Abstract
A growing number of preclinical and human studies demonstrate a disease-modifying effect of nutritional state in amyotrophic lateral sclerosis (ALS). The management of optimal nutrition in ALS is complicated, as physiological, physical, and psychological effects of the disease need to be considered and addressed accordingly. In this regard, multidisciplinary care teams play an integral role in providing dietary guidance to ALS patients and their carers. However, with an increasing research focus on the use of dietary intervention strategies to manage disease symptoms and improve prognosis in ALS, many ALS patients are now seeking or are actively engaged in using complementary and alternative therapies that are dietary in nature. In this article, we review the aspects of appetite control, energy balance, and the physiological effects of ALS relative to their impact on overall nutrition. We then provide current insights into dietary interventions for ALS, considering the mechanisms of action of some of the common dietary interventions used in ALS, discussing their validity in the context of clinical trials.
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease caused by the loss of motor neurons in the cerebral cortex, brainstem, and spinal cord. Death of these neurons results in progressive paralysis and disability, culminating in respiratory failure and death within 2–5 years following diagnosis.Citation1,Citation2 In the absence of an effective treatment for ALS, there is increased emphasis on interventions to improve the quality and extend the duration of life.
Engagement in ALS multidisciplinary care increases the quality of life while extending survival.Citation3 As part of this process, patients receive dietary guidance preferably by a dietician. While addressing dysphagia, nutritional management also seeks to address changes in appetite that can occur as part of the disease, possibly due to physiological and psychological effects of ALS. Worsening of nutritional state, as reflected by weight loss, is associated with worsening motor symptoms and survival.Citation4,Citation5 This highlights the critical need for patients to meet energy requirements. In this review, we summarize the merits and mechanisms of action of dietary interventions proposed for ALS. We discuss the variable impact of ALS on overall energy balance, noting the effects on appetite control and energy expenditure. We also discuss dietary interventions within the context of risk factors known to impact energy balance and dietary needs in ALS.
Meeting energy needs and maintaining energy homeostasis in ALS
Undernutrition is observed in ALS;Citation6 however, there is significant nutritional heterogeneity among individuals. This is of clinical importance as nutritional state may negatively impact survival,Citation7 whereas maintenance of body weight is an effective strategy to extend survival.Citation8 Moreover, recent discoveries of the compounding effects of coexisting neurodegenerative conditions (including behavioral variants of frontotemportal dementia [bvFTD]) on nutritional status and appetite control in ALS patients highlight additional complexities and heterogeneity of dietary control.Citation9 This heterogeneity indicates a need for well-directed treatments to improve nutrition in ALS while calling for a greater understanding of all factors that might impair energy homeostasis in ALS.
In ALS, a number of studies provide evidence for impairments in control of energy homeostasis (the balance of energy supply and use), which may compromise the capacity to meet energy needs. In particular, patients can experience reduced appetite, leading to reduced intake of food, and thus a failure to meet their energy requirements.
Complications of appetite control and ALS
Hunger represents our need to eat, a sensation that is generally guided by intrinsic mechanisms that promote behaviors that seek food. Appetite, by contrast, is a far more complex behavior, defined as our desire and capacity to consume energy. Many intrinsic and external factors may contribute to appetite, including emotions and/or our physical capacity to access food.Citation10 Considerable changes in appetite and eating behavior are observed in ALS.Citation11 Impaired appetite control in ALS could directly contribute to calorie deficit or excess, compounding weight loss, or weight gain. In ALS, appetite can be affected by physical difficulties, including difficulties in swallowing (dysphagia) or progressive disability that restricts access to food (including paralysis resulting in loss of use of hands), or dysfunction of endogenous processes that regulate hunger, satiety, and mood. Mechanisms of impaired appetite control discussed in this review are summarized in .
Figure 1 Mechanisms of impaired appetite control in amyotrophic lateral sclerosis (ALS).
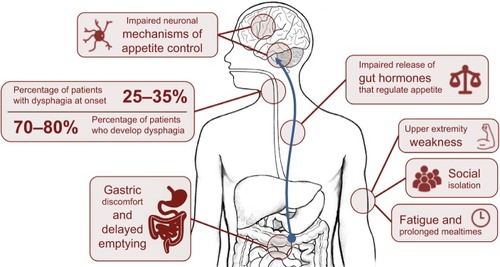
Progressive loss of cranial nerves contributes to difficulties in regulating the complex swallowing process.Citation12 This results in poor control of the food bolus during swallowing. When observed alongside declining respiratory status, weakening oral control can lead to fear of choking, a reduction in calorie and fluid consumption, and possibly malnutrition.Citation13 In ALS, 25%–35% of patients experience bulbar onset of weakness with dysphagia, and 70%–80% of patients develop dysphagia throughout the course of the disease.Citation14,Citation15 Initial management of dysphagia involves modification of food and fluid consistency (blending food, adding thickeners to liquids) and education of patients and carers in swallowing techniques.Citation16 Swallowing difficulties can be managed through enteral feeding.
Disabilities other than dysphagia can increase the risk of calorie deficit in ALS; upper extremity weakness can impair the capacity to prepare or manipulate food. This can be circumvented through assistance from a caregiver; however, prolonged mealtimes and fatigue increase the risk of developing dehydration and suboptimal nutrition.Citation17 Disability can also result in social isolation and embarrassment, further compromising a patient’s nutritional status. Social aspects of eating can be affected by dysphagia or time-consuming and exhausting practices associated with eating. Introduction of adapted eating utensils can help a patient to maintain autonomy.Citation18 Sialorrhoea (drooling or excessive salivation) can further burden patients during mealtimes, especially within social settings,Citation16,Citation19 lessening the desire to eat, and the enjoyment of meals. Treatment options range from anticholinergic drugs and botulinum toxin injections to reduce saliva production, through to radiological and surgical interventions.Citation19 Gastric emptying and colonic transit times can also be delayed in ALS.Citation20 While contributing to discomfort associated with eating, impairments in gut function or delayed gastric emptying can also impact the release of gut hormonesCitation21 associated with appetite control,Citation22 resulting in altered perception of hunger and satiety.
Appetite is controlled by peripheral and central mechanisms that sense and respond to changing energy balance. Energy homeostasis is achieved when circulating signals inform the brain of available fat stores and the brain then responds by stimulating corrective adjustments to food intake to maintain these stores. Various brain centers receive information on energy availability, calorie provision, and energy needs while also responding to behaviors and rewards (pleasure) associated with eating.Citation23 Dysfunction of any component within this network or changes in the way our brain responds to food could result in impairments in appetite control, leading to impairments in maintenance of body weight.
There is evidence of impairment of appetite-regulating pathways in ALS.Citation24 Hypothalamic neurons that promote satiety are less responsive in mouse models of ALS and in ALS patients than in controls.Citation25 Dysfunction of appetite control is further highlighted by TDP43 pathology in the lateral hypothalamus of some ALS patients,Citation26 with the accumulation of TDP43 thought to impair the function of, or contribute to the death of neurons that control appetite. TDP43 proteinopathy is also observed in areas of the brain associated with appetite motivation,Citation26 and thus impairments in appetite control in ALS may extend beyond hypothalamic control of energy homeostasis.
Clinical implications of altered appetite regulation in ALS, particularly with respect to weight management and prognosis, are still under investigation. Understanding of these pathways could lead to suggestions for therapy, and so it is important that we understand the changing energy needs of ALS patients.
Challenges to metabolic balance and ALS
Hypermetabolism in ALSCitation5,Citation27,Citation28 has been suggested to contribute energy deficit, weight loss,Citation29 and reduced survival.Citation27 However, weight loss in ALS occurs regardless of the metabolic status.Citation30 Therefore, hypermetabolism in ALS may only contribute to energy deficit and weight loss, should appetite be impaired. Given that hypermetabolism is linked to shorter survival,Citation27 attempts to define the cause of hypermetabolism and to manage hypermetabolism in ALS remains of interest.
Dietary interventions in ALS
There is evidence that ALS patients are adopting self-prescribed dietary strategies. Over 600 dietary strategies and/or supplements are reported to be used by ~9500 ALS patients currently registered with Patients Like Me, a web-based community where members share details about their treatments.Citation31 This is in line with findings that ~80% of ALS patients use high-dose vitamins, minerals, and other nutraceuticals.Citation32 Common dietary strategies include avoidance of certain dietary components, adoption of very specific dietary regimes, or use of dietary supplements. Of these strategies, the majority have not been tested in clinical trials, or have produced no positive effects when tested. Below, we discuss dietary interventions for which there is some evidence of benefit. Outcomes are summarized in .
Table 1 Dietary interventions proposed to improve capacity to meet energy needs or treat complications associated with ALS
Dietary interventions that address dysphagia in ALS
Customizing dietary strategies to suit ALS
Management of dysphagia needs to be progressively adjusted to accommodate the changing degree of impairment throughout the course of ALS. While dysphagia may not be observed early in disease course, early education regarding nutrition should be considered. At the onset of upper extremity and/or bulbar dysfunction, greater care should be taken to maintain stable body weight, and it is recommended that assessments of dietary efficiency occur at 3-month intervals. Weight and respiratory capacity should be assessed, and patients are encouraged to consult with speech pathologists for advice on improving swallowing techniques.Citation17 Patients may consider more effective dietary strategies to reduce the risk of choking or aspiration including altering the texture and consistency of food,Citation15,Citation16,Citation33 consuming smaller portions more frequently, consuming calorie-rich foods, and increasing mastication time.Citation17 With progressive bulbar dysfunction, the introduction of enteral nutrition should be offered. While not an attractive option to all patients,Citation17 this does improve survival.Citation34,Citation35
It is important that caregivers are actively engaged. Those who take on a caregiver role need to adapt to cater for the specific issue of ALS. Due to physical disabilities in ALS, carers may be responsible for the majority of meal preparationCitation36 and, thus, should be aware of dietary needs specific to ALS. Recently, the Motor Neurone Disease Association released information, recipes, and advice to assist patients and caregivers in sustaining optimal dietary intake while also providing advice on how to maintain enjoyment for food in social settings.Citation37
Enteral nutrition
As bulbar symptoms progress, or when patients lose >10% of their baseline body weight, enteral feeding is often indicated.Citation38 A recent prospective study assessing the use of gastrostomy in ALS suggests that enteral feeding should be considered when patients lose 5% of their baseline body weight, and when their nutritional intake does not match their energy requirements.Citation39,Citation40
Enteral feeding in ALS can be delivered via nasogastric tubing (NGT) or gastrostomy. Although feeding through NGT is a minor and noninvasive procedure, migration of the NGT, oropharyngeal secretion, nasopharyngeal discomfort, pain, and ulceration limits its long-term use in ALS.Citation16,Citation17 Gastrostomy is commonly used for patients who require long-term enteral nutrition,Citation41 and methods used in ALS include percutaneous endoscopic gastrostomy (PEG), radiologically inserted gastrostomy (RIG), and per-oral image-guided gastrostomy (PIG).Citation42 For more information on the use of gastrostomy in ALS, we encourage the reader to consult recent reviews.Citation41,Citation42
Recently, a double-blind, Phase II randomized clinical trial reported benefits of hypercaloric feeding via enteral nutrition. High-calorie supplementation improved survival outcome compared to an isocaloric intervention.Citation43 Importantly, it has also been found that placement of PEG in patients with dysphagiaCitation34 and early introduction and efficient use of PEG to prevent weight loss is able to extend survival in ALS.Citation35 Hence, refinements in the use of PEG might further improve disease outcome for those with ALS.
Dietary interventions that address weight loss in ALS
High-calorie diets
Use of high-calorie supplementation in ALS has stemmed from the finding that calorie-rich diets prevent or reverse weight loss in ALSCitation5,Citation44 by offsetting malnutrition and increasing energy requirements. In ALS, a limited number of studies have assessed caloric supplementation by modifying protein,Citation45 carbohydrate, or fatCitation43,Citation46 content in dietary regimens. While such studies report improvements or stabilization of body weightCitation45 or ALS Functional Rating Scale-Revised (ALSFRS-R),Citation43 only the prospective interventional study by Wills et al was able to demonstrate improvements in survival following high-calorie/high-carbohydrate, or high-calorie/high-fat supplementation.Citation43 Congruent with this, Dorst et al recently reported that high-calorie intake via PEG was associated with longer survival in ALS.Citation46 While these data are promising, small study sample size and limited experimental design necessitate that larger, double-blinded randomized clinical trials are conducted to clarify dietary strategies that improve prognosis in ALS. In this regard, a double-blinded randomized clinical trial to assess the efficacy, safety, and tolerability of high-lipid and -calorie supplementation in 200 ALS participants (NCT02306590) is currently underway. With the primary endpoint of the trial being survival time from randomization until death or date of study completion, data from this trial will shed some light on the utility of high-calorie supplementation as a treatment approach for ALS.
Dietary interventions that address reduced appetite and digestive health in ALS
Cannabis
Cannabis plants contain over 500 distinct chemical functional molecules, of which delta-9-tetrahydrocannabinol (THC) is considered the most biologically active.Citation47 Cannabinoids (CBs) such as THC modulate their effects via CB receptors expressed throughout the brain (predominantly CB1 receptors) and immune system (predominantly CB2 receptors).Citation48 The reader is encouraged to consult recent reviews on the use of cannabis and pharmacological targeting of the endocannabinoid system in treating disease.Citation49,Citation50 We limit the discussion to advances in the use of cannabis and cannabis-derived factors in treating symptoms associated with ALS, including appetite and spasticity.
ALS patients report improvements in appetite and sialorrhoea following the use of cannabis,Citation51 suggesting that CBs may negate some effects of ALS on appetite control.Citation52 Studies in mice show that CBs act via the CB1 receptor to promote food intake. Specifically, CBs promote the activity of hypothalamic pro-opiomelanocorti-(POMC)-expressing neurons (a neuronal population better known to promote satietyCitation53), stimulating β-endorphin release.Citation54 In turn, β-endorphins promote food intake, acting on additional brain circuitry.Citation55 These actions of CBs on POMC-expressing neurons are thought to occur via selective modification of mitochondrial activity.Citation54 Since mitochondrial dysfunctionCitation56–Citation58 and aberrant hypothalamic control of appetite regulation occurs in ALS,Citation25,Citation26 use of CBs may not improve appetite control across all ALS patients. There are potential adverse health effects of cannabis,Citation59 so there is a need for alternative compounds to promote hunger in ALS. Ghrelin is a potent endogenous orexigenic factor that stimulates appetite to increase calorie consumption.Citation60 Given the reduced circulating levels of ghrelin in ALS,Citation21,Citation61 treatment with ghrelin may improve appetite. Moreover, as ghrelin has been shown to protect spinal cord motoneurons from chronic glutamate excitotoxicity while inhibiting microglial activation,Citation62,Citation63 benefits of ghrelin treatment may extend beyond improvements in appetite control.Citation64 To our knowledge, no clinical trials have assessed the use of cannabis, CBs, or orexigenic factors such as ghrelin in promoting appetite in ALS. Of interest, a patent for the use of an analog that targets the ghrelin receptor as treatment in ALS has been filed (Patent Cooperation Treaty number JP2013/078743).
Dietary fiber
Fiber lowers the concentrations of inflammatory markers and can reduce the risk of mortality in some inflammatory diseases.Citation65,Citation66 Moreover, dietary fiber is known to promote gut health, thereby improving laxation.Citation67 Improvements in gut function following increased ingestion of fiber may also aid the production and release of key gut hormones (including ghrelin), thereby enhancing appetite control.Citation67 Comparing outcomes from five large cohort studies, no change in ALS risk relative to dietary fiber intake was observed.Citation68 Of interest, a diet rich in antioxidant nutrients, carotenoids and fiber, and vegetables is associated with improved function in ALS patients.Citation69 Whether improved function was due to increased fiber intake was not specifically addressed. It remains unclear whether dietary fiber led to improved gut health, reduced colonic transit times to improve laxation, or improved appetite control in ALS.
Dietary interventions directed toward physical disability in ALS
Dietary modification for cramps and fasciculations
Muscle cramping and fasciculations are widely reported in ALS,Citation70 with symptoms presenting prior to the onset of weakness and/or muscle wasting.Citation71,Citation72 Fasciculations occur as a consequence of abnormal excitation of the terminal branches of motor axons,Citation73 whereas cramps may occur as a consequence of mechanical excitation of the motor nerve terminal,Citation73 hyperexcitability or bistability of spinal motor neurons,Citation74 or through impairments in inhibitory mechanisms of interneuron activity.Citation75 Muscle cramping in ALS can be persistent and can cause debilitating pain. Various drugs have been proposed to treat cramps in ALS, including antispasmodics such as Baclofen or Zanaflex.Citation76 To manage cramping, patients report the use of quinine and increased consumption of dietary supplements including vitamin E and magnesium.
Quinine
Quinine is used for the treatment of malaria.Citation77 While showing some benefits in managing nocturnal leg cramps,Citation78 the occurrence of life-threatening complicationsCitation79 has resulted in a ruling by the FDA to prohibit the use of quinine for the treatment of cramps. Promotion of quinine for treatment of leg cramps has been prohibited in the USA since February 1995, with the marketing of quinine-containing preparations banned by the FDA since December 2006 (FDA, Docket No. 2006N-0476). ALS patients report reduced muscle cramps following the consumption of quinine as tonic water, with amounts of quinine ingested per liter of tonic water estimated to be ~25-fold lower than the proposed daily dosage for the treatment of malaria.Citation77
Vitamin E
Vitamin E is a group of lipid-soluble antioxidants commonly found in plant products.Citation80 Investigation of the effects of vitamin E on dermatological conditions led to the suggestion of treatment benefits on nocturnal leg cramping in individuals with possible vitamin E deficiency.Citation81 While vitamin E deficiency in ALS is not universal,Citation82–Citation84 a potential reduction in risk for ALS is suggested in those with higher vitamin E levels,Citation82 or those with low baseline vitamin E levels who are receiving vitamin E supplementation.Citation85–Citation87 Despite these positive associations, oral administration of vitamin E does not impact ALS survival or the quality of life.Citation88–Citation91 A randomized crossover trial to assess the impact of vitamin E on the number, duration, and severity of cramps was initiated in 2006 (NCT00372879). While this trial is now complete, the results have not been made public.
Magnesium
Magnesium deficiency can lead to neuronal excitability, enhancing neuromuscular transmission.Citation92 Accordingly, magnesium supplementation has been proposed for the treatment of cramps. A recent systematic review of randomized controlled trials with meta-analysis found no evidence for effective management of cramps using magnesium. A small effect in reducing nocturnal cramps was observed in pregnant women only.Citation93 Comparing the results from five large cohort studies, recent observations found no association between magnesium intake and ALS risk, or protective effects of magnesium on ALS,Citation94 although modest effects had been suggested previously.Citation95 We could not identify any studies assessing the specific use of magnesium to manage cramps in ALS.
Dietary modification for spasticity and stiffness
Spasticity, an increase in tonic stretch reflexes due to hyperexcitability, is observed in a number of neurological disorders as a result of loss of upper motor neurons.Citation96 Symptoms include increased tone, spasms, and/or clonus, and spasticity can lead to severe disability, pain, or incapacitation.Citation97 Pharmacological treatments include Baclofen, alpha-2 agonists such as clonidine, or use of anticonvulsants such as benzodiazepines.Citation96 Given the possible side effects of pharmacological treatment,Citation98 a multidisciplinary approach combining medication and physical therapy is recommended.Citation96 Use of nutraceuticals including l-threonine and CBs can be considered for treatment of spasticity.
l-Threonine
l-Threonine is an essential α-amino acid, acting as a glycine precursor to increase glycine levels in the central nervous system.Citation99 Potential use of glycine to treat spasticity has led to the assessment of l-threonine as a possible treatment for spasticity in ALS.Citation100 A double-blind, placebo-controlled, crossover study of oral l-threonine to treat spinal spasticity found a modest antispasmodic effect.Citation101 Similar treatment effects were not observed in ALS, with a pilot observationCitation100 and a double-blind, placebo-controlled, crossover study of l-threonine showing no improvements in ALS.Citation102 These observations were unexpected, given suggested positive treatment effects following short-term l-threonine treatment of ALS.Citation102 A recent Cochrane review found evidence for the effective use of l-threonine in the treatment of ALS to be of low quality,Citation103 and thus the use of l-threonine for treatment of spasticity in ALS remains unsupported.
Cannabis
Clinical use of cannabis and CBs in treating spasticity originates from observations in multiple sclerosis (MS), where synthetic THC treatment transiently improved spasticity scores.Citation104 This observation is in line with studies that implicate the endocanabinoid system in the pathophysiology of MS.Citation105 The use of CB agonists to ameliorate spasticity in MS is established.Citation106,Citation107 Evidence supporting the use of cannabis or cannabis-derived compounds in treating ALS is mostly limited to animal studies, and in particular observations of delayed disease progression following treatment of SOD1G93A mice with a CB1/CB2 agonist.Citation108 Moreover, the upregulation in CB2 receptor expressionCitation109 and potentiation of CB1 receptors controlling glutamatergic and GABAergic transmission in ALS miceCitation110 suggest the involvement of the endocannabinoid system in the pathophysiology of ALS. Unlike MS, clinical evidence showing positive treatment effects of CB use in ALS is mostly limited to patient self-reports of subjective improvement in spasticity.Citation51 These patient reports are contradicted by a small randomized double-blind crossover trial, where twice-daily treatment with THC did not improve cramp intensity.Citation111 Given the lack of evidence, use of THC in treating spasticity in ALS is not clinically supported, and more studies are needed to explore other treatment effects.Citation112
Dietary modification for low energy, fatigue, and weakness
Caffeine
Caffeine is a commonly consumed stimulant, acting within the central nervous system via various mechanisms to promote alertness, memory, performance, and coordination.Citation113 Chronic consumption of caffeine in the SOD1G93A mouse model negatively influenced motor performance and reduced survival duration,Citation114 prompting fears that caffeine might worsen disease survival. However, subsequent studies in humans found no association between caffeine and risk for developing ALS.Citation115 This contradicts findings from a case–control study, observing a reduction in ALS risk relative to increased coffee consumption.Citation116 Recent reviews discuss the possible mechanisms of action and perceived benefits of caffeine in ALS and other neurodegenerative diseases.Citation117–Citation119
Ketogenic diet
Introduced in 1921 for the treatment of epilepsy,Citation120 the ketogenic diet is a regimen that encompasses high dietary intake of fat alongside the restriction of protein and carbohydrates.Citation121 The ketogenic diet has been trialed as a nonpharmacological therapeutic approach across a number of neurological conditions.Citation122–Citation124 While its neuroprotective effects are proposed to range from mitigating inflammation to alleviating neuronal excitotoxicity, data remain contentious.Citation123 In ALS, the most pertinent mechanism by which the ketogenic diet could exert neuroprotection is through the provision of fat, which is converted into ketone bodies for use as a fuel substrate for the generation of cellular energy.Citation125 In light of defective mitochondrial function in skeletal muscleCitation126–Citation130 of ALS patients, and mouse data highlighting muscle weakness in the presence of defective skeletal muscle glucose metabolism,Citation131,Citation132 the ketogenic diet offers an avenue through which skeletal muscle can bypass mitochondrial dysfunction and the need for glucose to sustain function. Improved motor performance in SOD1G93A mice fed a ketogenic diet implies that this may be the case. However, given that improved motor performance was observed alongside the attenuation of motor neuron death,Citation133 it remains unknown whether the ketogenic diet has direct effects on muscle to mitigate weakness. To date, there is limited mouse data, and no human data to support the adoption of a ketogenic diet in ALS. While a clinical trial to assess the safety and tolerability of the ketogenic diet in ALS (NCT01016522) was initiated in 2009, the study was terminated, and no data have been disseminated. Critically, since the ketogenic diet promotes weight loss by reducing appetite and increasing the breakdown of endogenous fat stores,Citation134 this could exacerbate malnutrition and weight loss in ALS. Further research is needed to clarify the clinical efficacy of the ketogenic diet in ALS.
Deanna protocol
The Deanna protocol is a commercially available dietary supplement cocktail. Components of the Deanna protocol are thought to target pathophysiological processes of ALS including glutamate excitotoxicity and oxidative stress.Citation135 In addition, the key components of the Deanna protocol are l-arginine, which improves blood flow to promote protein synthesis,Citation136 and alpha-ketoglutarate, a fuel substrate that can be fed directly into mitochondrial pathways for the generation of cellular energy.Citation135,Citation137 Thus, it is plausible that l-arginine and alpha-ketoglutarate could have positive effects in ALS by promoting skeletal muscle growth and bypassing defects in skeletal muscle glucose metabolismCitation131 to improve energy production to sustain muscle function. Congruent with this, a 2014 study in SOD1G93A mice found that supplementation with arginine-alpha-ketoglutarate led to improved motor function.Citation137 However, this mouse data has not been independently replicated, and there are no published clinical trials on the use of the Deanna protocol in ALS. Given that the current clinical value of the Deanna protocol rests solely on anecdotal patient reportsCitation135, further research into the adoption of the Deanna protocol in ALS is needed.
Creatine
Creatine is a naturally occurring guanidine derivative that is synthesized in the liver and found predominantly in muscle and the brain.Citation138 Dietary supplementation with creatine is thought to increase energy output during anaerobic activity, increasing phosphocreatine availabilityCitation139 and stimulating mitochondrial respiration.Citation140 Creatine is also thought to act as an antioxidant, stabilizing mitochondrial membranes.Citation141 Study outcomes in SOD1G93A mice are mixed. Initial studies found that creatine was able to extend survivalCitation142 while reducing glutamate excitotoxicity;Citation143 however, improvements in muscle function were not observed.Citation144 Creatine use by ALS patients was reported to lead to improvements in muscle function,Citation145 with reduced muscle fatigue and improved endurance. Comprehensive studies, however, showed no positive outcomes on strength.Citation146,Citation147 A recent Cochrane review of trials in ALS found no improvement in ALSRFS-R scores following the use of creatine, suggesting no functional benefits of creatine use. The same review found no significant benefit of the use of creatine in ALS survival, noting studies are needed to assess the potential benefits of creatine when taken earlier in the disease course.Citation148
Protein
Few studies have assessed the impact of protein supplementation in ALS, despite the belief that protein supplementation could aid muscle recovery and slow loss of muscle mass. Supplementation with milk whey protein in a small cohort of ALS patients prevented weight loss, contributing to a modest but significant rise in BMI. Maintenance of body weight was associated with a stable ALSFRS-R score over the 4-month assessment period, whereas ALSRFS-R scores declined in individuals not supplemented with protein.Citation45 These results suggest that whey protein supplementation can improve the nutritional status of patients; specific effects of increased protein supply versus calorie supplementation remain unclear.
Vitamin D
Vitamin D is a secosteroid (fat-soluble sub-class of steroid-derived hormones) involved in a range of biological processes including calcium regulation. While some foods contain vitamin D, it is primarily produced endogenously following direct sun exposure to the skin.Citation149 Vitamin D supplementation alone, or in combination with calcium in aged individuals, improves musculoskeletal function,Citation149 suggesting possible benefits for treatment in ALS. Supplementation of vitamin D in SOD1G93A mice show mixed results, with studies documenting improvements in functional capacity but not survival. Treatment was found to be toxic in female mice.Citation150,Citation151 In patients, low vitamin D status correlates with a faster functional decline, with reduced life expectancy observed in those with vitamin D deficiency.Citation152 Assessment of ALS patients found vitamin D levels <30 ng/mL iñ80% of patients, and vitamin D levels <20 ng/mL in ~40% of patients. Noting a modest slowing of functional decline following vitamin D supplementation, the authors concluded that vitamin D supplementation was safe and may have some benefits.Citation153 Additional prospective control trials are required to study the effect of vitamin D on the progression of disability in ALS patients.
Dietary interventions that address pathological mechanisms in ALS
Antioxidants
A balance between oxidation/reduction states (redox homeostasis) allows for the generation of reactive oxygen species (eg, free radicals) and reactive nitrogen species that play essential roles in regulating cellular pathways that maintain function and survival. Naturally occurring antioxidant defense systems in cells protect against cell damage by preventing the oxidation of molecules and by scavenging free radicals. When the balance between free radical production and antioxidant defenses becomes unfavorable, oxidative stress ensues.Citation154 With oxidative stress considered to be one of the primary pathogenic mechanisms in ALS,Citation155 it is not surprising that dietary supplements with antioxidant effects are commonly used. Common antioxidants include vitamins, carnitine, and coenzyme Q10 (CoQ10). Despite the lack of bona fide clinical trial data to demonstrate positive effects in ALS for the majority of antioxidants,Citation156,Citation157 high tolerance and safety combined with ease of availability do not discourage their use by patients.
Vitamin B12
Vitamin B12 is a water-soluble vitamin that plays an essential role supporting the health of the nervous system.Citation158 Humans are unable to synthesize vitamin B12, and hence must obtain it through dietary sources.Citation159 Methylcobalamin, an active form of vitamin B12, has been trialed at high doses in ALS. In a double-blind controlled trial, Kaji et al found that compared to low-dose methylcobalamin, high-dose methylcobalamin was able to prevent the deterioration in the amplitude of the compound muscle action potential after 4 weeks of treatment.Citation160 A randomized double-blind, Phase III clinical trial (NCT00444613) conducted by the same group found that patients receiving high-dose methylcobalamin (intramuscularly) had a lesser decline in ALSFRS-R and prolonged survival if the compound was administered within 12 months of symptom onset.Citation161 Based on current clinical data, ALSUntangled have provided recommendations to encourage the replication of findings and the validation of the use of vitamin B12 in ALS.Citation162
Carnitine
Carnitine is an essential nutrient that plays an integral role in fatty acid metabolism. l-carnitine, the biologically active form of carnitine, can be synthesized endogenously and obtained through dietary sources. As an antioxidant, l-carnitine is proposed to protect against oxidative stress and damage.Citation163 A randomized double-blind placebo-controlled trial assessing the effects on disability and mortality of acetyl-l-carnitine in combination with riluzole showed that there was a slower decline in ALSFRS-R and an increase in median survival.Citation164
Coenzyme Q10 (CoQ10)
CoQ10, also known as ubiquinone, is a lipid-soluble antioxidant. Serving as a diffusible electron carrier in the mitochondrial electron transport chain, CoQ10 plays a vital role in cellular energy production.Citation165 Hence, CoQ10 could exert beneficial effects in ALS by boosting mitochondrial function, and by scavenging free radicals to protect against oxidative stress. In an open-label dose-escalation trial, doses of up to 3000 mg/day of CoQ10 were found to be safe and tolerable in ALS.Citation166 However, a subsequent Phase II double-blind randomized controlled trial assessing daily CoQ10 at 2700 mg/day demonstrated no significant benefit of CoQ10 over placebo.Citation167
Mitoquinone (MitoQ)
A synthetic derivative of CoQ10, MitoQ is a strong antioxidant that was designed to accumulate in and protect against oxidative damage in mitochondria.Citation168 Administration of MitoQ to symptomatic SOD1G93A mice slowed the decline in mitochondrial function in skeletal muscle and spinal cord, improved hindlimb strength, and extended survival.Citation169 These data suggest that specific targeting of antioxidants to mitochondria could be beneficial in ALS. A previous trial of MitoQ in Parkinson’s disease demonstrated no positive effects,Citation170 and there have been no clinical trials of MitoQ in ALS.
Edaravone
Currently being developed by Mitsubishi Tanabe Pharma Corporation (Japan), edaravone (MCI-186, Radicut®) is a potent antioxidant that scavenges reactive oxygen species.Citation171 Preclinical studies demonstrate that edaravone improves motor function, slows symptom progression, and attenuates motor neuron degeneration in rodent models of ALS.Citation172,Citation173 A confirmatory double-blind, parallel-group, placebo-controlled study of edaravone in ALS patients demonstrated a smaller reduction in ALSFRS-R in patients receiving edaravone over a 24-week treatment period.Citation174 More recently, Tanaka et al presented results from a 24-week, Phase III, double-blind, parallel-group study of edavarone in a defined cohort of patients (NCT01492686), revealing a lesser decline in ALSFRS-R at 6 months and less deterioration in quality of life.Citation175 Radicut® (The Japanese Pharmacopoeia Edaravone injection) is currently approved for use as a treatment for ALS in Japan and South Korea. Radicava™ (intravenous) received FDA approval for use in ALS in the USA in May 2017. A Phase I trial of an oral formulation of edaravone (TW001) in the Netherlands has returned positive results, and a Phase II/III trial is in progress. For a more detailed overview of Edaravone, we encourage the reader to consult a recent review.Citation176
Concluding remarks
In the absence of effective treatments for ALS, patients are increasingly adopting dietary regimens and using dietary supplements with the view to alleviate symptoms and to improve quality of life and disease outcome. While data suggest that the adoption of high-calorie or high-protein diets may have some benefit, larger clinical trials are needed to validate the effectiveness of these approaches in ALS. Although the majority of dietary supplements reviewed here have not been clinically validated, preliminary data from some trials suggest the potential usefulness of supplements that target specific pathogenic mechanisms in ALS. In these instances, further studies are needed.
Acknowledgments
STN acknowledges the support of a Scott Sullivan MND Research Fellowship funded by The MND and Me Foundation, The Royal Brisbane & Women’s Hospital Foundation, and the Queensland Brain Institute. The authors would like to acknowledge the valuable funding/salary support of Wesley Medical Research (Project number 2016-32) and The Motor Neurone Disease Research Institute of Australia.
Disclosure
The authors report no conflicts of interest in this work.
References
- MitchellJDBorasioGDAmyotrophic lateral sclerosisLancet200736995782031204117574095
- StrongMJAbrahamsSGoldsteinLHAmyotrophic lateral sclerosis – frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteriaAmyotroph Lateral Scler Frontotemporal Degener2017183–415317428054827
- TraynorBJAlexanderMCorrBFrostEHardimanOEffect of a multidisciplinary amyotrophic lateral sclerosis (ALS) clinic on ALS survival: a population based study, 1996–2000J Neurol Neurosurg Psychiatry20037491258126112933930
- JawaidAMurthySBWilsonAMA decrease in body mass index is associated with faster progression of motor symptoms and shorter survival in ALSAmyotrophic Lateral Sclerosis201011654254820500116
- MarinBDesportJCKajeuPAlteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patientsJ Neurol Neurosurg Psychiatry201182662863421097551
- GentonLViatteVJanssensJPHeritierACPichardCNutritional state, energy intakes and energy expenditure of amyotrophic lateral sclerosis (ALS) patientsClin Nutr201130555355921798636
- PaganoniSDengJJaffaMCudkowiczMEWillsAMBody mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosisMuscle Nerve2011441202421607987
- MillerRGJacksonCEKasarskisEJPractice parameter update: the care of the patient with amyotrophic lateral sclerosis: multidisciplinary care, symptom management, and cognitive/behavioural impairment (an evidence-based review)Neurology2009731227123319822873
- AhmedRMCagaJDevenneyECognition and eating behavior in amyotrophic lateral sclerosis: effect on survivalJ Neurol201626381593160327260291
- CastonguayTWApplegateEAUptonDESternJSHunger and appetite: old concepts/new distinctionsNutr Rev19834141011106346147
- HolmTMaierAWicksPSevere loss of appetite in amyotrophic lateral sclerosis patients: online self-assessment studyInteract J Med Res201321e823608722
- RobbinsJSwallowing in ALS and motor neuron disordersNeurol Clin19875221322911681400
- NeudertCOliverDWasnerMBorasioGDThe course of the terminal phase in patients with amyotrophic lateral sclerosisJ Neurol200124861261611518004
- MuscaritoliMKushtaIMolfinoAInghilleriMSabatelliMRossi FanelliFNutritional and metabolic support in patients with amyotrophic lateral sclerosisNutrition2012281095996622677356
- KornerSHendricksMKolleweKWeight loss, dysphagia and supplement intake in patients with amyotrophic lateral sclerosis (ALS): impact on quality of life and therapeutic optionsBMC Neurol2013131848423848967
- HeffernanCJenkinsonCHolmesTNutritional management in MND/ALS patients: an evidence based reviewAmyotroph Lateral Scler Other Motor Neuron Disord200452728315204009
- SilaniVKasarskisEJYanagisawaNNutritional management in amyotrophic lateral sclerosis: a worldwide perspectiveJ Neurol1998245Suppl 2S13S19 discussion S299747929
- GreenwoodDINutrition management of amyotrophic lateral sclerosisNutr Clin Pract201328339239923466470
- AndersenPMBorasioGDDenglerRGood practice in the management of amyotrophic lateral sclerosis: clinical guidelines. An evidence-based review with good practice points. EALSC Working GroupAmyotroph Lateral Scler20078419521317653917
- ToepferCFAKRLMGastrointestinal dysfunction in amyotrophic lateral sclerosisAmyotrophic Lateral Scler Other Motor Neuron Disord2009111519
- NgoSTSteynFJHuangLAltered expression of metabolic proteins and adipokines in patients with amyotrophic lateral sclerosisJ Neurol Sci20153571–2222726198021
- ChaudhriOSmallCBloomSGastrointestinal hormones regulating appetitePhilos Trans R Soc Lond B Biol Sci200636114711187120916815798
- AndrewsZBAbizaidANeuroendocrine mechanisms that connect feeding behavior and stressFront Neurosci2014831225324716
- AhmedRMIrishMPiguetOAmyotrophic lateral sclerosis and frontotemporal dementia: distinct and overlapping changes in eating behaviour and metabolismLancet Neurol201615333234226822748
- VercruyssePSinnigerJEl OussiniHAlterations in the hypothalamic melanocortin pathway in amyotrophic lateral sclerosisBrain2016139Pt 41106112226984187
- CykowskiMDTakeiHSchulzPEAppelSHPowellSZTDP-43 pathology in the basal forebrain and hypothalamus of patients with amyotrophic lateral sclerosisActa Neuropathol Commun2014217125539830
- KasarskisEJBerrymanSVanderleestJGSchneiderARMcClainCJNutritional status of patients with amyotrophic lateral sclerosis: relation to the proximity of deathAm J Clin Nutr19966311301378604660
- DesportJCPreuxPMMagyLFactors correlated with hypermetabolism in patients with amyotrophic lateral sclerosis.(Brief Article)Am J Clin Nutr200174332811522556
- IoannidesZANgoSTHendersonRDMcCombePASteynFJAltered metabolic homeostasis in amyotrophic lateral sclerosis: mechanisms of energy imbalance and contribution to disease progressionNeurodegener Dis2016165–638239727400276
- BouteloupCDesportJCClavelouPHypermetabolism in ALS patients: an early and persistent phenomenonJ Neurol200925681236124219306035
- FrostJOkunSVaughanTHeywoodJWicksPPatient-reported outcomes as a source of evidence in off-label prescribing: analysis of data from PatientsLikeMeJ Med Internet Res2011131e621252034
- BradleyWGAndersonFGowdaNMillerRGGroup ACSChanges in the management of ALS since the publication of the AAN ALS practice parameter 1999Amyotroph Lateral Scler Other Motor Neuron Disord20045424024415799554
- SalvioniCCStanichPAlmeidaCSOliveiraASNutritional care in motor neurone disease/amyotrophic lateral sclerosisArq Neuropsiquiatr201472215716324604371
- SpataroRFicanoLPiccoliFLa BellaVPercutaneous endoscopic gastrostomy in amyotrophic lateral sclerosis: effect on survivalJ Neurol Sci20113041–2444821371720
- FasanoAFiniNFerraroDPercutaneous endoscopic gastrostomy, body weight loss and survival in amyotrophic lateral sclerosis: a population-based registry studyAmyotroph Lateral Scler Frontotemporal Degener2017110
- MockfordCJenkinsonCFitzpatrickRA review: carers, MND and service provisionAmyotroph Lateral Scler20067313214116963402
- MNDAEating and drinking with motor neurone disease (MND)2017 Available from: https://www.mndassociation.org/wp-content/uploads/Eating-and-drinking-with-MND-final-web-PDF-2017.pdfAccessed June 16, 2017
- RioACawadiasENutritional advice and treatment by dietitians to patients with amyotrophic lateral sclerosis/motor neurone disease: a survey of current practice in England, Wales, Northern Ireland and CanadaJ Hum Nutr Diet200720131317241187
- KasarskisEJMendiondoMSMatthewsDEEstimating daily energy expenditure in individuals with amyotrophic lateral sclerosisAm J Clin Nutr201499479280324522445
- ProGas StudyGGastrostomy in patients with amyotrophic lateral sclerosis (ProGas): a prospective cohort studyLancet Neurol201514770270926027943
- Rahnemai-AzarAARahnemaiazarAANaghshizadianRKurtzAFarkasDTPercutaneous endoscopic gastrostomy: indications, technique, complications and managementWorld J Gastroenterol201420247739775124976711
- StavroulakisTWalshTShawPJMcDermottCJProgasSGastrostomy use in motor neurone disease (MND): a review, meta-analysis and survey of current practiceAmyotroph Lateral Scler Frontotemporal Degener20131429610422985431
- WillsA-MHubbardJMacklinEAHypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled phase 2 trialLancet201438399342065207224582471
- MattsonMPCutlerRGCamandolaSEnergy intake and amyotrophic lateral sclerosisNeuromolecular Med200791172017114821
- SilvaLBdCMourãoLFSilvaAAEffect of nutritional supplementation with milk whey proteins in amyotrophic lateral sclerosis patientsArquivos de Neuro-Psiquiatria20106826326820464297
- DorstJCypionkaJLudolphACHigh-caloric food supplements in the treatment of amyotrophic lateral sclerosis: a prospective interventional studyAmyotroph Lateral Scler Frontotemporal Degener2013147–853353623944684
- ThomasBFElSohlyMAChapter 1 – The botany of Cannabis sativa LThe Analytical Chemistry of CannabisElsevier2016126
- Di MarzoVBifulcoMDe PetrocellisLThe endocannabinoid system and its therapeutic exploitationNat Rev Drug Discov20043977178415340387
- GrotenhermenFMuller-VahlKThe therapeutic potential of cannabis and cannabinoidsDtsch Arztebl Int201210929–3049550123008748
- AlexanderSPTherapeutic potential of cannabis-related drugsProg Neuropsychopharmacol Biol Psychiatry20166415716626216862
- AmtmannDWeydtPJohnsonKLJensenMPCarterGTSurvey of cannabis use in patients with amyotrophic lateral sclerosisAm J Hosp Palliat Care20042129510415055508
- CarterGTAboodMEAggarwalSKWeissMDCannabis and amyotrophic lateral sclerosis: hypothetical and practical applications, and a call for clinical trialsAm J Hosp Palliat Care201027534735620439484
- CowleyMASmartJLRubinsteinMLeptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleusNature2001411683648048411373681
- KochMVarelaLKimJGHypothalamic POMC neurons promote cannabinoid-induced feedingNature20155197541455025707796
- KalraSPHorvathTLNeuroendocrine interactions between galanin, opioids, and neuropeptide Y in the control of reproduction and appetiteAnn N Y Acad Sci19988632362409928174
- ManfrediGXuZMitochondrial dysfunction and its role in motor neuron degeneration in ALSMitochondrion200552778716050975
- CozzolinoMCarriMTMitochondrial dysfunction in ALSProg Neurobiol2012972546621827820
- MuydermanHChenTMitochondrial dysfunction in amyotrophic lateral sclerosis – a valid pharmacological target?Br J Pharmacol201417182191220524148000
- VolkowNDBalerRDComptonWMWeissSRAdverse health effects of marijuana useN Engl J Med2014370232219222724897085
- WrenAMSealLJCohenMAGhrelin enhances appetite and increases food intake in humansJ Clin Endocrinol Metab20018612599211739476
- CzellDBaldingerRSchneiderUNeuwirthCWeberMThe role of the SenseWear device and ghrelin for metabolism in amyotrophic lateral sclerosisAmyotroph Lateral Scler Frontotemporal Degener2016173–4295296
- LimELeeSLiEKimYParkSGhrelin protects spinal cord motoneurons against chronic glutamate-induced excitotoxicity via ERK1/2 and phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3beta pathwaysExp Neurol2011230111412221530509
- LeeSKimYLiEParkSGhrelin protects spinal cord motoneurons against chronic glutamate excitotoxicity by inhibiting microglial activationKorean J Physiol Pharmacol2012161434822416219
- ColldénGTschopMHMullerTDTherapeutic potential of targeting the ghrelin pathwayInt J Mol Sci2017184E79828398233
- XuHHuangXRiserusUDietary fiber, kidney function, inflammation, and mortality riskClin J Am Soc Nephrol20149122104211025280496
- YoungRPHopkinsRJHigh dietary fiber lowers systemic inflammation: potential utility in COPD and lung cancerAm J Med20141278e13
- SlavinJFiber and prebiotics: mechanisms and health benefitsNutrients2013541417143523609775
- FondellEO’ReillyEJFitzgeraldKCDietary fiber and amyotrophic lateral sclerosis: results from 5 large cohort studiesAm J Epidemiol2014179121442144924816788
- NievesJWGenningsCFactor-LitvakPAssociation between dietary intake and function in amyotrophic lateral sclerosisJAMA Neurol201673121425143227775751
- HanischFSkudlarekABerndtJKornhuberMECharacteristics of pain in amyotrophic lateral sclerosisBrain Behav201553e0029625642388
- LayzerRBDiagnostic implications of clinical fasciculation and crampsAdv Neurol19823623297180684
- GubbaySSKahanaEZilberNCooperGPintovSLeibowitzYAmyotrophic lateral sclerosis. A study of its presentation and prognosisJ Neurol198523252953004056836
- LayzerRBThe origin of muscle fasciculations and crampsMuscle Nerve19941711124312497935546
- BaldisseraFCavallariPDworzakFMotor neuron ‘bistability’. A pathogenetic mechanism for cramps and myokymiaBrain1994117Pt 59299397953602
- ObiTMizoguchiKMatsuokaHTakatsuMNishimuraYMuscle cramp as the result of impaired GABA function–an electrophysiological and pharmacological observationMuscle Nerve19931611122812318413375
- MillerRGJacksonCEKasarskisEJQuality Standards Subcommittee of the American Academy of NeurologyPractice parameter update: the care of the patient with amyotrophic lateral sclerosis: multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of NeurologyNeurology200973151227123319822873
- AchanJTalisunaAOErhartAQuinine, an old anti-malarial drug in a modern world: role in the treatment of malariaMalar J20111014421609473
- Man-Son-HingMWellsGLauAQuinine for nocturnal leg cramps: a meta-analysis including unpublished dataJ Gen Intern Med19981396006069754515
- DysonEHProudfootATPrescottLFHeyworthRDeath and blindness due to overdose of quinineBr Med J (Clin Res Ed)198529164873133
- TuckerJMTownsendDMAlpha-tocopherol: roles in prevention and therapy of human diseaseBiomed Pharmacother200559738038716081238
- AyresSJrMihanRLeg cramps (systremma) and “restless legs” syndrome. Response to vitamin E (tocopherol)Calif Med1969111287915346435
- Michal FreedmanDKunclRWWeinsteinSJMalilaNVirtamoJAlbanesDVitamin E serum levels and controlled supplementation and risk of amyotrophic lateral sclerosisAmyotroph Lateral Scler Frontotemporal Degener201314424625123286756
- de BustosFJimenez-JimenezFJMolinaJACerebrospinal fluid levels of alpha-tocopherol in amyotrophic lateral sclerosisJ Neural Transm (Vienna)19981056–77037089826112
- IwasakiYIkedaKKinoshitaMVitamin A and E levels are normal in amyotrophic lateral sclerosisJ Neurol Sci199513221931948543947
- WangHO’ReillyEJWeisskopfMGVitamin E intake and risk of amyotrophic lateral sclerosis: a pooled analysis of data from 5 prospective cohort studiesAm J Epidemiol2011173659560221335424
- VeldinkJHKalmijnSGroeneveldGJIntake of polyunsaturated fatty acids and vitamin E reduces the risk of developing amyotrophic lateral sclerosisJ Neurol Neurosurg Psychiatry200778436737116648143
- AscherioAWeisskopfMGO’ReillyEJVitamin E intake and risk of amyotrophic lateral sclerosisAnn Neurol200557110411015529299
- KwiecinskiHJanikPJamrozikZOpuchlikAThe effect of selegiline and vitamin E in the treatment of ALS: an open randomized clinical trialsNeurol Neurochir Pol2001351 Suppl101106
- DesnuelleCDibMGarrelCFavierAA double-blind, placebo-controlled randomized clinical trial of alpha-tocopherol (vitamin E) in the treatment of amyotrophic lateral sclerosis. ALS riluzole-tocopherol Study GroupAmyotroph Lateral Scler Other Motor Neuron Disord20012191811465936
- GalbusseraATremolizzoLBrighinaLVitamin E intake and quality of life in amyotrophic lateral sclerosis patients: a follow-up case series studyNeurol Sci200627319019316897634
- GrafMEckerDHorowskiRHigh dose vitamin E therapy in amyotrophic lateral sclerosis as add-on therapy to riluzole: results of a placebo-controlled double-blind studyJ Neural Transm (Vienna)2005112564966015517433
- DurlachJBacPBaraMGuiet-BaraAPhysiopathology of symptomatic and latent forms of central nervous hyperexcitability due to magnesium deficiency: a current general schemeMagnes Res200013429330211153899
- SeboPCeruttiBHallerDMEffect of magnesium therapy on nocturnal leg cramps: a systematic review of randomized controlled trials with meta-analysis using simulationsFam Pract201431171924280947
- FondellEO’ReillyEJFitzgeraldKCMagnesium intake and risk of amyotrophic lateral sclerosis: results from five large cohort studiesAmyotroph Lateral Scler Frontotemporal Degener2013145–635636123777266
- LongneckerMPKamelFUmbachDMDietary intake of calcium, magnesium and antioxidants in relation to risk of amyotrophic lateral sclerosisNeuroepidemiology200019421021610859501
- ChangEGhoshNYanniDLeeSAlexandruDMozaffarTA review of spasticity treatments: pharmacological and interventional approachesCrit Rev Phys Rehabil Med2013251–2112225750484
- KhederANairKPSpasticity: pathophysiology, evaluation and managementPract Neurol201212528929822976059
- SimonOYelnikAPManaging spasticity with drugsEur J Phys Rehabil Med201046340141020927006
- MaherTJWurtmanRJl-threonine administration increases glycine concentrations in the rat central nervous systemLife Sci19802616128312866770208
- TestaDCaraceniTFetoniVGirottiFChronic treatment with l-threonine in amyotrophic lateral sclerosis: a pilot studyClin Neurol Neurosurg1992941791353011
- LeeAPattersonVA double-blind study of l-threonine in patients with spinal spasticityActa Neurol Scand19938853343388296531
- BlinOPougetJAubrespyGGueltonCCrevatASerratriceGA double-blind placebo-controlled trial of L-threonine in amyotrophic lateral sclerosisJ Neurol1992239279811313078
- NgLKhanFYoungCAGaleaMSymptomatic treatments for amyotrophic lateral sclerosis/motor neuron diseaseCochrane Database Syst Rev20171CD01177628072907
- PetroDJEllenbergerCJrTreatment of human spasticity with delta 9-tetrahydrocannabinolJ Clin Pharmacol1981218–9 Suppl413S416S6271839
- PryceGBakerDEndocannabinoids in multiple sclerosis and amyotrophic lateral sclerosisHandb Exp Pharmacol201523121323126408162
- FlacheneckerPHenzeTZettlUKNabiximols (THC/CBD oromucosal spray, Sativex(R)) in clinical practice--results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticityEur Neurol2014715–627127924525548
- FernandezOAdvances in the management of MS spasticity: recent observational studiesEur Neurol201472Suppl 1121425278118
- BilslandLGDickJRPryceGIncreasing cannabinoid levels by pharmacological and genetic manipulation delay disease progression in SOD1 miceFASEB J20062071003100516571781
- ShoemakerJLSeelyKAReedRLCrowJPPratherPLThe CB2 cannabinoid agonist AM-1241 prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis when initiated at symptom onsetJ Neurochem20071011879817241118
- RossiSDe ChiaraVMusellaAAbnormal sensitivity of cannabinoid CB1 receptors in the striatum of mice with experimental amyotrophic lateral sclerosisAmyotroph Lateral Scler2010111–2839019452308
- WeberMGoldmanBTrunigerSTetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trialJ Neurol Neurosurg Psychiatry201081101135114020498181
- Group ALALSUntangled No. 16: cannabisAmyotroph Lateral Scler201213440040422632446
- NehligADavalJLDebryGCaffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effectsBrain Res Brain Res Rev19921721391701356551
- PotenzaRLArmidaMFerranteAEffects of chronic caffeine intake in a mouse model of amyotrophic lateral sclerosisJ Neurosci Res201391458559223361938
- FondellEO’ReillyEIFitzgeraldKCIntakes of caffeine, coffee and tea and risk of amyotrophic lateral sclerosis: results from five cohort studiesAmyotroph Lateral Scler Frontotemporal Degener2015165–636637125822002
- BeghiEPupilloEMessinaPCoffee and amyotrophic lateral sclerosis: a possible preventive roleAm J Epidemiol201117491002100821946385
- Onatibia-AstibiaAFrancoRMartinez-PinillaEHealth benefits of methylxanthines in neurodegenerative diseasesMol Nutr Food Res2017
- YenisettiSCManjunathMJMuralidharaCNeuropharmacological properties of Withania somnifera – Indian Ginseng: an overview on experimental evidence with emphasis on clinical trials and patentsRecent Pat CNS Drug Discov201610220421527316579
- KolahdouzanMHamadehMJThe neuroprotective effects of caffeine in neurodegenerative diseasesCNS Neurosci Ther201723427229028317317
- ADBThe ketogenic diet in epilepsyCan Med Assoc J193124110610720318124
- PetermanMGThe ketogenic diet in epilepsyJAMA1925842619791983
- StafstromCERhoJMThe ketogenic diet as a treatment paradigm for diverse neurological disordersFront Pharmacol201235922509165
- GasiorMRogawskiMAHartmanALNeuroprotective and disease-modifying effects of the ketogenic dietBehav Pharmacol2006175–643143916940764
- PaoliABiancoADamianiEBoscoGKetogenic diet in neuromuscular and neurodegenerative diseasesBiomed Res Int2014201447429625101284
- VidaliSAminzadehSLambertBMitochondria: The ketogenic diet–A metabolism-based therapyInt J Biochem Cell Biol201563555925666556
- Echaniz-LagunaAZollJRiberaFMitochondrial respiratory chain function in skeletal muscle of ALS patientsAnn Neurol200252562362712402260
- WiedemannFRWinklerKKuznetsovAVImpairment of mitochondrial function in skeletal muscle of patients with amyotrophic lateral sclerosisJ Neurol Sci1998156165729559989
- VielhaberSWinklerKKirchesEVisualization of defective mitochondrial function in skeletal muscle fibers of patients with sporadic amyotrophic lateral sclerosisJ Neurol Sci19991691–213313910540022
- SicilianoGPastoriniEPasqualiLMancaMLIudiceAMurriLImpaired oxidative metabolism in exercising muscle from ALS patientsJ Neurol Sci20011911–2616511676993
- CrugnolaVLampertiCLucchiniVMitochondrial respiratory chain dysfunction in muscle from patients with amyotrophic lateral sclerosisArch Neurol201067784985420625092
- PalamiucLSchlagowskiANgoSTA metabolic switch toward lipid use in glycolytic muscle is an early pathologic event in a mouse model of amyotrophic lateral sclerosisEMBO Mol Med20157552654625820275
- PradatPFBruneteauGGordonPHImpaired glucose tolerance in patients with amyotrophic lateral sclerosisAmyotroph Lateral Scler2010111–216617120184518
- ZhaoZLangeDJVoustianioukAA ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosisBMC Neurosci200672916584562
- PaoliARubiniAVolekJSGrimaldiKABeyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) dietsEur J Clin Nutr201367878979623801097
- ALS Untangled GroupFournierCBedlackBALS Untangled No. 20: the Deanna protocolAmyotroph Lateral Scler Frontotemporal Degener201314431932323638638
- MoncadaSHiggsAThe L-arginine-nitric oxide pathwayN Engl J Med199332927200220127504210
- AriCPoffAMHeldHEMetabolic therapy with Deanna Protocol supplementation delays disease progression and extends survival in amyotrophic lateral sclerosis (ALS) mouse modelPLoS One201497e10352625061944
- WalkerJBCreatine: biosynthesis, regulation, and functionAdv Enzymol Relat Areas Mol Biol197950177242386719
- CaseyAGreenhaffPLDoes dietary creatine supplementation play a role in skeletal muscle metabolism and performance?Am J Clin Nutr2000722 Suppl607S617S10919967
- O’GormanEBeutnerGWallimannTBrdiczkaDDifferential effects of creatine depletion on the regulation of enzyme activities and on creatine-stimulated mitochondrial respiration in skeletal muscle, heart, and brainBiochim Biophys Acta1996127621611708816948
- StrongMJPatteeGLCreatine and coenzyme Q10 in the treatment of ALSAmyotroph Lateral Scler Other Motor Neuron Disord20001Suppl 4172011466954
- KlivenyiPFerranteRJMatthewsRTNeuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosisNat Med19995334735010086395
- AndreassenOAJenkinsBGDedeogluAIncreases in cortical glutamate concentrations in transgenic amyotrophic lateral sclerosis mice are attenuated by creatine supplementationJ Neurochem200177238339011299300
- DeraveWVan Den BoschLLemmensGEijndeBORobberechtWHespelPSkeletal muscle properties in a transgenic mouse model for amyotrophic lateral sclerosis: effects of creatine treatmentNeurobiol Dis200313326427212901841
- MazziniLBalzariniCColomboREffects of creatine supplementation on exercise performance and muscular strength in amyotrophic lateral sclerosis: preliminary resultsJ Neurol Sci20011911–213914411677005
- RosenfeldJKingRMJacksonCECreatine monohydrate in ALS: Effects on strength, fatigue, respiratory status and ALSFRSAmyotrophic Lateral Sclerosis20089526627218608103
- DroryVEGrossDNo effect of creatine on respiratory distress in amyotrophic lateral sclerosisAmyotroph Lateral Scler Other Motor Neuron Disord200231434612061948
- PastulaDMMooreDHBedlackRSCreatine for amyotrophic lateral sclerosis/motor neuron diseaseCochrane Database Syst Rev201212CD00522523235621
- KulieTGroffARedmerJHounshellJSchragerSVitamin D: an evidence-based reviewJ Am Board Fam Med200922669870619897699
- GianforcaroASolomonJAHamadehMJVitamin D(3) at 50× AI attenuates the decline in paw grip endurance, but not disease outcomes, in the G93A mouse model of ALS, and is toxic in femalesPLoS One201382e3024323405058
- GianforcaroAHamadehMJDietary vitamin D3 supplementation at 10x the adequate intake improves functional capacity in the G93A transgenic mouse model of ALS, a pilot studyCNS Neurosci Ther201218754755722591278
- CamuWTremblierBPlassotCVitamin D confers protection to motoneurons and is a prognostic factor of amyotrophic lateral sclerosisNeurobiol Aging20143551198120524378089
- KaramCBarrettMJImperatoTMacGowanDJScelsaSVitamin D deficiency and its supplementation in patients with amyotrophic lateral sclerosisJ Clin Neurosci201320111550155323815870
- McCordJMThe evolution of free radicals and oxidative stressAm J Med2000108865265910856414
- D’AmicoEFactor-LitvakPSantellaRMMitsumotoHClinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosisFree Radic Biol Med20136550952723797033
- OrrellRWLaneRJRossMA systematic review of antioxidant treatment for amyotrophic lateral sclerosis/motor neuron diseaseAmyotroph Lateral Scler20089419521118608090
- OrrellRWLaneRJRossMAntioxidant treatment for amyotrophic lateral sclerosis / motor neuron diseaseCochrane Database Syst Rev20071CD00282917253482
- ReynoldsEVitamin B12, folic acid, and the nervous systemLancet Neurol200651194996017052662
- WatanabeFVitamin B12 sources and bioavailabilityExp Biol Med (Maywood)2007232101266127417959839
- KajiRKodamaMImamuraAEffect of ultrahigh-dose methylcobalamin on compound muscle action potentials in amyotrophic lateral sclerosis: a double-blind controlled studyMuscle Nerve19982112177517789843082
- KajiRKuzuharaSIwasakiYUltra-high dose methylcobalamin (E0302) prolongs survival of ALS: report of 7 years’ randomised double-blind, phase 3 clinical trial (ClinicalTrials.gov NCT00444613)Paper presented at: AAN Annual Meeting2015
- ALS-UntangledALSUntangled No. 30: methylcobalaminAmyotroph Lateral Scler Frontotemporal Degener2015167–853653926203660
- GulcinIAntioxidant and antiradical activities of L-carnitineLife Sci200678880381116253281
- BeghiEPupilloEBonitoVRandomized double-blind placebo-controlled trial of acetyl-L-carnitine for ALSAmyotroph Lateral Scler Frontotemporal Degener2013145–639740523421600
- TurunenMOlssonJDallnerGMetabolism and function of coenzyme QBiochim Biophys Acta200416601–217119914757233
- FerranteKLShefnerJZhangHTolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALSNeurology200565111834183616344537
- KaufmannPThompsonJLLevyGPhase II trial of CoQ10 for ALS finds insufficient evidence to justify phase IIIAnn Neurol200966223524419743457
- KelsoGFPorteousCMCoulterCVSelective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic propertiesJ Biol Chem200127674588459611092892
- MiquelECassinaAMartinez-PalmaLNeuroprotective effects of the mitochondria-targeted antioxidant MitoQ in a model of inherited amyotrophic lateral sclerosisFree Radic Biol Med20147020421324582549
- SnowBJRolfeFLLockhartMMProtect Study GroupA double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s diseaseMov Disord201025111670167420568096
- WatanabeTTaharaMTodoSThe novel antioxidant edaravone: from bench to bedsideCardiovasc Ther200826210111418485133
- AokiMWaritaHMizunoHSuzukiNYukiSItoyamaYFeasibility study for functional test battery of SOD transgenic rat (H46R) and evaluation of edaravone, a free radical scavengerBrain Res2011138232132521276427
- ItoHWateRZhangJTreatment with edaravone, initiated at symptom onset, slows motor decline and decreases SOD1 deposition in ALS miceExp Neurol2008213244845518718468
- AbeKItoyamaYSobueGConfirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patientsAmyotroph Lateral Scler Frontotemporal Degener2014157–861061725286015
- TanakaMSakataTPalumboJAkimotoMA 24-week, phase III, double-blind, parallel-group study of edaravone (MCI-186) for treatment of amyotrophic lateral sclerosis (ALS)Paper presented at: AAN Annual Meeting2016Vancouver, Canada
- PetrovDMansfieldCMoussyAHermineOALS clinical trials review: 20 years of failure. are we any closer to registering a new treatment?Front Aging Neurosci201796828382000

