Abstract
Drug hypersensitivity is an inflammatory or immune reaction induced by drugs. It can be fatal if not appropriately treated and cause the risk of long-term complications. Sulfonamides are classified as antimicrobial drugs with a broad spectrum effective for gram-positive and gram-negative bacteria. This antibacterial agent works by competitively inhibiting folic acid synthesis, which prevents the growth and proliferation of microorganisms. In its use as antibiotics, sulfonamides can also cause adverse reactions in specific individuals. It has been widely reported that sulfonamide antimicrobials cause hypersensitivity reactions mediated by IgE or T cells. This review identifies symptoms or signs that can appear, as well as genes associated with sulfonamide hypersensitivity reactions, as sulfonamide may cause hypersensitivity in the form of uveitis, skin rash, Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN), parotitis, angioedema, drug reaction with eosinophilia and systemic symptoms (DRESS), and pruritus. In addition, several genes were found to be associated with sulfonamide hypersensitivity, including HLA-A29, HLA-B12, HLA-DR7, HLA-B44, and HLA A*11:01.
Introduction
Hypersensitivity is a term used to describe a symptom initiated by exposure to a stimulus that should be tolerated.Citation1 Drug hypersensitivity is an inflammatory or immune reaction induced by drugs. It can be fatal if not appropriately treated and cause the risk of long-term complications.Citation2,Citation3 According to the World Health Organization (WHO), drug hypersensitivity is a type B adverse drug reaction (ADR) in the form of a dose-independent, dangerous, and unwanted response with a wide range of clinical phenotypes onset and severity.Citation4 ADR occurs in all activities in the hospital in approximately 3–6% of hospitalized patients in about 10–15%.Citation5 In the United States in 2002, drug hypersensitivity reactions reached 6–10%, which mainly occurred in patients without inpatient treatment at the hospital.Citation6
The first synthetic antimicrobial drugs successfully used for various bacterial infections were sulfonamide antibiotics, which were first introduced in the 1930s.Citation7 Sulfonamides are classified as antimicrobial drugs with a broad spectrum effective for gram-positive and gram-negative bacteria.Citation8 This review will discuss about antibiotic and nonantibiotic sulfonamides. Sulfamethoxazole (SMX), sulfamerazine, sulfamethizole, sulfamoxole, sulfamethazine, sulfisoxazole, and sulfapyridine are antibiotic sulfonamides. Whereas furosemide, hydrochlorothiazide, sulfasalazine, acetazolamide, sumatriptan, and glyburide are classified as nonantibiotic sulfonamides.Citation9 There are chemical molecular structure differences between antibiotic and nonantibiotic sulfonamides. In the antibiotic structure, an arylamine group plays a role in skin reactions to drugs. The arylamine group will be metabolized, which causes an immune response by acting as a hapten and the presence of T lymphocytes that appear when a sulfonamide-induced skin reaction occurs.Citation10
Sulfonamides are usually prescribed to treat infectious diseases such as bronchitis, bacterial meningitis, pneumonia, diarrhea, urinary tract infections, eyes, and ears.Citation11 Sulfonamide antibacterial agents are derivatives of 4-aminobenzenesulfonamide or sulfanilamide.Citation7 This antibacterial agent works by competitively inhibiting folic acid synthesis, which prevents the growth and proliferation of microorganisms. Because of the mechanism, sulfonamides are included in the bacteriostatic antibiotic group.Citation8 As antibiotics, sulfonamides can also cause ADRs in specific individuals. In the general population, it is estimated that 3–8% of patients report a sulfonamide allergy.Citation12 It has been widely reported that sulfonamide antimicrobials cause hypersensitivity reactions mediated by IgE or T cells. This hypersensitivity can cause severe cutaneous adverse reactions (SCARs), such as Stevens-Johnson Syndrome (SJS)/Toxic Epidermal Necrolysis (TEN) Drug reaction with eosinophilia and systemic symptoms (DRESS).Citation13 There are risk factors associated with allergic reactions that cause sulfonamide, ie, HIV-positivity,Citation14 cross-reactivity,Citation15 genetic,Citation16 and drug administration.Citation5
HIV-positive patients and sulfonamide antimicrobial hypersensitivity have been detected by 3–8% of the population and 27% of the trimethoprim/sulfamethoxazole (TMP-SMX) treatment for Pneumocystis Pneumonia (PCP).Citation15 Then, the cutaneous administration of sulfonamide drugs can cause serious adverse effects, including allergic reaction.Citation5 After that, HLA-A*11:01 was responsible for Japanese patients causing hypersensitivity reactions of sulfonamide.Citation16 Unlike Japanese, a study published by Strom et al showed that cross-reactivity has a severe effect causing allergic reactions. The percentage of sulfonamide drugs with long-term uses has 9.9% for antimicrobial and 1.6% for non-antimicrobial.
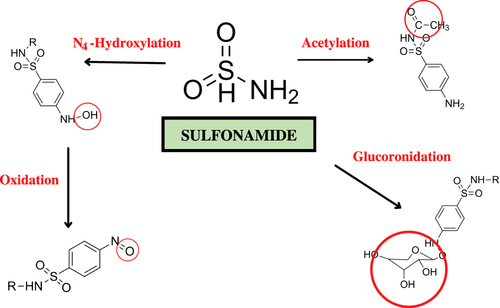
Three potentials can cause allergies due to sulfonamides, including drug molecules or their metabolites acting as haptens; the presence of molecular bonds with proteins that trigger an immune response; or cellular proteins that elicit direct cytotoxicity and induce an immune response. Based on sulfonamide metabolites, some parts are suspected of causing allergic reactions or hypersensitivity, marked with a red circle () when interacting with IgE. However, there is no specific explanation of the five metabolites that commonly cause allergic reactions or hypersensitivity. The NH2-SO2 cannot bind to IgE. Thus, the suspicion of this part causing an allergic reaction or hypersensitivity can be eliminated.Citation12
In a study by Reinhart et al, several candidate genes are involved in the pathogenesis of hypersensitivity to sulfonamides.Citation17 However, the pharmacogenetics for sulfonamide hypersensitivity is still being evaluated in a limited number of studies.Citation17 In 2001, a study demonstrated the association between the human leukocyte antigen (HLA)-A30 B13 Cw6 haplotype and TMP-SMX-induced skin lesions known as fixed drug eruption (FDE).Citation18 This indicates a link between hypersensitivity reactions with a person’s genetic condition. Therefore, this review aimed to discuss the immunopathology and clinical manifestations of sulfonamide hypersensitivity reactions, including description, reported case, mechanism, diagnosis, treatment, prevention of each clinical manifestation, and the associated genes are summarized in .
Table 1 Summary of Clinical Manifestations of Sulfonamide Hypersensitivity Reactions
Immunopathology
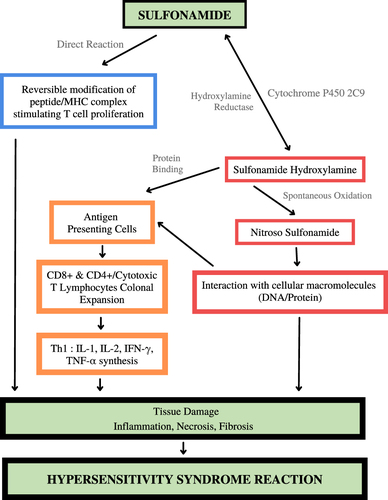
Normally, sulfonamides are metabolized by N-acetyltransferase. Sulfonamide toxicity is thought to be pioneered by drug bioactivation by forming hydroxylamine derivatives by the cytochrome P450 2C9 monooxygenase system, a cytotoxic metabolite produced by spontaneous oxidation to nitroso derivatives with a significant toxicity-enhancing effect. Hydroxylamine or nitroso compounds will interact with DNA or proteins to damage tissues and cause hypersensitivity syndrome reaction. Nitroso-derived compounds can also activate T cells through the major histocompatibility complex on antigen-presenting cells to produce cytotoxic T lymphocytes, which will later cause cell apoptosis and tissue damage. Unmetabolized sulfonamides can directly stimulate the immune system by activating T cell receptors via the major histocompatibility complex (MHC). The mechanism of sulfonamide hypersensitivity can be seen in .Citation19
Early Type Hypersensitivity Reaction
Angioedema
Angioedema is a sudden swelling in the skin and mucous membranes and the respiratory and digestive tracts.Citation20,Citation21 The process of this swelling usually lasts approximately 24 hours or more and can heal without changing skin color.Citation22 Therefore, angioedema is considered an early type of hypersensitivity and type I IgE-mediated hypersensitivity.Citation9 Approximately 25% of people in the United States will experience angioedema in their lives.Citation23,Citation24 Symptoms of angioedema include swelling around the eyes, lips, and tongue accompanied by shortness of breath and dizziness and can cause fainting.Citation25
In general, the mechanism of edema formation is an increase in the permeability of the local or subcutaneous submucosal capillaries, which causes extravasation of plasma, eventually causing temporary swelling. Plasma extravasation can be caused by various molecular mediators, one of which is mast cell mediators such as histamine. Hypersensitivity caused by sulfonamides can be associated with a classic allergic reaction that triggers histamine release, resulting in plasma extravasation.Citation20 The algorithm diagnostic for angioedema started by reviewing all patient medications. Then, stop all the medicines that cause angioedema. Measure the levels of C4 complement to determine whether it is hereditary angioedema (HAE) or not. Besides that, skin testing or IgE assays can be suggested.Citation24
The first line of therapy is antihistamines (H-1 blockers) because angioedema is associated with increased histamine levels. In addition, glucocorticoids (prednisolone) and sympathomimetics (norepinephrine) are used as second-line therapies if the first therapy does not provide significant results. It is necessary to pay attention to the patient’s condition first; if the swelling that is felt can interfere with the breathing process, intubation or oxygen therapy can be given.Citation20,Citation21 It is necessary to avoid triggers of angioedema, such as allergens or medications. The use of recommended drugs such as antihistamines or corticosteroids may not need to be given, but with these administrations, they can overcome symptoms more quickly.Citation25
Delayed-Type Hypersensitivity Reactions
Uveitis
Uveitis is an inflammatory process in the part of the eye known as the uvea (consisting of the iris, ciliary body, and choroid).Citation26 Uveitis has many prognoses and therapeutic approaches depending on its location, severity, and etiology. It can be infectious, neoplastic or traumatic, and immune-mediated.Citation27 Symptoms can include redness, painful eyes, conjunctival injection, and total vision loss.Citation26 Uveitis can be induced by using drugs both systemically and locally from drug-induced uveitis (DIU), which is a type III hypersensitivity reaction.Citation27 Sulfonamides are one of the drugs that can induce uveitis in humans through systemic administration.Citation28
The most prominent case series ever reported by Tilden et al, 1991 related to sulfonamide-induced uveitis, which included fourteen patients with bilateral anterior uveitis without hypopyon (twelve cases occurring after TMP-SMX administration, one after sulfadiazine administration, and one after unspecified sulfonamide derivative administration). Uveitis occurred an average of eight days after starting therapy, and four patients developed uveitis within the first 24 hours of treatment. Based on this case, uveitis is considered as a delayed hypersensitivity.Citation29 Potocnik et al, 2019 also reported uveitis as a form of ADR in the use of trimethoprim-sulfonamide in horses. Quarter horse mare has bilateral anterior uveitis with miosis. Both eyelids were swollen with edematous conjunctiva. There are yellowish-white annular opacities on the cornea originating from the limbus. A light flare increases in the anterior chamber. The left pupil was miotic, and the right pupil was slightly dilated. From horses’ clinical signs and symptoms, sulfonamide-induced uveitis is suspected to be due to the same syndrome described in humans.Citation19
In many cases, these ADRs were reported due to the combined administration of TMP-SMX. Trimethoprim, which is usually combined with sulfamethoxazole, has uveitogenic properties.Citation30 Inflammation may result from the direct immunogenicity of sulfonamides or the result of systemic necrotizing vasculitis in the case of Stevens-Johnson syndrome.Citation28 However, the exact mechanism of sulfonamide-induced uveitis remains unknown.Citation31 All patients with sulfonamide-induced uveitis were successfully treated with topical corticosteroids and drug discontinuation.Citation31 In the future, patients who have survived this uveitis should not be given another DIU, such as trimethoprim-sulfonamides.Citation19
Skin Rash
Drug-related hypersensitivity has a wide range of manifestations and can be fatal. The most common manifestation is skin rash, occurring in approximately 2–3% of hospitalized patients. The risk of this hypersensitivity depends on the structure of the drug, the patient’s immune system, the dose of the drugs, the route of administration, the duration of treatment, and the specific type of HLA.Citation32 A rash is an area of skin that is irritated or swollen. There is a rash with signs of redness, pain, itching, and irritation, and there is also a rash that causes blisters on the skin.Citation33 After beta-lactam antibiotics, sulfonamides are one of the most common causes of drug-related hypersensitivity.Citation34 Drug-induced hypersensitivity syndrome (DHS), such as skin rash, is a type IV hypersensitivity reaction. Sulfonamide antimicrobials have been implicated in various hypersensitivity reactions, such as T cell-mediated rash.Citation13
Schnyder and Pichler 2013 reported a 48-year-old man who developed a rash for three days and an intermittent fever with a high temperature for eight days. Three weeks before this incident, the patient took a series of drugs, including sulfasalazine (nonantibiotic sulfonamide group). During the initial evaluation, this man developed several other symptoms, and after six weeks, while in remission, the results of the patch test (10% petrolatum) with sulfasalazine, sulfapyridine, sulfamethoxazole, and other drugs were negative, but the lymphocyte transformation test (LTT) showed that there was an intense proliferation of the patient’s lymphocytes against sulfapyridine and sulfamethoxazole, the sulfonamide group. From these results, it can be seen that the patient has an allergy to sulfonamides.Citation35 Patients allergic to sulfamethoxazole (SMX) should avoid sulfasalazine, and conversely, patients allergic to sulfasalazine should no longer be given sulfonamide antibiotics.Citation36
The mechanism of sulfonamide-related hypersensitivity reactions, according to Schnyder and Pichler in 2013, involves Ig E and T cells and sometimes Ig G. Sulfamethoxazole, one of the sulfonamide antibiotics, is a prodrug that is metabolized to SMX-NOH (SMX hydroxylamine), which will then be oxidized to SMX-NO (Nitroso SMX). SMX-NO is highly reactive by binding to cysteine insoluble and cell-bound proteins. This results in an immune response. SMX can stimulate T cells directly without being metabolized before. SMX drugs can directly bind to HLA receptors and T cell receptors, which directly and indirectly cause T cell stimulation.Citation35 This mechanism is described in .Citation37
It is better to discontinue consuming drugs suspected of causing hypersensitivity that result in a rash. Methylprednisolone, which belongs to the class of corticosteroid drugs, can treat hypersensitivity symptoms with a rash. Corticosteroid treatment could be tapered off in a few weeks until the rash symptoms subside and can finally be stopped.Citation35
SJS/TEN
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are diseases that affect the skin and mucous membranes as a manifestation of adverse drug reactions.Citation38 These reactions are considered to have delayed-type hypersensitivity and represent an actual medical emergency.Citation39 SJS/TEN is a type IVc hypersensitivity reaction.Citation9 In addition to sulfonamides (eg, TMP-SMX), there are several other drug classes associated with SJS/TEN, such as anticonvulsants (phenobarbital, carbamazepine, phenytoin), antibiotics (penicillins, tetracyclines, macrolides), and other drugs (allopurinol, NSAIDs, sertraline).Citation40 Cases of drug-associated SJS/TEN are approximately 1.8 and 9.0 per million person-years.Citation41 In the 87 cases of TEN, 18% were thought to be TMP/SMX-related symptoms.Citation42 Symptoms associated with SJS/TEN are characterized by skin erythema with blisters, hemorrhagic erosion of mucous membranes, fever, and malaise.Citation38
Drugs are the most common etiologic factor in most cases of SJS/TEN. However, the relationship between drugs as a cause of epidermal necrosis is not known with certainty. There is a role for T cells, particularly CD8+ lymphocytes, identified and mediated by cytokines.Citation38 SJS generally results from widespread keratinocyte apoptosis induced by cytotoxic reactions and mediated by T lymphocytes.Citation43 Sulfonamides are metabolized by N-acetyltransferases, and their toxicity is associated with the formation of hydroxylamine derivatives by the cytochrome P450 (CYP)3 monooxygenase system, primarily via CYP2C9. Hydroxylamine will interact with cellular macromolecules such as DNA/protein, which will initiate the formation of tissue damage ().Citation19
In helping to classify the clinical conditions, the percentage of body surface area (BSA) with blisters or erosions in SJS/TEN is used.Citation45,Citation46 SJS will have erosions <10% of BSA, SJS-TEN overlaps with 10–30% of BSA, while TEN is about >30% of BSA.Citation44 In drug-induced hypersensitivity reactions, there are increased levels of TARC (serum thymus and activation regulated chemokine), including Th2 and Th1 chemokine. Therefore, those two values might be implicated in SJS/TEN.Citation46 Besides that, patch testing is used to identify the drugs that may cause the hypersensitivity reactions.Citation39
So far, there has been no specific therapy that is considered the best in the management of SJS/TEN. The most crucial aspect in the treatment of SJS/TEN is the prompt administration of the suspected drugCitation47 or by discontinuing the use of the drug suspected of triggering SJS/TEN. The patient can be given symptomatic therapy such as analgesics and rehydration. In addition, in the treatment of SJS/TEN, corticosteroids, cyclosporine A, IV immunoglobulin, plasmapheresis, and N-acetylcysteine can be administered.Citation48 Regarding the prevention of SJS/TEN, there is a need for screening related to pharmacogenetics. Based on Cummin et al, the HLA-B*1502 allele was found in all patients with drug-induced SJS/TEN, and only 3% were tolerant.Citation49 Then, preventive measures need to be taken to limit drugs known to cause hypersensitivity. In this case, the sulfonamides group induced SJS/TEN.
Parotitis
Parotitis is an inflammation that occurs in the parotid gland, an unusual condition caused by an autoimmune process, obstruction of salivary stones, or the presence of a viral/bacterial infection.Citation50 Commonly referred to include anticholinergics, antihistamines, and antipsychotics.Citation51 Based on Patel et al, this case of parotitis induced with TMP/SMX drugs was the first recorded in the literature. In this case, a 44-year-old man had bilateral parotid swelling with a tenderness that appeared seven days after treatment with TMP/SMX. There was no viral or bacterial cause, and when the drug was discontinued, the patient’s symptoms improved.Citation51 Until 2016, Baer et al identified 56 cases were salivary gland swelling with eosinophil-rich mucus.Citation52 Based on this case, parotitis is considered delayed-hypersensitivity because the reaction appears after several doses have been taken by the patient.Citation51 Based on Wu et al, an increased level of IgE in parotitis patients indicates type I hypersensitivity may be involved.Citation53
The exact mechanism by which TMP/SMX induces parotitis is yet unknown. Taguchi et al reported an association between methimazole causing pancreatitis and underlying parotitis associated with hypersensitivity reactions. This causes the possibility of a disease mechanism relationship that occurs between the pancreas and the parotid gland. Since sulfonamides are also associated with pancreatitis, it can be concluded that the correlation of TMP/SMX-induced parotitis is also the result of an immune response similar to Taguchi et al.Citation54,Citation55 In this case, the patient’s condition was resolved with the discontinuation of TMP/SMX. Thus, it is known that the patient’s parotitis was caused by hypersensitivity and caused by TMP/SMX.Citation51
Alternative therapy is given to the patient, such as an injection of methylprednisolone and increased fluid intake.Citation51 It is necessary to take preventive measures by limiting drugs known to cause SJS/TEN. In addition, it is important to maintain oral hygiene, which avoids parotitis caused by viruses or bacteria.
DRESS
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a drug-induced hypersensitivity reaction characterized by skin rashes. It can progress to internal organs such as the liver, lungs, kidneys, lymphadenopathy, and hematological manifestations. Dress syndrome is classified as idiosyncratic because this syndrome results from unexplained side effects, is independent of dose, and mainly occurs only in susceptible individuals.Citation56,Citation57 A study by Kardaun et al found that few drugs or classes of drugs that commonly cause DRESS are anti-epileptic drugs (AEDs) (35%), allopurinol (18%), sulfonamides and dapsone (12%), and other types of antibiotics (11%).Citation57 DRESS is associated with a delayed-type IVB hypersensitivity reaction by antiviral T cells that mediated it.Citation58
In this case, a 17-year-old male with high-risk same-sex sexual behavior was treated for one week for gonorrhea and one month with TMP-SMX for acne. After treatment, the patient had a fever, facial edema, papules on the buccal mucosa, and rash all over his body. Lab tests were also performed, and there were no signs of infection or Nikolsky’s indication. The patient was diagnosed with DRESS caused by sulfonamide antibiotics. The diagnosis can be made by examining skin rashes that occur in more than half of the body's surface area and skin histopathology. In addition, examination of internal organs, such as ultrasound hepatosplenomegaly, was performed because eosinophils can attack the body and cause damage. The most commonly damaged main organs are the liver, kidneys, and heart. Other organs that can also be damaged are the lungs and nervous system. Genetic testing and infection can also be performed if needed. The exact mechanism of DRESS is yet unknown, but it can be figured based on the etiology of genetics and viral reactivation ().Citation59,Citation60 Based on the report and publication, 23 cases of DRESS caused by sulfonamide have been reported.Citation61
So far, there has been no specific treatment for DRESS sufferers. The most crucial aspect of DRESS management is discontinuing suspected DRESS-inducing drugs and administering drugs to relieve symptoms such as antipyretic therapy and corticosteroids. Oral prednisolone is a commonly used corticosteroid at a dose of 0.5–1 mg/kg/day for 2–3 months. Other therapies include intravenous immunoglobulin (IVIg) and immunosuppressant agents, such as cyclosporine, cyclophosphamide, and rituximab. Other adjuvant therapies include rehydration, electrolyte balance, and high-calorie administration.Citation64,Citation65 In preventing DRESS, further research and investigation of DRESS are still needed.
Pruritus
Pruritus is an itchy disease that generally ranges from 6 weeks to a maximum of 6 months.Citation62 Pruritus is the most common dermatological disease, causing uncomfortable itching.Citation63,Citation64 Pruritus has various etiologies, one of which is a hypersensitivity reaction; in the case of Elzagallaai et al, pruritus induced by sulfonamide side effects reached 23% in 26 patients.Citation65 Pruritus has a variety of etiologies so that the diagnosis can be made by biopsy and examination of the history before the disease appears.Citation66 The most common signs of pruritus are xerosis (dry skin) or eczema. But, chronic pruritus can also induce sleep impairment, fatigue, and irritability.Citation66
There are many mechanisms of pruritus; histamine is one of them as a key that causes this disease. When something (chemical, biology, etc.) has activated, basophilic leukocyte and mast cells, histamine has been released and binds H1R. The activation of H1R by histamine causes the activation of phospholipase Cβ3 (PLCβ3) and phospholipase A2 (PLA2) that increase the development of the pruritus.Citation64
Pruritus is associated with type I delayed hypersensitivity that causes IgE immune system reactions.Citation67 The treatment can be stopped when it happens, and if it does not improve, antihistamines and corticosteroids can also be given necessarily. However, the treatment must be adjusted to the inducing drug and the route of administration.Citation68 Therapy with antihistamines or corticosteroids can be adjusted according to the patient’s needs and medical records because pruritus usually resolves on its own.Citation68
Genetic Influence
It has been explained in the previous discussion that sulfonamides can cause SCARS, including SJS/TEN and DRESS, or drug-induced hypersensitivity syndrome (DIHS).Citation35 Genetic susceptibility to the incidence of SCARs has long been suspected because of several family case reports and previous studies showing an association with serologically determined HLA.Citation69 Individuals who are susceptible to sulfonamide-induced SCARs can be seen by the presence of a characteristic HLA. This HLA was studied from patients who survived SCARs.Citation70
HLA is a membrane-bound glycoprotein that binds to antigenic peptides and presents them to T cells.Citation71 HLA is encoded by the major histocompatibility complex (MHC) and plays a vital role in maintaining the immunity.Citation72 The main function of HLA is to recognize foreign proteins that enter the body. When there is an interaction between the two, the protein complex will be brought to the cell surface to be recognized by T cells, resulting in an immune response.Citation73 HLA is divided into HLA class I and class II. HLA class I consists of HLA-A, HLA-B, HLA-C, and HLA class II consists of HLA-DR, HLA-DQ, and HLA-DP.Citation71 The mechanism of SCARs is suspected to be due to off-target drug binding to specific HLA molecules.Citation74
Roujeau et al, in 1987, studied HLA in patients who survived TEN. There were 13 patients with sulfonamide-induced TEN with nine cases induced by TMP-SMX. Of the 13 patients, HLA-A29 (6 of 13 patients), HLA-B12 (10 of 13), and HLA-DR7 (7 of 11) were associated with the incidence of sulfonamide-induced TEN. Fischer and Shigeoka’s study in 1983 also discussed the intervention of genetic factors in SCARs in families. There is a case of SJS in identical twins induced by the administration of erythromycin and sulfonamide. The mother turned out to have a history of urticarial rash after taking oral erythromycin for one day, and her maternal grandfather had experienced symptoms resembling SJS after taking sulfonamides. The same HLA A29, B44, and DR7 were found in four family members. These studies demonstrated that HLA was associated with an increased risk of SCARs after sulfonamide administration.Citation75
Nakamura et al, in 2020, also investigated the possibility of sulfonamide binding via the peptide-binding pathway with HLA. This study used 15 patients who had a history of sulfonamide-associated SCARs, eight patients had SJS/TEN, and seven patients had DIHS. Overall, 10 out of 15 patients had an HLA A A*11:01 excess, and the association was significant. Thus, this study found HLA-A*11:01 to be a risk factor for sulfonamide-induced SCARs in Japanese patients. From these studies, it can be learned that certain HLAs are susceptible to sulfonamides that cause SCARs in specific populations.Citation16 Therefore, prescreening genetic testing is thought to prevent severe hypersensitivity reactions.Citation14
Conclusion and Future Perspectives
In our study, we summarized and reviewed cases of sulfonamide-induced hypersensitivity reactions, such as uveitis, skin rash, SJS/TEN, parotitis, angioedema, methemoglobinemia, DRESS, and pruritus. This is a form of adverse drug reaction that does not occur in all individuals. There are some individuals with specific genes that can trigger this hypersensitivity reaction. Several genes were found that can be associated with sulfonamide hypersensitivity and need to be studied further regarding the mechanism that occurs, including HLA-A29, HLA-B12, HLA-DR7, HLA-B44, and HLA A*11:01. In the future, this study could assist in developing sulfonamide hypersensitivity research regarding the relationship between genes that have been mentioned to trigger this hypersensitivity reaction with a diagnosis of hypersensitivity that can be used.
Abbreviations
ADR, adverse drug reactions; SCARs, severe cutaneous adverse reactions; TMP-SMX, Trimethoprim-sulfamethoxazole; SMX, Sulfamethoxazole; DRESS, Drug Reaction with Eosinophilia and Systemic Symptoms; DIU, Drug-induced Uveitis; LTT, Lymphocyte Transformation Test; SNO, S-nitrosylation; AEDs, anti-epileptic drugs; DIHS, Drug-Induced Hypersensitivity Syndrome; HLA, Human Leukocyte Antigen; MHC, Major Histocompatibility Complex.
Author Contributions
All authors have contributed significantly in all aspects of the work reported, such as in the conception, design, execution, data retrieval, data analysis, and interpretation, as well as compiling, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the report has been submitted; and agreed to take responsibility and be accountable for all aspects of the work for this article.
Disclosure
All authors report no conflicts of interest in this work.
Acknowledgment
This study is supported by Universitas Padjadjaran, Indonesia.
References
- Johansson SGO, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: report of the nomenclature review committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113(5):832–836. doi:10.1016/j.jaci.2003.12.591
- Pichler WJ, Yerly D. Drug hypersensitivity: we need to do more. J Allergy Clin Immunol. 2018;141(1):89–91. doi:10.1016/j.jaci.2017.11.002
- Tsai YG, Chung WH, Abe R, Tassaneeyakul W. New advances in drug hypersensitivity research and treatment. J Immunol Res. 2018;2018:2–4. doi:10.1155/2018/9345078
- Tanno LK, Bierrenbach AL, Simons FER, et al. Critical view of anaphylaxis epidemiology: open questions and new perspectives. Allergy Asthma Clin Immunol. 2018;14(1):2. doi:10.1186/s13223-018-0234-0
- Thong BYH, Tan TC. Epidemiology and risk factors for drug allergy. Br J Clin Pharmacol. 2011;71(5):684–700. doi:10.1111/j.1365-2125.2010.03774.x
- Ratajczak HV. Drug-induced hypersensitivity: role in drug development. Toxicol Rev. 2004;23(4):265–280. doi:10.2165/00139709-200423040-00006
- Shah F, Bell IM. Cutaneous adverse events caused by sulfonamide-containing drugs: reality or perception? J Med Chem. 2020;63(14):7447–7457. doi:10.1021/acs.jmedchem.9b01932
- Tacic A, Nikolic V, Nikolic L, Savic I. Antimicrobial sulfonamide drugs. Adv Technol. 2017;6(1):58–71. doi:10.5937/savteh1701058t
- Castells M, Santos-Bonamichi R. Drug Hypersensitivity. Elsevier Ltd; 2019. Vol. 2. doi:10.1002/9781444300918.ch94
- Gruchalla RS. Drug Allergy. J Allergy Clin Immunol. 2003;111(2):548–559. doi:10.1067/mai.2003.93
- García-Galán MJ, Silvia Díaz-Cruz M, Barceló D. Identification and determination of metabolites and degradation products of sulfonamide antibiotics. TrAC - Trends Anal Chem. 2008;27(11):1008–1022. doi:10.1016/j.trac.2008.10.001
- Giles A, Foushee J, Lantz E, Gumina G. Sulfonamide Allergies. Pharmacy. 2019;7(3):132. doi:10.3390/pharmacy7030132
- Khan DA, Knowles SR, Shear NH. Sulfonamide Hypersensitivity: fact and Fiction. J Allergy Clin Immunol Pract. 2021;7(7):2116–2123. doi:10.1016/j.jaip.2019.05.034
- Jung J, Kim J, Park I, Choi B, Kang H. Genetic markers of severe cutaneous adverse reactions. Korean J Intern Med. 2018;33(5):867–875. doi:10.3904/kjim.2018.126
- Carr A, Swanson C, Penny R, Cooper DA. Clinical and laboratory markers of hypersensitivity to trimethoprim-sulfamethoxazole in patients with pneumocystis carinii pneumonia and AIDS. J Infect Dis. 1993;167(1):180–185. doi:10.1093/infdis/167.1.180
- Nakamura R, Ozeki T, Hirayama N, et al. Association of HLA-A*11:01 with sulfonamide-related severe cutaneous adverse reactions in Japanese patients. J Invest Dermatol. 2020;140:1659–1662.e6. doi:10.1016/j.jid.2019.12.025
- Reinhart JM, Motsinger-Reif A, Dickey A, Yale S, Trepanier LA. Genome-wide association study in immunocompetent patients with delayed hypersensitivity to sulfonamide antimicrobials. PLoS One. 2016;11(6):1–15. doi:10.1371/journal.pone.0156000
- Özkaya-Bayazit E, Akar U. Fixed Drug Eruption induced by trimethoprim-sulfamethoxazole: evidence for a Link to HLA-A30 B13 Cw6 Haplotype. J Am Acad Dermatol. 2001;45(5):712–717. doi:10.1067/mjd.2001.117854
- Potocnik E, Drozdzewska K, Schwarz B. Presumed sulfonamide-associated uveitis with Stevens-Johnson Syndrome in a quarter horse mare. J Equine Vet Sci. 2019;77:17–22. doi:10.1016/j.jevs.2019.02.004
- Kaplan AP, Greaves MW. Angioedema. J Am Acad Dermatol. 2005;53(3):373–388. doi:10.1016/j.jaad.2004.09.032
- Greaves M, Lawlor F. Angioedema: manifestations and management. J Am Acad Dermatol. 1991;25(1):155–165. doi:10.1016/0190-9622(91)70183-3
- Bas M, Hoffmann TK, Kojda G. Evaluation and management of angioedema of the head and neck. Curr Opin Otolaryngol Head Neck Surg. 2006;14(3):170–175. doi:10.1097/01.moo.0000193202.85837.7d
- Brown SGA. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114(2):371–376. doi:10.1016/j.jaci.2004.04.029
- Bernstein JA, Moellman J. Emerging concepts in the diagnosis and treatment of patients with undifferentiated angioedema. Int J Emerg Med. 2012;5(1):1. doi:10.1186/1865-1380-5-39
- Tarbox A, Bansal J, Peiris AN. Angioedema. JAMA. 2018;319(19):2054. doi:10.1001/jama.2018.4860
- Jabs D, Nussenblatt RB, Rosenbaum JT. Perspectives standardization of uveitis nomenclature for reporting clinical data. Am J Ophthalmol. 2005;140:509–516. doi:10.1016/j.ajo.2005.03.057
- Cordero-coma M, Salazar-m R, Garzo-garcı I. Drug-induced uveitis. Expert Opin Drug Saf. 2015:1–16. DOI:10.1517/14740338.2015.972363
- Mindel J, Uveitis D. Therapeutic Review. Clin Ther. 1998;42:6.
- Morris E, Rosenbaum JT, Fraunfelder FT. Systemic Sulfonamides as a Cause of Bilateral, Anterior Uveitis. Arch Ophthalmol. 2015;109:67–69.
- Agarwal M, Majumder P, Babu K, et al. Drug-Induced Uveitis: a Review. Indian J Ophthalmol. 2020;68(9):1799–1807. doi:10.4103/ijo.IJO_816_20
- Moorthy RS, London NJS, Garg SJ. Drug-Induced Uveitis. Curr Opin Ophthalmol. 2013:589–597. DOI:10.1097/01.icu.0000434534.32063.5c
- Pichler WJ. Drug Hypersensitivity. 5th ed. Elsevier Ltd; 2009. Vol. 2. doi:10.1002/9781444300918.ch94
- NIH. Rashes. Available from: https://medlineplus.gov/rashes.html. Accessed June 9, 2021.
- Jr DAD, Montanaro A. Allergies to sulfonamide antibiotics and sulfur- containing drugs. Ann Allergy Asthma Immunol. 2008;100(2):91–101. doi:10.1016/S1081-1206(10)60415-2
- Schnyder B, Pichler WJ. Allergy to Sulfonamides. J Allergy Clin Immunol. 2013;131(1):256–257.e5. doi:10.1016/j.jaci.2012.10.003
- Zawodniak A, Lochmatter P, Beeler A, Pichler WJ. Cross-reactivity in drug hypersensitivity reactions to sulfasalazine and sulfamethoxazole. Int Arch Allergy Immunol. 2010;153:152–156. doi:10.1159/000312632
- Neuman MG, Shear NH, Malkiewicz IM, et al. Immunopathogenesis of hypersensitivity syndrome. Transl Res. 2007:243–253. DOI:10.1016/j.trsl.2006.12.001
- Mockenhaupt M. The current understanding of Stevens-Johnson syndrome and Toxic Epidermal Necrolysis. Expert Rev Clin Immunol. 2011;7(6):803–815. doi:10.1586/eci.11.66
- Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, Harr T. Current perspectives on Stevens-Johnson Syndrome and toxic epidermal necrolysis. Clin Rev Allergy Immunol. 2018;54(1):147–176. doi:10.1007/s12016-017-8654-z
- Davis WD, Schafer PA. Stevens-Johnson Syndrome: a challenging diagnosis. Adv Emerg Nurs J. 2018;40(3):176–182. doi:10.1097/TME.0000000000000197
- Daniel BS, Joly P, Murrell DF. Management of erythema multiforme, Stevens-Johnson Syndrome and toxic epidermal necrolysis. Blistering Dis Clin Featur Pathog Treat. 2015;617–622. doi:10.1007/978-3-662-45698-9_66
- Guillaume J-C, Roujeau J-C, Revuz J, Penso D, Touraine R. The Culprit Drugs in 87 Cases of Toxic Epidermal Necrolysis (Lyell’s Syndrome). Arch Dermatol. 1987;123:1166–1170.
- Harris V, Jackson C, Cooper A. Review of Toxic Epidermal Necrolysis. Int J Mol Sci. 2016;17(12):1–11. doi:10.3390/ijms17122135
- Paquet P, Piérard GE, Quatresooz P. Novel treatments for drug-induced toxic epidermal necrolysis (Lyell’s Syndrome). Int Arch Allergy Immunol. 2005;136(3):205–216. doi:10.1159/000083947
- Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau J-C. Clinical classification of cases of toxic syndrome, and erythema multiforme. Arch Dermatol. 1993;129(1):92–96. doi:10.1001/archderm.1993.01680220104023
- Komatsu-Fujii T, Kaneko S, Chinuki Y, et al. Serum TARC levels are strongly correlated with blood eosinophil count in patients with drug eruptions. Allergol Int. 2017;66(1):116–122. doi:10.1016/j.alit.2016.06.003
- Wildermuth A. Stevens-Johnson Syndrome and toxic epidermal necrolysis. J Am Acad Physician Assist. 2020;33(8):48–49. doi:10.1097/01.JAA.0000684164.28009.4a
- Fernando SL. The management of toxic epidermal necrolysis. Australas J Dermatol. 2012;53(3):165–171. doi:10.1111/j.1440-0960.2011.00862.x
- Cummins JM, Rago C, Kohli M, Kinzler KW, Lengauer C, Vogelstein B. A marker for Stevens–Johnson Syndrome. Nature. 2004;428(6982):2501. doi:10.1038/nature02501
- Sehic A, Haenig C, Spear F. An unusual case of acute parotitis in a young adult. J Am Acad Physician Assist. 2017;30(8):27–29. doi:10.1097/01.JAA.0000521134.30915.a9
- Patel JS, Scheiner ED. Acute parotitis induced by trimethoprim/Sulfamethoxazole. Ear, Nose Throat J. 2011;90:2. doi:10.1177/014556131109000214
- Baer AN, Okuhama A, Eisele DW, Tversky JR, Gniadek TJ. Eosinophilic Sialodochitis: redefinition of ‘Allergic Parotitis’ and ‘Sialodochitis Fibrinosa. Oral Dis. 2017;23(7):840–848. doi:10.1111/odi.12595
- Wu S, Shi H, Cao N, Ye L, Yu C, Zheng L. The correlation of immunologic derangement and juvenile recurrent parotitis: an investigation of the laboratory immunological observation. Acta Otolaryngol. 2018;138(12):1112–1116. doi:10.1080/00016489.2018.1515498
- Taguchi M, Yokota M, Koyano H, Endo Y, Ozawa Y. Acute pancreatitis induced by methimazole in a patient with graves’ disease. Clin Endocrinol. 1999;51(1):667–670. doi:10.1089/thy.2011.0210
- Sasi S, Altarawneh H, Petkar MA, Nair AP. Drug reaction with eosinophilia and systemic symptoms secondary to naproxen: a case report and literature review. Case Reports Acute Med. 2020;3(2):63–72. doi:10.1159/000509712
- Zaccara G, Franciotta D, Perucca E. Idiosyncratic adverse reactions to antiepileptic drugs. Epilepsia. 2007;48(7):1223–1244. doi:10.1111/j.1528-1167.2007.01041.x
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169(5):1071–1080. doi:10.1111/bjd.12501
- Velema MS, Voerman HJ. DRESS Syndrome Caused by Nitrofurantoin. Neth J Med. 2009;67(4):147–149.
- Boyd A, Mills D, Hook K, Kaila R. Seventeen-year-old sexually active male with rash. Am J Emerg Med. 2016;34(4):764.e1–764.e3. doi:10.1016/j.ajem.2015.08.043
- Cho YT, Yang CW, Chu CY. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): an interplay among drugs, viruses, and immune system. Int J Mol Sci. 2017;18:6. doi:10.3390/ijms18061243
- Sharifzadeh S, Mohammadpour AH, Tavanaee A, Elyasi S. Antibacterial Antibiotic-Induced Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome: a literature review. Eur J Clin Pharmacol. 2021;77(3):275–289. doi:10.1007/s00228-020-03005-9
- Misery L, Stander S. Pruritus. London: Springer; 2010.
- Tivoli Y, Rubenstein R. Pruritus. J Clin Aesthet Dermatol. 2009;2(7):30–36.
- Song J, Xian D, Yang L, Xiong X, Lai R, Zhong J. Pruritus: progress toward pathogenesis and treatment. Biomed Res Int. 2018;2018:1–12. doi:10.1155/2018/9625936
- Elzagallaai A, Sultan E, Bend J, Abuzgaia A, Loubani E, Rieder M. Role of oxidative stress in hypersensitivity reactions to sulfonamides. J Clin Pharmacol. 2019;1–13. doi:10.1002/jcph.1535
- Nowak D, Yeung J. Diagnosis and Treatment of Pruritus. Can Fam Physician. 2017;63(12):918–924.
- Yang T-LB, Kim BS. Pruritus in allergy and immunology. J Allergy Clin Immunol. 2019;144(2):353–360. doi:10.1016/j.jaci.2019.06.016
- Ebata T. Drug-induced itch management. Curr Probl Dermatol. 2016;50:155–163. doi:10.1159/000446084
- Lonjou C, Borot N, Sekula P, et al. A European study of HLA-B in Stevens – Johnson Syndrome and Toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics. 2008;18:99–107. doi:10.1097/FPC.0b013e3282f3ef9c
- Roujeau J-C, Hundh T, Bracq C, Guillaume J-C, Revuz J, Touraine R. Genetic susceptibility to toxic epidermal necrolysis. Arch Dermatol. 2015;2015:8–10.
- Howell WM, Carter V, Clark B. The HLA system: immunobiology, HLA typing, antibody screening and crossmatching techniques. J Clin Pathol. 2010;63. 387–391. doi:10.1136/jcp.2009.072371
- Mosaad YM. Clinical role of human leukocyte antigen in health and disease. Scand J Immunol. 2015;82:283–306. DOI:10.1111/sji.12329
- Lund O, Nielsen M, Kesmir C, et al. Definition of supertypes for HLA molecules using clustering of specificity matrices. Immunogenetics. 2004;55:797–810. doi:10.1007/s00251-004-0647-4
- Pichler WJ. Delayed drug hypersensitivity reactions. PLoS One. 2013;683–693.
- Fischer PR, Shigeoka A. Familial occurrence of Steven Johnson Syndrome. AJDC. 1983;137:914–916. doi:10.1001/archpedi.1983.02140350086023