Abstract
Skin injuries caused by accidents and acute or chronic diseases place a heavy burden on patients and health care systems. Current treatments mainly depend on preventing infection, debridement, and hemostasis and on supplementing growth factors, but patients will still have scar tissue proliferation or difficulty healing and other problems after treatment. Conventional treatment usually focuses on a single factor or process of wound repair and often ignores the influence of the wound pathological microenvironment on the final healing effect. Therefore, it is of substantial research value to develop multifunctional therapeutic methods that can actively regulate the wound microenvironment and reduce the oxidative stress level at the wound site to promote the repair of skin wounds. In recent years, various bioactive nanomaterials have shown great potential in tissue repair and regeneration due to their properties, including their unique surface interface effect, small size effect, enzyme activity and quantum effect. This review summarizes the mechanisms underlying skin wound repair and the defects in traditional treatment methods. We focus on analyzing the advantages of different types of nanomaterials and comment on their toxicity and side effects when used for skin wound repair.
Introduction
As the body’s largest organ, skin constitutes the first barrier between the body and the environment. The main function of skin is to act as a protective barrier to protect the human body against harmful stimuli such as microorganisms, radiation, and chemical and physical stimuli in the external environment. Moreover, it has other important functions, such as maintaining body fluid balance and body temperature, synthesizing vitamin D, transmitting and detecting changes in the external environment and regulating immune responses.Citation1–4 In summary, as skin is an indispensable tissue in the human body, the integrity of its structure is a prerequisite for the performance of the above normal physiological functions.
In daily life, due to the large area of the skin and its direct exposure to the external environment, burns, wounds, infections, accidents and skin trauma resulting from medical surgery cannot always be avoided. According to reported statistics, tens of millions of people around the world suffer skin injuries from accidents or surgeries every year. In addition, with the increasing number of patients with diabetes, the number of patients with chronic or acute skin injuries resulting from diabetes or other causes is also increasing significantly.Citation5 According to the International Diabetes Federation (IDF), the number of people living with diabetes worldwide reached 537 million in 2021, and it is expected that the number will surpass 783 million by 2045, among whom approximately 10% suffer from foot ulcers, refractory wounds or other complications. Chronic diabetic skin wounds are one of the main diabetes-related causes of death or disability.Citation6,Citation7 Currently, acute and chronic skin wounds caused by various factors have become a public health problem of worldwide concern, bringing great economic and social burdens to patients’ families, medical and health systems and society. Additionally, the hyperplasia of scar tissue caused by wounds will also cause a great psychological burden for patients. Therefore, skin wounds caused by various factors are a serious challenge in clinical practice.
Due to the number of accidental injuries and the increasing number of patients with chronic or refractory wounds caused by diabetes, paraplegia or local radiation exposure and the skin being prone to trauma, wound repair has always been a research focus in the medical field. With social progress, people’s desires for beauty have gradually increased, the current level and quality of wound repair cannot meet the requirements of patients, and the contradiction between them has become increasingly sharp. Hence, it is necessary for researchers to further elucidate the mechanism and various influencing factors of wound repair, improve existing treatment methods and discover new treatment methods.Citation8–10
In recent years, with the joint efforts of medical workers and researchers, the basic research and clinical treatment of wound repair have made substantial progress. The development and implementation of a large number of drugs,Citation11 dressings,Citation12 skin grafts,Citation11,Citation13 physiotherapy,Citation14 other treatments have greatly enriched our options for repairing various acute and chronic injuries, and have achieved positive results.
At present, the clinical treatment methods for skin wound repair mainly include medical surgical sutures, medical limb adhesives, drug-carrying dressings, tissue-engineered skin, physical therapy and miRNA treatments promoting skin wound repair. However, each treatment method has a certain application scope and side effects that cannot be ignored. For instance, (1) surgical suture is the most commonly used clinical treatment for acute skin wounds, but it may cause secondary damage to the skin and other soft tissues. In addition, it shows low efficiency; it is difficult to control the absorption period and ensure that the sutures begin to degrade after wound healing.Citation15–17 (2) Medical adhesives, widely used in clinical practice, are prone to promoting rash or skin abnormalities after use, and some of them exhibit defects, such as a brittle adhesive layer, formaldehyde production during decomposition and poor air permeability.Citation18,Citation19 (3) Although wound dressings can temporarily protect the wound, prevent contamination, promote healing and provide a suitable environment for the process of wound healing and treatment, they have the defects of a short efficacy duration and uneven sustained release.Citation20 (4) Tissue-engineered skin has good application prospects in the study of treatment of skin wounds caused by acute or chronic skin trauma, but it also displays some disadvantages, such as immature multicell coculture technology and a simpler structure than that of real skin.Citation21–23 (5) Physical therapy is noninvasive and has the advantages of noninvasiveness, simple operation, no complications, wide indications and others but is usually only an auxiliary means to other therapies to promote wound repair.Citation24–26 (6) Recently, miRNA technology that promotes skin wound repair has attracted the attention of researchers, and some progress has been made in research on the role of miRNA in the repair of acute skin wounds, chronic diabetic injury, scar hyperplasia and other pathophysiological processes, but there is still a long way to go before clinical application will be possible.Citation27–29 In view of the above problems and challenges that have not been completely solved clinically, the design and development of new multifunctional wound treatment methods to accelerate and improve wound repair has important clinical application value.
Nanomaterials have attracted increasing attention from researchers and have been widely studied in tissue engineering and biomedicine due to their good biocompatibility, controllable preparation methods, and unique physical, chemical, and biological properties.Citation30–32 In the past few years, the research and development of nanomaterials and nanotechnology have provided many novel solutions and strategies for the treatment of acute or chronic skin wounds, which not only effectively overcome the defects of traditional treatments but also utilize the unique functions and properties of many nanomaterials.Citation33,Citation34 Compared with traditional materials, nano materials can effectively enter the human body by changing the drug dosage form, improving the drug concentration in blood, controlling the drug release rate or delivering the drug to the wound site, and the special physical and chemical properties of nanomaterials show more advantages in the field of wound repair.Citation35,Citation36 For example, modified nanomaterials with pH, charge and microenvironmental effect and other characteristics are conducive to eliminating the obstacles of physiological barriers to drug transport, enhancing the targeting of drug delivery system and reducing drug dosage, thus improving drug absorption, regulating drug release rate, enhancing drug targeting, improving bioavailability and reducing drug toxicity and side effects.Citation37–39 With research efforts deepening and improvements in the understanding of the mechanism of action and biological safety, the use of nanomaterials will likely continue to be developed, and there is hope that nanomaterials may be the next generation of medical materials for acute or chronic skin wound therapy. This review summarizes the mechanisms underlying and key factors influencing skin wound repair, and we focus on the promotion and development of skin wound repair by nanomaterials.
Mechanisms Underlying and Factors Influencing Skin Wound Repair
Wound repair is a complex dynamic form of skin repair after an injury and is important for maintaining the integrity of the skin structure and its normal function; both the speed and quality of repair are affected by various factors.
Mechanisms Underlying Skin Wound Repair
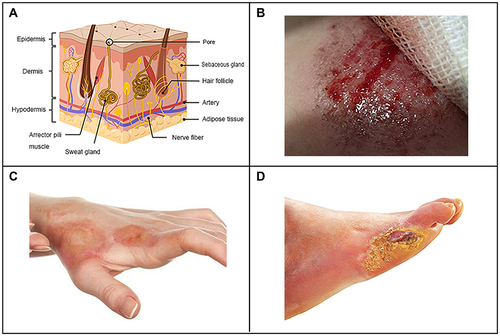
The skin is the largest organ and covers the outermost layer of the human body, and it is mainly composed of three parts: epidermis, dermis and hypodermis.Citation40,Citation41 Keratinocytes are the main components of the epidermis, accounting for more than 80% of epidermal cells, and keratin can be produced in the process of differentiation. Keratinocytes and their substructures are connected by desmosomes and hemidesmosomes. According to the differentiation stage and characteristics, the epidermis can be divided into five layers, which from inside to outside are the basal layer, spinous layer, granular layer, transparent layer and horny layer.Citation42,Citation43 The hypodermis accounts for most of the skin structure, and the dermis has a thickness of approximately 0.2 cm and can be divided into two layers, namely, the papillary layer and the reticular layer, which are mostly composed of proteins including collagen and elastin, with the addition of nerves, sweat glands, sebaceous glands and hair follicles.Citation41 Subcutaneous tissue is the loose connective tissue and adipose tissue below the skin, which connects the skin and muscle. It is vulnerable to trauma and ischemia, especially during inflammation, which can cause degeneration and necrosis ().Citation41 Skin wounds caused by various accidents and medical surgery constitute common phenomenon due to the large area of the skin and its direct exposure to the external environment. Additionally, skin diseases and diabetes can also cause skin wounds. The common skin wounds mainly include physical wounds (), chemical wounds () and pathological wounds (). Most physical wounds in daily life are caused by scratching, scraping or superficial cuts, which damage the epidermis and dermis, while hair follicles and sweat glands are intact. Different degrees of wounds correspond to different skin injuries.Citation44 Chemical damage is the acute skin damage caused by skin contact with certain chemicals, such as erythema, blisters, eschar and so on. Some studies have found that the skin irritation by anionic detergent sodium lauryl sulphate which causes chemical damage of the stratum corneum and interrupts skin barrier function.Citation45 Pathological wounds, such as diabetic foot ulcers, can be classified according to their size (area and depth), infection and ischemia. Ulcers in the early stages are limited to the skin and subcutaneous tissue, and in severe cases they can reach muscles, tendons or deeper.Citation46
Figure 1 Schematic diagram of skin structure (A), physical trauma (B), chemical trauma (C) and pathological trauma (D).
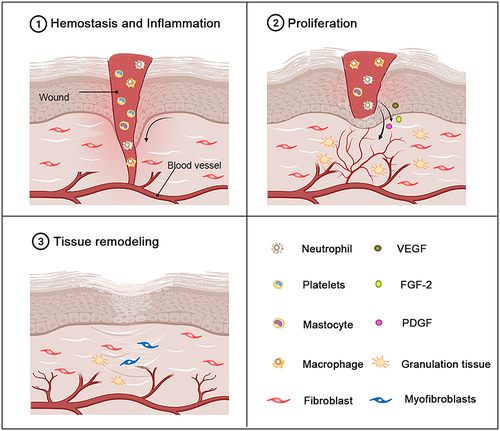
Wound repair is a complex dynamic self-repairing process of the skin (or other organ tissues) affected by interactions among multiple repair cells, growth factors and the extracellular matrix after an injury occurs, and it usually comprises three stages.Citation47,Citation48 The first stage is hemostasis and inflammation.Citation49 Vasoconstriction, platelet aggregation and fibrin clot formation occur immediately after skin trauma and participate in wound hemostasis. Inflammation is caused by the formation of fibrin clots and the degranulation of aggregated platelets, which release chemokines. These chemokines recruit white blood cells (mainly neutrophils), macrophages or mast cells from the blood into the wound.Citation50–53 The inflammatory response begins to decrease a few days after trauma, accompanied by apoptosis of inflammatory cells, and tissue fragments and invasive microorganisms are removed during the inflammatory response.Citation11,Citation48,Citation54 The second stage is cell proliferation and differentiation: in the inflammatory stage, the remaining inflammatory cells, migrating epithelial cells and dermal cells induce and maintain cell proliferation and start cell migration via autocrine, paracrine and other growth factors.Citation11,Citation55,Citation56 In this stage, vascular endothelial cells begin to form, and vascular endothelial cells degrade the basement membrane under the mediating effects of vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF-2) and platelet-derived growth factor (PDGF); the cells migrate to the wound, proliferate, form intercellular contacts, and finally form blood vessels. At the same time, fibroblasts proliferate in the wound, synthesize extracellular matrix and form granulation tissue with rich neovascularization.Citation48,Citation57,Citation58 The entire stage is characterized by dynamic and interactive feedback between cells and the extracellular matrix mediated by growth factors and cytokines.Citation59 Tissue reconstruction or scar formation is the final stage of wound repair. The newly formed granulation tissue is reconstructed into scar tissue. The matrix temporarily formed in the above stage mainly contains collagen III, fibronectin and hyaluronic acid and is gradually replaced by the extracellular matrix, which mainly contains collagen I. Then, the migration of fibroblasts and myofibroblasts and the subsequent reconstruction of granulation tissue matrix leads to wound contraction.Citation58 Finally, fibroblasts undergo apoptosis to form scar tissue with very few cells, and the tensile strength of the scar tissue is similar to that of normal skin tissue ().Citation60
Figure 2 The different stages of normal wound healing. Normal wound healing is a complex biological process that can be divided into three stages: the hemostasis and inflammatory stage, the proliferation stage and the tissue remodeling stage. The inflammatory stage occurs shortly after injury and is characterized by the influx of inflammatory factors. In response to inflammatory signals, neutrophils, macrophages and mast cells are migrate to the wound. As the inflammatory stage subsides and the proliferative phase of tissue repair begins, the dermis and epidermal cells migrate and proliferate excessively in the wound bed. Epithelialization, collagen deposition, angiogenesis, and formation of granulation tissue occur during this stage. The beginning of the stage of tissue remodeling is characterized by matrix remodeling and decreased cell density. At this stage, the wound undergoes contraction to form a scar.
Factors Influencing Skin Wound Repair
Key Influence Factors
The speed and quality of wound repair are affected by the interaction of various factors, and further study of these factors could lead to the development of new and effective treatments. In general, the influencing factors can be divided into local factors and systemic factors. Local factors can directly affect the characteristics of the wound itself to influence the wound repair process, while systemic factors mainly influence the pathological, psychological and physiological states of the body, thereby affecting wound repair ability.Citation61,Citation62 Local factors affecting wound repair mainly include temperature,Citation63,Citation64 infection,Citation62,Citation65 oxidative stress levels.Citation66 Systemic factors mainly include age,Citation67 healthy state,Citation68,Citation69 autoimmune disease,Citation70 genetic factors,Citation71 psychological factors,Citation72 living habitsCitation73 and so on. Normally, these factors are interrelated, and systemic factors affect the wound repair process mainly through their effect on local wounds. The above factors can delay or promote the process of wound healing by affecting one or more aspects of the process and thus become the focus of attention in the process of wound treatment.
Influence of the Local Microenvironment of Wounds
In recent years, a number of studies have shown that skin wound repair not only is regulated by growth factors, nucleic acids, cells and other active components but also is affected by the microenvironment of the wound site.Citation74 In the process of wound healing, these active components of the wound site interact with the wound microenvironment in a continuous and bidirectional way, which plays a key role in accelerating wound repair speed and driving regenerative repair.Citation75 For example, the accumulation of excessive reactive oxygen species (ROS) caused by hyperglycemic stimulation and a chronic inflammatory response is one of the characteristics of skin wounds in patients with chronic diabetes and hinders the repair process.Citation76 Moreover, continuous exposure of endogenous proteins, nucleic acids, lipids and other important biomacromolecules at the wound site to high concentrations of ROS may lead to irreversible damage to their structure and function, thus leading to impaired wound repair.Citation77 Studies have shown that in a microenvironment characterized by inflammation and oxidative stress, the proliferation and redifferentiation of stem cells is significantly reduced and apoptosis can be induced, limiting their regenerative function.Citation78,Citation79 A large number of studies have found that reducing the level of excessive ROS and oxidative damage at the wound site and improving the wound microenvironment are significantly conducive to repairing various skin wounds.Citation80
Influence of Pathogen Infection
In the process of wound repair, the infection of external bacteria will lead to the delay of wound healing.Citation81,Citation82 Therefore, the prevention and treatment of wound infection is one of the focal issues in the process of skin wound repair, especially the wounds caused by burns and scalds, which are prone to the infection of Gram-positive bacteria such as Staphylococcus aureus.Citation83,Citation84 Moreover, the use of inadequately sterilized surgical instruments increases the risk of infection during wound repair surgery. For instance, infections caused by Gram-negative bacteria such as Pseudomonas aeruginosa, Enterococcus and Acinetobacter are more common in the healing of chronic wounds, while inflammation caused by bacterial infection will delay the healing of wounds, form chronic wounds, increase the medical costs of patients, and affect the physical and mental health of patients.Citation83,Citation85,Citation86
Biological Nanomaterials for Skin Wound Repair
Nanomaterials are natural or artificial materials consisting of basic particles in powder form or clumps. One or more dimensions of this basic particle are between 1 nm and 100 nm, and the total number of this basic particle accounts for more than 50% of the total number of all particles in the material as a whole. Furthermore, nanomaterials composed of nanocrystals have a set of innovative physical properties, such as a small size effect,Citation87 interface effect,Citation88 quantum effect and quantum tunneling effect.Citation89,Citation90 Naturally, nanomaterials used in skin wound repair have different functions according to their physical and chemical properties and structures.
Nanomaterials with Biological Activity
Bioactive nanomaterials, usually with relatively clear chemical structures and surface properties, can interact with proteins, cells or tissues and cause biological reactions, which directly affect the interaction between materials and tissues or cells. In recent years, many bioactive nanomaterials have been developed for wound repair or tissue regeneration, and these nanomaterials have antibacterial, antioxidant, nano adhesion and other biological activities and have good effects with regard to accelerating wound healing, eliminating ROS on the wound surface and inhibiting infection.Citation91–94
Metal and Metal Oxide Nanomaterials
Silver Nanomaterials
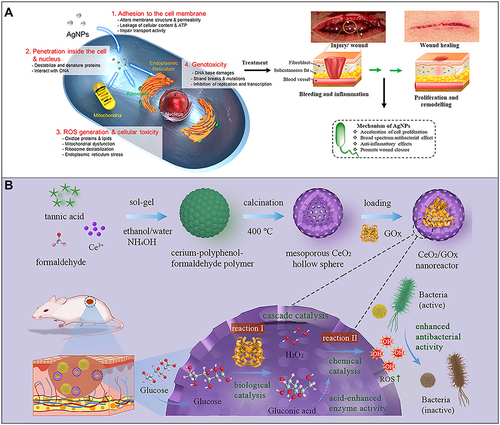
Silver nanomaterials (AgNPs) are used as therapeutic agents for wound healing mainly because of their significant anti-inflammatory and antibacterial properties.Citation95,Citation96 The main mechanism underlying the antibacterial activity of AgNPs can be simplified to the high surface area of Ag+ released by them. AgNPs in the aqueous environment are oxidized in the presence of oxygen and protons, and Ag+ is released when the surface of a particle dissolves. Ag+ can interact with mercaptan groups in key bacterial enzymes and proteins and disrupt cell respiration, resulting in cell death.Citation97,Citation98 The specific mechanisms are considered to be as follows. 1. AgNPs adhere to the surface of the cell membrane, which leads to the destruction of the cell membrane and changes in transport activity. 2. AgNPs enter the cell and interact with many organelles (mitochondria, etc.) and biomolecules (protein, DNA, etc.), resulting in its dysfunction. 3. AgNPs induce cytotoxicity and oxidative stress by producing ROS and free radicals. 4. Genotoxicity is induced by AgNPs and Ag+.Citation97,Citation99,Citation100 A large number of studies have shown that by reducing the expression levels of proinflammatory cytokines, such as interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α) and so on, AgNPs can inhibit a variety of pathogenic microorganisms, including fungi, different types of bacteria and even viruses and have shown excellent anti-inflammatory activity, which means that they can be used to prevent or reduce wound infection.Citation101–104 Similarly, in diabetic wounds, AgNPs accelerate wound healing by activating the proliferation and migration of keratinocytes and helping fibroblasts differentiate into myofibroblasts, thus promoting wound contraction and accelerating the healing of diabetic ulcers. AgNPs are widely used in wound healing because they accelerate cell proliferation, possess broad-spectrum antibacterial and anti-inflammatory activities, and promote wound healing ().Citation105–107 Additionally, unlike conventional antibiotic therapy which faces the challenges of multiple resistance and biofilm formation, silver nanoparticles do not pose these difficult problems.Citation108
Figure 3 (A) Schematic diagram of the antibacterial mechanism and wound healing mechanism of silver nanomaterials. Exposure to silver nanomaterials (AgNPs) prevents bacterial colonization and inflammation in the wound, thereby promoting wound closure. Adapted with permission from: Lee SH, Jun BH. Silver nanoparticles: synthesis and application for nanomedicine. Int J Mol Sci. 2019;20(4):E865. doi:10.3390/ijms20040865.Citation97 © 2019 by the authors. Licensee MDPI, Basel, Switzerland (http://creativecommons.org/licenses/by/4.0/). And from: Nqakala ZB, Sibuyi NRS, Fadaka AO, Meyer M, Onani MO, Madiehe AM. Advances in nanotechnology towards development of silver nanoparticle-based wound-healing agents. Int J Mol Sci. 2021;22(20):11272. doi:10.3390/ijms222011272.Citation125 Copyright © 2021 by the authors. Licensee MDPI, Basel, Switzerland. Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/). (B) Establishment of a mesoporous CeO2 hollow sphere/enzyme nanoreactor and schematic diagram of cascade catalytic antibacterial therapy. Reproduced with permission from: Qin J, Feng Y, Cheng D, et al. Construction of a mesoporous ceria hollow sphere/enzyme nanoreactor for enhanced cascade catalytic antibacterial therapy. ACS Appl Mater Interfaces. 2021;13(34):40302–40314. doi:10.1021/acsami.1c10821.Citation124 Copyright © 2021, American Chemical Society.
Copper Nanoparticles (CuNPs)
Compared to other metallic nanomaterials such as silver and gold nanoclusters, CuNPs with good biocompatibility can be prepared quickly and produced at a low cost.Citation109,Citation110 Moreover, CuNPs may interfere with viral activity and have a broad spectrum of antibacterial properties by producing oxidative stress that leads to the breakdown of viral or bacterial membranes.Citation111,Citation112 It has been found that 1 μM 80 nm CuNPs promote acute full-layer skin defect wound healing quickly by accelerating skin cell migration, proliferation, and new blood vessels.Citation113 In addition, phenytoin-loaded copper nanoparticles have been shown to accelerate epidermal regeneration and stimulate granulation and tissue formation in rats.Citation114 Therefore, CuNPs is a material with great potential for effective and rapid wound healing.
Ceria Nanomaterials
Ceria nanomaterials display good biocompatibility and high antioxidant capacity and can be used to remove ROS in the lesion site. As a nano antioxidant enzyme with excellent performance, ceria materials have a large number of oxygen defects on the surface, and Ce3+ and Ce4+ can be rapidly transformed into each other, which confers on them antioxidant enzyme activity and a renewable ability to remove a variety of ROS.Citation115–118 Numerous in vitro and in vivo studies have shown that ceria nanomaterials can protect tissue cells from oxidative damage, prolong cell life in the oxidative microenvironment, promote cell proliferation and migration, and reduce the activity of functional enzymes such as those involved in inflammation.Citation119–121 Ceria is considered an antibacterial agent that kills bacteria by a Fenton-like reaction in the presence of H2O2.Citation122,Citation123 Qin et alCitation124 established a mesoporous ceria hollow sphere/enzyme nanoreactor by preparing ceria hollow sphere nanozymes loaded with glucose oxidase. The nanoreactor can effectively convert nontoxic glucose into highly toxic hydroxyl radicals through a cascade catalytic reaction, thus seriously destroying the cell structure of bacteria and preventing the formation of biofilms. In addition, gluconic acid can reduce the local pH value and further improve the peroxidase-like catalytic performance of mesoporous ceria. At the same time, in vivo experiments showed that the nanoreactor eliminated up to 99.9% of the bacteria in the wound tissue to prevent persistent inflammation and did not damage normal tissue ().
Metal-Organic Frameworks (MOFs)
MOFs are inorganic-organic hybrid porous nanomaterials self-assembled from metal ions/clusters and multi-tooth organic ligands.Citation126 Compared with traditional organic and inorganic drug delivery systems, drug carriers based on MOFs have higher drug load and the possibility of chemical functionalization, which can enhance drug affinity.Citation127,Citation128 They are widely used in biological fields such as drug delivery and biomarker detection due to their large pore volume, pore size and surface area, as well as adjustable surface functionality, and the metal-ligand bond strength is determined by the properties of metal ions and ligands, and is also the key to determining the hydrothermal stability of MOFs.Citation127,Citation129,Citation130 Recently, Yao et alCitation131 prepared a Zn-MOF encapsulated methacrylated hyaluronic acid (MeHA) microneedles (MNs) array. This MNs array has excellent antibacterial activity and considerable biocompatibility. It can significantly accelerate epithelial regeneration and neovascularization, promote wound healing, and play an important role in promoting wound healing.
Upconversion Nanoparticles (UCNPs)
Among many nanomaterials, UCNPs with luminescent properties show unique advantages, which are light stability, persistent luminescence, deep tissue penetration and self-fluorescence inhibition and so on in medical diagnosis and treatment.Citation132 In order to make UCNPs have good water dispersion and biocompatibility, a large number of studies have been conducted to functionalize them by exchanging oleic acid ligands with DNA, which has great advantages in drug delivery targeting and imaging function in recent years.Citation133,Citation134 Sun et alCitation135 reported an antibacterial nanocomposite film containing UCNPs. After 5min was irradiated by near-infrared light, UCNP in the nanocomposite film could trigger the release of ROS by photosensitizer, thus rapidly killing Gram-positive Staphylococcus aureus (94.5%) and Gram-negative Escherichia coli (93.2%). This work provides a new strategy for designing new antibacterial materials for anti-infection and wound healing.
Nonmetallic Nanomaterials
Polymer Nanomaterials
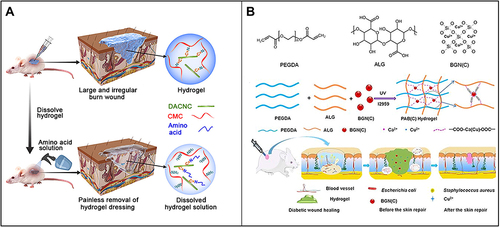
Nanopolymers (especially natural ones), which have excellent biocompatibility and biodegradation, have been widely used in biomedical research.Citation136–138 For instance, as a kind of representative polymer nanomaterial, chitosan nanoparticles have good application prospects in the field of wound repair due to their excellent mucosal adhesion, anti-infection activity and hemostatic activity.Citation139–141 Huang et alCitation142 developed a nanocomposite self-healing hydrogel using water-soluble carboxymethyl chitosan (CMC) and rigid rod-like dialdehyde-modified cellulose nanocrystal (DACNC), which can be injected into irregular and deep burn wound beds. Once injected, it quickly heals, reforms a complete hydrogel, thoroughly fills the wound area and protects the wound from the external environment ().
Figure 4 (A) Carboxymethyl chitosan (CMC) combined with dialdehyde-modified cellulose nanocrystal (DACNC) nanocomposite hydrogel for irregular and deep burn wounds. Reproduced with permission from: Huang W, Wang Y, Huang Z, et al. On-demand dissolvable self-healing hydrogel based on carboxymethyl chitosan and cellulose nanocrystal for deep partial thickness burn wound healing. ACS Appl Mater Interfaces. 2018;10(48):41076–41088. doi:10.1021/acsami.8b14526.Citation142 Copyright © 2018, American Chemical Society. (B) Synthesis of bioactive silica-based nanocomposite (PABC) hydrogel scaffold and its potential application and hypothetical mechanism in diabetic wound healing. Reproduced with permission from: Li Y, Xu T, Tu Z, et al. Bioactive antibacterial silica-based nanocomposites hydrogel scaffolds with high angiogenesis for promoting diabetic wound healing and skin repair. Theranostics. 2020;10(11):4929–4943. doi:10.7150/thno.41839.Citation146 Copyright © The author(s). Creative commons attribution license (https://creativecommons.org/licenses/by/4.0/).
Silicon Oxide Nanomaterials
Meddahi-Pellé et alCitation143 discovered and demonstrated that aqueous silica nanomaterials had a nanobridge effect that enabled rapid and strong bonding in both skin wounds and organic deep wounds. Silica nanoparticle adhesive aqueous solution for wound repair has the advantages of quick and convenient use and it does not require complex and time-consuming operation.Citation144 Unlike polymer tissue adhesives, silica nanoparticle adhesives do not require complex cross-linking reactions; furthermore, they still have a good adhesion effect under the humid environment in the body.Citation19 Moreover, unlike traditional suture, suture nail and other closed wound treatment methods, nano aqueous solution does not readily cause secondary damage to soft tissues or organs, such as the skin, liver and lung, and does not require subsequent stitch removal.Citation145 Li et alCitation146 reported a bioactive silica-based nanocomposite hydrogel scaffold (PABC scaffold) with high angiogenesis-promoting abilities for supporting diabetic wound healing and skin repair, including the main network formed by polyethylene glycol diacrylate (PEGDA) and an auxiliary dynamic network formed between copper-containing bioactive glass nanoparticles containing copper (BGNC) and alginate (ALG). The scaffold can significantly promote the viability, proliferation and angiogenesis of endothelial progenitor cells (EPCs) in vitro. In vivo, it can promote early angiogenesis/neovascularization, effectively repair the vascular network and significantly accelerate wound healing and skin tissue regeneration by increasing the expression levels of hypoxia-inducible factor-1α (HIF-1α)/VEGF and the deposition of collagen matrix in diabetic full-thickness wounds ().
Carbon Nanomaterials
Carbon nanomaterials have the advantages of large drug load, good biocompatibility and strong targeting, especially which are modified.Citation147 Carbon nanotubes are ideal drug carriers for their large surface area, excellent optical properties and good compatibility with cells. Their water dispersion and biocompatibility coated with polymer materials are greatly improved, and their precious metal coordination and binding abilities are enhanced, which have significant advantages in drug delivery, biosensor and wound diagnosis.Citation148 Magnetic hollow carbon nanospheres with acorn as carbon source are prepared via high temperature calcination method, and the drug loading and release of ibuprofen show that they have good drug loading and release ability.Citation149 Carbon nanotubes have also been studied as potential antimicrobial agents with inherent antibiotic activity. Carbon nanotubes have effective bactericidal activity against Enterobacter and Escherichia coli, which can cause cell aggregation and microbial death.Citation150 This may be expected to be used in the process of wound repair.
Nanofiber Materials
Nanofiber materials have a large specific surface area, high porosity and facilitate the transport of nutrients and oxygen, which led researchers to the idea of using them for drug delivery.Citation151,Citation152 In recent years, breakthroughs have been made in the research and preparation of starch nanofibers for drug loading and external application. For example, starch nanofibers prepared by crosslinking method have the advantages of controllable sustained release and good biocompatibility.Citation153 Xuan et alCitation154 prepared an injectable nanofiber polysaccharide self-healing hydrogel, which can significantly promote wound healing.
Biological Nanomaterials for Topical Therapeutic Drug Delivery Carriers
Biological nanomaterials used for local therapeutic drug delivery carriers are mainly employed to transport nitric oxide, antioxidants, growth factors, nucleic acids, antibiotics, and other active ingredients to promote wound repair. Here, we focus on the following four types of active ingredients loaded in nanomaterials.
Nanomaterials Loaded with Growth Factors
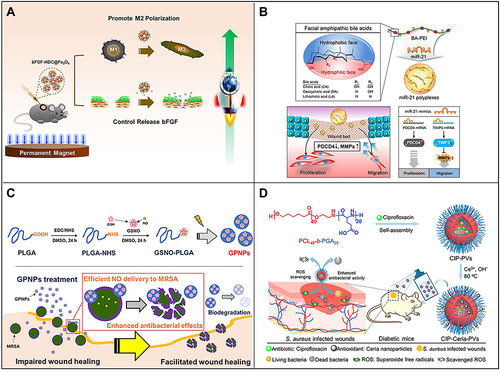
It is well known that growth factors play a key role in all stages of wound repair.Citation56 For example, recombinant human platelet-derived growth factor has been approved by the American FDA for the treatment of chronic diabetic foot.Citation155 Under normal physiological conditions, growth factors are not stable and are easily degraded by enzymes in the wound microenvironment. In addition, high local concentrations of these factors may even cause disorder in the wound repair process; however, loading growth factors into nanomaterials can effectively prevent these problems. Furthermore, they can achieve sustained release and improve the therapeutic effect of growth factors in the process of wound repair.Citation156–158 Choi et alCitation159 designed and established an electrospun nanocomposite containing human recombinant epidermal growth factor (EGF), which achieved continuous and effective release of EGF and achieved good results in diabetic wound repair. In addition, Wu et alCitation160 based on the Fe3O4 nanoparticle, developed a basic fibroblast growth factor (bFGF) loaded Fe3O4 nanoparticle (bFGF-HDC@Fe3O4) using a simple mussel-inspired surface immobilization method, which can stabilize bFGF under different conditions and exhibit a sustained release effect. It has been found that bFGF-HDC@Fe3O4 can greatly promote wound healing by promoting M2 macrophage polarization and cell proliferation. Therefore, the continuous release of growth factors with regulatory behavior is achieved by modifying nanoparticles, which provides a broad prospect for their application to tissue regeneration ().
Figure 5 (A) Fe3O4 nanoparticles loaded with basic fibroblast growth factor (bFGF) can greatly promote wound healing by polarizing M2 macrophages and promoting cell proliferation. Reproduced with permission from: Wu J, Zhu J, Wu Q, et al. Mussel-inspired surface immobilization of heparin on magnetic nanoparticles for enhanced wound repair via sustained release of a Growth Factor and M2 Macrophage Polarization. ACS Appl Mater Interfaces. 2021;13(2):2230–2244. doi:10.1021/acsami.0c18388.Citation160 Copyright © 2021, American Chemical Society. (B) A schematic diagram of the use of miRNA-21 mimic nanocarriers in the treatment of skin wounds and the role of miRNA-21 mimics in wound repair by activating cell proliferation and migration. Reproduced with permission from: Wang SY, Kim H, Kwak G, et al. Development of microRNA-21 mimic nanocarriers for the treatment of cutaneous wounds. Theranostics. 2020;10(7):3240–3253. doi:10.7150/thno.39870.Citation166 Copyright © The author(s). Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). (C) Schematic diagram of S-nitrosoglutathione-conjugated poly(lactic acid-glycolic acid) nanoparticles (GPNPs) synthesis and treatment of methicillin-resistant Staphylococcus aureus (MRSA)-infected skin wounds. Reproduced with permission from: Lee J, Kwak D, Kim H, et al. Nitric Oxide-Releasing S-Nitrosoglutathione-Conjugated Poly(Lactic-Co-Glycolic Acid) Nanoparticles for the Treatment of MRSA-Infected Cutaneous Wounds. Pharmaceutics. 2020;12(7):E618. doi:/10.3390/pharmaceutics12070618.Citation183 Copyright © 2020 by the authors. Licensee MDPI, Basel, Switzerland. Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/). (D) Preparation of CIP-loaded and ceria-decorated polymer vesicles (CIP-Ceria-PVs) and combination of antioxidants-antibiotics in the treatment of diabetic infected wounds. Reproduced with permission from: Wang T, Li Y, Cornel EJ, Li C, Du J. Combined Antioxidant-Antibiotic Treatment for Effectively Healing Infected Diabetic Wounds Based on Polymer Vesicles. ACS Nano. 2021;15(5):9027–9038. doi:10.1021/acsnano.1c02102.Citation195 Copyright © 2021, American Chemical Society.
Nanomaterials Loaded with Nucleic Acids
MicroRNAs (miRNAs) have been shown to be potentially involved in the entire wound healing process in a highly decisive and coordinated manner.Citation161–164 However, exogenous miRNA therapy has several limitations, including poor stability, low cell uptake rate, limited selectivity for target tissues and so on. To improve the local release of miRNAs and improve the efficiency of wound healing, the method of introducing nanomaterials to load nucleic acid molecules has been continuously studied.Citation165–167 Nanomaterials can also deliver DNA, siRNA, miRNA and other nucleic acid molecules to upregulate or inhibit the expression of target genes, thereby regulating of the skin wound repair process.Citation168,Citation169 Randeria et alCitation170 connected thiolated siRNA to the surface of gold nanoparticles with a diameter of 13 nm, which was used to downregulate monosialoganglioside 3 synthase expression in diabetic ulcer sites. The experimental results showed that siRNA-loaded nanocomposites can promote keratinocyte migration and proliferation and thus effectively promote the repair of diabetic lesions. Wang et alCitation166 introduced miRNA-21 nanocarriers electrostatically complexed with three types of bile acid-attached polyethyleneimine (BA-PEI) conjugates, including cholic acid (CA), deoxycholic acid (DA), and lithocholic acid (LA) for treatment of skin wounds. This nanocarrier delivery system has been found to regulate programmed cell death protein 4 (PDCD4) and matrix metalloproteinases (MMPs) after transcription to promote cell migration and proliferation, which in turn helps to accelerate wound repair ().
Nanomaterials Loaded with Therapeutic Gases
Endogenous gas signaling molecules, such as nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S), can affect vascular dilation, transfer information among cells, participate in neural development and regulate gene expression.Citation171–173 At the site of skin wounds, severe trauma often leads to damage to blood vessels, nerves and tissues, and wound repair requires the active participation of these gas signaling molecules.Citation174,Citation175 As the earliest discovered and studied gas signaling molecule, NO has been applied in a large number of products for the clinical treatment of various diseases.Citation176 NO is an endogenous fungicide produced by a large number of macrophages and has broad-spectrum antibacterial properties. It has been reported that NO can destroy bacterial cell membranes, proteins and DNA through the formation of active nitrogen species or direct nitrosation, resulting in bacterial cell death.Citation177,Citation178 In addition to the antibacterial activity of NO, NO has attracted much attention as a new type of infectious wound healing drug because of its beneficial role in regulating inflammation and promoting wound healing, such as through promoting cell proliferation and tissue remodeling.Citation176,Citation179 In the wound repair field, NO can promote angiogenesis at the wound site by activating signaling pathways related to vascular endothelial growth factor, basic fibroblast growth factor and transforming growth factor-β.Citation180,Citation181 It has been found that NO loaded in poly(lactic acid-glycolic acid)(PLGA)-doped polyethyleneimine (PEI) (PLGA-PEI) composite nanoparticles significantly improved the repair speed of wounds infected with methicillin-resistant Staphylococcus aureus.Citation182 Lee et alCitation183 synthesized S-nitrosoglutathione (GSNO)-conjugated PLGA (GSNO-PLGA) and used this new polymer to prepare GSNO-PLGA nanoparticles (GPNPs) for the treatment of methicillin-resistant Staphylococcus aureus (MRSA)-infected wounds. GPNPs had a higher NO load and showed stronger antibacterial activity against MRSA than did GSNO ().
Nanomaterials Loaded with Antibiotics
Traditional wound dressings mainly play a role in hemostasis, infection control, wound cleaning and exudate absorption. This method requires large doses of antibiotics and takes weeks to months. In addition, this treatment can produce drug-resistant pathogens in the body.Citation184–186 More effective antibiotic strategies have been developed. For example, antibiotics can be loaded in functional nanoparticles, such as micelles,Citation187 vesicles,Citation188 nanosheetsCitation189 and so on, which can achieve slow release of antibiotics, resulting in a continuous antibacterial effect, and can also be functionalized through targeted modifications. These characteristics reduce the required dose of antibiotics, thereby reducing the chance of developing antibiotic resistance.Citation190–192 Due to their intrinsic biological activity and therapeutic efficacy, nanomaterials can regulate different stages of wound repair, which promises to be a new approach to wound treatment. Evidence verified that mesoporous silica nanoparticles (MSNs) loaded with the broad-spectrum antibiotic tetracycline in their channels and mixed with carboxymethyl cellulose hydrogel synchronously absorbed wound exudate and released antibiotics to prevent infection, which could effectively promote the skin wound repair process.Citation193,Citation194 Wang et alCitation195 proposed a method to treat diabetic infected wounds based on polymer vesicles combined with antioxidant-antibiotic therapy. Polymer vesicles are self-assembled by amphiphilic block copolymers poly(ε-caprolactone)-block-polyglutamic acid. In this self-assembly process, ciprofloxacin (CIP) is loaded into the vesicles, and ceria nanoparticles grow in situ on the CIP-loaded polymer vesicles after self-assembly. These CIP-loaded and ceria-decorated polymer vesicles (CIP-Ceria-PVs) showed strong antibacterial activity and scavenging ability of superoxide anion free radicals in the treatment of diabetic infected wounds ().
Therefore, based on the various factors influencing the process of skin wound repair, a variety of multifunctional composite nanobioactive materials have been designed at the micro and nano scales to combine the unique advantages of each material, and when these composites are loaded with the appropriate therapeutic drugs, this approach is expected to result in new safe and effective wound treatment methods. In recent years, nanomaterials used as local drug delivery carriers have become a research hotspot in the biomedical field, but there are still some problems and challenges in the clinical transformation application. Although drug-carrying nanoparticles have some advantages in terms of particle size, synthesis method, surface modification and biocompatibility, it is still a challenge to study the behavior, toxicity, biological distribution and clearance mode of these nano-delivery systems in vivo before they can be used in clinic.Citation196 Therefore, the clinical transformation of making drug-loaded nanoparticles as drug delivery system is a key problem to be solved in the development of drug-loaded nanomaterials.
Multifunctional Composite Biological Nanomaterials
In recent decades, considering that a single functional material or therapeutic has difficulty addressing the multiple factors of skin wound repair, the strategies of wound tissue repair and regeneration and local drug delivery, based on multifunctional nanocomposite materials, have gradually attracted the attention of researchers.Citation197 The establishment of multifunctional nanocomposites and local drug delivery systems can not only retain the biological activity of nanomaterials but also overcome the deficiency of the poor efficacy of single materials when carrying therapeutic drugs. Moreover, the overall performance of composite materials is optimized, and this approach is expected to open a new direction for skin wound repair and local drug delivery.Citation146,Citation198,Citation199
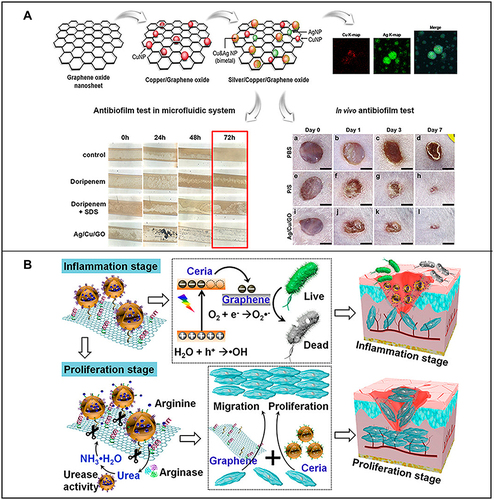
For example, due to high surface charge density, silicate nanosheets can induce coagulation factor aggregation to promote coagulation; moreover, they can also greatly improve the physiological stability, injectability and hemostatic ability of nanocomposite hydrogels and have a good synergistic effect with a gelatin matrix.Citation200 A study showed that nanocomposite hydrogels with shear thinning properties can be used as injectable hemostatic agents by loading silicate nanosheets into a gelatin matrix.Citation201 Castleberry et alCitation202 established a nanocoating containing siRNA based on a nylon matrix using layer-layer self-assembly technology, which achieved the sustained release of siRNA in the wound site, thus successfully downregulating the expression of target gene metalloproteinase-9 and greatly promoting the repair of diabetic wounds. Jang et alCitation203 prepared silver and copper bimetallic nanoparticles on the surface of graphene oxide (GO) by a chemical reduction method (Ag/Cu/GO). To evaluate the potential of the antibacterial treatment in vivo, the effect of Ag/Cu/GO on the skin of mice subjected to trauma and infected with Pseudomonas aeruginosa was analyzed. It was found that local application of Ag/Cu/GO could effectively remove antibiotic-resistant bacteria and quickly and effectively heal skin or wound diseases caused by bacterial infection and biofilm formation (). Cheng et alCitation204 designed a arginine-loaded and detachable ceria-graphene nanocomposites (ACG NCS). In the inflammatory stage, ACG NCS can effectively produce ROS and kill bacteria under white light irradiation. During the proliferative phase, ceria nanoparticles can be isolated from ACG NCS and absorbed by cells to remove intracellular ROS and promote cell proliferation, while the isolated graphene can be used as a scaffold to promote fibroblast migration to the wound site (). With the advantages of good biocompatibility and small particle size, nanomaterials can be combined with traditional dressings, thus showing advantages of easy fixation and removability in wound repair.Citation205
Figure 6 (A) Preparation of silver/copper/graphene oxide nanocomposites and their antibacterial film effect and promotion of wound healing after Pseudomonas aeruginosa infection. Reproduced with permission from: Jang J, Lee JM, Oh SB, Choi Y, Jung HS, Choi J. Development of Antibiofilm Nanocomposites: Ag/Cu Bimetallic Nanoparticles Synthesized on the Surface of Graphene Oxide Nanosheets. ACS Appl Mater Interfaces. 2020;12(32):35826–35834. doi: 10.1021/acsami.0c06054.Citation203 Copyright © 2020, American Chemical Society. (B) Schematic diagram of the wound healing mechanism of arginine-loaded and detachable ceria-graphene nanocomposites (ACG NCS). Reproduced with permission from: Cheng Y, Chang Y, Feng Y, et al. Hierarchical acceleration of wound healing through intelligent nanosystem to promote multiple stages. ACS Appl Mater Interfaces. 2019;11(37):33725–33733. doi:10.1021/acsami.9b13267.Citation204 Copyright © 2019, American Chemical Society.
Polymer-based nanocomposites, as one of the multifunctional composites and mainly including hyperbranched polymers, liposomes and polymer micelles, have great application value in drug delivery systems due to their good biocompatibility and ability to load hydrophobic/hydrophilic drugs, proteins and other bioactive agents.Citation206,Citation207 For example, polymer micelles are nano-agglomerates with shell/core structure formed by self-assembly of amphiphilic block copolymer in aqueous solution, and widely used in the field of wound repair.Citation208,Citation209 Their hydrophobic core can encapsulate a variety of hydrophobic anti-inflammatory and antibacterial drugs and improve their stability and solubility without changing their chemical structure, so the hydrophilic shell and nano particle size are conducive to drug release and enrichment at the trauma site.Citation209 Another multifunctional nanocomposite materials are hydrogel nanocomposites used for drug sustained-release material, wound dressing and scaffold for tissue engineering during wound repair treatment, with good biocompatibility, shaping ability and performance characteristics similar to extracellular matrix.Citation210,Citation211 However, the further development of hydrogels is still limited because of their poor mechanical properties and self-healing properties, which has great potential for improvement. The introduction of different types of nanoparticles into hydrogel nanocomposites, such as carbon-based, polymer-based, inorganic and metal-based nanoparticles, is a common method to obtain nano-composite hydrogels with excellent properties and customized functions, which can not only enhance the mechanical properties, self-healing properties and chemical properties, but also improve their stability.Citation197,Citation198,Citation212 Therefore, multifunctional nanocomposites have a good application potentiality in the field of wound repair.
Therefore, nanomaterials and physical materials with different physical and chemical properties, biological activities and drug loading capacities are synergistically integrated into nanocomposites, providing a new approach to wound repair, tissue regeneration and local drug delivery. Multifunctional nanocomposites take full advantage of the unique physicochemical properties and synergistic gains of therapeutic drugs, biomaterials and nanomaterials, and this approach is beneficial for addressing the diverse pathological mechanisms discovered in research and advancing the field of wound repair and tissue regeneration.
Toxicity and Side Effects and the Challenges and Perspectives of Biological Nanomaterials in Wound Repair
At present, with the increasing frequency of the usage of nanomaterials, there is an urgent need to evaluate their biological safety. However, the establishment of a nanomaterial biosafety evaluation system is still in the exploratory stage, and the biosafety evaluation of nanomaterials mainly focuses on the toxicological study of their health effects, but there is still a lack of follow-up observation and evaluation after clinical wound repair. Nanomaterials can enter cells and affect the transmembrane process, cell division, proliferation, apoptosis and other basic life processes and the regulation of related signal transduction pathways, so as to produce certain biological effects at the cellular level.Citation91,Citation213 It has been found that carbon nanotubes can easily enter human cells and affect the cell structure. At low doses, carbon nanotubes can stimulate the phagocytosis of macrophages, but at high doses, they can seriously reduce the phagocytosis of macrophages to exogenous poisons.Citation214,Citation215 Cells treated with nano TiO2 can be detected to leak large amounts of Ca2+, and studies have shown that nano TiO2 can attack cell membranes, causing them to rupture, leading to cell death.Citation216,Citation217 What’s more, after entering human cytoplasm by virtue of the tiny properties, nano TiO2 can oxidize and damage cellular genetic material depending on their high chemical activity. Research found that 8-hydroxyguanosine could be detected in RNA, which were separated from the cells treated by nano TiO2, thus resulting in that the expression of cell genetic information is affected indirectly via RNA.Citation218 ROS generation and oxidative stress response are important mechanisms of nanomaterials to induce a variety of biological toxicity effects.Citation219 The toxicity study of nano-ZnO shows that nano-ZnO has higher cytotoxicity and could significantly increase intracellular ROS levels, deplete glutathione, and significantly reduce malondialdehyde and superoxide dismutase contents.Citation220 Therefore, oxidative stress may be a major manifestation of the cytotoxicity of nanomaterials.
Many consumer products such as sunscreens and cosmetics may contain nano-TiO2, so it is meaningful to study the skin absorption of these nanoparticles. In one study, pig skin, considered most similar to human skin, was treated by nano-TiO2, and particle-induced X-ray fluorescence analysis was used to observe the distribution of nano-TiO2 in the skin structure. The results showed that nano-TiO2 can enter the granular layer below the epidermis through the cuticle, especially in the epidermal germinal layer.Citation221 Moreover, deposits of ultrafine TiO2 particles in sunscreen were found in the cuticle and dermal papilla of hair follicles.Citation222,Citation223
According to the current research results, nanomaterials have three main characteristics promoting skin penetration. The first is related to the particle size of nanomaterials, and the smaller the particle size, the easier it is for it to penetrate the skin. Second, the degree of skin irritation is determined by the properties of the nanomaterial entering the dermis. Third, nanoparticles or other extracts containing soluble substances and metals easily penetrate human skin.Citation224–226 Due to the use of various nanoparticle types and their different physical and chemical properties, even the same nanomaterial with different particle sizes will display different biological effects. Therefore, there is a particularly urgent need to evaluate the biosafety of the new nanomaterials emerging every year, and researchers and designers should try to avoid or lessen these toxic side effects when designing related nanomaterials.
Although some biological effects of nanomaterials have been assessed, the potential toxicity and possible effects are not clear. On account of the high yield of nanomaterials and the urgency of screening potential hazards, the current priority is to reach an international convention on the impact of nanotechnology on the human body and the environment, and establish scientific and perfect detection methods. However, there is no systematic study on the metabolism and biochemical reaction of nanomaterials toxicity in vivo. Therefore, the preliminary establishment of a structure-activity relationship model provides a scientific basis for comprehensive and accurate evaluation of the toxicity of nanomaterials. In recent years, flow cytometry, high throughput virtual screening and metabonomics all have been widely used in toxicological research, but the biggest problem is the uncertainty and randomness of evaluation methods, and there is no objective index that can directly reflect the toxicity of nanomaterials. Although nanomaterials may produce some toxic effects, the current research results and data are not sufficient to indicate that these toxic effects will become a major problem in wound repair, and they have great potential clinical application value, we should treat the impact of nanomaterials positively and objectively.
Conclusions and Perspectives
Various acute and chronic skin wounds are common clinical problems; moreover, the mortality and disability rates caused by chronic diabetes wounds are high, which all result in substantial health problems and inconvenience for patients. Although the existing clinical wound treatment methods can control infection, stop bleeding and absorb exudates to some extent, various difficulties still exist, and the ideal wound repair effect still cannot be achieved. In recent years, nanomaterials with good biocompatibility, controllable preparation methods and unique physicochemical and biological properties have not only effectively overcome the shortcomings of traditional therapeutic methods but also show unique functions and properties.
This review summarizes three types of nanomaterials currently used in the treatment of skin wounds, namely, nanomaterials with inherent biological activity, nanomaterials used as local delivery carriers for therapeutic drugs, and multifunctional composite nanomaterials. Nanomaterials currently used in skin wound repair show ideal efficacy and advantages over traditional treatment methods. Moreover, the toxicity and side effects of related nanomaterials are also emphasized, and a sound biosafety evaluation system is expected to be established. We believe that with the development of biomedicine and materials science, additional innovations in skin wound repair nanomaterials with ideal repair functions and few toxic side effects will emerge, resulting in considerable reparative effects in skin wound patients.
Author Contributions
Yue Lin and Zheyan Chen contributed equally. Investigation, data curation, writing - original draft, writing - review and editing: Yue Lin and Zheyan Chen; supervision, writing - review and editing: Yinai Liu, Jiawen Wang and Wang Lv; conceptualization, project administration: Renyi Peng. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in relation to this work.
Acknowledgments
This work was supported by the Basic Medical Technology Project of Wenzhou Science and Bureau (Y20190457) and Natural Science Foundation of Zhejiang Province (LQ20C020003).
References
- Biggs LC, Kim CS, Miroshnikova YA, Wickström SA. Mechanical forces in the skin: roles in tissue architecture, stability, and function. J Invest Dermatol. 2020;140(2):284–290. doi:10.1016/j.jid.2019.06.137
- Swaney MH, Kalan LR, Richardson AR. Living in your skin: microbes, molecules, and mechanisms. Infect Immun. 2021;89(4):e00695–e00720. doi:10.1128/IAI.00695-20
- Lee YI, Choi S, Roh WS, Lee JH, Kim TG. Cellular senescence and inflammaging in the skin microenvironment. Int J Mol Sci. 2021;22(8):3849. doi:10.3390/ijms22083849
- Araviiskaia E, Berardesca E, Bieber T, et al. The impact of airborne pollution on skin. J Eur Acad Dermatol Venereol. 2019;33(8):1496–1505. doi:10.1111/jdv.15583
- Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17(12):E2085. doi:10.3390/ijms17122085
- Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
- Sen CK. Human wound and its burden: updated 2020 compendium of estimates. Adv Wound Care. 2021;10(5):281–292. doi:10.1089/wound.2021.0026
- Zomer HD, Trentin AG. Skin wound healing in humans and mice: challenges in translational research. J Dermatol Sci. 2018;90(1):3–12. doi:10.1016/j.jdermsci.2017.12.009
- Wang Y, Beekman J, Hew J, et al. Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev. 2018;123:3–17. doi:10.1016/j.addr.2017.09.018
- Chouhan D, Dey N, Bhardwaj N, Mandal BB. Emerging and innovative approaches for wound healing and skin regeneration: current status and advances. Biomaterials. 2019;216:119267. doi:10.1016/j.biomaterials.2019.119267
- Kim HS, Sun X, Lee JH, Kim HW, Fu X, Leong KW. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev. 2019;146:209–239. doi:10.1016/j.addr.2018.12.014
- Qu J, Zhao X, Liang Y, Zhang T, Ma PX, Guo B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 2018;183:185–199. doi:10.1016/j.biomaterials.2018.08.044
- Dai C, Shih S, Khachemoune A. Skin substitutes for acute and chronic wound healing: an updated review. J Dermatolog Treat. 2020;31(6):639–648. doi:10.1080/09546634.2018.1530443
- Rosińczuk J, Taradaj J, Dymarek R, Sopel M. Mechanoregulation of wound healing and skin homeostasis. Biomed Res Int. 2016;2016:3943481. doi:10.1155/2016/3943481
- Ashraf I, Butt E, Veitch D, Wernham A. Dermatological surgery: an update on suture materials and techniques. Part 1. Clin Exp Dermatol. 2021;46(8):1400–1410. doi:10.1111/ced.14770
- Butt E, Ashraf I, Veitch D, Wernham A. Dermatological surgery: an update on suture materials and techniques. Part 2. Clin Exp Dermatol. 2021;46(8):1411–1419. doi:10.1111/ced.14812
- Byrne M, Aly A. The surgical suture. Aesthet Surg J. 2019;39(Suppl_2):S67–S72. doi:10.1093/asj/sjz036
- Yag-Howard C. Sutures, needles, and tissue adhesives: a review for dermatologic surgery. Dermatol Surg. 2014;40(Suppl 9):S3–S15. doi:10.1097/01.DSS.0000452738.23278.2d
- Nam S, Mooney D. Polymeric Tissue Adhesives. Chem Rev. 2021;121(18):11336–11384. doi:10.1021/acs.chemrev.0c00798
- Dong R, Guo B. Smart wound dressings for wound healing. Nano Today. 2021;41:101290. doi:10.1016/j.nantod.2021.101290
- Sierra-Sánchez Á, Kim KH, Blasco-Morente G, Arias-Santiago S. Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries. NPJ Regen Med. 2021;6(1):35. doi:10.1038/s41536-021-00144-0
- Przekora A. A concise review on tissue engineered artificial skin grafts for chronic wound treatment: can we reconstruct functional skin tissue in vitro? Cells. 2020;9(7):E1622. doi:10.3390/cells9071622
- Vig K, Chaudhari A, Tripathi S, et al. Advances in skin regeneration using tissue engineering. Int J Mol Sci. 2017;18(4):E789. doi:10.3390/ijms18040789
- Qureshi AA, Ross KM, Ogawa R, Orgill DP. Shock wave therapy in wound healing. Plast Reconstr Surg. 2011;128(6):721e–727e. doi:10.1097/PRS.0b013e318230c7d1
- Luo R, Dai J, Zhang J, Li Z. Accelerated skin wound healing by electrical stimulation. Adv Healthc Mater. 2021;10(16):e2100557. doi:10.1002/adhm.202100557
- Chen B, Kao HK, Dong Z, Jiang Z, Guo L. Complementary effects of negative-pressure wound therapy and pulsed radiofrequency energy on cutaneous wound healing in diabetic mice. Plast Reconstr Surg. 2017;139(1):105–117. doi:10.1097/PRS.0000000000002909
- Meng Z, Zhou D, Gao Y, Zeng M, Wang W. miRNA delivery for skin wound healing. Adv Drug Deliv Rev. 2018;129:308–318. doi:10.1016/j.addr.2017.12.011
- Ban E, Jeong S, Park M, et al. Accelerated wound healing in diabetic mice by miRNA-497 and its anti-inflammatory activity. Biomed Pharmacother. 2020;121:109613. doi:10.1016/j.biopha.2019.109613
- Fahs F, Bi X, Yu FS, Zhou L, Mi QS. New insights into microRNAs in skin wound healing. IUBMB Life. 2015;67(12):889–896. doi:10.1002/iub.1449
- Kang HJ, Chen N, Dash BC, Hsia HC, Berthiaume F. Self-assembled nanomaterials for chronic skin wound healing. Adv Wound Care. 2021;10(5):221–233. doi:10.1089/wound.2019.1077
- Bellu E, Medici S, Coradduzza D, Cruciani S, Amler E, Maioli M. Nanomaterials in skin regeneration and rejuvenation. Int J Mol Sci. 2021;22(13):7095. doi:10.3390/ijms22137095
- Bai Q, Han K, Dong K, et al. Potential applications of nanomaterials and technology for diabetic wound healing. Int J Nanomedicine. 2020;15:9717–9743. doi:10.2147/IJN.S276001
- Stoica AE, Chircov C, Grumezescu AM. Nanomaterials for wound dressings: an up-to-date overview. Molecules. 2020;25(11):E2699. doi:10.3390/molecules25112699
- Wang M, Huang X, Zheng H, et al. Nanomaterials applied in wound healing: mechanisms, limitations and perspectives. J Control Release. 2021;337:236–247. doi:10.1016/j.jconrel.2021.07.017
- Jain AK, Thareja S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif Cells Nanomed Biotechnol. 2019;47(1):524–539. doi:10.1080/21691401.2018.1561457
- Sayes CM, Aquino GV, Hickey AJ. Nanomaterial drug products: manufacturing and analytical perspectives. AAPS J. 2017;19(1):18–25. doi:10.1208/s12248-016-0008-x
- Farjadian F, Ghasemi A, Gohari O, Roointan A, Karimi M, Hamblin MR. Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine. 2019;14(1):93–126. doi:10.2217/nnm-2018-0120
- Li X, Tsibouklis J, Weng T, et al. Nano carriers for drug transport across the blood-brain barrier. J Drug Target. 2017;25(1):17–28. doi:10.1080/1061186X.2016.1184272
- Moon JH, Moxley JW, Zhang P, Cui H. Nanoparticle approaches to combating drug resistance. Future Med Chem. 2015;7(12):1503–1510. doi:10.4155/fmc.15.82
- Arda O, Göksügür N, Tüzün Y. Basic histological structure and functions of facial skin. Clin Dermatol. 2014;32(1):3–13. doi:10.1016/j.clindermatol.2013.05.021
- Wong R, Geyer S, Weninger W, Guimberteau JC, Wong JK. The dynamic anatomy and patterning of skin. Exp Dermatol. 2016;25(2):92–98. doi:10.1111/exd.12832
- Baroni A, Buommino E, De Gregorio V, Ruocco E, Ruocco V, Wolf R. Structure and function of the epidermis related to barrier properties. Clin Dermatol. 2012;30(3):257–262. doi:10.1016/j.clindermatol.2011.08.007
- Menon GK. New insights into skin structure: scratching the surface. Adv Drug Deliv Rev. 2002;54(Suppl 1):S3–S17. doi:10.1016/s0169-409x(02)00121-7
- Bornes L, Windoffer R, Leube RE, Morgner J, van Rheenen J. Scratch-induced partial skin wounds re-epithelialize by sheets of independently migrating keratinocytes. Life Sci Alliance. 2021;4(1):e202000765. doi:10.26508/lsa.202000765
- Leskur D, Perišić I, Romac K, et al. Comparison of mechanical, chemical and physical human models of in vivo skin damage: randomized controlled trial. Skin Res Technol. 2021;27(2):208–216. doi:10.1111/srt.12932
- Monteiro-Soares M, Russell D, Boyko EJ, et al. Guidelines on the classification of diabetic foot ulcers (IWGDF 2019). Diabetes Metab Res Rev. 2020;36(Suppl 1):e3273. doi:10.1002/dmrr.3273
- Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: a Cellular Perspective. Physiol Rev. 2019;99(1):665–706. doi:10.1152/physrev.00067.2017
- Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223. doi:10.1098/rsob.200223
- Yussof SJM, Omar E, Pai DR, Sood S. Cellular events and biomarkers of wound healing. Indian J Plast Surg. 2012;45(02):220–228. doi:10.4103/0970-0358.101282
- Ridiandries A, Tan JTM, Bursill CA. The role of chemokines in wound healing. Int J Mol Sci. 2018;19(10):E3217. doi:10.3390/ijms19103217
- Komi DEA, Khomtchouk K, Santa Maria PL. A review of the contribution of mast cells in wound healing: involved molecular and cellular mechanisms. Clin Rev Allergy Immunol. 2020;58(3):298–312. doi:10.1007/s12016-019-08729-w
- Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184(7):3964–3977. doi:10.4049/jimmunol.0903356
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi:10.1038/nri3399
- Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370–378. doi:10.1111/bjd.13954
- Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci. 2017;18(7):E1545. doi:10.3390/ijms18071545
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. doi:10.1111/j.1524-475X.2008.00410.x
- Stone RC, Pastar I, Ojeh N, et al. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016;365(3):495–506. doi:10.1007/s00441-016-2464-0
- Olczyk P, Mencner Ł, Komosinska-Vassev K. The role of the extracellular matrix components in cutaneous wound healing. Biomed Res Int. 2014;2014:e747584. doi:10.1155/2014/747584
- Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36(6):1031–1037. doi:10.1016/j.biocel.2003.12.003
- Grieb G, Steffens G, Pallua N, Bernhagen J, Bucala R. Circulating fibrocytes–biology and mechanisms in wound healing and scar formation. Int Rev Cell Mol Biol. 2011;291:1–19. doi:10.1016/B978-0-12-386035-4.00001-X
- El-Ashram S, El-Samad LM, Basha AA, El Wakil A. Naturally-derived targeted therapy for wound healing: beyond classical strategies. Pharmacol Res. 2021;170:105749. doi:10.1016/j.phrs.2021.105749
- Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229. doi:10.1177/0022034509359125
- Romanovsky AA. Skin temperature: its role in thermoregulation. Acta Physiol. 2014;210(3):498–507. doi:10.1111/apha.12231
- Power G, Moore Z, O’Connor T. Measurement of pH, exudate composition and temperature in wound healing: a systematic review. J Wound Care. 2017;26(7):381–397. doi:10.12968/jowc.2017.26.7.381
- Smith R, Russo J, Fiegel J, Brogden N. Antibiotic delivery strategies to treat skin infections when innate antimicrobial defense fails. Antibiotics. 2020;9(2):E56. doi:10.3390/antibiotics9020056
- Deng L, Du C, Song P, et al. The role of oxidative stress and antioxidants in diabetic wound healing. Oxid Med Cell Longev. 2021;2021. doi:10.1155/2021/8852759
- Gould L, Abadir P, Brem H, et al. Chronic wound repair and healing in older adults: current status and future research. Wound Repair Regen. 2015;23(1):1–13. doi:10.1111/wrr.12245
- Basu Mallik S, Jayashree BS, Shenoy RR. Epigenetic modulation of macrophage polarization- perspectives in diabetic wounds. J Diabetes Complications. 2018;32(5):524–530. doi:10.1016/j.jdiacomp.2018.01.015
- Wilson JA, Clark JJ. Obesity: impediment to postsurgical wound healing. Adv Skin Wound Care. 2004;17(8):426–435. doi:10.1097/00129334-200410000-00013
- Siddle HJ, Firth J, Waxman R, Nelson EA, Helliwell PS. A case series to describe the clinical characteristics of foot ulceration in patients with rheumatoid arthritis. Clin Rheumatol. 2012;31(3):541–545. doi:10.1007/s10067-011-1886-z
- Götz A, Eckert F, Landthaler M. Ataxia-telangiectasia (Louis-Bar syndrome) associated with ulcerating necrobiosis lipoidica. J Am Acad Dermatol. 1994;31(1):124–126. doi:10.1016/S0190-9622(09)80245-4
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62(12):1377–1384. doi:10.1001/archpsyc.62.12.1377
- Ahn C, Mulligan P, Salcido RS. Smoking-The bane of wound healing: biomedical interventions and social influences. Adv Skin Wound Care. 2008;21(5):227–236; quiz 237–238. doi:10.1097/01.ASW.0000305440.62402.43
- Kirchner S, Lei V, MacLeod AS. The cutaneous wound innate immunological microenvironment. Int J Mol Sci. 2020;21(22):E8748. doi:10.3390/ijms21228748
- Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19(2):134–148. doi:10.1111/j.1524-475X.2011.00673.x
- Bryan N, Ahswin H, Smart N, Bayon Y, Wohlert S, Hunt JA. Reactive oxygen species (ROS)–a family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur Cell Mater. 2012;24:249–265. doi:10.22203/ecm.v024a18
- Gonçalves RV, Costa AMA, Grzeskowiak L. Oxidative stress and tissue repair: mechanism, biomarkers, and therapeutics. Oxid Med Cell Longev. 2021;2021:6204096. doi:10.1155/2021/6204096
- Dizdaroglu M, Jaruga P. Mechanisms of free radical-induced damage to DNA. Free Radic Res. 2012;46(4):382–419. doi:10.3109/10715762.2011.653969
- Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. 2013;19(32):5695–5703. doi:10.2174/1381612811319320005
- Zhou X, Li M, Xiao M, et al. ERβ accelerates diabetic wound healing by ameliorating hyperglycemia-induced persistent oxidative stress. Front Endocrinol. 2019;10:499. doi:10.3389/fendo.2019.00499
- Bowler PG. Wound pathophysiology, infection and therapeutic options. Ann Med. 2002;34(6):419–427. doi:10.1080/078538902321012360
- Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis. 2004;17(2):91–96. doi:10.1097/00001432-200404000-00004
- Serra R, Grande R, Butrico L, et al. Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev Anti Infect Ther. 2015;13(5):605–613. doi:10.1586/14787210.2015.1023291
- Roy S, Santra S, Das A, et al. Staphylococcus aureus biofilm infection compromises wound healing by causing deficiencies in granulation tissue collagen. Ann Surg. 2020;271(6):1174–1185. doi:10.1097/SLA.0000000000003053
- Chong KKL, Tay WH, Janela B, et al. Enterococcus faecalis modulates immune activation and slows healing during wound infection. J Infect Dis. 2017;216(12):1644–1654. doi:10.1093/infdis/jix541
- Rahim K, Saleha S, Zhu X, Huo L, Basit A, Franco OL. Bacterial contribution in chronicity of wounds. Microb Ecol. 2017;73(3):710–721. doi:10.1007/s00248-016-0867-9
- Li L, Xi WS, Su Q, et al. Unexpected size effect: the interplay between different-sized nanoparticles in their cellular uptake. Small. 2019;15(38):e1901687. doi:10.1002/smll.201901687
- Asuri P, Bale SS, Karajanagi SS, Kane RS. The protein-nanomaterial interface. Curr Opin Biotechnol. 2006;17(6):562–568. doi:10.1016/j.copbio.2006.09.002
- Alavi M, Jabari E, Jabbari E. Functionalized carbon-based nanomaterials and quantum dots with antibacterial activity: a review. Expert Rev Anti Infect Ther. 2021;19(1):35–44. doi:10.1080/14787210.2020.1810569
- Al-Mamun M, Orlowski M. Electron tunneling between vibrating atoms in a copper nano-filament. Sci Rep. 2021;11(1):7413. doi:10.1038/s41598-021-86603-6
- Liu XQ, Tang RZ. Biological responses to nanomaterials: understanding nano-bio effects on cell behaviors. Drug Deliv. 2017;24(sup1):1–15. doi:10.1080/10717544.2017.1375577
- He W, Wamer W, Xia Q, Yin J, Fu PP. Enzyme-like activity of nanomaterials. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2014;32(2):186–211. doi:10.1080/10590501.2014.907462
- Kant V, Kumari P, Jitendra DK, Ahuja M, Kumar V. Nanomaterials of natural bioactive compounds for wound healing: novel drug delivery approach. Curr Drug Deliv. 2021;18(10):1406–1425. doi:10.2174/1567201818666210729103712
- Bramhill J, Ross S, Ross G. Bioactive nanocomposites for tissue repair and regeneration: a review. Int J Environ Res Public Health. 2017;14(1):E66. doi:10.3390/ijerph14010066
- Berthet M, Gauthier Y, Lacroix C, Verrier B, Monge C. Nanoparticle-based dressing: the future of wound treatment? Trends Biotechnol. 2017;35(8):770–784. doi:10.1016/j.tibtech.2017.05.005
- Huang L, Yu L, Yin X, Lin Y, Xu Y, Niu Y. Silver nanoparticles with vanadium oxide nanowires loaded into electrospun dressings for efficient healing of bacterium-infected wounds. J Colloid Interface Sci. 2022;622:117–125. doi:10.1016/j.jcis.2022.04.026
- Lee SH, Jun BH. Silver nanoparticles: synthesis and application for nanomedicine. Int J Mol Sci. 2019;20(4):E865. doi:10.3390/ijms20040865
- Kim JS, Kuk E, Yu KN, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3(1):95–101. doi:10.1016/j.nano.2006.12.001
- Tang S, Zheng J. Antibacterial activity of silver nanoparticles: structural effects. Adv Healthcare Mater. 2018;7(13):1701503. doi:10.1002/adhm.201701503
- Xu L, Wang YY, Huang J, Chen CY, Wang ZX, Xie H. Silver nanoparticles: synthesis, medical applications and biosafety. Theranostics. 2020;10(20):8996–9031. doi:10.7150/thno.45413
- Abdellatif AAH, Alsharidah M, Al Rugaie O, Tawfeek HM, Tolba NS. Silver nanoparticle-coated ethyl cellulose inhibits tumor necrosis factor-α of breast cancer cells. Drug Des Devel Ther. 2021;15:2035–2046. doi:10.2147/DDDT.S310760
- Fehaid A, Taniguchi A. Silver nanoparticles reduce the apoptosis induced by tumor necrosis factor-α. Sci Technol Adv Mater. 2018;19(1):526–534. doi:10.1080/14686996.2018.1487761
- Tyavambiza C, Elbagory AM, Madiehe AM, Meyer M, Meyer S. The antimicrobial and anti-inflammatory effects of silver nanoparticles synthesised from cotyledon orbiculata aqueous extract. Nanomaterials. 2021;11(5):1343. doi:10.3390/nano11051343
- Ali EM, Abdallah BM. Effective inhibition of invasive pulmonary aspergillosis by silver nanoparticles biosynthesized with Artemisia sieberi leaf extract. Nanomaterials. 2021;12(1):51. doi:10.3390/nano12010051
- Choudhury H, Pandey M, Lim YQ, et al. Silver nanoparticles: advanced and promising technology in diabetic wound therapy. Mater Sci Eng C. 2020;112:110925. doi:10.1016/j.msec.2020.110925
- Liu X, Lee PY, Ho CM, et al. Silver nanoparticles mediate differential responses in keratinocytes and fibroblasts during skin wound healing. ChemMedChem. 2010;5(3):468–475. doi:10.1002/cmdc.200900502
- Kumar S, Majhi RK, Singh A, et al. Carbohydrate-coated gold–silver nanoparticles for efficient elimination of multidrug resistant bacteria and in vivo wound healing. ACS Appl Mater Interfaces. 2019;11(46):42998–43017. doi:10.1021/acsami.9b17086
- Bruna T, Maldonado-Bravo F, Jara P, Caro N. Silver Nanoparticles and Their Antibacterial Applications. Int J Mol Sci. 2021;22(13):7202. doi:10.3390/ijms22137202
- Ermini ML, Voliani V. Antimicrobial nano-agents: the copper age. ACS Nano. 2021;15(4):6008–6029. doi:10.1021/acsnano.0c10756
- Gawande MB, Goswami A, Felpin FX, et al. Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem Rev. 2016;116(6):3722–3811.
- Sánchez-López E, Gomes D, Esteruelas G, et al. Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials. 2020;10(2):E292. doi:10.3390/nano10020292
- Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013;11(6):371–384. doi:10.1038/nrmicro3028
- Alizadeh S, Seyedalipour B, Shafieyan S, Kheime A, Mohammadi P, Aghdami N. Copper nanoparticles promote rapid wound healing in acute full thickness defect via acceleration of skin cell migration, proliferation, and neovascularization. Biochem Biophys Res Commun. 2019;517(4):684–690. doi:10.1016/j.bbrc.2019.07.110
- Saddik MS, Alsharif FM, El-Mokhtar MA, et al. Biosynthesis, characterization, and wound-healing activity of phenytoin-loaded copper nanoparticles. AAPS Pharm Sci Tech. 2020;21(5):175. doi:10.1208/s12249-020-01700-5
- Pirmohamed T, Dowding JM, Singh S, et al. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun. 2010;46(16):2736–2738. doi:10.1039/b922024k
- Thakur N, Manna P, Das J. Synthesis and biomedical applications of nanoceria, a redox active nanoparticle. J Nanobiotechnology. 2019;17(1):84. doi:10.1186/s12951-019-0516-9
- Xu C, Qu X. Cerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014;6(3):e90–e90. doi:10.1038/am.2013.88
- Singh S. Cerium oxide based nanozymes: redox phenomenon at biointerfaces. Biointerphases. 2016;11(4):04B202. doi:10.1116/1.4966535
- Ribeiro FM, de Oliveira MM, Singh S, et al. Ceria nanoparticles decrease UVA-induced fibroblast death through cell redox regulation leading to cell survival, migration and proliferation. Front Bioeng Biotechnol. 2020;8. doi:10.3389/fbioe.2020.577557
- Chigurupati S, Mughal MR, Okun E, et al. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials. 2013;34(9):2194–2201. doi:10.1016/j.biomaterials.2012.11.061
- Peloi KE, Contreras Lancheros CA, Nakamura CV, et al. Antioxidative photochemoprotector effects of cerium oxide nanoparticles on UVB irradiated fibroblast cells. Colloids Surf B Biointerfaces. 2020;191:111013. doi:10.1016/j.colsurfb.2020.111013
- Heckert EG, Seal S, Self WT. Fenton-like reaction catalyzed by the rare earth inner transition metal cerium. Environ Sci Technol. 2008;42(13):5014–5019. doi:10.1021/es8001508
- Fisher TJ, Zhou Y, Wu TS, Wang M, Soo YL, Cheung CL. Structure-activity relationship of nanostructured ceria for the catalytic generation of hydroxyl radicals. Nanoscale. 2019;11(10):4552–4561. doi:10.1039/c8nr09393h
- Qin J, Feng Y, Cheng D, et al. Construction of a mesoporous ceria hollow sphere/enzyme nanoreactor for enhanced cascade catalytic antibacterial therapy. ACS Appl Mater Interfaces. 2021;13(34):40302–40314. doi:10.1021/acsami.1c10821
- Nqakala ZB, Sibuyi NRS, Fadaka AO, Meyer M, Onani MO, Madiehe AM. Advances in Nanotechnology Towards Development Of Silver Nanoparticle-Based Wound-Healing Agents. Int J Mol Sci. 2021;22(20):11272. doi:10.3390/ijms222011272
- Vaitsis C, Sourkouni G, Argirusis C. Metal Organic Frameworks (MOFs) and ultrasound: a review. Ultrason Sonochem. 2019;52:106–119. doi:10.1016/j.ultsonch.2018.11.004
- Saeb MR, Rabiee N, Mozafari M, Mostafavi E. Metal-organic frameworks-based nanomaterials for drug delivery. Materials. 2021;14(13):3652. doi:10.3390/ma14133652
- Safdar Ali R, Meng H, Li Z. Zinc-based metal-organic frameworks in drug delivery, cell imaging, and sensing. Molecules. 2021;27(1):100. doi:10.3390/molecules27010100
- Huxford RC, Della Rocca J, Lin W. Metal-organic frameworks as potential drug carriers. Curr Opin Chem Biol. 2010;14(2):262–268. doi:10.1016/j.cbpa.2009.12.012
- Liu X, Liang T, Zhang R, et al. Iron-based metal-organic frameworks in drug delivery and biomedicine. ACS Appl Mater Interfaces. 2021;13(8):9643–9655. doi:10.1021/acsami.0c21486
- Yao S, Chi J, Wang Y, Zhao Y, Luo Y, Wang Y. Zn-MOF encapsulated antibacterial and degradable microneedles array for promoting wound healing. Adv Healthc Mater. 2021;10(12):e2100056. doi:10.1002/adhm.202100056
- Chang H, Kim J, Lee SH, et al. Luminescent Nanomaterials (II). Adv Exp Med Biol. 2021;1309:97–132. doi:10.1007/978-981-33-6158-4_5
- Wang S, Xi W, Wang Z, et al. Nanostructures based on vanadium disulfide growing on UCNPs: simple synthesis, dual-mode imaging, and photothermal therapy. J Mater Chem B. 2020;8(27):5883–5891. doi:10.1039/d0tb00993h
- Zhang L, Jin D, Stenzel MH. Polymer-functionalized upconversion nanoparticles for light/imaging-guided drug delivery. Biomacromolecules. 2021;22(8):3168–3201. doi:10.1021/acs.biomac.1c00669
- Sun J, Zhang P, Fan Y, et al. Near-infrared triggered antibacterial nanocomposite membrane containing upconversion nanoparticles. Mater Sci Eng C Mater Biol Appl. 2019;103:109797. doi:10.1016/j.msec.2019.109797
- Shanmuganathan R, Edison TN, LewisOscar F, Kumar P, Shanmugam S, Pugazhendhi A. Chitosan nanopolymers: an overview of drug delivery against cancer. Int J Biol Macromol. 2019;130:727–736. doi:10.1016/j.ijbiomac.2019.02.060
- Mohammadi Nasr S, Rabiee N, Hajebi S, et al. Biodegradable nanopolymers in cardiac tissue engineering: from concept towards nanomedicine. Int J Nanomedicine. 2020;15:4205–4224. doi:10.2147/IJN.S245936
- Vijayan A, James PP, Nanditha CK, Kumar GSV. Multiple cargo deliveries of growth factors and antimicrobial peptide using biodegradable nanopolymer as a potential wound healing system. Int J Nanomedicine. 2019;14:2253–2263. doi:10.2147/IJN.S190321
- Rashki S, Asgarpour K, Tarrahimofrad H, et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr Polym. 2021;251:117108. doi:10.1016/j.carbpol.2020.117108
- Biranje SS, Madiwale PV, Patankar KC, Chhabra R, Dandekar-Jain P, Adivarekar RV. Hemostasis and anti-necrotic activity of wound-healing dressing containing chitosan nanoparticles. Int J Biol Macromol. 2019;121:936–946. doi:10.1016/j.ijbiomac.2018.10.125
- He J, Liang Y, Shi M, Guo B. Anti-oxidant electroactive and antibacterial nanofibrous wound dressings based on poly(ε-caprolactone)/quaternized chitosan-graft-polyaniline for full-thickness skin wound healing. Chem Eng J. 2020;385:123464. doi:10.1016/j.cej.2019.123464
- Huang W, Wang Y, Huang Z, et al. On-demand dissolvable self-healing hydrogel based on carboxymethyl chitosan and cellulose nanocrystal for deep partial thickness burn wound healing. ACS Appl Mater Interfaces. 2018;10(48):41076–41088. doi:10.1021/acsami.8b14526
- Meddahi-Pellé A, Legrand A, Marcellan A, Louedec L, Letourneur D, Leibler L. Organ repair, hemostasis, and in vivo bonding of medical devices by aqueous solutions of nanoparticles. Angew Chem Int Ed Engl. 2014;53(25):6369–6373. doi:10.1002/anie.201401043
- Rose S, Prevoteau A, Elzière P, Hourdet D, Marcellan A, Leibler L. Nanoparticle solutions as adhesives for gels and biological tissues. Nature. 2014;505(7483):382–385. doi:10.1038/nature12806
- Dumville JC, Coulthard P, Worthington HV, et al. Tissue adhesives for closure of surgical incisions. Cochrane Database Syst Rev. 2014;(11):CD004287. doi:10.1002/14651858.CD004287.pub4
- Li Y, Xu T, Tu Z, et al. Bioactive antibacterial silica-based nanocomposites hydrogel scaffolds with high angiogenesis for promoting diabetic wound healing and skin repair. Theranostics. 2020;10(11):4929–4943. doi:10.7150/thno.41839
- Gaur M, Misra C, Yadav AB, et al. Biomedical applications of carbon nanomaterials: fullerenes, quantum dots, nanotubes, nanofibers, and graphene. Materials. 2021;14(20):5978. doi:10.3390/ma14205978
- Tran PA, Zhang L, Webster TJ. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv Drug Deliv Rev. 2009;61(12):1097–1114. doi:10.1016/j.addr.2009.07.010
- Zhou W, Gao P, Shao L, et al. Drug-loaded, magnetic, hollow silica nanocomposites for nanomedicine. Nanomedicine. 2005;1(3):233–237. doi:10.1016/j.nano.2005.06.005
- Karimi M, Solati N, Amiri M, et al. Carbon nanotubes part I: preparation of a novel and versatile drug-delivery vehicle. Expert Opin Drug Deliv. 2015;12(7):1071–1087. doi:10.1517/17425247.2015.1003806
- Chen S, John JV, McCarthy A, Xie J. New forms of electrospun nanofiber materials for biomedical applications. J Mater Chem B. 2020;8(17):3733–3746. doi:10.1039/d0tb00271b
- Madhukiran DR, Jha A, Kumar M, Ajmal G, Bonde GV, Mishra B. Electrospun nanofiber-based drug delivery platform: advances in diabetic foot ulcer management. Expert Opin Drug Deliv. 2021;18(1):25–42. doi:10.1080/17425247.2021.1823966
- Liu G, Gu Z, Hong Y, Cheng L, Li C. Electrospun starch nanofibers: recent advances, challenges, and strategies for potential pharmaceutical applications. J Control Release. 2017;252:95–107. doi:10.1016/j.jconrel.2017.03.016
- Xuan H, Wu S, Fei S, Li B, Yang Y, Yuan H. Injectable nanofiber-polysaccharide self-healing hydrogels for wound healing. Mater Sci Eng C Mater Biol Appl. 2021;128:112264. doi:10.1016/j.msec.2021.112264
- Embil JM, Papp K, Sibbald G, et al. Recombinant human platelet-derived growth factor-BB (becaplermin) for healing chronic lower extremity diabetic ulcers: an open-label clinical evaluation of efficacy. Wound Repair Regen. 2000;8(3):162–168. doi:10.1046/j.1524-475x.2000.00162.x
- Park JW, Hwang SR, Yoon IS. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules. 2017;22(8):1259. doi:10.3390/molecules22081259
- Zhang L, Zhou Y, Li G, Zhao Y, Gu X, Yang Y. Nanoparticle mediated controlled delivery of dual growth factors. Sci China Life Sci. 2014;57(2):256–262. doi:10.1007/s11427-014-4606-5
- Koria P. Delivery of growth factors for tissue regeneration and wound healing. BioDrugs. 2012;26(3):163–175. doi:10.2165/11631850-000000000-00000
- Choi JS, Leong KW, Yoo HS. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials. 2008;29(5):587–596. doi:10.1016/j.biomaterials.2007.10.012
- Wu J, Zhu J, Wu Q, et al. Mussel-inspired surface immobilization of heparin on magnetic nanoparticles for enhanced wound repair via sustained release of a growth factor and M2 macrophage polarization. ACS Appl Mater Interfaces. 2021;13(2):2230–2244. doi:10.1021/acsami.0c18388
- Li D, Landén NX. MicroRNAs in skin wound healing. Eur J Dermatol. 2017;27(S1):12–14. doi:10.1684/ejd.2017.3040
- Petkovic M, Sørensen AE, Leal EC, Carvalho E, Dalgaard LT. Mechanistic actions of microRNAs in diabetic wound healing. Cells. 2020;9(10):E2228. doi:10.3390/cells9102228
- Li X, Guo L, Liu Y, et al. MicroRNA-21 promotes wound healing via the Smad7-Smad2/3-Elastin pathway. Exp Cell Res. 2018;362(2):245–251. doi:10.1016/j.yexcr.2017.11.019
- Miscianinov V, Martello A, Rose L, et al. MicroRNA-148b targets the TGF-β pathway to regulate angiogenesis and endothelial-to-mesenchymal transition during skin wound healing. Mol Ther. 2018;26(8):1996–2007. doi:10.1016/j.ymthe.2018.05.002
- Wang H, Agarwal P, Zhao S, Yu J, Lu X, He X. A near-infrared laser-activated “Nanobomb” for breaking the barriers to MicroRNA delivery. Adv Mater. 2016;28(2):347–355. doi:10.1002/adma.201504263
- Wang SY, Kim H, Kwak G, et al. Development of microRNA-21 mimic nanocarriers for the treatment of cutaneous wounds. Theranostics. 2020;10(7):3240–3253. doi:10.7150/thno.39870
- Scheideler M, Vidakovic I, Prassl R. Lipid nanocarriers for microRNA delivery. Chem Phys Lipids. 2020;226:104837. doi:10.1016/j.chemphyslip.2019.104837
- Berger AG, Chou JJ, Hammond PT. Approaches to modulate the chronic wound environment using localized nucleic acid delivery. Adv Wound Care. 2021;10(9):503–528. doi:10.1089/wound.2020.1167
- Cheema SK, Chen E, Shea LD, Mathur AB. Regulation and guidance of cell behavior for tissue regeneration via the siRNA mechanism. Wound Repair Regen. 2007;15(3):286–295. doi:10.1111/j.1524-475X.2007.00228.x
- Randeria PS, Seeger MA, Wang XQ, et al. siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown. Proc Natl Acad Sci U S A. 2015;112(18):5573–5578. doi:10.1073/pnas.1505951112
- Lei J, Vodovotz Y, Tzeng E, Billiar TR. Nitric oxide, a protective molecule in the cardiovascular system. Nitric Oxide. 2013;35:175–185. doi:10.1016/j.niox.2013.09.004
- Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90(2):E17–24. doi:10.1161/hh0202.104530
- Kondo K, Bhushan S, King AL, et al. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127(10):1116–1127. doi:10.1161/CIRCULATIONAHA.112.000855
- Olas B. Gasomediators (·NO, CO, and H2S) and their role in hemostasis and thrombosis. Clin Chim Acta. 2015;445:115–121. doi:10.1016/j.cca.2015.03.027
- Mistry RK, Brewer AC. Redox regulation of gasotransmission in the vascular system: a focus on angiogenesis. Free Radic Biol Med. 2017;108:500–516. doi:10.1016/j.freeradbiomed.2017.04.025
- Malone-Povolny MJ, Maloney SE, Schoenfisch MH. Nitric oxide therapy for diabetic wound healing. Adv Healthcare Mater. 2019;8(12):1801210. doi:10.1002/adhm.201801210
- Jones ML, Ganopolsky JG, Labbé A, Wahl C, Prakash S. Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Appl Microbiol Biotechnol. 2010;88(2):401–407. doi:10.1007/s00253-010-2733-x
- Carpenter AW, Schoenfisch MH. Nitric oxide release: part II. Therapeutic applications. Chem Soc Rev. 2012;41(10):3742–3752. doi:10.1039/c2cs15273h
- Schwentker A, Vodovotz Y, Weller R, Billiar TR. Nitric oxide and wound repair: role of cytokines? Nitric Oxide. 2002;7(1):1–10. doi:10.1016/s1089-8603(02)00002-2
- Dulak J, Józkowicz A. Regulation of vascular endothelial growth factor synthesis by nitric oxide: facts and controversies. Antioxid Redox Signal. 2003;5(1):123–132. doi:10.1089/152308603321223612
- Efron DT, Most D, Shi HP, Tantry US, Barbul A. Modulation of growth factor and cytokine expression by nitric oxide during rat colon anastomotic healing. J Gastrointest Surg. 2003;7(3):393–399. doi:10.1016/s1091-255x(02)00433-x
- Hasan N, Cao J, Lee J, et al. PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Mater Sci Eng C Mater Biol Appl. 2019;103:109741. doi:10.1016/j.msec.2019.109741
- Lee J, Kwak D, Kim H, et al. Nitric oxide-releasing S-Nitrosoglutathione-Conjugated Poly(Lactic-Co-Glycolic Acid) nanoparticles for the treatment of MRSA-infected cutaneous wounds. Pharmaceutics. 2020;12(7):E618. doi:10.3390/pharmaceutics12070618
- Lipsky BA, Peters EJG, Senneville E, et al. Expert opinion on the management of infections in the diabetic foot. Diabetes Metab Res Rev. 2012;28(S1):163–178. doi:10.1002/dmrr.2248
- Wukich DK, Armstrong DG, Attinger CE, et al. Inpatient management of diabetic foot disorders: a clinical guide. Diabetes Care. 2013;36(9):2862–2871. doi:10.2337/dc12-2712
- Commons RJ, Robinson CH, Gawler D, Davis JS, Price RN. High burden of diabetic foot infections in the top end of Australia: an emerging health crisis (DEFINE study). Diabetes Res Clin Pract. 2015;110(2):147–157. doi:10.1016/j.diabres.2015.09.016
- Liu Y, van der Mei HC, Zhao B, et al. Eradication of multidrug-resistant staphylococcal infections by light-activatable micellar nanocarriers in a murine model. Adv Funct Mater. 2017;27(44):1701974. doi:10.1002/adfm.201701974
- Zhu Y, Yang B, Chen S, Du J. Polymer vesicles: mechanism, preparation, application, and responsive behavior. Prog Polym Sci. 2017;64:1–22. doi:10.1016/j.progpolymsci.2015.05.001
- Sun B, Xi Z, Wu F, et al. Quaternized chitosan-coated montmorillonite interior antimicrobial metal–antibiotic in situ coordination complexation for mixed infections of wounds. Langmuir. 2019;35(47):15275–15286. doi:10.1021/acs.langmuir.9b02821
- Gao W, Zhang L. Nanomaterials arising amid antibiotic resistance. Nat Rev Microbiol. 2021;19(1):5–6. doi:10.1038/s41579-020-00469-5
- Makabenta JMV, Nabawy A, Li CH, Schmidt-Malan S, Patel R, Rotello VM. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat Rev Microbiol. 2021;19(1):23–36. doi:10.1038/s41579-020-0420-1
- Gupta A, Mumtaz S, Li CH, Hussain I, Rotello VM. Combatting antibiotic-resistant bacteria using nanomaterials. Chem Soc Rev. 2019;48(2):415–427. doi:10.1039/c7cs00748e
- Rakhshaei R, Namazi H. A potential bioactive wound dressing based on carboxymethyl cellulose/ZnO impregnated MCM-41 nanocomposite hydrogel. Mater Sci Eng C-Mater Biol Appl. 2017;73:456–464. doi:10.1016/j.msec.2016.12.097
- Namazi H, Rakhshaei R, Hamishehkar H, Kafil HS. Antibiotic loaded carboxymethylcellulose/MCM-41 nanocomposite hydrogel films as potential wound dressing. Int J Biol Macromol. 2016;85:327–334. doi:10.1016/j.ijbiomac.2015.12.076
- Wang T, Li Y, Cornel EJ, Li C, Du J. Combined antioxidant-antibiotic treatment for effectively healing infected diabetic wounds based on polymer vesicles. ACS Nano. 2021;15(5):9027–9038. doi:10.1021/acsnano.1c02102
- Siddiqui L, Bag J, Mittal D, et al. Assessing the potential of lignin nanoparticles as drug carrier: synthesis, cytotoxicity and genotoxicity studies. Int J Biol Macromol. 2020;152:786–802. doi:10.1016/j.ijbiomac.2020.02.311
- Asadi N, Pazoki-Toroudi H, Del Bakhshayesh AR, Akbarzadeh A, Davaran S, Annabi N. Multifunctional hydrogels for wound healing: special focus on biomacromolecular based hydrogels. Int J Biol Macromol. 2021;170:728–750. doi:10.1016/j.ijbiomac.2020.12.202
- Clasky AJ, Watchorn JD, Chen PZ, Gu FX. From prevention to diagnosis and treatment: biomedical applications of metal nanoparticle-hydrogel composites. Acta Biomater. 2021;122:1–25. doi:10.1016/j.actbio.2020.12.030
- Yang N, Zhu M, Xu G, Liu N, Yu C. A near-infrared light-responsive multifunctional nanocomposite hydrogel for efficient and synergistic antibacterial wound therapy and healing promotion. J Mater Chem B. 2020;8(17):3908–3917. doi:10.1039/d0tb00361a
- Baker SE, Sawvel AM, Zheng N, Stucky GD. Controlling bioprocesses with inorganic surfaces: layered clay hemostatic agents. Chem Mater. 2007;19(18):4390–4392. doi:10.1021/cm071457b
- Gaharwar AK, Avery RK, Assmann A, et al. Shear-thinning nanocomposite hydrogels for the treatment of hemorrhage. ACS Nano. 2014;8(10):9833–9842. doi:10.1021/nn503719n
- Castleberry SA, Almquist BD, Li W, et al. Self-assembled wound dressings silence MMP-9 and improve diabetic wound healing in vivo. Adv Mater. 2016;28(9):1809–1817. doi:10.1002/adma.201503565
- Jang J, Lee JM, Oh SB, Choi Y, Jung HS, Choi J. Development of antibiofilm nanocomposites: Ag/Cu bimetallic nanoparticles synthesized on the surface of graphene oxide nanosheets. ACS Appl Mater Interfaces. 2020;12(32):35826–35834. doi:10.1021/acsami.0c06054
- Cheng Y, Chang Y, Feng Y, et al. Hierarchical acceleration of wound healing through intelligent nanosystem to promote multiple stages. ACS Appl Mater Interfaces. 2019;11(37):33725–33733. doi:10.1021/acsami.9b13267
- Naskar A, Kim KS. Recent advances in nanomaterial-based wound-healing therapeutics. Pharmaceutics. 2020;12(6):E499. doi:10.3390/pharmaceutics12060499
- Gobi R, Ravichandiran P, Babu RS, Yoo DJ. Biopolymer and synthetic polymer-based nanocomposites in wound dressing applications: a review. Polymers. 2021;13(12):1962. doi:10.3390/polym13121962
- Bonferoni MC, Sandri G, Dellera E, et al. Ionic polymeric micelles based on chitosan and fatty acids and intended for wound healing. Comparison of linoleic and oleic acid. Eur J Pharm Biopharm. 2014;87(1):101–106. doi:10.1016/j.ejpb.2013.12.018
- Lin M, Dai Y, Xia F, Zhang X. Advances in non-covalent crosslinked polymer micelles for biomedical applications. Mater Sci Eng C Mater Biol Appl. 2021;119:111626. doi:10.1016/j.msec.2020.111626
- Wang Y, Sun H. Polymeric nanomaterials for efficient delivery of antimicrobial agents. Pharmaceutics. 2021;13(12):2108. doi:10.3390/pharmaceutics13122108
- Ianchis R, Ninciuleanu CM, Gifu IC, et al. Hydrogel-clay nanocomposites as carriers for controlled release. Curr Med Chem. 2020;27(6):919–954. doi:10.2174/0929867325666180831151055
- Barrett-Catton E, Ross ML, Asuri P. Multifunctional hydrogel nanocomposites for biomedical applications. Polymers. 2021;13(6):856. doi:10.3390/polym13060856
- Ebhodaghe SO. A short review on chitosan and gelatin-based hydrogel composite polymers for wound healing. J Biomater Sci Polym Ed. 2022;1–28. doi:10.1080/09205063.2022.2068941
- Lee KC, Lo PY, Lee GY, Zheng JH, Cho EC. Carboxylated carbon nanomaterials in cell cycle and apoptotic cell death regulation. J Biotechnol. 2019;296:14–21. doi:10.1016/j.jbiotec.2019.02.005
- Li Y, Cao J. The impact of multi-walled carbon nanotubes (MWCNTs) on macrophages: contribution of MWCNT characteristics. Sci China Life Sci. 2018;61(11):1333–1351. doi:10.1007/s11427-017-9242-3
- Harik VM. Geometry of carbon nanotubes and mechanisms of phagocytosis and toxic effects. Toxicol Lett. 2017;273:69–85. doi:10.1016/j.toxlet.2017.03.016
- Wu Q, Guo D, Du Y, Liu D, Wang D, Bi H. UVB irradiation enhances TiO2 nanoparticle-induced disruption of calcium homeostasis in human lens epithelial cells. Photochem Photobiol. 2014;90(6):1324–1331. doi:10.1111/php.12322
- Tong T, Wilke CM, Wu J, et al. Combined toxicity of Nano-ZnO and Nano-TiO2: from single- to multinanomaterial systems. Environ Sci Technol. 2015;49(13):8113–8123. doi:10.1021/acs.est.5b02148
- Pelclova D, Zdimal V, Fenclova Z, et al. Markers of oxidative damage of nucleic acids and proteins among workers exposed to TiO2 (nano) particles. Occup Environ Med. 2016;73(2):110–118. doi:10.1136/oemed-2015-103161
- Yuan X, Zhang X, Sun L, Wei Y, Wei X. Cellular toxicity and immunological effects of carbon-based nanomaterials. Part Fibre Toxicol. 2019;16(1):18. doi:10.1186/s12989-019-0299-z
- Sahu D, Kannan GM, Vijayaraghavan R. Size-dependent effect of zinc oxide on toxicity and inflammatory potential of human monocytes. J Toxicol Environ Health A. 2014;77(4):177–191. doi:10.1080/15287394.2013.853224
- Sadrieh N, Wokovich AM, Gopee NV, et al. Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol Sci. 2010;115(1):156–166. doi:10.1093/toxsci/kfq041
- Melquiades FL, Ferreira DD, Appoloni CR, et al. Titanium dioxide determination in sunscreen by energy dispersive X-ray fluorescence methodology. Anal Chim Acta. 2008;613(2):135–143. doi:10.1016/j.aca.2008.02.058
- Kertész Z, Szikszai Z, Gontier E, et al. Nuclear microprobe study of TiO2-penetration in the epidermis of human skin xenografts. Nucl Instrum Methods Phys Res B. 2005;231(1):280–285. doi:10.1016/j.nimb.2005.01.071
- Baroli B. Penetration of nanoparticles and nanomaterials in the skin: fiction or reality? J Pharm Sci. 2010;99(1):21–50. doi:10.1002/jps.21817
- Liang XW, Xu ZP, Grice J, Zvyagin AV, Roberts MS, Liu X. Penetration of nanoparticles into human skin. Curr Pharm Des. 2013;19(35):6353–6366. doi:10.2174/1381612811319350011
- Friedman N, Dagan A, Elia J, Merims S, Benny O. Physical properties of gold nanoparticles affect skin penetration via hair follicles. Nanomedicine. 2021;36:102414. doi:10.1016/j.nano.2021.102414

