Abstract
A comparative clinical study was conducted to evaluate the safety and tolerability of two commonly used fixed dose artemisinin-based combinations for the treatment of uncomplicated Plasmodium falciparum malaria in the second and third trimester of pregnancy. To achieve this, a total of 155 participants were recruited for the study. Eighty of these were drawn from pregnant women who came for routine antenatal care while 40 nonpregnant participants were recruited from apparently healthy females in the community. Eighty pregnant participants with uncomplicated P. falciparum malaria were randomized into artesunate/amodiaquine (AA) and artemether/lumefantrine (AL) treatment arms while 40 nonpregnant and 35 nonmalarious pregnant women were used as control. The interventional groups received standard fixed dose combinations of AA (100/270 mg) daily or AL (20/120 mg) twice daily for 3 days. Blood samples were collected on day 4 and patients were followed-up closely to ascertain the safety of the drugs. The study showed a significant (p<0.0001) elevation of alkaline phosphatase in the AA and AL group compared to the nonpregnant control and a significant (p<0.05) elevation of alanine transaminase and aspartate transaminase level in the AL combination group when compared with the AA group. The elevated hepatic enzymes were within the normal range for pregnancy and were not clinically significant. Adverse event rate was higher in the AA group (n=28 [70%]) when compared to the AL group (n=4 [10%]) although the drugs were well-tolerated in both treatment arms. In conclusion, the use of these combinations is safe in the second and third trimester of pregnancy. However, we recommend active pharmacovigilance and spontaneous drug reporting of the agents in order to continuously monitor safety in the vastly heterogeneous population.
Introduction
Artemisinin and its derivatives are known to be effective against multidrug resistant Plasmodium falciparum malaria with a more rapid clearance of sensitive parasites from the blood than any other antimalarial agent.Citation1,Citation2 This activity is, generally, attributed to the rapid schizonticidal action of the drugs. The drug is, generally, well-tolerated despite reports of neurotoxicity and embryotoxicity from animal studies.Citation3,Citation4
However, reported cases of adverse reactions include a few case reports of anaphylaxis.Citation5,Citation6 Currently the recommended antimalarial policy requires the use of combination therapy which includes an artemisinin to delay the emergence of drug resistance as well as improve cure rates. In this treatment, the choice of partner drugs used in combination with artemisinin derivatives depends on the pattern of parasite resistance in the region.Citation1,Citation5 For example, in Nigeria the combinations commonly used for treatment of uncomplicated P. falciparum malaria are artesunate/amodiaquine (AA) and artemether/lumefantrine (AL) combinations. This is based on a research report from the Nigerian Ministry of Health which revealed that the combinations have an acceptable cure rate of >90% in some of the geopolitical zones of the country.Citation7
Artemether is a semisynthetic derivative of artemisinin whereas lumefantrine also known as benflumetol is a synthetic product. Both drugs were first developed in China. Artesunate is a hemisuccinate derivative of dihydroartemisinin obtained by the reduction of artemisinin, a sesquiterpene lactone endoperoxide extracted from a plant known as Artemisia annua. This is used as traditional herbal preparation in the treatment of malaria.Citation8 The report that lethal agranulocytosis and severe hepatic impairment is associated with the use of amodiaquine as a chemoprophylactic agentCitation9 and the fact that the safety of artemisinins still remains a questionable in pregnancy due to insufficient data, forms the rationale for this study.
In malaria endemic regions, malaria infection may occur repeatedly in individuals within months which warrants the use of repeated doses of antimalarials. This situation occurs more prominently in individuals with increased susceptibility such as children, pregnant women and immunocompromised patients.Citation10,Citation11 Hence, the issue of safety cannot be overemphasized. Also, there is a dearth of safety data on the use of these combinations in the treatment of uncomplicated malaria in pregnancy particularly among Africans. This forms the basis for this study, which aims to comparatively assess the safety of the combinations in the management of uncomplicated falciparum malaria in pregnancy.
Study design
The study is an open label randomized control trial comparing the safety and tolerability of artesunate + amodiaquine and artemether + lumefantrine combinations in a population of pregnant women with uncomplicated P. falciparum malaria. The minimum sample size required to determine an inter-group difference of <10% in a population with type 1 error of 5% and a power of 80% was statistically estimated to be 35 per group. A total of 155 participants were recruited for the study. Eighty participants were drawn from the pool of pregnant women who came for routine antenatal care visits to a busy hospital in Ekpoma, a semi-urban community endemic for malaria. Eligibility for the study was based on the following inclusion criteria:
age of >18≤35 years,
positive rapid diagnostic test (RDT) for P. falciparum + microscopic confirmation of asexual parasitemia in Giemsa stained thick and thin blood film,
parasite density ≥1000/µL,
acute manifestation of malaria (e.g., history of fever in the preceding 24 hours, a temperature of >37.5°C at baseline, malaise, generalized body pains, chills, rigors, etc.),
ability to tolerate oral therapy,
second and third trimester of pregnancy (i.e., >12 weeks gestation),
informed consent.
The exclusion criteria for the study were as follows:
adequate antimalarial treatment within the previous 4 weeks,
use of hematinics in the last 4 weeks,
use of herbal medications in the last 4 weeks,
antibiotic treatment for a concurrent infection,
mixed plasmodial infection,
severe malaria,
severe underlying disease,
other diseases associated with fever.
Written informed consent was obtained from the participants and approval was granted by the Ethical Review Board of the Edo State Ministry of Health. The study was conducted in accordance with the principles of the Helsinki Declaration and its Hong Kong amendment and according to the principles of good clinical practice. Recruited participants with malaria were randomly assigned in a 1:1 ratio to either AA or AL treatment arm. On day 0 the treatment groups were placed on oral doses of fixed dose combination of AA (100/270 mg) and AL (20/120 mg), respectively. The drugs were administered orally for 3 days (AA 3 tablets once daily and AL 4 tablets twice daily). Dosing was based on the recommended WHO schedule for uncomplicated malaria in pregnancy.Citation12 The other comparator groups consisted of 35 pregnant and 40 nonpregnant women who had no malaria and were not on any antimalarial drug.
Patients on treatment were followed-up consecutively on days 1–3, 7, 14, 21 and 28. At each visit, patients were clinically evaluated and adverse events were monitored and documented. Venous blood samples were taken on study day 4 to determine the hemoglobin level, hematocrit, differential white blood cell count and some biochemical parameters such as total and conjugated bilirubin, alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP) and serum lipid profile. Dried thick blood smears were stained with 10% Giemsa solutions at pH 7.2 for 10 minutes. Thin blood smears were also made. Parasite species were identified using standard morphological characteristics, and the presence of asexual parasitemia was confirmed accordingly. Parasite density was evaluated using standard procedure in which parasites were counted per 200 white blood cells (WBC) multiplied by leukocytes count/µL. Statistical analyses of the data were performed using graph pad prism 6.0. Student’s t-test and one way analysis of variance were used to compare the mean of the laboratory data between groups. This was followed by Tukey’s post hoc test for multiple comparisons. The statistical significance level was set at 95% confidence interval and p value <0.05 was considered significant. A flow chart was developed for the study based on the CONSORT 2010 statement for the reporting of clinical trials.Citation13
Results
The baseline characteristics of the study population () showed that most of the recruited pregnant patients were multiparous with body mass index and blood pressures within normal range (body mass index [BMI]: AA; 21.76±0.21 kg/m2, AL; 20.98±0.56 kg/m2). The mean systolic and diastolic blood pressures in the AA and AL group were 106.81±0.91/68.76±1.21 mmHg and 112.13±0.86/69.83±0.45 mmHg, respectively. Of the 80 participants assigned into both treatment arms, five (AA, n=3 [3.75%]; AL, n=2 [2.5%]) were lost to follow-up on day 14 ().
Table 1 Baseline characteristics of the study population
In addition, there was a consistent reduction (, ) in mean temperature (AA, 38.08°C±0.10°C; AL, 38.51°C±0.12°C) and parasite density (AA, 3634±537/µL; AL, 4884±386.59/µL) after day 0 of treatment. However, the late parasitological failure observed on day 14 (AA, n=1 [2.63%]; AL, n=2 [5.41%]) in both treatment arms may be due to a reinfection because this was not polymerase chain reaction-corrected to rule out recrudescence. Clinical response was adequate throughout the follow-up period.
Figure 2 Temperature and parasite density following intervention in AA and AL treatment arm.
Abbreviations: AA, artesunate/amodiaquine; AL, artemether/lumefantrine.
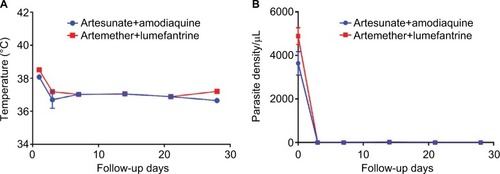
Figure 3 Alkaline phosphatase and transaminase profile in treated and control groups.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase.
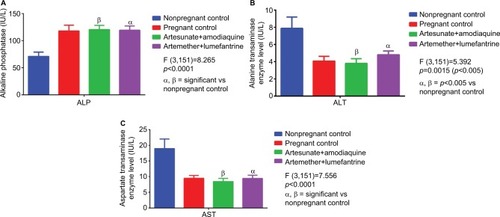
There was no significant change (p>0.05) in ALP levels () between treatment groups and pregnant control. Although ALP values were within normal reference range in the test groups (AA, 120.27±7.81 IU/L; AL, 119.27±7.81 IU/L) they were significantly (p<0.0001) different from the nonpregnant control (70.97±7.87 IU/L). In addition, the serum levels of ALT and AST () though significantly higher (p<0.05) in AL (4.85±0.44 and 9.47±1.01 IU/L) compared to the AA (3.80±0.54 and 8.43±1.02 IU/L) group, were within normal range in pregnancy. Also, the serum transaminase levels though normal in the treated groups were significantly different from the nonpregnant control.
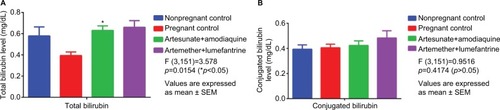
Although, total bilirubin level () was significantly (p<0.05) higher in AA and AL groups when compared with pregnant control there was no significant change (p>0.05) between the treatment groups (AA, 0.66±0.06 mg/dL; AL, 0.63±0.04 mg/dL). In addition, there was no significant change () in conjugated bilirubin level between the treatment groups and control groups.
Figure 4 Comparative serum bilirubin levels in treated vs control groups.
Abbreviation: SEM, standard error of mean.
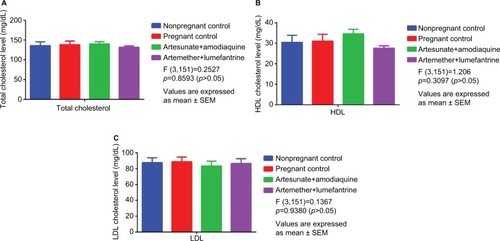
A comparative serum lipid assessment () showed a nonsignificant (p>0.05) reduction of low-density lipoprotein (LDL) cholesterol in the AA group when compared with the control and AL combination groups. In addition, high-density lipoprotein (HDL) cholesterol level was nonsignificantly (p>0.05) increased in the AA combination group (34.60±3.20 mg/dL) when compared with the control groups (31.00±3.39 and 30.45±3.37 mg/dL) and AL (27.60±1.20 mg/dL) groups.
Figure 5 Comparative lipid profile in treated versus control groups.
Abbreviations: HDL, high density lipoprotein; LDL, low density lipoprotein; SEM, standard error of mean.
Packed cell volume (PCV) and hemoglobin concentration () was markedly and significantly (p<0.01) reduced in the AA (30.44%±0.12%; 10.12±0.21 g/dL) and AL (31.33%±0.23%; 10.87±0.17 g/dL) treatment groups when compared with nonpregnant control (35.81%±0.21%; 12.21±0.15 g/dL). This was not significantly (p>0.05) different from the pregnant control group. WBC count was significantly (p<0.01) increased in the AA (6072.31±224.32/mm3) group when compared with the pregnant control (5436.00±121.24/mm3) which also showed a significant (p<0.01) decrease in WBC compared to the nonpregnant control (6890.00±213.21/mm3). However, the values were well within the range of normal (4000–8000/mm3) in the environment under consideration.Citation14 Eosinophil, monocyte and basophil differential count was significantly (p<0.01) increased in the AA group when compared with nonpregnant control. There was a significant (p<0.01) reduction in the monocyte and basophil differential counts in the AL group when compared with the AA group. The neutrophil and basophil count in the pregnant control were also significantly (p<0.01) increased compared to the nonpregnant control.
Table 2 Hematological profile of pregnant women on artesunate + amodiaquine and artemether + lumefantrine combination for uncomplicated malaria
The adverse event rate was higher in the AA group (n=28 [70%]) when compared to the AL group (n=4 [10%]). Adverse events () in the AA group were more predominant in the central nervous system (30%) with effects ranging from headaches, dizziness and drowsiness. This was followed closely by gastrointestinal tract disturbances (20%). Most of the reported adverse effects were mild. AL combination showed a comparatively safe profile with fewer side effects reported by patients on treatment with the combination. Adverse events ranged from GIT disturbances (7.5%), to fatigue and muscle weakness (2.5%) and were mostly mild.
Table 3 Adverse events profile of pregnant patients on artesunate + amodiaquine and artemeter + lumefantrine for uncomplicated malaria
Discussion
Due to the emergence of resistance to previously used antimalarials there has been a change in the treatment policy for malaria in Africa. Based on WHO recommendation most African countries have resorted to the use of artemisinin-based combination for the treatment of uncomplicated P. falciparum malaria. However, one major challenge has been the issue of safety and tolerability of the drugs in the second and third trimesters of pregnancy. Despite the scarcity of data about the safe use of these agents in pregnancy, the combination is still in use for the treatment of uncomplicated P. falciparum malaria in the second and third trimesters of pregnancy in Nigeria due to its efficacy.Citation15 This has remained so because clinicians have not been able to pin-point any severe adverse effect based on the clinical experience accrued from the use of the drugs over the years.Citation16
Recent preclinical safety data for amodiaquine revealed that there was no significant elevation of liver enzymes in rats exposed to high doses (120 mg/kg) except when coadministered with sulfadoxine-pyrimethamine.Citation17 Additionally, the drug has also been linked to agranulocytosis and hepatotoxicity following prophylactic use.Citation18 However, other reports have linked amodiaquine to serious hepatic injury with hepatocellular pattern of liver enzyme elevation often associated with agranulocytosis.Citation19 In addition, AL use has also been associated with some degree of hepatotoxicity. Recent studies suggest an elevation of AST and ALT levels in patients treated with AL.Citation20 Other studies have also shown hepatorenal and hepatobiliary toxicity in pregnant albino rats.Citation21,Citation22
In this study, AST and ALT levels although within normal range were significantly higher in the AL group compared to the AA group, but not significantly different from the pregnant control group. Similarly, ALP levels were within normal range and the elevation in both AA and AL group is likely to be physiological since it was not significantly different from pregnant control. The outcome of a recent animal study showed that the mean serum concentrations of ALP, ALT and AST were not significantly elevated in animals treated with AL.Citation23 However, the results from this animal study may be due to the fact that the animals used were not parasitized. Although most data from clinical trials suggest elevation of liver enzymes, none has shown any clinically significant outcome.Citation1 This study suggests that alteration in hepatic enzyme levels may be influenced by physiological changes in pregnancy and not necessarily because of the hepatotoxic effect of the drug as reported by other researchers.
In addition, available facts suggest that many patients show some degree of hepatic impairment following episodes of acute malaria.Citation24 Therefore, making an inference that the agents are hepatotoxic without correlating it with any clinical outcome of toxicity makes the finding inconclusive. Hence WHO has recommended that caution should be exercised only in clinical settings that suggest hepatitis, particularly in conditions associated with the development of jaundice and clinical features suggestive of agranulocytosis, such as fever, tonsillitis and mouth ulcers.Citation14 The marked elevation of total bilirubin in both the AA and AL groups may not be unconnected with increased hemolysis associated with malaria infection.Citation25,Citation26 This is corroborated by a study conducted in Colombia which showed anemia and elevated bilirubin in patients who received AA.Citation27 In addition, this study showed a nonsignificant reduction in LDL-cholesterol and a nonsignificant change in HDL-cholesterol in the AA group compared to the AL group. Some studies have shown that AA and AL caused an insignificant decrease in total cholesterol levels when administered at therapeutic doses.Citation28 However, there are reports that artesunate has significant hypolipidemic effect when coadministered with ursolic acid in rats.Citation29
In this study, hematological changes in AA and AL groups were not significantly different from the pregnant control group. However, there was a significant reduction in and hemoglobin concentration in both AA and AL groups and a significant reduction in monocyte and basophil differential count in AL group compared to nonpregnant control. Other studies have shown that administration of AL in Wistar rats caused a significant dose-dependent and reversible decrease in red blood cells (RBC) and WBC count as well as Hb concentration.Citation30 In another study, AL did not cause any significant change in hematological parameters when used at recommended doses and duration but only caused significant reduction in RBC count, Hb concentration and PCV when used for a more prolonged period of 7 days.Citation31 In a recent study conducted in Nigeria, anemia was reported as an adverse event following the administration of AL in children.Citation32
In addition, this finding was attributed to be part of the manifestation of malaria rather than an effect caused by the drug. Other reports have also linked maternal anemia to malaria infection in pregnancyCitation25,Citation26 thus making it difficult to determine the actual effect of the drugs on PCV and Hb indices. This therefore implies that physiological changes in pregnancy and hemolysis associated with malaria infection may have influenced the findings in this study. A Colombian study reported the occurrence of neutropenia and leukopenia. In the study, severe neutropenia developed in 8.3% of subjects who received treatment with AA.Citation27 However, the findings of this study showed a significant increase in WBC and differential count in the AA and AL groups. In addition, the monocyte and basophil count were significantly decreased in the AL group compared to the AA group. This was not clinically significant as values remained within the normal range for the population.
Also, the adverse event rate was higher in the AA group when compared to the AL group. In the AA group, adverse events involved mainly the central nervous system and the gastrointestinal tract. However, some patients presented with fatigue and muscle weakness and dermatological reactions such as rash and pruritus during follow-up. Gastrointestinal disturbances were the predominant adverse event reported in the AL group. In a similar study, mild to moderate fatigue occurred more frequently in the AA treatment group than the AL group.Citation33 This corroborates the findings of this study. However, other studies revealed no difference in fatigue between the AA and AL treatment groups.Citation34–Citation36 In this study, most of the observed adverse effects were mild and well tolerated which is in keeping with other reports. According to Ndiaye et al,Citation37,Citation38 dizziness and somnolence were the predominant neurological effects reported in the AA group which corroborates our findings. In a systematic review conducted by Manyando et al,Citation39 AL use in second and third trimester of pregnancy was not associated with adverse pregnancy outcome in 890 clinical studies evaluated. However, this study is limited by the small sample size and the fact that the safety of the drugs was not evaluated in the fetus.
In Nigeria, the practice of pharmacovigilance and adverse drug reporting is low among health personnel in tertiary health centers.Citation40 It is therefore important to reawaken the culture of active spontaneous adverse drug reporting in our setting with regards to artemisinin-based combination therapy use in pregnancy. With active monitoring of the safety and tolerability of these agents it will be possible to accumulate data over time that will help to sufficiently address the issue of safety and tolerability of ACTs in pregnancy. Recently, concerted effort was made to monitor the safety of ACTs in the six geopolitical areas of Nigeria. However, challenges that may affect the reliability of data from the project include; dearth of local expertise on pharmacovigilance, the method and approach used in the collection of data, and failure to include researchers with requisite knowledge and skill in the field.Citation41 The safety of artemisinin-based combination drugs in pregnancy still remains a pressing issue that needs to be addressed in our setting. Also, there is a problem of under-reporting of adverse events by physicians due to difficulty in establishing a causal relationship between the drugs and the suspected reaction.Citation42 However, there is need to continue to emphasize the adoption of spontaneous adverse drug reporting as a means of creating a reliable platform for the early detection of untoward reactions to ACTs in pregnancy.
Conclusion
In conclusion, the differences observed in hepatic enzyme levels between treatment groups were not clinically significants though statistically significant. The elevations were within the normal range for the second and third trimesters of pregnancy and no new safety issues were identified with the use of the two ACTs in the treatment of malaria in pregnancy. However, the limitation of the study was the small sample size; hence a large scale multicenter study will have to be conducted in different endemic foci in our region. In addition, a robust pharmacovigilance tool is needed to obtain more safety data in this vulnerable population because we were not able to assess for safety of the ACTs in the fetus.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We acknowledge the management and staff of Faithdome Medical Center and all the nurses who assisted in the recruitment of participants for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- BremanJGAlilioMSWhiteNJDefining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives: Supplement to Volume 77(6) of American Journal of Tropical Medicine and HygieneNorthbrook, ILAmerican Society of Tropical Medicine and Hygiene2007
- EyasuMAntimalarial drug resistance: in the past, current status and future perspectivesBr J Pharmacol Toxicol201561115
- O’NeillPMPosnerGHA medicinal chemistry perspective on artemisinin and related endoperoxidesJ Med Chem200447122945296415163175
- AngusBNovel anti-malarial combinations and their toxicityExpert Rev Clin Pharmacol20147329931624716844
- RosenthalPJArtesunate for the treatment of severe Falciparum malariaN Engl J Med20083581829183618434652
- DubeSKPandaPSAgrawalGRSinghDKAnaphylaxis to artesunate?Indian J Crit Care Med2012161555722557837
- Federal Ministry of Health Nigeria (FMOH)National Antimalarial Treatment Policy2005131 Available from: http://apps.who.int/medicinedocs/documents/s18401en/s18401en.pdfAccessed August 14, 2016
- WoodrowCJHaynesRKKrishnaSArtemisininsPostgrad Med J200581952717815701735
- ThomasFErhartAD’AlessandroUAmodiaquine in pregnancy: can amodiaquine be used safely during pregnancy?Lancet Infect Dis20044423523915050942
- DiagneNRogierCSokhnaCSIncreased susceptibility to malaria during the early postpartum periodN Engl J Med2000343959860310965006
- TionoABGuelbeogoMWSagnonNFDynamics of malaria transmission and susceptibility to clinical malaria episodes following treatment of Plasmodium falciparum asymptomatic carriers: results of a cluster-randomized study of community-wide screening and treatment, and a parallel entomology studyBMC Infect Dis20131353524215306
- World Health OrganizationGuidelines for the Treatment of Malaria2nd edGenevaWHO20101921 http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdfAccessed June 21, 2016
- SchulzKFAltmanDGMoherDCONSORT GroupCONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trialsBMJ2010340c33220332509
- Miri-DasheTOsaweSTokdungMComprehensive reference ranges for hematology and clinical chemistry laboratory parameters derived from normal Nigerian adultsPLoS One201495e9391924832127
- AjayiNAUkwajaKNPossible artemisinin based combination therapy-resistant malaria in Nigeria: a report of three casesRev Soc Bras Med Trop201346452552723982103
- WHOGlobal Malaria ProgramArtemisinin and Artemisinin-Based Combination Therapy Resistance: Status ReportWHO/HTM/GMP/2016.5 Available from: https://www.apps.who.int/iris/bitstream/10665/208820/1/WHO_HTM_GMP_2016.5_eng.pdfAccessed August 14, 2016
- MishraSKSinghPRathSKA study of the toxicity and differential gene expression in murine liver following exposure to anti-malarial drugs: amodiaquine and sulfadoxine-pyrimethamineMalar J20111010921529379
- NostenFWhiteNJArtemisinin-based combination treatment of fal-ciparum malariaAm J Trop Med Hyg200777Suppl 618119218165491
- United States National Library of MedicineAmodiaquine: clinical and research information on drug induced liver injury Available from: https://www.livertox.nih.gov/amodiaquine.htmlAccessed September 24, 2016
- TaylorWRWhiteNJAntimalarial drug toxicity a reviewDrug Saf2004271256114720085
- UmohIUEkanamTBEluwaMAFetal Hepatorenal toxicity of artemether/lumefantrine (COARTEMR) in second trimester of pregnancy of Albino ratsEJPMR201742240246
- UgianEADasofunjoKNwangwaJNEffect of artemisinin-based combination therapy on some selected liver function indices of pregnant Wistar Albino ratsJ Appl Pharm Sci201339152154
- KhandaveSSJoshiSSSawantSVOnkarSVEvaluation of bio-equivalence and cardio-hepatic safety of a single dose of fixed dose combination of artemether/lumefantrineJ Bioequiv Availab201024081085
- EffiongOOUdoNVNsaAAComparative effects of two antimalarial drugs (P-Alaxin & Coartem) on serum electrolytes and serum enzymes in Albino Wistar ratsRes J Pharm Biol Chem Sci (RJPBCS)2014515463
- KocharDKAgarwalPKocharSKHepatocyte dysfunction and hepatic encephalopathy in Plasmodium falciparum malariaQJM200396750551212881593
- DesaiMter KuileFONostenFEpidemiology and burden of malaria in pregnancyLancet Infect Dis2007729310417251080
- HuynhBTCottrellGCotMBriandVBurden of malaria in early pregnancy: a neglected problem?Clin Infect Dis201560459860425362205
- OsorioLGonzalezIOlliaroPTaylorWRArtemisinin based combination therapy for uncomplicated Plasmodium falciparum malaria in ColombiaMalar J200762517328806
- OtuechereCAEdeworGKaleOEEkorMSubacute therapeutic dosing of AL and AA combinations preserves plasma cholesterol, renal antioxidant status and organ weights in ratsMalar Res Treat20122579865
- YuliangWZejianWHaulinSMingYKexieanTThe hypolipidemic effect of artesunate and ursolic acid in ratsPak J Pharm Sci201528387187426004719
- OsunugaIOOsunugaOAOsonugaAOnadekoAAOsunugaAAEffect of the artemether on hematological parameters of healthy and uninfected adult Wistar ratsAsian Pac J Trop Biomed20122649349523569957
- OfemOEEssienNMOkonUAEffects of chloroquine and coartem on hematological parameters in ratsAfr J Biomed Res20131613946
- EgunsolaOOshikoyaKAComparative safety of artemether-lumefantrine and other artemisinin-based combination in children: a systemic reviewMalar J20131238524175945
- SchrammBValehPBaudinETolerability and safety of artesunate-amodiaquine and artemether-lumefantrine fixed dose combination for the treatment of uncomplicated P. falciparum malaria: two open-label randomized trials in Nimba County LiberiaMalar J20131225023866736
- AdjeiGOKurtzhalsJARodriguesOPAmodiaquine-artesunate vs artemether-lumefantrine for uncomplicated malaria in Ghanaian children: a randomized efficacy and safety trial with one year follow-upMalar J2008712718620577
- FayeBOffiananATNdiayeJLEfficacy and tolerability of artesunate-amodiaquine (Camoquine+) versus artemether-lumefantrine against uncomplicated P. falciparum malaria: multisite trial in Senegal and Ivory CoastTrop Med Int Health201015560861320214761
- NdiayeJLFayeBGueyeARepeated treatment of recurrent uncomplicated Plasmodium falciparum malaria in Senegal with fixed-dose artesunate plus amodiaquine versus fixed-dose artemether plus lumefantrine: a randomized, open-label trialMalar J20111023721838909
- NdiayeJLRandrianaRMSagaraIRandomized, multicentre assessment of the efficacy and safety of ASAQ–a fixed-dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated Plasmodium falciparum malariaMalar J2009812519505304
- ManyandoCKayentaoKD’AllessandroUOkaforHUJumaEHamedKA systematic review of the safety and efficacy of artemether-lumefantrine against uncomplicated Plasmodium falciparum malaria during pregnancyMalar J20121114122548983
- Ohaju-ObodoJOIribhogbeOIExtent of pharmacovigilance among resident doctors in Edo and Lagos states of NigeriaPharmacoepidemiol Drug Saf201019219119520014358
- OshikoyaKAAdverse event monitoring of artemisinin combination therapy in Nigeria: the challenges and limitation of the studyWest Afr J Med201029422122420931507
- PalleriaCLeporiniCClumirnSLimitations and obstacles of the spontaneous adverse drug reaction reporting: two challenging case reportsJ Pharmacol Pharmacother20134Suppl 1S66S7224347986