICS withdrawal safe? and 2) How to withdraw ICS in appropriate patients?
Current guideline recommendations and real-life utilization of ICS in COPD
Although ICS have been long used in the management of COPD, evidence supporting their use has been considered equivocal and their positioning in guidelines as controversial. According to the latest 2015 update of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) report, ICS in combination with a long-acting β2-agonist (LABA) and/or long-acting muscarinic antagonist (LAMA) is the first recommended choice for patients with a high-risk of exacerbations (Groups C and D; ).Citation2 In addition to this international “gold standard” guideline, a number of national and multinational guidelines exist; however, their recommendations pertaining to the use of ICS are inconsistent with those of the GOLD report, in part, due to the varying criteria for categorizing patients with COPD and different interpretations of the equivocal evidence supporting ICS use.Citation1,Citation3–Citation7 Despite this, there is consensus that ICS are indicated in combination with long-acting bronchodilators (LABDs) for patients with COPD at risk of exacerbations and/or in those with asthma–COPD overlap syndrome (ACOS), but never as monotherapy.
Figure 1 GOLD recommendations for the pharmacologic management of stable COPD according to the four GOLD groups of COPD, which are based on a combined assessment of symptoms and exacerbation risk.
Abbreviations: CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; mMRC, modified Medical Research Council Dyspnea Scale; PDE-4i, phosphodiesterase-4 inhibitor; prn, as needed; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic antagonist.
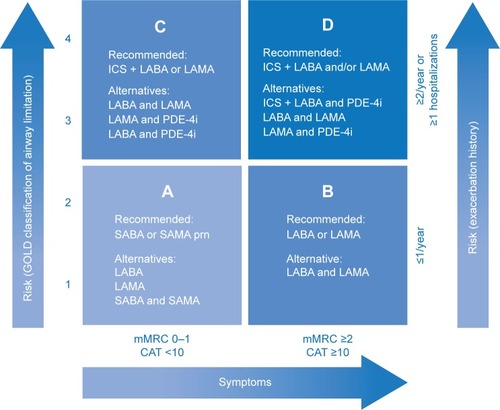
It is not surprising that there is a tremendous amount of confusion surrounding the use of ICS for the management of COPD in clinical practice. Several studies in multiple countries found discrepancies between guideline recommendations and real-life practice regarding ICS use in patients with COPD.Citation8–Citation10 In fact, ICS are being widely prescribed to the majority of patients with COPD, many of whom do not meet the recommendation criteria for ICS use (ie, 38.8% and 51.8% of GOLD Groups A and B, respectively).Citation10 Market research estimates suggest that ICS are used by >70% of patients with COPD, and given as initial therapy to >50% of newly diagnosed patients, mostly in combination with a LABA.Citation11 These high real-world utilization rates are also corroborated by the treatment profiles of patients entering recent randomized controlled trials (RCTs), where ~35% of patients classified into either GOLD Group A or B were receiving ICS at baseline.Citation12,Citation13
Evidence for the use of ICS in COPD: is there a benefit?
The introduction of ICS in the management of COPD was rather unorthodox and unsubstantiated for nearly 20 years (summarized in and previously reviewed in detail elsewhere).Citation1,Citation11,Citation14,Citation15 Briefly, in the 1980s, ICS were adopted in COPD on the basis of their effectiveness in asthma rather than scientific evidence.Citation11,Citation16 The earliest RCTs of ICS therapy in mild COPD were conducted in the late 1990s; however, their outcomes were negative.Citation11,Citation15,Citation17,Citation18 ISOLDE (Inhaled Steroids in Obstructive Lung Disease in Europe) was the first trial to demonstrate a beneficial effect of ICS on the occurrence of exacerbations in patients with moderate-to-severe COPD; albeit, no benefit on the rate of lung function decline was observed.Citation19 In 2001, a large population-based cohort study of 22,620 patients with COPD and a very recent hospitalization found that those who received ICS within 90 days postdischarge had 24% fewer rehospitalizations and a 29% risk reduction for mortality during a 1-year follow-up.Citation20 Although these findings are not definitive due to their observational nature, they suggest that patients who have had a hospitalization in the past year should continue receiving ICS. From 2002 onward, the subsequent cohort of RCTs began evaluating ICS in combination with LABDs.Citation11,Citation21,Citation22
Table 1 History of evidence for the use of ICS-containing treatment regimens in COPD
In the landmark TORCH (TOwards a Revolution in COPD Health) trial, it was found that ICS plus a LABA (ICS + LABA) resulted in significantly fewer exacerbations, slower rate of decline in lung function, and improved health status; however, there was only a trend toward improved survival.Citation22 These findings formed the initial basis for the inclusion of ICS-containing treatment regimens in clinical guidelines.Citation2,Citation11 At this time, the importance of exacerbations as a key determinant of health status and its association with a faster decline in lung function was being recognized, and the prevention of exacerbations became a key objective of COPD management.Citation16 Of note, a post hoc analysis of the TORCH data, which evaluated the independent contribution of ICS and LABA, found that the benefits of ICS + LABA were actually provided by the LABA.Citation1,Citation15,Citation23 Soon after TORCH, the INSPIRE (Investigating New Standards for Prophylaxis in Reduction of Exacerbations) trial was the first to show that ICS + LABA was no more effective than a LAMA in preventing exacerbations in patients with severe COPD, which initiated the discussion of whether adding ICS to LABDs is beneficial.Citation24 Indeed, a recent Cochrane meta-analysis questioned the superiority of ICS + LABA combinations over LABA alone in preventing exacerbations.Citation25 Lastly, the findings of the SUMMIT (Study to Understand Mortality and MorbidITy in COPD) trial were recently presented at the 2015 European Respiratory Society Congress.Citation26 The SUMMIT trial to evaluate the impact of a once-daily ICS + LABA (fluticasone furoate/vilanterol) versus the monocomponents on the survival of 16,485 patients with moderate COPD and either a history or increased risk of cardiovascular disease (CVD). After a 44-month follow-up, it was found that the risk of mortality on ICS + LABA was 12.2% lower than on placebo over the study period; however, this difference was not statistically significant (P=0.137). While the findings of the TORCH trial suggested that ICS + LABA may reduce mortality in patients with COPD, particularly those with CVD risk, no study to date has been able to demonstrate a benefit.Citation22,Citation26
What are the risks associated with ICS?
A number of local and systemic adverse events have been associated with ICS, particularly with long-term and high-dose use.Citation1,Citation15 The local adverse events include pneumonia,Citation27,Citation28 oropharyngeal candidiasis,Citation29 and tuberculosis,Citation30,Citation31 and the systemic adverse events include cataracts,Citation32,Citation33 glaucoma,Citation15 osteoporosis and bone fractures,Citation34 easy bruising,Citation18 type 2 diabetes,Citation35 and even cases of adrenal suppression.Citation36 While there have always been concerns about the risks of developing osteoporosis, cataracts, and diabetes in patients with COPD, risk of pneumonia is arguably the most notable and well-established adverse event associated with ICS use in COPD, as supported by consistent evidence from RCTs.Citation1,Citation14,Citation15,Citation24,Citation27,Citation37 Corroborating this, a recent Canadian population-based cohort study of 103,386 patients with COPD who were treated with ICS demonstrated that discontinuation of ICS was associated with a 37% reduction in the risk of serious pneumonia.Citation38 Indeed, in the latest update of the GOLD report, it is cautioned that long-term ICS-containing treatment should not be prescribed outside their indication due to risk of pneumonia and the possibility of a slight increased risk of fractures following long-term exposure.Citation2 As patients with COPD are more likely to be older and often have several comorbidities for which they receive multiple medications, they are more susceptible to ICS-associated adverse events, and their potential risks need to be weighed against the likely benefits on a case-by-case basis, even in those already on ICS.Citation1 Given the concerns about the risk–benefit profile of ICS, there has been a renewed interest in reevaluating the role of ICS in COPD and identifying which patients can be managed as well with alternate therapies.Citation37
Evidence regarding ICS withdrawal
Early observational studies and RCTs investigating the implications of ICS withdrawal in patients with COPD found that an abrupt cessation of ICS therapy precipitates exacerbations, and results in a deterioration in lung function, symptoms, and health status (summarized in ).Citation39–Citation44 However, a meta-analysis of three of these RCTs (ie, COPE, COSMIC [COPD and Seretide: a Multi-center Intervention and Characterization], and WISP), the only trials deemed to be acceptable in terms of quality and level of bias, determined that withdrawal of ICS was not associated with any statistically significant increase in the exacerbation rate, and that the effects on other outcomes, such as lung function and health status, were inconclusive.Citation45 The contradictory findings of these studies may be due to a number of methodological issues, including heterogeneity in patients’ characteristics, disease severity, outcome definitions (eg, exacerbations), concomitant treatments (eg, run-in period treatment, maintenance with LABDs vs placebo), and setting (ie, primary vs secondary care).Citation45,Citation46 Of note, since patient inclusion in all trials was based on spirometry while receiving ICS therapy, this does not rule out a diagnosis of concomitant asthma, even if spirometry was of no significant reversibility.Citation42–Citation44 Additionally, in COSMIC, the forced expiratory volume in 1 second (FEV1)/forced vital capacity ratios used do not match the diagnostic criteria for COPD (ie, <70%), suggesting that these patients may have had an asthma component, and thereby, cessation of ICS could have been detrimental.Citation2,Citation43
Table 2 Summary of studies evaluating the withdrawal of ICS in patients with COPD
According to the latest GOLD recommendations, patients with COPD and a low risk of exacerbations should not be prescribed ICS-containing regimens.Citation2 Given that a large proportion of patients are already initiated on such regimens, it would be helpful to know if there are consequences associated with ICS withdrawal in such populations (ie, GOLD Groups A and B). Recently, in the first real-life prospective study (OPTIMO [Real-Life study On the aPpropriaTeness of treatment in MOderate COPD patients]), it was demonstrated that withdrawal of ICS in patients with symptomatic, moderate COPD (ie, FEV1 >50% predicted) at a low risk of exacerbations (ie, <2/year) was not associated with any deterioration in lung function, symptoms, and exacerbation rate over a 6-month observation period.Citation47 These findings were further confirmed by the recent INSTEAD (Indacaterol: Switching Non-exacerbating Patients with Moderate COPD from Salmeterol/Fluticasone to Indacaterol) trial, the first RCT with a clearly defined patient population with moderate COPD (ie, FEV1 50%–80% predicted) and no prior exacerbation history, which found that switching from a fixed-dose combination of ICS + LABA to a LABA was not associated with any differences in lung function, symptoms, health status, and exacerbations.Citation48 In addition to supporting the current GOLD recommendations (ie, no need for ICS in Groups A and B), the results from both studies suggest that ICS therapy can be safely withdrawn from patients with moderate COPD and a low risk of exacerbations provided that they are left on maintenance treatment with LABDs.
In all studies to date, withdrawal of ICS has been abrupt. It is well known that an abrupt cessation of chronic corticosteroid therapy precipitates rebound systemic effects due to steroid withdrawal symptoms.Citation11,Citation46 Currently, there is no clear evidence on whether abrupt ICS withdrawal is safe; however, similar to the approach used to down-titrate ICS in patients with asthma, a stepwise withdrawal of ICS may minimize the potential risk of rebound steroid effects.Citation11,Citation46,Citation49
Adding to the growing clinical evidence-base on the impact of ICS withdrawal against a background of LABDs, the WISDOM (Withdrawal of Inhaled Steroids During Optimized Bronchodilator Management) trial, which is larger than all of the previous ICS withdrawal RCTs combined, was the first to assess the question of whether a stepwise withdrawal of ICS on top of maintenance therapy with dual bronchodilation (tiotropium/salmeterol) had a similar risk of exacerbations in patients with COPD and a history of exacerbations (ie, GOLD Groups C and D, in whom GOLD recommendations support the addition of ICS to LABD therapy).Citation2,Citation46,Citation50 In this, 12-month, randomized, double-blind, parallel-group, active-controlled trial, 2,485 patients with COPD and a history of exacerbations received triple therapy (ie, fluticasone propionate/tiotropium/salmeterol) during a 6-week run-in period. Afterward, patients were randomized to either continue or withdraw ICS therapy. In the ICS withdrawal group, the dose of ICS was gradually reduced by approximately half in a stepwise fashion every 6 weeks, such that after 12 weeks, ICS was completely withdrawn. It was found that there was no difference in the occurrence of moderate or severe exacerbations between the two groups.Citation50 Additionally, subgroup analyses revealed no notable differences in the occurrence of exacerbations based on age, sex, smoking status, body mass index, ICS or β-blocker therapy at screening, chronic bronchitis, GOLD stage and group, and prior therapy with antibiotics or systemic glucocorticoids. While there was a small, but significant, reduction in lung function when ICS was completely withdrawn, the clinical importance of this is unclear. Taken together, these findings suggest that even patients with severe COPD and a high-risk of exacerbations can be safely withdrawn from ICS therapy as long as they are clinically stable and maintained on a background of LABDs. Even with ICS use being in line with the GOLD recommendations, the WISDOM results also indicate that not all patients with severe-to-very-severe COPD seem to benefit from including ICS in their treatment regimen.
With the intent of identifying potential markers of ICS responsiveness, a substudy on ~500 patients was also performed in the WISDOM trial.Citation46 These patients were evaluated for emphysema, bronchiectasis, diffusing capacity for carbon monoxide, and the following biomarkers: adiponectin, leptin, C-reactive protein, interleukin-6, interleukin-8, tumor necrosis factor-α, fibrinogen, soluble interleukin adhesion molecule-1, serum amyloid A, procalcitonin, and B-type natriuretic peptide. Airway inflammation through differential cell count in sputum and fraction of exhaled nitric oxide (FeNO) was also assessed. Unfortunately, no subgroups or biomarkers were found to be associated with an increased likelihood of exacerbations after ICS withdrawal;Citation46,Citation51 albeit, certain phenotypes and potential biomarkers that have been previously shown to be associated with ICS responsiveness were not evaluated (discussed in the next section).Citation16
COPD phenotypes: which patients benefit from ICS?
Because COPD is a complex and heterogeneous disease with several different pathophysiological mechanisms, it is likely that ICS may have an effect on some components of the disease, particularly when airway inflammation is present.Citation11,Citation15 Increasing evidence suggests that patients with certain COPD phenotypes appear to benefit from ICS treatment, including patients with ACOS, frequent exacerbators, and those with eosinophilia.Citation1,Citation6,Citation15,Citation52
ACOS was only formally recognized by the Global Initiative for Asthma (GINA) and GOLD for the first time in 2014. It has been estimated that ACOS occurs in up to 25% of patients with COPD; however, establishing its prevalence has been difficult because there is no universally accepted definition for this syndrome.Citation1,Citation52–Citation56 According to the GINA/GOLD Consensus Statement, ACOS is characterized by persistent airflow limitation and identified by the features that it shares with both asthma and COPD.Citation56 In addition to having a history or clinical features of asthma, the presence of a large bronchodilator response (ie, >12% and 400 mL) and eosinophilic inflammation may also point to ACOS in patients with persistent airway obstruction.Citation15,Citation56 Due to the presence of an asthma component, patients with ACOS are likely to benefit from ICS therapy. Recently, a large observational study reported a survival benefit in patients with COPD starting on ICS + LABA compared with LABA alone; however, this survival benefit was only observed in those who had a codiagnosis of asthma.Citation57
Frequent exacerbators account for approximately one-third of patients with COPD.Citation52,Citation58 The definition of an exacerbator is based on expert opinion rather than rigorous phenotypic assessment, with a history of exacerbations being the best predictor of exacerbations across all GOLD stages.Citation2 In the current GOLD report, exacerbation risk is based on either GOLD classification of airway limitation or history of exacerbations (ie, high exacerbation risk is defined as ≥2 exacerbations per year or ≥1 hospitalization); however, not all patients with moderate-to-severe COPD (ie, GOLD Groups C and D) are necessarily exacerbators, and therefore, require ICS.Citation2,Citation59 Indeed, in a 26-week, randomized, double-blind, parallel-group trial of patients with moderate-to-severe COPD and no history of exacerbations, it was found that dual bronchodilation (ie, LABA + LAMA) provided significantly better and clinically relevant improvements in lung function versus ICS + LABA.Citation12 The findings from the ENERGITO trial, which were recently reported at the 2015 European Respiratory Society Congress, further corroborate this point.Citation60 In this randomized, double-blind, double-dummy, four-period crossover trial, lung function was evaluated in patients with moderate-to-severe COPD after treatment with LABA + LAMA (ie, tiotropium/olodaterol) versus ICS + LABA (ie, fluticasone propionate/salmeterol). After 6 weeks of treatment, it was found that LABA + LAMA significantly improved lung function compared with ICS + LABA. Thus, subclassification within GOLD Groups C and D may be warranted to take into account nonexacerbators. To complicate matters further, while both history of exacerbations and COPD severity are risk factors for repeat exacerbations, there are a number of other risk factors associated with repeat exacerbations that should be considered, including eosinophilic inflammation, comorbidities and extrapulmonary manifestations (eg, CVD, anxiety, depression, myopathy, reflux disease), chronic bronchitis, and increasing age.Citation6
Lastly, it has been suggested that up to 30% of patients with COPD have eosinophilia.Citation16,Citation61 Eosinophilia has been suggested to be predictive of exacerbations in patients with mild-to-moderate COPD following withdrawal of ICS,Citation62 and both sputum and blood eosinophil levels have been shown to be promising biomarkers of ICS responsiveness in patients with COPD.Citation63,Citation64 In one RCT, a management strategy for patients with COPD that suppressed sputum eosinophil levels ≤3% was found to reduce severe exacerbations.Citation64 Although it has been suggested that sputum eosinophil levels may be the most reliable predictor of ICS responsiveness in patients with COPD to date, obtaining sputum samples is technically demanding and not feasible in routine clinical practice.Citation15 Since there is a reasonable correlation between sputum and blood levels, blood eosinophil levels may offer a more practical alternative.Citation63 Consequently, blood eosinophil levels are currently a topical issue and defining the appropriate cutoff for determining ICS responsiveness in patients with COPD is a subject of intense discussion. In a recent post hoc analysis of two replicate, 12-month, double-blind RCTs comparing a LABA with ICS + LABA in a total of 3,177 patients with moderate-to-severe COPD and a history of ≥1 exacerbation, it was found that across all doses of ICS, ICS + LABA significantly reduced exacerbations by 29% versus LABA alone in patients with blood eosinophil levels of ≥2%.Citation63 Conversely, in another post hoc analysis of two replicate, 26-week, double-blind, double-dummy, parallel-group RCTs, an absolute blood eosinophil cutoff of >300 cells/mm3 at baseline as opposed to a percent cutoff appeared to best differentiate patients with moderate-to-severe COPD who benefited from ICS + LABA versus LABA + LAMA therapy in terms of reduction in exacerbation risk.Citation65 This observation was corroborated by another post hoc analysis of the randomized, double-blind, parallel-group FORWARD (FOsteR 48-week trial to reduce exAceRbations in COPD) trial, which concluded that a greater reduction in exacerbations was observed when ICS was added to a LABA in patients with severe COPD and a history of exacerbations who had an eosinophil count ≥279.8 cells/mm3.Citation66 Using an absolute as opposed to a percent cutoff was further supported by the findings of the Copenhagen General Population Study, which evaluated 7,225 patients with COPD over a median follow-up of 3.3 years and found that an absolute blood eosinophil count of ≥340 cells/mm3 was a better predictor of both moderate and severe exacerbation risk than a percent cutoff of 2%.Citation67 Accordingly, ≥300 cells/mm3 can be tentatively used as a cutoff until this value is further validated in prospective trials. Meanwhile, it will suffice to recognize that blood eosinophil counts can be easily measured in clinical practice as they are more accessible than sputum eosinophil levels.
In addition to eosinophil levels, it has been suggested that FeNO levels can be used as a surrogate marker of eosinophilic inflammation in patients with COPD exacerbations.Citation68,Citation69 A recent review on ACOS suggested the following FeNO levels for comparing between patients with asthma, ACOS, and COPD: >50, 25–50, and <25 ppb, respectively.Citation55 These cutoffs are in line with the 2011 American Thoracic Society FeNO guidelines, which suggest that FeNO <25 ppb provides a strong indication for an unlikely ICS response and FeNO >50 ppb provides a strong indication for a likely ICS response, but FeNO between 25 and 50 ppb should be interpreted with caution.Citation70,Citation71
Safely withdrawing ICS from patients with COPD: a proposed algorithm for clinical practice
Since there is an overall paucity of evidence about when and how ICS can be safely withdrawn, the criteria for continuing or withdrawing ICS in patients with COPD are uncertain and more studies are warranted to characterize patients in whom withdrawal is safe. In a recent Spanish consensus document on the appropriate use of ICS in COPD, a panel of 25 experts voted on statements developed by a coordinator group that systematically reviewed scientific evidence on the efficacy and safety of ICS, and criteria for ICS withdrawal.Citation52 Consensus was reached on the following statements concerning ICS withdrawal: withdrawal of ICS in COPD is feasible, patients who discontinue ICS should be evaluated in the short-term, and ICS withdrawal should be tapered. ICS withdrawal was thought to be possible if there is no evidence of ACOS, no exacerbations in the previous 2 years, and, with a lower level of agreement, in the absence of a positive bronchodilator test and decline after switching from high to low ICS doses. However, it should be kept in mind that these criteria are based on expert opinion and need to be validated in RCTs. Of note, there were also a significant number of statements for which it was difficult to reach a consensus, thereby identifying several areas of uncertainty.
Currently, no national or international clinical guidelines advocate withdrawal of ICS nor do they provide recommendations regarding the safe withdrawal of ICS in patients with COPD. For reasons previously described, there is an urgent need for a step-by-step algorithm that can be applied in real-life clinical practice. proposes such an algorithm and attempts to address the following questions: 1) In which patients is ICS withdrawal safe? and 2) How to withdraw ICS in appropriate patients? This algorithm takes into account not only exacerbation risk, as per GOLD, but also the emerging, neglected ACOS phenotype, as per the GINA/GOLD Consensus Statement. Potential markers of eosinophilia are also considered and noted as optional, as these are still theoretical and need to be tested in RCTs. Furthermore, the stepwise ICS withdrawal protocol on top of maintenance therapy with dual bronchodilation is primarily based on the WISDOM trial, although, instead of down-titrating ICS every 6 weeks, it is proposed that physicians consider stepping down ICS dose every 6–12 weeks. The rationale for this is to ensure that the effects of ICS in the inflammation cascade of COPD have been optimized on a physiological level,Citation72 and from a practical perspective, permit enough time to monitor for potential exacerbations. In regards to optimizing bronchodilation following ICS withdrawal, we are now more equipped than ever before with a larger armamentarium of novel LABA + LAMA combinations that clinicians can consider for more effective bronchodilation maintenance ().
Table 3 LABA + LAMA combinations currently available or in development
Figure 2 A proposed step-by-step algorithm for safely withdrawing ICS from patients with COPD in real-life clinical practice.
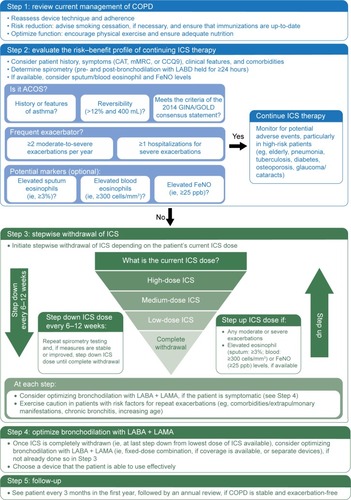
Lastly, it is fully acknowledged that the proposed algorithm will need to be validated, particularly in the real-life setting. Additionally, there are a number of potential barriers to using the proposed algorithm that need to be considered. Health care resources, such as physician time, access to spirometry, and measurement of eosinophil and FeNO levels, may be limited or unavailable; certain countries may not have multiple doses of ICS approved to allow for a stepwise reduction; and patients and physicians may be reluctant to change therapy if it is sufficient in terms of current disease control as opposed to avoiding future risks, such as side effects. Patients may also be resistant to changing therapy, as it will require more effort on their part and there is a potential risk of symptom worsening, especially if they end up having a phenotype that should be receiving ICS therapy.
Conclusion
It is now becoming evident that maintaining or initiating certain patients with COPD on ICS therapy solely on the basis of reducing their risk of exacerbations may not be necessary, particularly if they are maintained on effective dual bronchodilation with a LABA and LAMA. Clinicians need to carefully tailor therapy on a case-by-case basis, and determine who is an appropriate patient for ICS therapy, and if a patient is already on ICS therapy, how to carefully step down ICS therapy without doing harm. Currently, no clinical guidelines provide any recommendations regarding the safe withdrawal of ICS in patients with COPD. Accordingly, until this need is met, this perspective article proposes an algorithm for the stepwise withdrawal of ICS in real-life clinical practice based on the evidence available to date.
Acknowledgments
Under Dr Kaplan’s direction, medical writing assistance was provided by Marina Komolova, PhD, of Sage Medica Inc.; this assistance was supported by Boehringer Ingelheim (Canada) Ltd., which had no role in the preparation of this manuscript.
Disclosure
AGK has received travel grants from AstraZeneca, Boehringer Ingelheim, and Novartis. He has been on the speaker’s bureau for AstraZeneca, Boehringer Ingelheim, Griffols, Merck Frosst, Pfizer, Purdue, Novartis, Sanofi, and Takeda. He has been on advisory boards for AstraZeneca, Boehringer Ingelheim, Aerocrine, GSK, Pfizer, Purdue, Meda, and Novartis. The author (AGK) declares no conflicts of interest in this work, and has received no funding for preparing this manuscript.
References
- PriceDYawnBBrusselleGRossiARisk-to-benefit ratio of inhaled corticosteroids in patients with COPDPrim Care Respir J20132219210023135217
- Global Initiative for Chronic Obstructive Lung Disease (GOLD)Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease; updated 2015 Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015.pdfAccessed April 28, 2015
- O’DonnellDEHernandezPKaplanACanadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2008 update – highlights for primary careCan Respir J200815Suppl A1A8A
- National Institute for Health and Care Excellence (NICE)Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care; 2010 Available from: https://www.nice.org.uk/guidance/cg101/resources/cg101-chronic-obstructive-pulmonary-disease-update-full-guideline2Accessed April 29, 2015
- QaseemAWiltTJWeinbergerSEDiagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory SocietyAnn Intern Med2011155317919121810710
- MiravitllesMCalleMSoler-CataluñaJJClinical phenotypes of COPD: identification, definition and implications for guidelinesArch Bronconeumol2012483869822196477
- KankaanrantaHHarjuTKilpelainenMDiagnosis and pharmacotherapy of stable chronic obstructive pulmonary disease: the Finnish guidelinesBasic Clin Pharmacol Toxicol2015116429130725515181
- PriceDWestDBrusselleGManagement of COPD in the UK primary-care setting: an analysis of real-life prescribing patternsInt J Chron Obstruct Pulmon Dis2014988990425210450
- KoblizekVPecenLZatloukalJReal-life GOLD 2011 implementation: the management of COPD lacks correct classification and adequate treatmentPLoS One2014911e11107825380287
- VestboJVogelmeierCSmallMHigginsVUnderstanding the GOLD 2011 strategy as applied to a real-world COPD populationRespir Med2014108572973624675239
- SuissaSBarnesPJInhaled corticosteroids in COPD: the case againstEur Respir J2009341131619567599
- VogelmeierCFBatemanEDPallanteJEfficacy and safety of once-daily QVA149 compared with twice-daily salmeterol–fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group studyLancet Respir Med201311516024321804
- YawnBKleerupEZhangJKianifardFWilliamsJInhaled corticosteroid use and GOLD severity stage among patients with chronic obstructive pulmonary disease in different regions [abstract]Am J Respir Crit Care Med2012182A2944
- BabuKSKastelikJAMorjariaJBInhaled corticosteroids in chronic obstructive pulmonary disease: a pro-con perspectiveBr J Clin Pharmacol201478228230025099256
- ErnstPSaadNSuissaSInhaled corticosteroids in COPD: the clinical evidenceEur Respir J201545252553725537556
- HalpinDMGQuintJKThe WISDOM of inhaled corticosteroids in COPDThorax201469121071107225323619
- VestboJSorensenTLangePBrixATorrePViskumKLong-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trialLancet199935391671819182310359405
- PauwelsRALofdahlCGLaitinenLALong-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society study on chronic obstructive pulmonary diseaseN Engl J Med1999340251948195310379018
- BurgePSCalverleyPMJonesPWSpencerSAndersonJAMaslenTKRandomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trialBMJ200032072451297130310807619
- SinDDTuJVInhaled corticosteroids and the risk of mortality and readmission in elderly patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2001164458058411520719
- SzafranskiWCukierARamirezAEfficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary diseaseEur Respir J2003211748112570112
- CalverleyPMAndersonJACelliBSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med2007356877578917314337
- SuissaSErnstPVandemheenKLAaronSDMethodological issues in therapeutic trials of COPDEur Respir J200831592793318216056
- WedzichaJACalverleyPMASeemungalTAHaganGAnsariZStockleyRAThe prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromideAm J Respir Crit Care Med20081771192617916806
- NanniniLJLassersonTJPoolePCombined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20129CD00682922972099
- VestboJAndersonJBrookRDStudy to understand mortality and morbidity in COPD (SUMMIT) [abstract]Eur Respir J201546Suppl 59OA3476
- CrimCCalverleyPMAAndersonJAPneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study resultsEur Respir J200934364164719443528
- SuissaSPatenaudeVLapiFErnstPInhaled corticosteroids in COPD and the risk of serious pneumoniaThorax201368111029103624130228
- YangIAClarkeMSSimEHFongKMInhaled corticosteroids for stable chronic obstructive pulmonary diseaseCochrane Database Syst Rev20127CD00299122786484
- KimJHParkJSKimKHJeongHCKimEKLeeJHInhaled corticosteroid is associated with an increased risk of TB in patients with COPDChest201314341018102423079688
- BrassardPSuissaSKezouhAErnstPInhaled corticosteroids and risk of tuberculosis in patients with respiratory diseasesAm J Respir Crit Care Med2011183567567820889902
- CummingRGMitchellPLeederSRUse of inhaled corticosteroids and the risk of cataractsN Engl J Med199733718149203425
- ErnstPLow-dose inhaled and nasal corticosteroid use and the risk of cataractsEur Respir J20062761168117416481387
- LokeYKCavallazziRSinghSRisk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studiesThorax201166869970821602540
- SuissaSKezouhAErnstPInhaled corticosteroids and the risks of diabetes onset and progressionAm J Med2010123111001100620870201
- HayCMSprattDIAdrenal insufficiency in a woman secondary to standard-dose inhaled fluticasone propionate therapyEndocrinol Diabetes Metab Case Rep2014201413008024683484
- CalverleyPMWhat to use INSTEAD of inhaled corticosteroids in COPD?Eur Respir J20144461391139325435522
- SuissaSCoulombeJErnstPDiscontinuation of inhaled corticosteroids in COPD and the risk reduction of pneumoniaChest Epub2015625
- JaradNAWedzichaJABurgePSCalverleyPMAn observational study of inhaled corticosteroid withdrawal in stable chronic obstructive pulmonary disease. ISOLDE Study GroupRespir Med199993316116610464871
- O’BrienARusso-MagnoPKarkiAEffects of withdrawal of inhaled steroids in men with severe irreversible airflow obstructionAm J Respir Crit Care Med2001164336537111500334
- SchermerTRHendriksAJChavannesNHProbability and determinants of relapse after discontinuation of inhaled corticosteroids in patients with COPD treated in general practicePrim Care Respir J2004131485516701637
- van der ValkPMonninkhofEvan der PalenJZielhuisGvan HerwaardenCEffect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE studyAm J Respir Crit Care Med2002166101358136312406823
- WoutersEFMWithdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trialThorax200560648048715923248
- ChoudhuryABDawsonCMKilvingtonHEWithdrawal of inhaled corticosteroids in people with COPD in primary care: a randomised controlled trialRespir Res200789318162137
- NadeemNJTaylorSJCEldridgeSMWithdrawal of inhaled corticosteroids in individuals with COPD – a systematic review and comment on trial methodologyRespir Res20111210721838890
- MagnussenHWatzHKirstenAStepwise withdrawal of inhaled corticosteroids in COPD patients receiving dual bronchodilation: WISDOM study design and rationaleRespir Med2014108459359924477080
- RossiAGuerrieroMCorradoAOPTIMO/AIPO Study GroupWithdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO)Respir Res2014157725005873
- RossiAvan der MolenTOlmoRdINSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPDEur Respir J20144461548155625359348
- LeuppiJDSalomeCMJenkinsCRPredictive markers of asthma exacerbation during stepwise dose reduction of inhaled corticosteroidsAm J Respir Crit Care Med2001163240641211179114
- MagnussenHDisseBRodriguez-RoisinRWithdrawal of inhaled glucocorticoids and exacerbations of COPDN Engl J Med2014371141285129425196117
- MagnussenHTetzlaffKCalverleyPMInhaled glucocorticoids and COPD exacerbationsN Engl J Med20153721939425551535
- Alcazar NavarreteBCasanovaCMiravitllesM“Correct use of inhaled corticosteroids in chronic obstructive pulmonary disease”: a consensus documentArch Bronconeumol201551419319825540900
- BramanSSThe chronic obstructive pulmonary disease-asthma overlap syndromeAllergy Asthma Proc2015361111825562551
- BujarskiSParulekarADSharafkhanehAHananiaNAThe Asthma COPD Overlap Syndrome (ACOS)Curr Allergy Asthma Rep201515319
- LouieSZekiAASchivoMThe asthma-chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerationsExpert Rev Clin Pharmacol20136219721923473596
- Global Initiative for Asthma (GINA)Global Initiative for Chronic Obstructive Lung Disease (GOLD)Diagnosis of Diseases of Chronic Airflow Limitation: Asthma, COPD and Asthma-COPD Overlap Syndrome (ACOS); 2015 Available from: http://www.goldcopd.org/uploads/users/files/AsthmaCOPDOverlap.pdfAccessed May 4, 2015
- GershonASCampitelliMACroxfordRCombination long-acting β-agonists and inhaled corticosteroids compared with long-acting β-agonists alone in older adults with chronic obstructive pulmonary diseaseJAMA2014312111114112125226477
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- AgustiAFabbriLInhaled steroids in COPD: when should they be used?Lancet Respir Med201421186987125439566
- BeehKMDeromEEchave-SustaetaJENERGITO: efficacy and safety of once-daily combined tiotropium + olodaterol versus twice-daily combined fluticasone propionate + salmeterol [abstract]Eur Respir J201546Suppl 59PA4366
- BafadhelMMcKennaSTerrySAcute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkersAm J Respir Crit Care Med2011184666267121680942
- LieskerJJBathoornEPostmaDSVonkJMTimensWKerstjensHASputum inflammation predicts exacerbations after cessation of inhaled corticosteroids in COPDRespir Med2011105121853186021802933
- PascoeSLocantoreNDransfieldMTBarnesNCPavordIDBlood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trialsLancet Respir Med20153643544225878028
- SivaRGreenRHBrightlingCEEosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trialEur Respir J200729590691317301099
- WedzichaJAPriceDMezziKFogelRBanerjiDQVA149 compared with salmeterol/fluticasone (SFC) on exacerbations and its correlation with baseline blood eosinophils: a pooled analysis of LANTERN and ILLUMINATE [abstract]Eur Respir J201546Suppl 59PA1005
- SiddiquiSHGuasconiAVestboJBlood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2015192452352526051430
- Vedel-KroghSNielsenSFLangePVestboJNordestgardBGBlood eosinophils and exacerbations in chronic obstructive pulmonary disease: the Copenhagen General Population Study [abstract]Eur Respir J201546Suppl 59OA2934
- AntusBBartaIHorvathICsiszerERelationship between exhaled nitric oxide and treatment response in COPD patients with exacerbationsRespirology201015347247720210889
- SoterSBartaIAntusBPredicting sputum eosinophilia in exacerbations of COPD using exhaled nitric oxideInflammation20133651178118523681903
- BjermerLAlvingKDiamantZCurrent evidence and future research needs for FeNO measurement in respiratory diseasesRespir Med2014108683084124636813
- DweikRABoggsPBErzurumSCAn official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applicationsAm J Respir Crit Care Med2011184560261521885636
- BarnesPJInhaled corticosteroidsPharmaceuticals201033514540
- Lung Health Study Research GroupEffect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary diseaseN Engl J Med2000343261902190911136260
- AlsaeediASinDDMcAlisterFAThe effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review of randomized placebo-controlled trialsAm J Med20021131596512106623
- AaronSDVandemheenKLFergussonDTiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trialAnn Intern Med2007146854555517310045
- WelteTMiravitllesMHernandezPEfficacy and toler-ability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2009180874175019644045

