Abstract
In primates, adequate growth of the fetus depends on the development of the uteroplacental unit. On the fetal side, this is achieved by the creation of the vascular network of the placenta. On the maternal side, the transformation of the spiral arteries into saccular nonreactive vessels by the trophoblast provides high blood flow to the intervillous space. Apart from the changes in the uterine arteries, the mother expands her plasma volume – at the expense of stimulating the renin-angiotensin-aldosterone system – and her cardiac output. In the maintaining of normotension in the face of an increased cardiac output and plasma volume, the renin-angiotensin-aldosterone system requires an enhanced vasodilator synthesis. Finally, in the late stages of pregnancy, a normal endothelial function is required to provide an ample margin to the activation provoked by deportation of syncytiotrophoblast fragments/factors to the maternal circulation. These four adaptative processes require various interrelated vasodilator systems. Deficient adaptations cause isolated or proteinuric arterial hypertension, intrauterine growth restriction, preterm delivery, and stillbirths, among others. Moreover, a normal or a defective adaptation to pregnancy influences maternal cardiovascular health in later life, as evidenced by various studies, most of them epidemiological; thus, pregnancy is now considered a stress test to the maternal cardiovascular system. Because of this, women planning to become pregnant should be screened for clinical and biochemical cardiovascular risks. Inversely, women presenting with hypertension in pregnancy should be thoroughly studied to detect and correct cardiovascular risks. The incorporation of the predictive value of a hypertensive pregnancy should help reduce cardiovascular disease in women.
Introduction
In primates, adequate growth of the fetus depends on the morphological and functional development of the uteroplacental unit. On the fetal side, this is achieved by the creation of the vascular network of the placenta from hemangioblasts.Citation1 On the maternal side, this is achieved by (1) the transformation of the uterine arteries by extravillous trophoblasts that destroy their smooth muscle layer, change their tubular conformation into a saccular one, and make them nonreactive to vasomotor stimuli;Citation2,Citation3 and (2) the elevation of the mother’s plasma volume and cardiac output to bathe the placental villi with increasing fractions of the cardiac output.Citation4,Citation5
The expansion of plasma volume is due to the renin-angiotensin (Ang)-aldosterone system; plasma renin activity (PRA) and aldosterone levels increase progressively to attain values 7–8-fold higher than basal ones. In this context, the normotension of approximately 90% of pregnant women, the reduction of blood pressure in the second trimester, and the decreased sensitivity to endogenous Ang II infusionCitation6 are difficult to understand, unless we invoke a potent vasodilatory response. The functional relevance of vasodilators to increase maternal vascular compliance in pregnancy is supported by the association of preeclampsia and intrauterine growth retardation to a reduced plasma volume that precedes the clinical expression of the syndrome.Citation5,Citation7 Moreover, in preeclampsia, PRA and Ang II levels are reduced,Citation8 and the increase of vascular reactivity to Ang II contrasts with the decreased sensitivity observed in normal pregnancy.Citation6
Convinced that an orchestrated network of vasodilator systems participates in the systemic and local changes of pregnancy, the authors of this paper have strived to understand their temporal profiles, localization, and potential roles. The following analysis will include a brief description of the main vasodilator agents/factors, the description of their systemic and local expression in human normal pregnancy and preeclampsia, and lastly an analysis of the implications of a defective adaptation over late cardiovascular morbi-mortality. (For a review that includes animal models, see Valdés et al.)Citation9
Vasodilator factors and their systemic response in normotensive and preeclamptic pregnancy
Prostanoids
Prostanoids are derived from arachidonic acid, which is mobilized from the cell membrane by phospholipase A2 and is metabolized by cyclooxygenases (COXs) (constitutive [COX-1] and inducible [COX-2]) into vasodilatory or vasoconstrictor prostaglandins (PGs) (PGE2/prostacyclin [PGI2] and PGF2α/thromboxane [TXA] respectively). PGI2, the main vasodilatory and antiaggregating PG, is predominantly synthesized by the endotheliumCitation10 and represents the first vasoactive factor studied in this condition.Citation11,Citation12
For more than two decades, there has been evidence that the metabolites of PGI2 rise progressively to achieve a fivefold increment at term. Since this increment is not associated to increased TXA, the balance favors vasodilatation.Citation11 Women with severe preeclampsia have a reduced excretion of PGI2 metabolites by weeks 13–16 of pregnancy, while TXA remains stable up to week 21, to rise later causing a predominance of vasoconstriction and platelet aggregation. This imbalance could contribute to the main characteristics of preeclampsia, as hypertension, platelet aggregation, and reduced uteroplacental blood flow. Due to the disturbed PGI2/TXA balance several trials have tested low dose aspirin administration in women at risk of preeclampsia, with the intention of reducing synthesis of TXA in platelets, while that of endothelial PGI2 remains intact. However, the incidence of preeclampsia has only been reduced modestly (12% in the Collaborative Low-dose Aspirin Study in Pregnancy)Citation13, suggesting that there is more to preeclampsia than an increment of TXA.
Nitric oxide (NO)
NO, a potent vasodilator, is derived from the transformation of L-arginine into NO and citruline by NO synthase (NOS). The three cognate forms of NOS are the constitutive endothelial (eNOS) and neuronal (nNOS) forms, and the inducible (iNOS) form.Citation14 In pregnant rats, NO synthesis increases, as demonstrated by plasma levels, urinary nitrites/nitrates, and cyclic guanosine monophosphate, second messenger of NO;Citation15 moreover, the blockade of NO synthesis induces marked preeclampsia-like effects.Citation16,Citation17 In human pregnancy, changes in the urinary excretion of nitrites/nitrates have been discordant, and this has been attributed to different content of these metabolites in the diet, and to a poor equivalence with NO production. However, elevated asymmetric dimethylarginine (ADMA), an endogenous inhibitor of NOS is associated to endothelial dysfunction, alterations of the uterine artery blood flow, and later development of preeclampsia.Citation18
Kallikrein-kinin system (KKS)
The KKS system includes a couple of serine proteases: tissue and plasma kallikrein, which generate kallidin and bradykinin from low and high molecular kininogen respectively. The effects of kinins are mediated by bradykinin receptors (B1R and B2R), of which the B2R induces vasodilatation and increased vascular permeability and platelet antiaggregation.Citation19
The authors of this paper initially postulated that in pregnancy the KKS represented a counterpart to the vasoconstrictor renin-Ang system. However, the nadir attained by urinary kallikrein excretion in normal pregnancy between 8 and 12 weeksCitation20 precedes the highest levels of vasoconstrictors. In hypertensive pregnancies, urinary kallikrein is reduced, and low values are a good predictor of preeclampsia.Citation8,Citation21,Citation22
Vasodilator components of the renin-Ang system (RAS)
The RAS has traditionally been considered the paradigmatic vasoconstrictor system.Citation23 More recently, various vasodilator pathways have been described within the RAS. One is represented by Ang-(1–7), the Mas receptor and the Ang converting enzyme-2 (ACE2);Citation24 another by the stimulation of the receptor 2 of Ang II (AT-2-R) causes vasodilatation, antiproliferation, antifibrosis, and antiangiogenesis by activating eNOS and kinins;Citation25–Citation27 and lastly, by Ang-(3–8) or Ang IV, which provokes hypertrophy, vasodilatation, and vascular inflammation.Citation28
Generation of Ang-(1–7) is increased in normotensive human gestation as demonstrated by increasing urinary excretion starting from 12 to 14 weeks, and by the elevation of plasma levels that result in late-pregnancy values 1.5-fold greater than those of nonpregnant women.Citation29,Citation30 In preeclampsia, the circulating levels of Ang-(1–7) are reduced, a circulating agonistic antibody for the vasoconstrictor AT-1-R appears,Citation31 and heterodimers of the AT-1-R–B2R receptors are increased in platelets and omental vessels;Citation32 these heterodimers increase the vasoconstrictor effect of Ang II and reduce the vasodilatation of bradykinin. More recently, Zhou and collaborators have shown that oxidation of angiotensinogen, which has been observed in preeclampsia, renders this substrate more effective at liberating Ang by renin activity.Citation33
Vasodilator role of vascular endothelial growth factor (VEGF)
VEGF, considered the most potent angiogenic stimulus, increases vascular permeability, cell migration, synthesis of metalloproteinases, and most importantly for this review, vasodilatation through NO and PGI2.Citation34–Citation36 These effects are exerted through its receptors: VEGF receptor (VEGF-R)1 or fms-like tyrosine kinase (FLT)-1, and VEGF-R2 or kinase domain receptor (KDR).Citation37,Citation38
In women submitted to in-vitro fertilization, circulating levels of VEGF increase approximately 30 days after embryo transfer,Citation39 and continue to increase up to weeks 34–36.Citation40 Placental lobes liberate VEGF to the fetal and the maternal compartment, with a predominance to the latter.Citation41
The binding of VEGF to its receptors is reduced by a soluble form (sFLT-1) generated by alternative splicing of FLT-1. The minimal circulating levels in the nonpregnant condition rise in pregnancy and are extremely elevated in preeclampsia, probably derived from the ischemic placenta.Citation42,Citation43 Mice transfected with the gene coding sFLT-1 present a preeclampsia-like syndrome in the absence of pregnancy.Citation42
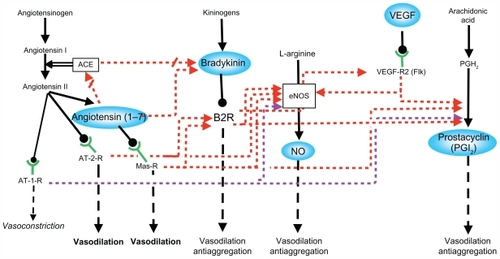
The studies described above support the participation of several interrelated vasodilator systems in the maternal hemodynamic changes of pregnancy as a partially redundant network (). It is highly likely that this network provides chained relay mechanisms in order to exert permanent vasodilatation along the changing hormonal milieu of pregnancy.
Figure 1 Interactions between the different vasodilator systems.
Abbreviations: ACE, angiotensin converting enzyme; AT-1-R, angiotensin II type 1 receptor; AT-2-R, angiotensin II type 2 receptor; B2R, bradykinin 2 receptor; eNOS, endothelial nitric oxide synthase; Flk, fetal liver kinase 1; Mas-R, Mas receptor; NO, nitric oxide; PGH2, prostaglandin H2; VEGF, vascular endothelial growth factor; VEGF-R2, VEGF receptor 2.
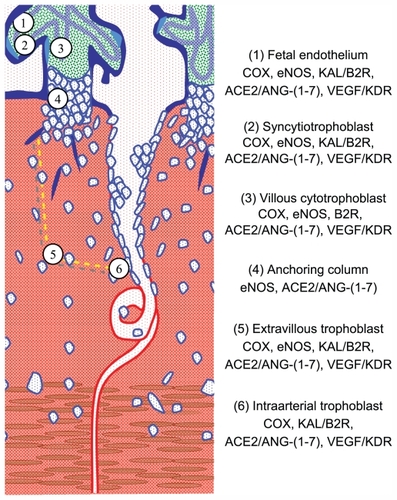
Expression of vasodilator factors in the uteroplacental interface
Since the vasodilator factors display paracrine/autocrine effects, their expression in reproductive human tissues is crucial to understand their participation in trophoblast invasion and in the development and maintenance of uteroplacental circulation.
Prostanoids
COX-1 is expressed in trophoblasts, endothelial cells, macrophages, smooth muscle, and decidual cells. COX-2 is expressed in macrophages, trophoblasts, fibroblasts, smooth muscle, and endothelial cells, and the expression of its mRNA does not differ between normal and preeclamptic pregnancies. The expression of COX-1 mRNA does not differ in placentas of normal or preeclamptic pregnancies, but is elevated in preeclampsia in the placental bed.Citation44 The TXA/PGI2 ratio, as well as that of lipid peroxides, is elevated in the cytotrophoblast and the stroma of placental villi.Citation44 These results are in agreement with the first studies of prostanoids in human placenta,Citation45 which indicate that the placenta, in particular the cytotrophoblast, contributes to the imbalance of the TXA/PGI2 ratio in preeclampsia, favoring platelet aggregation and the greater vascular reactivity of this condition.
NO
In the first trimester, eNOS is expressed in syncytiotrophoblast, anchoring columns and the extravillous trophoblast. It is highly likely that NO liberated by the extravillous trophoblast primes the spiral arteries for their morphological transformation.Citation46 This preconditioning is supported by the fact that in guinea pigs, only dilated arteries surrounded by trophoblasts expressing eNOS are subsequently invaded.Citation47
The expression of eNOS in human reproductive tissues in women with preeclampsia is discordant. However, the local and systemic improvement observed with L-arginine supplementation in hypertensive pregnant women (reduction of blood pressure, uterine artery resistance, synthesis of NO) supports the participation of NO in the systemic and local adaptation of pregnancy.Citation48–Citation51 In addition, in preeclampsia the expression of placental arginase II – the enzyme that degrades arginine into ornithine – is elevated in placental villi, and the concentration of L-arginine is reduced in cord blood and in villi.Citation52
KKS
The mRNAs that code for kallikrein and the B2R are expressed in syncytiotrophoblast, cytotrophoblast, villous fetal endothelium, decidual cells of the basal and chorionic plate, and in intravascular and extravillous trophoblast. Kallikrein is present in these same cell types, with the exception of the anchoring columns and the extravillous trophoblast.Citation53,Citation54 In placenta accreta, a condition of exaggerated trophoblast invasion, the expression of kallikrein is increased in syncytiotrophoblast, and that of the B2R in the fetal endothelium and the extravillous trophoblast; in contrast to normal pregnancy, kallikrein is expressed in extravillous trophoblast. The only difference observed in preeclampsia – the increase of the B2R in the extravillous trophoblast – has been interpreted as support to the formation of AT-1-R and B2R heterodimers.Citation55
The B2R-induced migration and invasion in immortalized extravillous trophoblasts recently described by the authors of this paperCitation56 supports the role of the KKS in placentation.
Vasodilator components of the RAS
The functional importance of the RAS in pregnancy is highlighted by the catastrophic effects of converting enzyme inhibitors on fetal wellbeing,Citation57,Citation58 by the association of preeclampsia to autoantibodies against the AT-1-R, and to the M235T polymorphism of the gene that codes for angiotensinogen,Citation59 and finally, by the preeclamptic syndrome presented by female mice previously transfected with the human angiotensinogen mated with males transfected with the human renin gene.Citation60 Ang-(1–7) and ACE2, its main generating enzyme, are expressed in syncytio and cytotrophoblast, villous smooth vascular muscle, extravillous and intraarterial trophoblast, decidual cells, umbilical cord endothelium, and vascular smooth muscle. Ang-(1–7) is no different in placental villi from normotensive and preeclamptic pregnancies, but Ang II is increased, suggesting a balance that favors vasoconstriction.Citation61 In preeclamptic uterine placental bed, Ang II peptide levels, and renin- and Ang-converting enzyme mRNA expression were elevated compared with normal pregnancy.Citation62 Angiotensinogen and the AT-1-R are increased in villi and decidua of preeclampsia.Citation61 Herse et al found an overexpression of the vasoconstrictor AT-1-R mRNA in the decidua of preeclamptic pregnancies while that of the vasodilator AT-2-R in decidua and placenta was exceptionally present in preeclampsia and prevalent in control pregnancies.Citation31
The expression of the vasodilator receptor of Ang IV, AT-4-R, is increased in normal term placentas and is reduced in preeclampsia. Placental explants incubated with Ang IV or Ang II show increased trophoblast invasion and reduced apoptosis.Citation63
VEGF
During the menstrual cycle, VEGF, and its receptors FLT-1 and KDR, are expressed in the endometrium.Citation64,Citation65 In pregnancy, VEGF is mainly expressed in villous cyto- and syncytiotrophoblast, the invading front of the anchoring columns, and extravillous and endovascular trophoblast.Citation66–Citation68 VEGF in the perivascular trophoblast could prime the spiral arteries to invasion, and in placental villi, enhance the blood flow in fetal capillaries. In a dual perfusion model of term placental lobes, physiological concentrations of VEGF exert a potent vasodilator effect on vascular fetoplacental vessels, partially mediated by KDR-induced NO stimulation.Citation41 Transfection of the VEGF gene to uterine arteries provokes vasodilatation, reduces the response to phenylephrine, and potentiates the response to bradykinin.Citation69
The sites of expression of paracrine vasodilator factors, their generating enzymes, and their receptors () support their participation not only in blood flow regulation in fetal and maternal vessels but suggest other functions as well. Due to the cognate pleitropic effects of these factors,Citation70 they could participate in platelet aggregation and in nonvasoactive processes such as: (1) angiogenesis, (2) interstitial trophoblast invasion, (3) transformation of spiral arteries, and (4) the replacement of their endothelium by intra-arterial trophoblast. However, it must be taken into account that the expression of vasodilator pleiotropic factors in late pregnancy does not represent their expression during the determinant first 20 weeks of pregnancy.
Figure 2 Diagram of the uteroplacental interface, showing its main cell types and the vasodilator repertoire of each type.Citation9 The discontinuous yellow line depicts the path of trophoblasts that detach from the anchoring column to migrate through the uterine stroma, destroy the vascular smooth muscle, and colonize the lumen of the spiral arteries.
Abbreviation: NK, natural killer.
Hypertensive pregnancy and its association with late cardiovascular disease in women
Epidemiological studies associate preeclampsia, other hypertensive gestations, and diseases related to defective placentation to greater cardiovascular risk,Citation71–Citation75 so that pregnancy is considered a stress test to cardiovascular health. In addition, preeclampsia also increases the risk of end-stage renal disease.Citation76
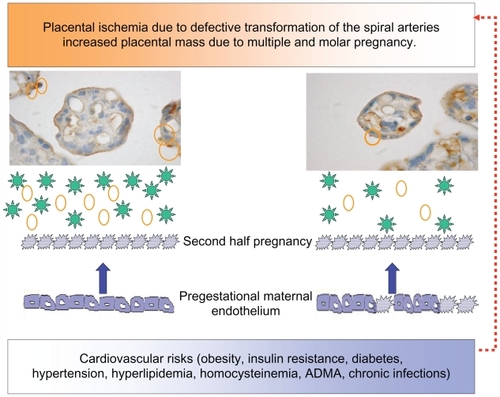
In a study performed by the authors of this paper, in women submitted to coronary angiography because of clinical suspicion of coronary artery disease, it was found that women with a previous hypertensive pregnancy presented a greater number of arteries with significant lesions according to age than women with normotensive gestation.Citation77 This same study underscored the increased risk of a familial history of premature cardiovascular disease both for hypertension in pregnancy and coronary disease. Many are the risks shared by hypertensive pregnancy and cardiovascular disease (eg, obesity, metabolic syndrome, diabetes, premature cardiovascular disease, endothelial dysfunction, thrombophilias, hyperhomocistinemia, inflammation, ADMA, and oxidative stress). These factors provoke the first stage of atheromatous disease, endothelial dysfunction, which has been demonstrated far from parturition in subjects with prior preeclampsia,Citation18 and especially with early onset preeclampsia.Citation78 As women with recurrent miscarriages also present endothelial dysfunction,Citation79 this alteration probably lies at the root of placentation defects and is not derived from the hypertensive phase of preeclampsia. It is feasible that the association between hypertensive pregnancies and late cardiovascular disease is given by risk factors within high normal limits in the reproductive stage, which do not provide an ample margin to the metabolic, hemodynamic, and inflammatory changes provoked by gestation. Based on the hypothesis that hypertension in pregnancy expresses a combination of underlying maternal conditions, and the placental debris/factors that are shed into the maternal circulation, the authors of this paper propose that proteinuric hypertension (preeclampsia) derives from combinations of placental and preexisting maternal factors (). Nonproteinuric hypertension (eg, transient hypertension or exacerbation of a preexistent hypertension) on the contrary may derive from a reduced capacity to stimulate vasodilator/antiaggregating factors (eg, PGI2, kallikrein, and Ang-(1–7)), a limitation that impinges the maternal hemodynamic adaptation to pregnancy, and later favors the development of cardiovascular disease.
Figure 3 Diagram showing the relation between the placenta and the maternal endothelium, in ischemic conditions, increased placental mass, and underlying cardiovascular risks. The microphotographs of the placental villi show syncytial knots prior to being deported into the maternal circulation (orange circles). In addition, the placenta sheds factors to the maternal circulation (green stars: sFLT-1, agonist autoantibodies to the AT-1-R, ADMA, and reactive oxygen species). Both syncytiotrophoblast microparticles and the soluble factors provoke endothelial dysfunction in pregestational healthy (smooth borders) or dysfunctional endothelial cells (spiky borders). Pregestational endothelial dysfunction also hinders uterine artery transformation (red broken arrow).
The authors of this paper cannot avoid referring to the lasting hemodynamic and metabolic effects exerted on the offspring by severe reduced perfusion of the intervillous space either in intrauterine or later life (alterations in the fetal arteries in order to preserve cerebral blood flow, intrauterine growth restriction, oligamnion, and spontaneous or induced preterm delivery). Be it by genetic determinants of trophoblast invasion, vasoactive adaptation, epigenetic changes, or intrauterine programming, the offspring will have a higher cardiovascular and metabolic risk,Citation80,Citation81 which needs to be addressed.
Conclusion
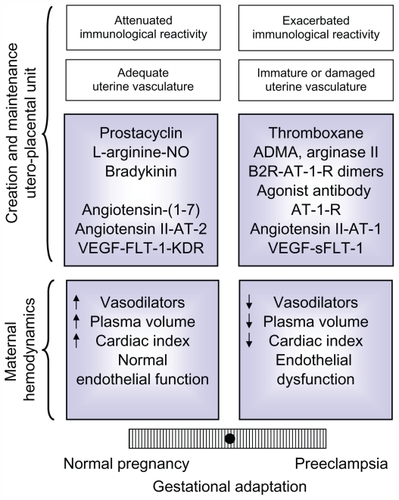
The data analyzed in this review shows that normal gestation represents a paradigm of a finely tuned vasodilator/vasoconstrictor balance, which is tipped to the vasoconstrictor arm, in preeclampsia (). On the other hand, the multiple factors that determine an adequate systemic and local adaptation also provide a diversity of pathways that could go awry and generate preeclampsia, transforming what was initially considered a disease into a syndrome.
Figure 4 Balance of factors that determine an adequate or defective adaptation to pregnancy in the maternal hemodynamics and in the development and maintenance of the uteroplacental unit,Citation9 based on the equilibrium initially proposed for PGI2 and TXA.Citation45 The modulation of the maternal immune reaction and the state of the maternal vasculature have also been included, because though not analyzed in this review, they influence the adaptation to pregnancy.
Abbreviations: ADMA, asymmetric dimethylarginine; AT-1-R, angiotensin II type 1 receptor; AT-1, angiotensin II type 1; AT-2, angiotensin II type 2; B2R, bradykinin 2 receptor; KDR, kinase domain receptor; FLT-1, fms-like tyrosine kinase 1; NO, nitric oxide; sFLT-1, soluble fms-like tyrosine kinase 1; VEGF, vascular endothelial growth factor.
Having focused on the equilibrium of vasoactive systems, we cannot disregard the morphological changes of the cardiovascular system induced by gestation. The high flow arteriovenous fistula represented by the intervillous space, and the increases in aortic valve area, ventricular mass,Citation82 and aortic complianceCitation83 underscore the extraordinary cardiovascular plasticity demanded by pregnancy.
The authors of this paper wish to emphasize that an adequate or a defective adaptation to pregnancy is expressed in several stages. The first silent stage is determined by an extensive or a shallow trophoblast invasion, and is only detected by ultrasonographic study of uterine arteries. The second is expressed clinically as a normal, preeclamptic, or a hypertensive pregnancy depending on the amount of placental deportation and the conditions of the maternal vasculature. Lastly, when the protection provided by estrogen is lost, cardiovascular diseases will be absent or present depending on the underlying risks that influenced pregnancy and the variations induced by lifestyle. No wonder gestation, which is equivalent to a prolonged moderate exercise,Citation82 is a powerful and lasting stress test.Citation84
Acknowledgments
The studies of the vasodilator factors have been supported by Fondo Nacional de Ciencia y Tecnología, Chile (Grants 1940636, 1980958, 1020705, 1050707, and 1080228).
Disclosure
The authors have no conflicts of interest to report in relation to this paper.
References
- Charnock-JonesDSKaufmannPMayhewTMAspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular regulationPlacenta20042510311314972443
- KaufmannPBlackSHuppertzBEndovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsiaBiol Reprod2003691712620937
- Red-HorseKZhouYGenbacevOTrophoblast differentiation during embryo implantation and formation of the maternal-fetal interfaceJ Clin Invest200411474475415372095
- HyttenFBlood volume changes in normal pregnancyClin Haematol1985146016124075604
- SalasSPRossoPEspinozaRRobertJAValdésGDonosoEMaternal plasma volume expansion and hormonal changes in women with idiopathic fetal growth retardationObstet Gynecol199381102910338497346
- GantNFDaleyGLChandSWhalleyPJMacDonaldPCA study of angiotensin II pressor response throughout primigravid pregnancyJ Clin Invest197352268226894355997
- SalasSPMarshallGGutierrezBLRossoPTime course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restrictionHypertension20064720320816380519
- KarlbergBERydénGWichmanKChanges in the renin-angiotensin-aldosterone and kallikrein-kinin systems during normal and hypertensive pregnancyActa Obstet Gynecol Scand19841181724
- ValdésGKaufmannPCorthornJEricesRBrosnihanKBJoyner-GranthamJVasodilator factors in the systemic and local adaptations to pregnancyReprod Biol Endocrinol200977919646248
- GryglewskiRJProstacyclin among prostanoidsPharmacol Rep20086031118276980
- BussolinoFBenedettoCMassobrioMCamussiGMaternal vascular prostacyclin activity in pre-eclampsiaLancet198027026106815
- YlikorkalaOPekonenFViinikkaLRenal prostacyclin and thromboxane in normotensive and preeclamptic pregnant women and their infantsJ Clin Endocrinol Metab198663130713123536979
- CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant womenCLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative GroupLancet19943436196297906809
- KnowlesRGMoncadaSNitric oxide synthases in mammalsBiochem J19942982492587510950
- ConradKPJoffeGMKruszynaHIdentification of increased nitric oxide biosynthesis during pregnancy in ratsFASEB J199375665717682524
- MolnarMSutoTTothTHertelendyFProlonged blockade of nitric oxide synthesis in gravid rats produces sustained hypertension, proteinuria, thrombocytopenia, and intrauterine growth retardationAm J Obstet Gynecol1994170145814667909994
- YallampalliCGarfieldREInhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsiaAm J Obstet Gynecol1993169131613208238200
- SavvidouMDHingoraniADTsikasDFrolichJCVallancePNicolaidesKHEndothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsiaLancet20033611511151712737861
- MoreauMEGarbackiNMolinaroGBrownNJMarceauFAdamAThe kallikrein-kinin system: current and future pharmacological targetsJ Pharmacol Sci20059963816177542
- ValdésGForadoriAOyarzúnEUrinary kallikrein along cycle, pregnancy and lactationPrenatal Neonatal Medicine19983474481
- ElebuteOAMillsIHUrinary kallikrein in normal and hypertensive pregnanciesPerspect Nephrol Hypertens197653293381005045
- KylePMCampbellSBuckleyDA comparison of the inactive urinary kallikrein:creatinine ratio and the angiotensin sensitivity test for the prediction of pre-eclampsiaBr J Obstet Gynaecol19961039819878863695
- FyhrquistFSaijonmaaORenin-angiotensin system revisitedJ Intern Med200826422423618793332
- FerrarioCMBrosnihanKBDizDIAngiotensin-(1–7): a new hormone of the angiotensin systemHypertension1991185 SupplIII126III1331937675
- TsutsumiYMatsubaraHMasakiHAngiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilationJ Clin Invest199910492593510510333
- WiddopREMatrouguiKLevyBIHenrionDAT2 receptor-mediated relaxation is preserved after long-term AT1 receptor blockadeHypertension20024051652012364356
- YayamaKHiyoshiHImazuDOkamotoHAngiotensin II stimulates endothelial NO synthase phosphorylation in thoracic aorta of mice with abdominal aortic banding via type 2 receptorHypertension20064895896417000928
- ColemanJKKrebsLTHamiltonTAAutoradiographic identification of kidney angiotensin IV binding sites and angiotensin IV-induced renal cortical blood flow changes in ratsPeptides1998192692779493859
- MerrillDCKarolyMChenKFerrarioCMBrosnihanKBAngiotensin-(1–7) in normal and preeclamptic pregnancyEndocrine20021823924512450315
- ValdésGGermainAMCorthornJUrinary vasodilator and vasoconstrictor angiotensins during menstrual cycle, pregnancy, and lactationEndocrine20011611712211887932
- HerseFDechendRHarsemNKDysregulation of the circulating and tissue-based renin-angiotensin system in preeclampsiaHypertension20074960461117261642
- AbdAllaSLotherHel MassieryAQuittererUIncreased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsivenessNat Med200171003100911533702
- ZhouACarrellRWMurphyMPA redox switch in angiotensinogen modulates angiotensin releaseNature201046810811120927107
- DvorakHFBrownLFDetmarMDvorakAMVascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesisAm J Pathol1995146102910397538264
- BrownbillPMillsTASoydemirDFSibleyCPVasoactivity to and endogenous release of vascular endothelial growth factor in the in vitro perfused human placental lobule from pregnancies complicated by preeclampsiaPlacenta20082995095518845336
- Wheeler-JonesCAbu-GhazalehRCospedalRHoulistonRAMartinJZacharyIVascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase A2 in endothelial cells via p42/p44 mitogen-activated protein kinaseFEBS Lett199742028329450544
- TammelaTEnholmBAlitaloKPaavonenKThe biology of vascular endothelial growth factorsCardiovasc Res20056555056315664381
- OlssonAKDimbergAKreugerJClaesson-WelshLVEGF receptor signalling – in control of vascular functionNat Rev Mol Cell Biol2006735937116633338
- EvansPWWheelerTAnthonyFWOsmondCA longitudinal study of maternal serum vascular endothelial growth factor in early pregnancyHum Reprod199813105710629619570
- BosioPMWheelerTAnthonyFConroyRO’HerlihyCMcKennaPMaternal plasma vascular endothelial growth factor concentrations in normal and hypertensive pregnancies and their relationship to peripheral vascular resistanceAm J Obstet Gynecol200118414615211174494
- BrownbillPMcKeemanGCBrockelsbyJCCrockerIPSibleyCPVasoactive and permeability effects of vascular endothelial growth factor-165 in the term in vitro dually perfused human placental lobuleEndocrinology20071484734474417640983
- MaynardSEMinJYMerchanJExcess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsiaJ Clin Invest200311164965812618519
- LevineRJQianCMaynardSEYuKFEpsteinFHKarumanchiSASerum sFlt1 concentration during preeclampsia and mid trimester blood pressure in healthy nulliparous womenAm J Obstet Gynecol20061941034104116580293
- WetzkaBNusingRCharnock-JonesDSSchaferWZahradnikHPSmithSKCyclooxygenase-1 and -2 in human placenta and placental bed after normal and pre-eclamptic pregnanciesHum Reprod199712231323209402302
- WalshSWPreeclampsia: an imbalance in placental prostacyclin and thromboxane productionAm J Obstet Gynecol19851523353403923838
- CravenCMMorganTWardKDecidual spiral artery remodelling begins before cellular interaction with cytotrophoblastsPlacenta1998192412529639319
- NanaevAChwaliszKFrankHGKohnenGHegele-HartungCKaufmannPPhysiological dilation of uteroplacental arteries in the guinea pig depends on nitric oxide synthase activity of extravillous trophoblastCell Tissue Res19952824074218581935
- FacchinettiFLongoMPiccininiFNeriIVolpeAL-arginine infusion reduces blood pressure in preeclamptic women through nitric oxide releaseJ Soc Gynecol Investig19996202207
- FacchinettiFSaadeGRNeriIPizziCLongoMVolpeAL-arginine supplementation in patients with gestational hypertension: a pilot studyHypertens Pregnancy20072612113017454224
- GermainAMValdésGRomanikMCReyesMSEvidence supporting a beneficial role for long-term L-arginine supplementation in high-risk pregnanciesHypertension200444e115218885
- NeriIJasonniVMGoriGFBlasiIFacchinettiFEffect of L-arginine on blood pressure in pregnancy-induced hypertension: a randomized placebo-controlled trialJ Matern Fetal Neonatal Med20061927728116753767
- NorisMTodeschiniMCassisPL-arginine depletion in preeclampsia orients nitric oxide synthase toward oxidant speciesHypertension20044361462214744923
- ValdésGChacónCCorthornJFigueroaCDGermainAMTissue kallikrein in human placenta in early and late gestationEndocrine20011419720411394637
- ValdésGGermainAMCorthornJChacónCFigueroaCDMuller- EsterlWTissue kallikrein and bradykinin B2 receptor in human uterus in luteal phase and in early and late gestationEndocrine20011620721511954665
- FuruyaMKurasawaKNagahamaKDisrupted balance of angiogenic and antiangiogenic signalings in preeclampsiaJ Pregnancy2011201112371721490787
- EricesRCorthornJLisboaFValdésGBradykinin promotes migration and invasion of human immortalized trophoblastsReprod Biol Endocrinol201199721729302
- Kreft-JaisCPlouinPFTchobroutskyCBoutroyMJAngiotensin-converting enzyme inhibitors during pregnancy: a survey of 22 patients given captopril and nine given enalaprilBr J Obstet Gynaecol1988954204222838069
- HanssensMKeirseMJVankelecomFVan AsscheFAFetal and neonatal effects of treatment with angiotensin-converting enzyme inhibitors in pregnancyObstet Gynecol1991781281352047053
- InoueIRohrwasserAHelinCA mutation of angiotensinogen in a patient with preeclampsia leads to altered kinetics of the renin-angiotensin systemJ Biol Chem199527011430114367744780
- TakimotoEIshidaJSugiyamaFHoriguchiHMurakamiKFukamizuAHypertension induced in pregnant mice by placental renin and maternal angiotensinogenScience19962749959988875944
- AntonLMerrillDCNevesLAActivation of local chorionic villi angiotensin II levels but not angiotensin (1–7) in preeclampsiaHypertension2008511066107218259034
- AntonLMerrillDCNevesLAThe uterine placental bed renin-angiotensin system in normal and preeclamptic pregnancyEndocrinology20091504316432519520788
- WilliamsPJMistryHDInnesBABulmerJNPipkinFBExpression of AT1R, AT2R and AT4R and their roles in extravillous trophoblast invasion in the humanPlacenta3144845520304486
- KrusselJSCasanEMRagaFExpression of mRNA for vascular endothelial growth factor transmembraneous receptors Flt1 and KDR, and the soluble receptor sflt in cycling human endometriumMol Hum Reprod1999545245810338368
- MollerBLindblomBOlovssonMExpression of the vascular endothelial growth factors B and C and their receptors in human endometrium during the menstrual cycleActa Obstet Gynecol Scand20028181782412225295
- DemirRKayisliUASevalYSequential expression of VEGF and its receptors in human placental villi during very early pregnancy: differences between placental vasculogenesis and angiogenesisPlacenta20042556057215135240
- CampbellSRoweJJacksonCJGalleryEDInteraction of cocultured decidual endothelial cells and cytotrophoblasts in preeclampsiaBiol Reprod20047124425215028631
- ShiraishiSNakagawaKKinukawaNNakanoHSueishiKImmunohistochemical localization of vascular endothelial growth factor in the human placentaPlacenta1996171111218730881
- DavidALTorondelBZacharyILocal delivery of VEGF adenovirus to the uterine artery increases vasorelaxation and uterine blood flow in the pregnant sheepGene Ther2008151344135018563186
- ValdésGCorthornJReview: the angiogenic and vasodilatory uteroplacental networkPlacenta201132Suppl 2S170S17521295852
- BellamyLCasasJPHingoraniADWilliamsDJPre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysisBMJ200733597417975258
- CraiciIMWagnerSJHaymanSRGarovicVDPre-eclamptic pregnancies: an opportunity to identify women at risk for future cardiovascular diseaseWomens Health20084133135
- SmithGCPellJPWalshDPregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129, 290 birthsLancet20013572002200611438131
- WilsonBJWatsonMSPrescottGJHypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort studyBMJ200332684512702615
- IrgensHUReisaeterLIrgensLMLieRTLong term mortality of mothers and fathers after pre-eclampsia: population based cohort studyBMJ20013231213121711719411
- VikseBEIrgensLMLeivestadTSkjaervenRIversenBMPreeclampsia and the risk of end-stage renal diseaseN Engl J Med200835980080918716297
- ValdésGQuezadaFMarchantEAssociation of remote hypertension in pregnancy with coronary artery disease: a case-control studyHypertension20095373373819204177
- YinonYKingdomJCOdutayoAVascular dysfunction in women with a history of preeclampsia and intrauterine growth restriction: insights into future vascular riskCirculation20101221846185320956209
- GermainAMRomanikMCGuerraIEndothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events?Hypertension200749909517116761
- BarkerDJThe developmental origins of adult diseaseJ Am Coll Nutr2004236 Suppl588S595S15640511
- GluckmanPDHansonMACooperCThornburgKLEffect of in utero and early-life conditions on adult health and diseaseN Engl J Med2008359617318596274
- RobsonSCHunterSBoysRJDunlopWSerial study of factors influencing changes in cardiac output during human pregnancyAm J Physiol1989256H1060H10652705548
- PoppasAShroffSGKorcarzCESerial assessment of the cardiovascular system in normal pregnancy. Role of arterial compliance and pulsatile arterial loadCirculation199795240724159170404
- WilliamsDPregnancy: a stress test for lifeCurr Opin Obstet Gynecol20031546547114624211
