Abstract
The quest for the right combination of bronchodilators with different mechanisms of action such as long-acting muscarinic antagonists and long-acting β-agonists in the management of stable moderate-to-severe chronic obstructive pulmonary disease (COPD) is a topic of intense research activity currently, given the rising morbidity and mortality due to this disease. The fixed-dose combination of aclidinium bromide and formoterol fumarate in a single inhaler seems to offer superior advantages over either drugs given alone or as separate inhalers concurrently. Since the fixed-dose combination needs to be given twice daily, it is likely to achieve control of symptoms most crucial to the quality of life in COPD, namely, the morning hours. This is reflected in significant trough FEV1 (forced expiratory volume in 1 second) improvements after the dose. This paper reviews the various studies related to this combination put in the perspective of its safety and efficacy and potential benefits over other therapeutic options. However, there is a dearth of data on the long-term safety and efficacy in terms of improvement in lung function. This combination could emerge as an excellent option in the management of stable COPD if data on exacerbation rates and patient-reported outcomes become available from longer-term studies. Moreover, we need some more studies to define the ideal phenotype of COPD best suited for the use of this combination.
Introduction
Chronic obstructive pulmonary disease (COPD) is a recalcitrant inflammatory disease of the lungs with irreversible and progressive airflow limitation and parenchymal destruction with significant systemic inflammatory components. It is the third most severe disease in terms of mortality and morbidity globally, and the World Health Organization (WHO) predicts that it would step up to the second leading cause of mortality by 2030.Citation1–Citation6 The disease is manifested by dynamic hyperinflation, and the inflammation in COPD is steroid-nonresponsive. The inhaled corticosteroids (ICS) are the mainstay of treatment across all categories of asthma. However, in COPD, the therapeutic use of ICS is perhaps limited to reducing the rate of frequent exacerbations. The role of steroids in controlling the inflammation in COPD seems to be lacking the same class of evidence as compared to their role in asthma inflammation. Of note, ICS has no effect on dynamic hyperinflation in COPD as compared to the bronchodilators. Therefore, the only treatment that has shown significant merit in COPD management is the bronchodilators.Citation7 Bronchodilators act by either stimulating β2 agonist receptors or blocking muscarinic receptors. The long-acting bronchodilators are naturally the preferred drugs due to reduced frequency of dosing, which induces better compliance, reducing the symptoms for prolonged duration. The Global Initiative for Chronic Obstructive Lung Diseases (GOLD)Citation8 guidelines recommend combining the two types of long-acting bronchodilators with differing mechanisms of action if monotherapy is ineffective in controlling the disease. Several combination formulation compounds of long-acting muscarinic antagonists (LAMAs) and long-acting β2 agonists (LABAs) have been clinically tested or are in the process of formulation, such as glycopyrrolate-formoterol, glycopyrronium-indacaterol, tiotropium-olodaterol, umeclidinium-vilanterol, and aclidinium-formoterol, in the management of obstructive airway disease. The pharmaceutical industries are investing in developing several once- or twice-daily LABA/LAMA combinations to improve COPD treatment in future either as free combinations in different devices or as a fixed-dose combination (FDC) in a single inhaler.Citation9,Citation10 It is hoped that FDCs could offer advantages of better compliance, adherence, and cost-efficacy in addition to synergistic action of the components in free combinations in separate devices. presents recent evidences of the efficacy of these newer LAMAs and LABAs on the onset of action and improvement of trough forced expiratory volume in 1 second (FEV1) among COPD patients. presents results of some LABA and LAMA combinations as free combinations and FDCs.
Table 1 Comparison of various drugs in development of combination therapy with respect to frequency of dosage, rapidity of action, and quantum of improvement in trough FEV1
Table 2 Currently available LABA and LAMA combinations
Formoterol (LABA) and aclidinium bromide (LAMA) have shown significant individual efficacy in COPD management, and combination of these two drugs raises the promise of prospective therapeutic application in the management of COPD, although clinical evidences are still emerging. In this paper, we have taken an approach to revisit the evidences critically how the combination of these drugs could be useful in clinical practice.
Formoterol fumarate – an effective LABA with unique advantages
Formoterol is being used as a preferred bronchodilator in obstructive airway diseases over a long time. It has a stronger affinity to the receptors in contrast to other LABAs such as salmeterol. In a comparative study between salmeterol and formoterol, it was found that formoterol protected against methacholine-induced bronchial hyperresponsiveness in a dose–response manner and that effect was higher than that of salmeterol, which also suggested that salmeterol has properties of a partial agonist of β2 receptors.Citation11 Aalbers et alCitation12 conducted a randomized, controlled study and demonstrated that COPD patients who received 9 and 18 μg formoterol twice a day had reduced symptoms and increased number of symptom-free days; they also found that formoterol at a dose of 4.5 μg or higher could significantly improve lung function in COPD patients. Gross et alCitation13 also reported that formoterol fumarate delivered through nebulizers had improved lung function and Saint George’s Respiratory Questionnaire (SGRQ) score, and compared to any short-acting β2 agonist or short-acting muscarinic antagonist, formoterol imparts its action within 5 minutes of administration via any metered dose inhaler or dry powder inhaler.Citation14 There is a huge body of evidence suggesting the salvaging properties of formoterol in COPD in clinical practice, which is beyond the scope of this review. However, because of its acute and prolonged action, formoterol provides one of the best LABA options to be used in various combination therapies.
The new LAMA: aclidinium bromide – pharmacology and clinical evidences
Chemical composition
Aclidinium is a quaternary ammonium derivative of a (3R)-quinuclidinol ester containing two thiophene rings, and the chemical signature of aclidinium bromide is (3R)-3-y-1-(3-phenoxypropyl)-1-azoniabicyclo[2.2.2] octane bromide.Citation15,Citation16 The compound was developed by Almirall S.A. (Barcelona, Spain) and Forest Laboratories (New York, NY, USA). It is a muscarinic antagonist and has high binding affinity for the M3 receptor. Although it has a long duration of action and preliminary safety profile, quaternization of its tertiary amino function imparts a low oral bioavailability and low blood–brain barrier permeability,Citation17 thereby reducing systemic exposure, especially via the inhaled route, and this has made it a drug of choice with low side effect profile compared to other muscarinic antagonists such as tiotropium.Citation16
Physiological effects
Aclidinium has a high kinetic selectivity for M3 receptors in preference to other types of muscarinic receptors and is recommended as twice-a-day (BID) therapy in clinical practice. Some detailed analyses of the kinetics and receptor-binding activities have elucidated interesting results.Citation17 Although the half-life of aclidinium at muscarinic receptors in guinea pig lung was found shorter when compared to tiotropium (29 hours vs 34 hours), aclidinium had a faster onset of action.Citation18 In an in vitro study on isolated guinea pig trachea, Gavaldà et alCitation18 had shown that the onset of action of aclidinium (t1/2 =6.8±1.5 minutes; tmax =35.9±8.2 minutes) was faster than that of tiotropium (t1/2 =13.6±2.7 minutes; tmax =61.2±10.6 minutes), but similar to that of ipratropium (t1/2 =5.1±1.5 minutes; tmax =24.1±3.5 minutes) (). In their study, they reported that when compared to tiotropium, aclidinium had significantly faster hydrolysis, with an extremely short half-life in human plasma (2 minutes).Citation19 Another recent report has reconfirmed this previous finding and has shown that aclidinium had a shorter plasma half-life than glycopyrronium (2 minutes vs 12 hours).Citation20 This rapid plasma clearance of aclidinium suggests lower systemic and central nervous system side effects profile compared to other LAMAs.Citation18 The systemic side effects of any drug remains a major concern in COPD because of its elderly population predominance with an increased propensity to comorbidities such as cardiovascular disease and altered metabolic profile.
Figure 1 Onset of action of aclidinium, ipratropium, and tiotropium in isolated guinea pig trachea.
Abbreviation: SE, standard error.
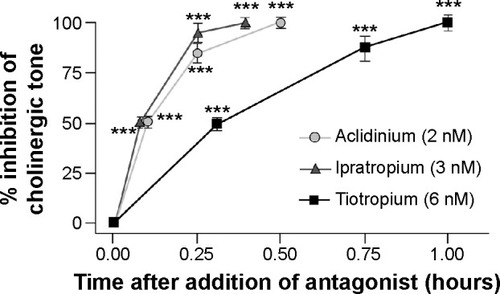
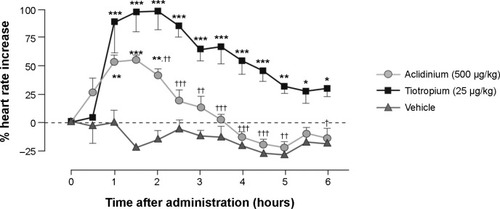
Antimuscarinics are known to have significant cardiac side effects as a class effect.Citation21 However, cardiac effects associated with aclidinium are much lower compared to other currently available antimuscarinics. In one study, tiotropium was shown to induce a significant increase in heart rate lasting for 6 hours, while aclidinium-induced increased heart rate lasted barely for 2.5 hours ().Citation18 Another preclinical cardiovascular safety study of the use of aclidinium further exemplified the lower side effects of aclidinium in comparison with tiotropium.Citation22
Figure 2 Effect of aclidinium and tiotropium on heart rate in conscious beagle dogs.
Abbreviation: SE, standard error.
Efficacy and safety of aclidinium: evidence from studies
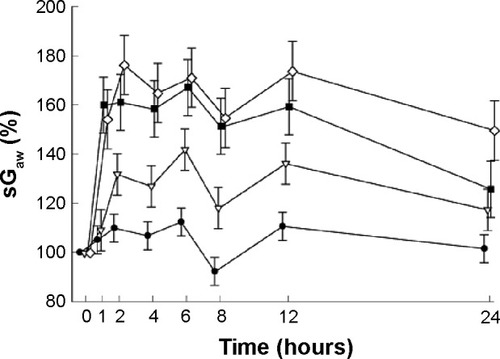
Extensive clinical studies have been conducted to determine the efficacy of aclidinium in COPD. Aclidinium bromide has demonstrated significant bronchodilator potential in obstructive airway diseases.Citation23–Citation33 However, discussion on each of those studies is out of the scope of this paper. A Phase I trial showed that low to very high doses of aclidinium increased specific airway conductance (sGaw) of healthy adult individuals in a dose-dependent manner ().Citation26 Apart from its direct action on bronchoconstriction, aclidinium has been shown to contribute to a number of other favorable outcomes in obstructive airway diseases. Aclidinium has been found to reduce carbachol- and tobacco smoke-induced overexpression of MUC5AC,Citation34 resulting in minimized secretion of mucin from goblet cells in COPD patients.Citation35,Citation36 Some of the major causes of exacerbation in COPD patients are exposure to airborne allergens and other environmental insults. These aeroallergens trigger an inflammatory response, which cannot be relieved by bronchodilators. Aclidinium, however, seems to be a better option than the other conventional bronchodilators because of its possible additional anti-inflammatory action. In a preclinical study, aclidinium has been shown to reduce Aspergillus fumigatus-induced eosinophil trafficking in bronchoalveolar lavage of mice in addition to complete abrogation of methacholine-induced increased airway resistance.Citation37 This demonstrates significant additional clinical advantage of aclidinium in COPD as Aspergillus is a very ubiquitous saprophytic fungus.
Figure 3 Mean (± SE) changes in sGaw (%) over 24 hours as a percentage of baseline value.
Abbreviations: sGaw, specific airway conductance; SE, standard error.
A couple of Phase II and Phase III clinical trials investigated the safety aspects of the administration of aclidinium bromide in COPD patients. The ACCORD I (AClidinium in Chronic Obstructive Respiratory Disease I) study recruited 561 patients in that Phase III trial and stated that administration of 200 and 400 μg aclidinium (BID) significantly improved bronchodilation, health status, and symptoms in moderate-to-severe COPD patients and that both the doses were well tolerated without untoward adverse effects for 12 weeks.Citation38 Two more studies by Fuhr et alCitation29 (Phase IIb trial) and Jones et alCitation30 (Phase III trial – the ATTAIN (Aclidinium To Treat Airway obstruction In COPD patieNts) study) also strongly advocated the administration of 200 and 400 μg aclidinium twice daily as safe doses in management of moderate-to-severe COPD. Later, Gelb et alCitation31 and Beier et alCitation32 also stated that either of the two doses (200 and 400 μg) of aclidinium twice daily was well tolerated by moderate-to-severe COPD patients. However, the safety and efficacy of aclidinium were significantly established before (2011) by Jones et alCitation39 when the investigators reviewed pooled evidences from two Phase III clinical trials (AClidinium CLinical trial Assessing efficacy and safety In Moderate to severe COPD patients – the ACCLAIM study).
Aclidinium bromide/formoterol fixed-dose combination therapy – evidences from clinical trials
In a very recent study, Cazzola et alCitation40 probed the therapeutic effects of aclidinium and formoterol combination on isolated human bronchial experiments. Interestingly, the combination model indicated a synergistic action at the low doses of aclidinium and formoterol in inducing smooth muscle relaxation in acetylcholine-induced bronchial contraction. The combination therapy induced more additive response compared with the expected additive response of the individual drug (in segment bronchi: +18.4%±2.7%; P<0.05 vs expected effect; in bronchioles: +19.7%±0.9%; P<0.05 vs expected effect). This is one of the very few published preclinical studies on aclidinium/formoterol combinations that clearly highlights the bronchodilation potential of the combination formulation at different doses.
Almirall S.A. and Forest Laboratories have developed a aclidinium bromide/formoterol fumarate FDC. These two companies have been conducting a series of Phase II and Phase III clinical trials to establish the clinical efficacy of the combination. These clinical trials included parallel arms including monotherapy by either of the two drugs (aclidinium and formoterol) at various doses and placebo to compare the efficacy, tolerance, and safety of the combination drug.Citation41 Although many of those trials have been completed, results are yet to be published. elucidates the list of the trials that looked into different aspects of this combination drug in the management of COPD.
Table 3 Recent clinical trials of aclidinium/formoterol fixed-dose combination therapies
Apart from the aforementioned clinical trials, there are some studies that merit discussion, as some results are available in the form of published abstracts. Sliwinski et alCitation42 reported a dose–response clinical trial that was aimed to assess the efficacy, safety, and pharmacokinetics of three different doses of formoterol (6, 12, and 18 μg) combined with aclidinium bromide 200 μg and compared against aclidinium bromide 200 μg monotherapy and formoterol 12 μg monotherapy.Citation42 This was a large study in which treatment was administered daily for 4 weeks to 566 stable moderate-to-severe COPD patients. The investigators reported that aclidinium combined with formoterol exhibited greater improvements in pulmonary parameters than did either drug alone or placebo, and all combinations were significantly superior to placebo (P<0.001) and to both the monotherapies (P<0.001).Citation42 Another Phase IIa clinical trial by Magnussen et alCitation43 was designed to investigate the pharmacokinetics, safety, tolerability, and lung function efficacy of aclidinium bromide and formoterol combination delivered through different inhalers.Citation43 In that randomized, single-blinded, crossover study, 24 moderate-to-severe COPD patients obtained either an FDC of aclidinium bromide (200 μg) and formoterol (12 μg) once daily through Genuair® (Almirall S.A.), or formoterol (12 μg) twice daily through Aerolizer® or, once daily through two different inhalers (Aerolizer® and Genuair®, Almirall S.A.).Citation43 Each of the 4-day treatment periods was separated by a 7-day washout period, and all four treatments were found to be safe and well tolerated and improve the lung function.
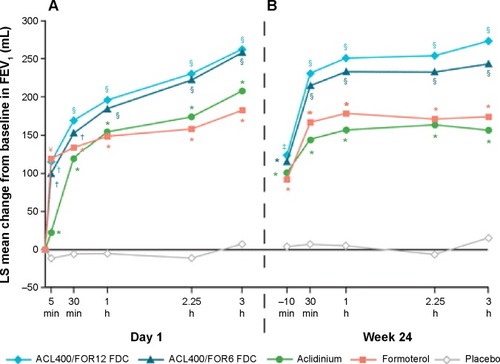
The efficacy and long-term safety of aclidinium bromide/formoterol fumarate combination therapy in the management of COPD has been advocated in two recently published large-scale clinical trials – the AUGMENT COPD study and the ACLIFORM-COPD (ACLIdinium FORMoterol-COPD) study. Aclidinium/formoterol fUmarate combination for investiGative use in the treatMENT of moderate-to-severe COPD (AUGMENT COPD) study (trial registration id: NCT01437397) was a 24-week double-blind study in which 1,692 patients with stable COPD were equally randomized to twice-daily treatment with an FDC of aclidinium 400 μg/formoterol 12 μg (ACL400/FOR12 FDC), FDC aclidinium 400 μg/formoterol 6 μg (ACL400/FOR6 FDC), aclidinium 400 μg, formoterol 12 μg, or placebo. All the drugs were administered by a multidose dry powder inhaler (Genuair®/Pressair®, Almirall S.A.).Citation44 The primary end points of this study were change from baseline to week 24 in 1-hour morning postdose FEV1 (FDCs vs aclidinium) and change from baseline to week 24 in morning predose (trough) FEV1 (FDCs vs formoterol), while the secondary end points were change from baseline in SGRQ total score and improvement in Transition Dyspnea Index (TDI) focal score at week 24. The study also assessed the safety and tolerability of the FDCs. The study was completed in 2012. In accordance to the results, COPD patients treated with ACL400/FOR12 FDC or ACL400/FOR6 FDC had exhibited greater 1-hour postdose improvement in FEV1 from baseline than did those patients who received aclidinium alone (108 and 87 mL, respectively; P<0.001). Similarly, patients who received ACL400/FOR12 FDC had a significant (P=0.01) 45 mL improvement in trough FEV1 than did those who received formoterol 12 μg alone, although ACL400/FOR6 FDC showed only an insignificant 26 mL change over formoterol alone. Both the ACL/FOR FDCs induced rapid bronchodilation with significant improvement in FEV1 within 5 minutes of the morning dose on day 1 than aclidinium alone or formoterol alone or placebo (). FEV1 at 3-hours postdose at week 24 also showed results similar to what was observed on day 1 (). Both SGRQ total and TDI focal scores also showed significant improvement at the end of the study in the ACL400/FOR12 FDC group over placebo with differences over placebo exceeding the minimal clinically important difference of ≥4 points and ≥1 unit, respectively. The investigators concluded that treatment with twice-daily aclidinium 400 μg/formoterol 12 μg FDC could help provide rapid and sustained bronchodilation over monotherapy with either drugs, which also helped in improving dyspnea and the health status of the COPD patients.Citation44 This was a conventional clinical trial and there were hardly any limitations in the study design.
Figure 4 Mean changes from baseline in FEV1 0–3 hours (A) on day 1 and (B) at week 24.
Abbreviations: ACL, aclidinium; FOR, formoterol; LS, least squares; FEV1, forced expiratory volume in 1 second; FDCs, fixed-dose combinations; ACL400/FOR12 FDC, FDC of aclidinium 400 μg and formoterol 12 μg; ACL400/FOR6 FDC, FDC of aclidinium 400 μg and formoterol 6 μg.
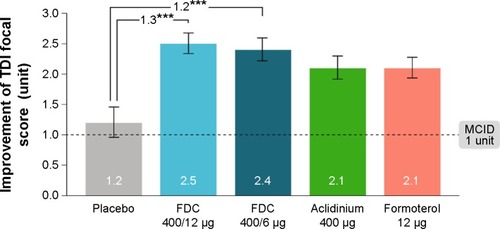
Another study published interesting outcomes of aclidinium bromide/formoterol FDC therapy, which had end points similar to those of the aforementioned study. The ACLIFORM-COPD study (NCT01462942) was a double-blind, randomized, parallel group, active- and placebo-controlled, multicenter study conducted at 193 centers in 22 countries.Citation45 In this study, patients with stable, moderate-to-severe COPD were randomized with a double-blind treatment of twice-daily aclidinium/formoterol FDC 400/12 μg or FDC 400/6 μg, aclidinium 400 μg and formoterol 12 μg or placebo. All medications were administered via a breath-actuated, multiple-dose dry powder inhaler (Genuair®/Pressair®, Almirall S.A.). The investigators reported that when compared to aclidinium monotherapy, both the FDCs of aclidinium and formoterol led to significant improvements in 1-hour postdose FEV1 from baseline (125 mL in ACL400/FOR12 [95% CI: 90–160, P<0.001] and 69 mL in ACL400/FOR6 [95% CI: 34–105, P<0.001]). The results were very close to what the other group had shown (108 and 87 mL, respectively).Citation44 Changes in trough FEV1 in the FDC groups in contrast to the formoterol alone were found to be 85 mL (95% CI: 51–119; P<0.001) and 53 mL (95% CI: 19–87; P<0.01), respectively, which were higher than those observed in the other study. In addition to that, ACL400/FOR12 and ACL400/FOR6 provided significant improvements in TDI focal score compared with placebo (1.29 units [95% CI: 0.73, 1.86; P<0.001] and 1.16 units [95% CI: 0.59, 1.73; P<0.001], respectively ()). This study also concluded that both the FDCs of aclidinium and formoterol significantly improved bronchodilation when compared with monotherapy, without any additional risk.Citation45
Figure 5 Improvement in TDI focal score at 24 weeks (ITT population).
Abbreviations: FDC, aclidinium/formoterol fixed-dose combination; ITT, intent-to-treat; MCID, minimum clinically important difference; SE, standard error; TDI, Transition Dyspnea Index.
Discussion
These clinical trials have strongly advocated the potential therapeutic advantages of the use of aclidinium/formoterol FDC therapies, as they are superior to either drugs alone and safe over long periods of time. What could be next? The latest update by GOLDCitation8 also does not settle all the questions. A new combination therapy always raises the concern of efficacy and safety.Citation46 The efficacy of aclidinium + formoterol in reducing exacerbations would need a 6- or 12-month-long trial. Patient-reported outcomes also would require large multicentric trials possibly involving all phenotypes of COPD. It is definitely a great challenge to formulate the right LABA/LAMA combination that could be delivered along with a corticosteroid, and here the evidence of safety and efficacy of aclidinium/formoterol combination raises a potential option to be delivered as a triple-drug therapy (either separately or as a mixture with ICS) in the management of COPD globally, although such combination therapies need to be tested in patients with frequent exacerbations. Although it may be assumed that such combination therapies would help improve the quality of life of the patients and increase the patient adherence, the availability of such drugs is still very limited.Citation47
Conclusion
The FDC of aclidinium bromide and formoterol fumarate holds the promise of round-the-clock control of symptoms of stable moderate-to-severe COPD with significant lung function improvement. However, the effect of this combination in reducing risk of exacerbations in relevant phenotypes of COPD and in improving patient-reported outcome measures and health-related quality-of-life measures in the long term remains to be established. It is worth waiting for further investigations of this FDC and also potentially its incorporation into triple-drug therapy as a free combination or single-inhaler FDC.
Disclosure
The authors report no conflicts of interest in this work.
References
- CasaburiRMahlerDAJonesPWA long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J200219221722411866001
- RabeKFHurdSAnzuetoAGlobal Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2007176653255517507545
- ChapmanKRManninoDMSorianoJBEpidemiology and costs of chronic obstructive pulmonary diseaseEur Respir J200627118820716387952
- MathersCDLoncarDProjections of global mortality and burden of disease from 2002 to 2030PLoS Med2006311e44217132052
- ManninoDMBuistASGlobal burden of COPD: risk factors, prevalence, and future trendsLancet2007370958976577317765526
- VestboJHurdSSAgustíAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med201318734736522878278
- CazzolaMPageCPCalzettaLPharmacology and therapeutics of bronchodilatorsPharmacol Rev20126445050422611179
- Global Initiative for Chronic Obstructive Lung Disease (GOLD)Global strategy for the diagnosis, management and prevention of COPD2015 Available from: http://www.goldcopd.org/Accessed February 4, 2015
- CazzolaMBrusascoVCentanniSProject PriMo: sharing principles and practices of bronchodilator therapy monitoring in COPD: a consensus initiative for optimizing therapeutic appropriateness among Italian specialistsPulm Pharmacol Ther20132621822823147424
- van der MolenTCazzolaMBeyond lung function in COPD management: effectiveness of LABA/LAMA combination therapy on patient-centred outcomesPrim Care Respir J20122110110822222945
- PalmqvistMIbsenTMellenALotvallJComparison of the relative efficacy of formoterol and salmeterol in asthmatic patientsAm J Respir Crit Care Med199916024424910390407
- AalbersRAyresJBackerVFormoterol in patients with chronic obstructive pulmonary disease: a randomized, controlled, 3-month trialEur Respir J200219593694312030736
- GrossNJNelsonHSLapidusRJFormoterol Study GroupEfficacy and safety of formoterol fumarate delivered by nebulization to COPD patientsRespir Med2008102218919718363201
- TashkinDPFabbriLMLong-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agentsRespir Res20101114921034447
- NormanPLong-acting muscarinic M3 receptor antagonistsExpert Opin Ther Pat2006161315132020144059
- PratMFernandezDBuilMADiscovery of novel quaternary ammonium derivatives of (3R)-quinuclidinol esters as potent and long-acting muscarinic antagonists with potential for minimal systemic exposure after inhaled administration: identification of (3R)-3-hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phenoxypropyl)-1-azoniabicyclo[2.2.2]octane bromide (aclidinium bromide)J Med Chem200952165076509219653626
- MoultonBCFryerADMuscarinic receptor antagonists, from folklore to pharmacology; finding drugs that actually work in asthma and COPDBr J Pharmacol2011163445221198547
- GavaldàAMiralpeixMRamosICharacterization of aclidinium bromide, a novel inhaled muscarinic antagonist, with long duration of action and a favorable pharmacological profileJ Pharmacol Exp Ther2009331274075119710368
- GavaldàAMiralpeixMRamosIAclidinium bromide, a novel muscarinic receptor combining long residence at M3 receptors and rapid plasma clearanceEur Respir J200730Suppl 51209S210S
- GavaldàARamosICarcasonaCThe in vitro and in vivo profile of aclidinium bromide in comparison with glycopyrronium bromidePulm Pharmacol Ther20142811412124928173
- BeasleyRSinghSLokeYKEnrightPFurbergCDCall for worldwide withdrawal of tiotropium Respimat mist inhalerBMJ2012345e739023144209
- GrasJGavaldàALlenasJThe preclinical cardiovascular safety profile of aclidinium bromide, a novel long-acting anticholinergic drugAm J Respir Crit Care Med2008177A654
- CazzolaMMateraMGNovel long-acting bronchodilators for COPD and asthmaBr J Pharmacol200815529129918604231
- ChanezPBurgePSDahlRAclidinium bromide provides long-acting bronchodilation in patients with COPDPulm Pharmacol Ther201023152119683590
- JoosGFSchelfhoutVJPauwelsRABronchodilatory effects of aclidinium bromide, a long-acting muscarinic antagonist, in COPD patientsRespir Med201010486587220044242
- SchelfhoutVJFerrerPJansatJMActivity of aclidinium bromide, a new long-acting muscarinic antagonist: a phase I studyBr J Clin Pharmacol201069545846420573081
- MaltaisFCelliBCasaburiRAclidinium bromide improves exercise endurance and lung hyperinflation in patients with moderate to severe COPDRespir Med201110558058721183326
- SinghDMagnussenHKirstenAA randomized, placebo-and active-controlled dose-finding study of aclidinium bromide administered twice a day in COPD patientsPulm Pharmacol Ther20122524825322497752
- FuhrRMagnussenHSaremKEfficacy of aclidinium bromide 400 μg twice daily compared with placebo and tiotropium in patients with moderate to severe COPDChest2012141374575221903737
- JonesPWSinghDBatemanEDEfficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN studyEur Respir J20124083083622441743
- GelbAFTashkinDPMakeBJLong-term safety and efficacy of twice-daily aclidinium bromide in patients with COPDRespir Med20131071957196523916502
- BeierJKirstenAMMrózREfficacy and safety of aclidinium bromide compared with placebo and tiotropium in patients with moderate-to-severe chronic obstructive pulmonary disease: results from a 6-week, randomized, controlled phase IIIb studyCOPD20131051152223819698
- RottenkolberMRottenkolberDFischerRInhaled beta-2 agonists/muscarinic antagonists and acute myocardial infarction in COPD patientsRespir Med20141081075109024950946
- CortijoJMataMMilaraJAclidinium inhibits cholinergic and tobacco smoke-induced MUC5AC in human airwaysEur Respir J201137224425420525722
- CaramoriGCasolariPDi GregorioCMUC5AC expression is increased in bronchial submucosal glands of stable COPD patientsHistopathology200955332133119723147
- InnesALWoodruffPGFerrandoREEpithelial mucin stores are increased in the large airways of smokers with airflow obstructionChest200613041102110817035444
- DameraGJiangMZhaoHAclidinium bromide abrogates allergen-induced hyperresponsiveness and reduces eosinophilia in murine model of airway inflammationEur J Pharmacol20106491–334935320868661
- KerwinEMD’UrzoADGelbAFLakkisHGarcia GilECaractaCFACCORD I study investigatorsEfficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I)COPD2012929010122320148
- JonesPWRennardSIAgustiAEfficacy and safety of once-daily aclidinium in chronic obstructive pulmonary diseaseRespir Res20111215521518460
- CazzolaMCalzettaLPageCPPharmacological characterization of the interaction between aclidinium bromide and formoterol fumarate on human isolated bronchiEur J Pharmacol201474513514325446566
- CazzolaMRoglianiPMateraMGAclidinium bromide/formoterol fumerate fixed-dose combination for the treatment of chronic obstructive pulmonary diseaseExpert Opin Pharmacother201314677578123472632
- SliwinskiPPerngD-WChuchalinAJonesPWEfficacy and safety of once-daily aclidinium bromide 200 μg in combination with formoterol in patients with COPDThorax201065A136
- MagnussenHWatzHKretschmarGPharmakokinetik, Sicherheit und Aktivitat von formoterol verabreicht uber den Genuair Inhalator® mit und ohne Aclidinium-BromidPneumologie201165V446 German
- D’UrzoADRennardSIKerwinEMMergelVLeselbaumARCaractaCFAUGMENT COPD Study InvestigatorsEfficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD studyRespir Res201415112325756831
- SinghDJonesPWBatemanEDEfficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised studyBMC Pulm Med20141417825404569
- CazzolaMSegretiARoglianiPComparative effectiveness of drugs for chronic obstructive pulmonary diseaseDrugs Today2012481278579423243635
- SalamaROYoungPMRoguedaPAdvances in drug delivery: is triple therapy the future for the treatment of chronic obstructive pulmonary disease?Expert Opin Pharmacother2011121913193221714776
- MoenMDIndacaterol: in chronic obstructive pulmonary diseaseDrugs2010702269228021080743
- van NoordJAKorduckiLHamiltonAKokerPFour weeks once daily treatment with BI 1744 CL, a novel long-acting β2-agonist, is effective in COPD patients [abstract]Am J Respir Crit Care Med2009179A6183
- HananiaNAFeldmanGZachgoWDose-related efficacy of vilanterol trifenatate (VI) in COPD [abstract]Eur Respir J201036Suppl 54217s
- VestboJVogelmeierCCreemersJFalquesMRiberaAGarcia GilEOnset of effect of aclidinium, a novel, long-acting muscarinic antagonist, in patients with COPDCOPD2010733133620854047
- VerkindreCFukuchiYFlémaleASustained 24-h efficacy of NVA237, a once-daily long-acting muscarinic antagonist, in COPD patientsRespir Med20101041482148920541381
- KerwinEMHebertJPedinoffANVA237 once daily provides rapid and sustained bronchodilation in COPD patients, with efficacy similar to tiotropium: the GLOW2 trial [abstract]Am J Respir Crit Care Med2012185A2920
- BatemanEFeldmanGKilbrideSEfficacy and safety of the long-acting muscarinic antagonist GSK233705 delivered once daily in patients with COPDClin Respir J2012624825722329914
- CasaburiRBriggsDDJrDonohueJFSerbyCWMenjogeSSWitekTJJrfor the US Tiotropium Study GroupThe spirometric efficacy of once-daily dosing with tiotropium in stable COPD. A 13-week multicenter trialChest20001181294130211083677
- TashkinDPFergusonGTCombination bronchodilator therapy in the management of chronic obstructive pulmonary diseaseRespir Res2013144923651244
- BeierJvan NoordJDeansASafety and efficacy of dual therapy with GSK233705 and salmeterol versus monotherapy with salmeterol, tiotropium, or placebo in a crossover pilot study in partially reversible COPD patientsInt J Chron Obstruct Pulmon Dis2012715316422419863
- TashkinDPDonohueJFMahlerDAEffects of arformoterol twice daily, tiotropium once daily, and their combination in patients with COPDRespir Med200910351652419208459
- HananiaNABootaAKerwinETomlinsonLDenis-MizeKEfficacy and safety of nebulized formoterol as add-on therapy in COPD patients receiving maintenance tiotropium bromide: results from a 6-week, randomized, placebo-controlled, clinical trialDrugs2009691205121619537837
- TashkinDPPearleJIezzoniDVargheseSTFormoterol and tiotropium compared with tiotropium alone for treatment of COPDCOPD20096172519229704
- VogelmeierCKardosPHarariSGansSJStengleinSThirlwellJFormoterol mono- and combination therapy with tiotropium in patients with COPD: a 6-month studyRespir Med20081021511152018804362
- van NoordJAAumannJ-LJanssensECombining tiotropium and salmeterol in COPD: effects on airflow obstruction and symptomsRespir Med2010104995100420303247
- ReisnerCFogartyCSpangenthalSNovel combination of glycopyrrolate and formoterol MDI (GFF-MDI) provides superior bronchodilation compared to its components administered alone, tiotropium DPI, and formoterol DPI in a randomized, double-blind, placebo-controlled Phase 2b study in patients with COPD [abstract]Am J Respir Crit Care Med2011183A6453
- ReisnerCSt RoseEStromSFixed combination of glycopyrrolate and formoterol MDI (GFF-MDI) demonstrates superior inspiratory capacity (IC) compared to tiotropium DPI (Tio) following 7 days dosing, in a randomized, double-blind, placebo-controlled phase 2b study in patients with COPD [abstract]Eur Respir J201138Suppl 55150s
- van NoordJABuhlRLaForceCQVA149 demonstrates superior bronchodilation compared with indacaterol or placebo in patients with chronic obstructive pulmonary diseaseThorax2010651086109120978028
- Van de MaeleBFabbriLMMartinCHortonRDolkerMOverendTCardiovascular safety of QVA149, a combination of indacaterol and NVA237, in COPD patientsCOPD2010741842721166630
- BatemanEDFergusonGTBarnesNDual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE studyEur Respir J2013421484149423722616
- VogelmeierCFBatemanEDPallanteJEfficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group studyLancet Respir Med20131516024321804
- DahlRChapmanKRudolfMQVA149 administered once daily provides significant improvements in lung function over 1 year in patients with COPD: the ENLIGHTEN study [abstract]Eur Respir J201240Suppl 56P2896
- MaltaisFBeckEWebsterDFour weeks once daily treatment with tiotropium + olodaterol (BI 1744) fixed dose combination compared with tiotropium in COPD patients [abstract]Eur Respir J201036Suppl 541014s
- AalbersRMaleki-YazdiMRHamiltonADose-finding study for tiotropium and olodaterol when administered in combination via the Respimat® inhaler in patients with COPDEur Respir J201240Suppl 56525s
- FeldmanGWalkerRRBrooksJMehtaRCraterGSafety and tolerability of the GSK573719/vilanterol combination in patients with COPD [abstract]Am J Respir Crit Care Med2012185A2938

