Abstract
Background:
Trastuzumab plus fluoropyrimidine and cisplatin have been established as the standard first-line chemotherapy for human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer (AGC). While a survival benefit of continuing trastuzumab beyond progression (TBP) has been shown for HER2-positive breast cancer, efficacy for HER2-positive AGC has yet to be demonstrated. We examined the efficacy of TBP as the second-line chemotherapy for HER2-positive AGC.
Patients and methods:
We retrospectively reviewed the medical records of 21 patients with HER2-positive AGC treated with trastuzumab plus fluoropyrimidine and cisplatin as the first-line therapy during the period from June 2011 to May 2015 in our department. A total of 13 patients received TBP with chemotherapy as the second-line therapy. The Kaplan–Meier method with a log-rank test was applied to evaluate overall survival (OS) and progression-free survival (PFS).
Results:
The median OS and PFS with TBP after administration of the second-line therapy were 9.2 months (95% CI, 4.3–11.9 months) and 3.6 months (95% CI, 1.5–3.9), respectively. The median OS after the first-line therapy was 13.3 months (95% CI, 8.2–19.5 months) in this population, significantly shorter than those in the remaining 10 patients not given TBP as the second-line therapy (not reached; 95% CI, 11.5 months–not available [NA]; p=0.025). Four patients with good tumor shrinkage after the first-line therapy received conversion surgery and had a good survival rate, which was markedly superior to those of patients without surgery, including the TBP population.
Conclusion:
TBP for HER2-positive AGC seems unlikely to provide a survival benefit in patients with tumors refractory to trastuzumab-containing first-line therapies.
Introduction
Trastuzumab, a humanized monoclonal antibody targeting human epidermal growth factor receptor 2 (HER2), has been shown to improve the survivals of patients with HER2-positive advanced gastric cancer (AGC) when combined with fluoropyrimidine plus cisplatin in the first-line setting.Citation1,Citation2 Several randomized studies have shown continuing trastuzumab beyond progression (TBP) after failure of trastuzumab-containing chemotherapy to improve survivals of patients with HER2-positive metastatic breast cancer.Citation3,Citation4 A previous cohort study suggested that TBP was feasible and safe in patients with HER2-positive AGC as well;Citation5 however, the survival benefit of TBP is still under debate.
The present study aimed to examine the clinical outcomes of patients with HER2-positive AGC who received TBP as the second-line chemotherapy.
Patients and methods
Study eligibility
We reviewed 21 consecutive patients with HER2-positive AGC treated with an oral fluoropyrimidine derivative, capecitabine/S-1, cisplatin and trastuzumab as the first-line therapy during the period from June 2011 to May 2015. Their data were obtained from a prospectively constructed database in the Department of Gastrointestinal Surgery, The University of Tokyo. HER2 status was evaluated as positive based on immunohistochemistry (IHC) 3+, or IHC 2+ and dual-color in-situ hybridization (DISH) positivity (defined as HER2/CEP17 ratio ≥2.0). Three patients lacking IHC scores but with DISH-positive results were also included in this study. Sites of metastasis were recorded based on Japanese classification of gastric carcinoma.Citation6
Statistical analysis
We determined overall survival (OS) and progression-free survival (PFS) in 11 patients who received TBP. We also calculated their OS, 1-year survival rate and PFS after the first-line therapy comparing to those patients not receiving TBP as the second-line therapy. In the case of patients receiving conversion surgery after first-line therapy, surgeries were evaluated as censors in PFS. We depicted their PFS after the first-line chemotherapy, the duration of TBP and their OS as bar charts, in addition to the clinical outcomes of the first-line chemotherapy. Their hematologic adverse events were collected from the database and assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0.
We compared patient characteristics between the patients with and without TBP using Fisher’s exact tests except the Student’s t-test for age and Welch’s t-test for HER2 status (DISH). Owing to the anonymous nature of the data, the requirement for informed consent was waived. Our study was approved by the ethics committee of the Faculty of Medicine, The University of Tokyo.
Survivals were estimated using the Kaplan–Meier method. Data were censored on October 19, 2016. Data of patients who were alive on the day of last contact/follow up were censored for the OS analysis. OS was calculated from the date of starting chemotherapy to the date of death from any cause. PFS was calculated from the date of starting chemotherapy to the date of disease progression.
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics.Citation7
Results
Patient characteristics
Of the 21 patients, 11 received the second-line chemotherapy containing TBP. No selection criteria were established for TBP in this study period.
Trastuzumab was given by intravenous infusion at a dose of 6 mg/kg every 3 weeks with chemotherapy until disease progression. Four patients showed a good response (partial response [PR], three; complete response [CR], one) after the first-line therapy and then underwent conversion surgery. Three patients received the second-line chemotherapy without trastuzumab, and two patients were given best supportive care. One patient was observed without the second-line therapy.
No characteristic features distinguished the 11 patients who received TBP from the other 10 patients (). IHC status in the TBP group was IHC 3+ in nine patients (82%) and IHC 2+/DISH+ in two patients (18%).
Table 1 Characteristics of the 21 patients with HER2-positive AGC receiving (11) versus not receiving (10) TBP
Survivals after the second-line TBP therapy
The median follow-up time was 13.3 months after starting the first-line therapy in the 11 patients who received TBP as the second-line therapy. Paclitaxel (PTX), docetaxel (DTX) and irinotecan (IRI) were used as the second-line chemotherapy in nine (82%), one (9%) and one (9%) patients, respectively.
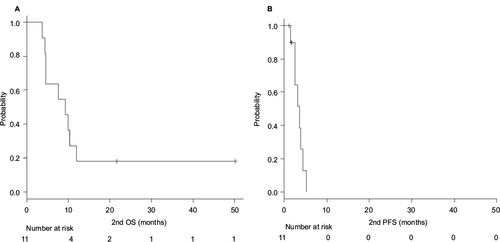
The median OS and PFS after starting the second-line therapy with TBP were 9.2 months (95% CI, 4.3–11.9 months) and 3.6 months (95% CI, 1.5–3.9 months), respectively (, respectively).
Figure 1 Median OS (A) and PFS (B) of the second-line TBP therapy.
Abbreviations: OS, overall survival; PFS, progression-free survival; TBP, trastuzumab beyond progression.
Survivals after the first-line therapy in all HER2-positive patients
The median follow-up time was 16.5 months (range, 7.2–61.7 months) in all 21 patients. The median OS from the first-line therapy for all 21 patients reached 16.8 months (95% CI: 13.1 months–not available [NA]), and their 1-year survival rate was 75.2% (95% CI: 50.9–88.9%; ).
Figure 2 Median OS (A and B) and PFS (C) after starting the first-line therapy.
Abbreviations: OS, overall survival; PFS, progression-free survival; TBP, trastuzumab beyond progression.
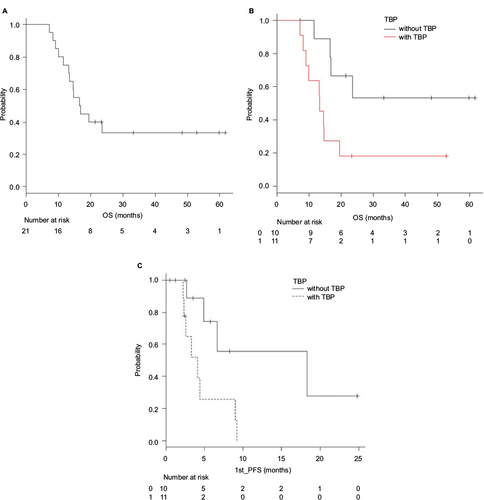
The median OS after starting the first-line therapy was 13.3 months (95% CI, 8.2–19.5 months) in patients receiving TBP and was not reached (95% CI, 11.5 months–NA) in the remaining 10 patients not given TBP (p=0.025). Their 1-year survival rates were 63.6% (95% CI: 29.7–84.5%) and 88.9% (95% CI, 43.3–98.3%), respectively ().
The median PFS from the first-line therapy for all 21 patients reached 4.4 months (95% CI: 2.6–9.0 months). The median PFS after starting the first-line therapy was 4.1 months (95% CI, 2.2–9.0 months) in patients receiving TBP and was 18.3 months (95% CI, 2.7 months–NA) in the remaining 10 patients not given TBP (p=0.018), respectively ().
In this study, four patients showing good tumor shrinkage after the first-line capecitabine+cisplatin (XP)/S-1+cisplatin (SP)+trastuzumab underwent conversion surgery, and R0 resection was achieved in three of these patients (including two patients with CR).
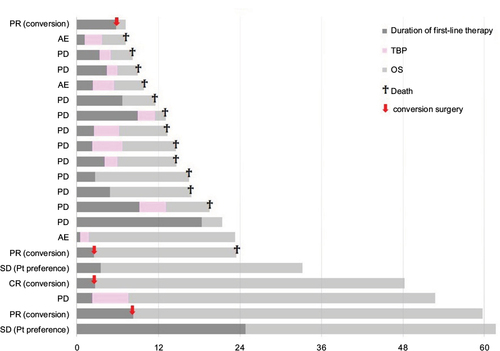
The PFS after the first-line chemotherapy, the duration of TBP and the OS are depicted as bar charts, in addition to the clinical outcomes of the first-line chemotherapy, in . Patients with shorter OS mostly received TBP, in contrast to those with longer OS in whom TBP was not used.
Figure 3 The PFS after the first-line chemotherapy, the duration of TBP and the OS are depicted as bar charts, in addition to the clinical outcomes of the first-line chemotherapy.
Adverse events
Grade 3 or 4 hematological adverse events in patients receiving TBP were neutropenia (18%) and hypoalbuminemia (9%; ). Serious hematologic adverse event was not reported.
Table 2 Hematologic adverse events of patients receiving TBP
Discussion
TBP was deemed to be feasible but provided no benefit in terms of OS in our cohort. Median OS and PFS ranges in the previous studies evaluating the second-line single drug chemotherapy in AGC patients were 5.2–9.5 months and 2.3–3.6 months, respectively.Citation8–Citation11 PFS of the TBP group in this study might be equivalent but certainly not superior to these prior results.
In all 21 patients, the median OS after starting the first-line therapy was similar to the survivals obtained in previous clinical trials ().Citation1,Citation2 However, the median OS after starting the first-line therapy was significantly shorter in patients with TBP than in those without TBP. This is partially due to the longer survival in the population selected to undergo conversion surgery after good tumor shrinkage in response to the first-line therapy, while most of the patients with shorter PFS after the first-line therapy received TBP. Our results showing no survival benefit by TBP in patients with early progression after trastuzumab, including the first-line therapy, might be supported by the fact that short PFS in the first-line therapy has negative prognostic value of the second-line therapy.Citation12
Table 3 Outcomes of clinical trials for patients with HER2-positive AGC
There is no evidence, to our knowledge, supporting a survival benefit with anti-HER2 therapy beyond progression in HER2-positive AGC. Thuss-Patience et al reported the outcome of the Phase II/III GATSBY trial, which compared ado-trastuzumab emtansine (T-DM1) with taxane alone as the second-line therapy in patients with HER2-positive advanced gastroesophageal cancer. In their trial, 415 patients (77.4% had received prior HER2-targeted therapy) were randomized into two groups, and there were no significant differences in OS (median 7.9 vs. 8.6 months, HR 1.15 [95% CI: 0.87–1.51], p=0.86) or PFS (median 2.7 vs. 2.9 months, HR 1.13 [95% CI: 0.89–1.43], p=0.30) between the two.Citation13 Their results might support our observation that anti-HER2 therapy beyond progression offers no apparent survival advantage in patients with HER2-positive AGC. On the other hand, in a prospective observational cohort study of 59 HER2-positive AGC patients by Li et al,Citation14 median PFS and OS from the first-line therapy was significantly longer in 32 patients with TBP than in 27 patients without TBP. Further studies are warranted to reach a firm conclusion about the possible survival benefit of TBP.
Limitations
The limitations of this study include its retrospective design, the small number of patients and selection bias arising from physicians subjectively deciding whether or not patients received TBP.
Accordingly, a long-term double-blind trial should be performed using well-matched groups of patients to confirm these preliminary observations. A randomized Phase II study comparing TBP plus PTX with PTX alone as the second-line therapy for patients with HER2-positive AGC is now underway in Japan, and the results are anticipated to provide a final conclusion regarding the efficacy of TBP.
Conclusion
TBP for HER2-positive AGC provides no apparent survival benefit in patients with tumors refractory to trastuzumab-containing the first-line therapy. TBP is not a viable treatment option, at least for poor responders to the first-line chemotherapy with trastuzumab, although further study is warranted.
Author contributions
All authors contributed to data collection and the statistical analysis, drafting and revising of the manuscript and take responsibility for all aspects of the work.
Acknowledgments
This article was written primarily by the first author, with input from the coauthors. The manuscript was written in English, then proofread by a native speaker of English (Bierta Barfod, MD, MPH) and then corrected accordingly by the first author.
Disclosure
Y Seto received commercial research grants from Taiho Pharmaceutical Co., Ltd. and Yakult Honsha Co., Ltd. The authors report no other conflicts of interest in this work.
References
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697.
- Kurokawa Y, Sugimoto N, Miwa H, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer. 2014;110(5):1163–1168.
- von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German breast group 26/breast international group 03-05 study. J Clin Oncol. 2009;27(12):1999–2006.
- Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124–1130.
- Al-Shamsi HO, Fahmawi Y, Dahbour I, et al. Continuation of trastuzumab beyond disease progression in HER2-positive metastatic gastric cancer: the MD Anderson experience. J Gastrointest Oncol. 2016;7(4):499–505.
- Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14(2):97–100.
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458.
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–1235.
- Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31(35):4438–4444.
- Ford HER, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15(1):78–86.
- Kang JH, Lee SI, Lim do H, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30(13):1513–1518.
- Fanotto V, Cordio S, Pasquini G, et al. Prognostic factors in 868 advanced gastric cancer patients treated with second-line chemotherapy in the real world. Gastric Cancer. Epub 2016 Dec 27.
- Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18(5):640–653.
- Li Q, Jiang H, Li H, et al. Efficacy of trastuzumab beyond progression in HER2 positive advanced gastric cancer: a multicenter prospective observational cohort study. Oncotarget. 2016;7(31):50656–50665.
