Abstract
The Food and Drug Administration Amendments Act of 2007 mandated that sponsors of applicable studies must provide results within one year of study completion. We aimed to analyze the factors associated with reporting of results from interventional studies registered on ClinicalTrials.gov. On May 20, 2010, we retrieved 20 available fields from 57,233 closed studies on the website and identified 31,161 interventional studies that were required to post results. We compared the proportion of studies with results versus studies without results by age, gender, and disease status of participants, by interventions, sponsors, phase of clinical trials, and completion dates. The results of studies were reported for 4.7% of applicable studies, 8% of industry-sponsored studies, 7.5% of Phase II and 6.5% of Phase IV clinical trials, 4.9% of drug studies, and 0% of genetic studies. Withdrawn (n = 486) and suspended (n = 414) interventions did not provide results. The percentage of studies with results varied from 0% to 21% among different sponsors. The first studies with results were completed in 1992. The proportion of studies with results increased over time. Completion dates were not available for 7446 studies. The database does not have fields available to facilitate routine analysis of the rate of compliance with federal law for posting results. The analysis of accuracy of the protocols in relation to the results and publications is not possible without time-consuming evaluation of individual postings and individual publications.
Introduction
Transparency in designing, conducting, and reporting of human experiments and observational studies is essential to guarantee the integrity of clinical research.Citation1 Registration of clinical trials with information about study sponsors and protocols makes clinical research more transparent. In 2000, the National Institutes of Health (NIH) requested that clinical trials assessing pharmacologic treatments for serious or life-threatening diseases be registered in the ClinicalTrials.gov online database. This website was developed by the National Library of Medicine as an information service for the NIH and the US Department of Health and Human Services.Citation2 However, at that time, NIH policy did not require mandatory registration of all human studies. In 2005, the World Association of Medical Editors started to require mandatory registration of all clinical studies as a condition of publication.Citation3 Sponsors must now provide the World Health Organization (WHO) with a minimum dataset of information, including details of study design, recruitment activities, ethics review of research, target sample size, conditions of eligibility for subjects to participate in the study, and primary and secondary outcomes.Citation4 Stakeholders can find detailed information about study protocols on ClinicalTrials.gov, but study results are not yet consistently available online.
Selective publication of positive results and outcomesCitation5–Citation7 led to further scrutiny and called for public disclosure of study results. The Food and Drug Administration Amendments Act (FDAAA) of 2007 mandated that sponsors of applicable studies must provide study flow, baseline subject characteristics, and outcomes after active and control interventions within one year of study completion.Citation8 The FDAAA regulations defined the following clinical trials as needing to adhere to the requirements: Phase II–IV interventional studies, studies involving any drugs, biological products, or medical devices regulated by the FDA, studies having at least one site in the US or which are conducted under an investigational new drug application or investigational device exemption; and studies initiated or ongoing as of September 27, 2007, or later.Citation9–Citation12 Results can be posted within three years of completion of the study for trials investigating off-label use of previously approved drugs.
Registration of clinical trials on ClinicalTrials.gov improved the transparency of clinical research tremendously. Citation13–Citation15 Stakeholders can find the WHO minimum dataset for the design of 92,385 studies.Citation16 Harvard University has recognized the achievement of this website with their Innovations in American Government Award.Citation17 The degree of sponsor compliance with federal law in providing results of studies on ClinicalTrials.gov has not been examined as yet. We aimed to examine the completeness of the posted study designs and factors associated with reporting study results.
Methods
We retrieved all closed studies from ClinicalTrials.gov as of May 20, 2010. We retrieved all 20 available fields, including the ClinicalTrials.gov identifier, age group, gender, disease status of the eligible subjects, and examined interventions, recruitment status, study sponsors, study type and design, phase of clinical trials, start and completion dates, and posting of study results. Field locations were as described online at http://prsinfo.clinicaltrials.gov/definitions.html. We used the exact data provided by the sponsors. We further categorized interventions as behavioral, biological, device, dietary supplements, disease management, drug, education, genetic, procedural, and exercise. We also redefined conditions into larger diagnostic categories with the first disease stated. For example, when the condition was defined as “arthralgia, pain assessment”, we analyzed it under the category of “arthralgia”. The available data do not have a single field to define the studies that are applicable to the US Public Law 110-85 (FDAAA), Title VIII, Section 801 for mandatory reporting of results. Therefore, we defined closed not-recruiting interventional trials, excluding Phase 0–I trials, as applicable to comply with FDAAA regulations.
We calculated descriptive frequency statistics without formal hypothesis testing because we did not sample the data but rather analyzed all closed studies available. We then compared the proportions of studies having results with the proportions of studies without results in categories of age, gender, disease status, interventions, study sponsorship, types, and completion dates. All calculations were performed with frequency procedure using SAS 9.1 software (SAS Institute Inc., Cary, NC).
Results
We retrieved 57,299 records but eliminated 66 records with misplaced fields, leaving a total of 57,233 records for analysis. We analyzed the completeness of the minimum dataset and found that 393 studies did not provide the gender for eligible subjects, 287 studies did not specify the type of study, and 5908 did not specify intervention or exposure (). We noticed a marked inconsistency in the classification and reporting of patient conditions, which made statistical analysis difficult. For example, “non small cell lung cancer” was reported variously as “non-small cell lung cancer”, “non small cell lung carcinoma”, or “NSCLC”. More than 50% of the studies included adult subjects, and 85% of all studies recruited both genders. Children and seniors were included in a very small proportion of studies. More than 60% of closed studies were completed, and more than 65% of closed studies examined the effects of pharmacologic treatments. A total of 39% of all studies were sponsored by industry. Most of the studies (97%) did not have their results posted on the website.
Table 1 Distribution of the studies closed in www.clinicaltrials.gov on 20 May 2010
Of 31,161 applicable closed interventional studies, 4.5% had results available. Proportions of studies with results contained similar age and gender groups (). Studies with children as subjects tended to report results more frequently. Industry-sponsored studies reported results more often (8.1%) than nonindustry-funded studies (). Phase III and IV clinical trials reported results more often (total 14%) compared with Phase II trials (3%, ). Suspended interventions (n = 414) and withdrawn interventions (n = 486) did not provide results or reasons for the cause of suspension or withdrawal (). The first studies containing results (n = 3) were sponsored by Merck and added to the database in 2009. These three studies are listed as NCT00882440, NCT00886600, and NCT00887250, and were completed in 1992. Among the applicable closed interventional studies, 7446 did not provide a completion date. The proportion of the studies with results increased over time (see ).
Figure 1 Time trend in percentage of interventional studies with results among all applicable closed studies.
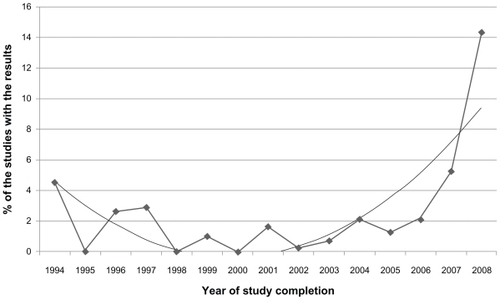
Table 2 Age and gender distribution of the interventional, active, not recruiting studies applicable to reporting the results (Phase 0–I excluded)
Table 3 Funding distribution, phases, and completion status of the interventional, active, not recruiting studies applicable to reporting of results (Phase 0–I excluded)
Among the applicable 31,161 closed interventional studies, the studies of hypertension and influenza reported results more often (7.5% and 9.7%, respectively, ). The most commonly reported examined disease states included breast cancer, schizophrenia, and pain. Most of the closed studies of these conditions did not report the results (). The proportion of studies with results varied substantially between different sponsors (). Several pharmaceutical firms, including GlaxoSmithKline, Pfizer, Merck, and Eli Lilly and Company, sponsored more than 50 studies each and provided the results for more than 10% of the total sponsored studies. Two pharmaceutical firms, ie, Alcon Research and Eli Lilly and Company, provided results for more than 20% of applicable sponsored studies. Several sponsors did not provide results for funded studies. For instance, the National Cancer Institute sponsored 208 closed interventions without results and Memorial Sloan-Kettering Cancer Center sponsored 190 closed interventions without results. Genetic treatments were examined in 48 closed studies. None of the genetic studies provided results (). The studies of several drug, including zidovudine, risperidone, and vildagliptin, did not report their results.
Table 4 Distribution of conditions of subjects in closed interventional, active, not recruiting studies applicable to reporting of results (Phase 0–I excluded)
Table 5 Sponsors of the closed interventional, active, not recruiting studies applicable to reporting of results (Phase 0–I excluded)
Table 6 Treatments that were examined in the closed interventional, active, not recruiting studies applicable to reporting of results (Phase 0–I excluded)
Of 31,161 applicable closed interventional studies, 2860 were terminated and 5.3% of 2860 reported results (). In terminated studies, the most common diseases involved were diabetes and human immunodeficiency virus. Of the terminated studies in prostate cancer, anemia, asthma, and rheumatoid arthritis, more than 10% reported results. The terminated studies of subjects with lymphoma, pain, obesity, heart failure, and colorectal cancer did not provide results. The majority of interventions that were suspended or withdrawn examined the effects of drugs (). Breast cancer and prostate cancer were among the most common conditions for trials which were suspended or had interventions withdrawn.
Table 7 Terminated interventional, active, not recruiting studies applicable to reporting of results (Phase 0–I excluded) by type of condition (shown for ≥20 total studies)
Table 8 Patient conditions in withdrawn and suspended interventions applicable to reporting of results by type of treatment (shown if ≥10 interventions). The results are not available for all studies
Discussion
We found that a statistical analysis of compliance with mandatory reporting of results was difficult to perform. Missing or inconsistently reported study details, including patient diseases, study completion dates, and reported interventions may lead to wrong conclusions about a sponsor’s compliance with federal law regarding study registration and reporting of outcomes. We could not identify a single well-defined field that has applicability status of individual studies to provide results. One variable provides information about the posting of the results. The changes in protocols and deviations from the planned presentation of the primary outcomes and safety outcomes were not easy to analyze without time-consuming evaluation of each study. Reporting of studies completed before September 2007 was available in a small proportion of the interventions, when the sponsors decided to comply.
Both clinicians and the general public need complete and accurate information about study protocols and results.Citation9 Stakeholders should be able to find a clear description of interventions, including prior FDA approvals, off-label evaluations, investigational new drug applications and numbers, and investigational device exemptions, as well as the applicability of reported results.Citation18–Citation20 Critical appraisal of the protocols and reported results on a regular basis by clinical epidemiologists may be worthwhile to ensure integrity of the clinical research reported on ClinicalTrials.gov. Clinicians and patients need independent access to protocols and market approval status of the tested interventions. Finally, our analysis found that none of the suspended or withdrawn studies reported either the baseline characteristics of enrolled subjects or the exact reasons for terminating the study. Posting the results of a study should be mandatory for all trials, regardless of prior FDA approval.Citation9,Citation11
Our study had several limitations. We defined the applicability of studies without evaluation of the market status of individual studies. We did not analyze deviations from the protocols when reporting the results. We did not analyze whether the sponsors posted the study results in a timely manner according to the expected date. Future research should analyze time intervals between completion of the study, posting of results, and publication of the results in peer-reviewed journals.
We conclude that compliance with the requirements to post results of closed studies is low for both industry- and nonindustry-sponsored studies. The need for studies to report the results should be identified in the database during the registration of the studies.
Acknowledgment
The authors would like to thank Marilyn Eells and Michele Rockne for editing and formatting the manuscript.
Disclosure
The author reports no conflict of interest in this work.
References
- The Office of Research Integrity (ORI)Responsible Conduct of Research (RCR) Available at: http://ori.dhhs.gov/education/Accessed on Jun 16, 2010
- Food and Drug AdministrationModernization Act (FDAMA) of 1997. Pub L No. 105-115, 111 Stat 2296, 2310, 113 (Nov 21, 1997) Available at: http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDAMA/default.htmAccessed on Jun 16, 2010
- World Association of Medical Editors (WAME) Editorial Policy CommitteeThe Registration of Clinical Trials Available at: http://www.wame.org/resources/policies#trialregAccessed Jul 15, 2010
- The World Health OrganizationInternational Clinical Trials Registry Platform Available at: http://www.who.int/ictrp/en/Accessed on Jun 16, 2010
- DwanKAltmanDGArnaizJASystematic review of the empirical evidence of study publication bias and outcome reporting biasPLoS One200838e308118769481
- SterneJAGavaghanDEggerMPublication and related bias in meta-analysis: Power of statistical tests and prevalence in the literatureJ Clin Epidemiol200053111119112911106885
- MathieuSBoutronIMoherDAltmanDGRavaudPComparison of registered and published primary outcomes in randomized controlled trialsJAMA2009302997798419724045
- Food and Drug AdministrationAmendments Act of 2007. Pub L No. 110-85, 121 Stat 823, 904, Title VIII (Sep 27, 2007), 42 USC 282(j) (Supp 2009) Available at: http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FoodandDrugAdministrationAmendmentsActof2007/default.htmAccessed on Jun 16, 2010
- ChanAWLaupacisAMoherDRegistering results from clinical trialsJAMA20103032121382139 author reply 213920516411
- ManciniJReynierCJRegistering results from clinical trialsJAMA2010303212139 author reply 213920516413
- MillerJDRegistering clinical trial results: The next stepJAMA2010303877377420179288
- TseTWilliamsRJZarinDAUpdate on registration of clinical trials in ClinicalTrials.govChest2009136130430519584213
- ZarinDAKeselmanARegistering a clinical trial in ClinicalTrials. govChest2007131390991217303677
- ZarinDAIdeNCTseTHarlanWRWestJCLindbergDAIssues in the registration of clinical trialsJAMA2007297192112212017507347
- ZarinDATseTIdeNCTrial registration at ClinicalTrials.gov between May and October 2005N Engl J Med2005353262779278716382064
- MojaLPMoschettiINurbhaiMCompliance of clinical trial registries with the World Health Organization minimum data set: A surveyTrials2009105619624821
- National Institutes of Health’s ClinicalTrials.gov web site wins prestigious awardJ Investig Med2004527432
- TseTWilliamsRJZarinDAReporting “basic results” in ClinicalTrials.govChest2009136129530319584212
- HirschLTrial registration and results disclosure: Impact of US legislation on sponsors, investigators, and medical journal editorsCurr Med Res Opin20082461683168918462565
- PihlstromBLReporting clinical trial resultsJ Am Dent Assoc20091401–121415
