Abstract
Anticoagulation therapy is essential for the effective treatment and secondary prevention of venous thromboembolism (VTE). For many years, anticoagulation for acute VTE was limited to the use of initial parenteral heparin, overlapping with and followed by a vitamin K antagonist. Although highly effective, this regimen has several limitations and is particularly challenging when given in an ambulatory setting. Current treatment pathways for most patients with deep-vein thrombosis typically involve initial hospital or community-based ambulatory care with subsequent follow-up in a secondary care setting. With the introduction of non-vitamin K antagonist oral anticoagulants (NOACs) into routine clinical practice, it is now possible for the initial acute management of patients with deep-vein thrombosis to be undertaken by primary care. As hospital admissions associated with VTE become shorter, primary care will play an increasingly important role in the long-term management of these patients. Although the NOACs can potentially simplify patient management and improve clinical outcomes, primary care physicians may be less familiar with these new treatments compared with traditional therapy. To assist primary care physicians in further understanding the role of the NOACs, this article outlines the main differences between NOACs and traditional anticoagulation therapy and discusses the benefit–risk profile of the different NOACs in the treatment and secondary prevention of recurrent VTE. Key considerations for the use of NOACs in the primary care setting are highlighted, including dose transition, risk assessment and follow-up, duration of anticoagulant therapy, how to minimize bleeding risks, and the importance of patient education and counseling.
Introduction
Anticoagulation therapy is essential for the effective treatment and secondary prevention of venous thromboembolism (VTE), comprising deep-vein thrombosis (DVT) and pulmonary embolism (PE), but is associated with a risk of bleeding.Citation1 Current treatment pathways for most patients with DVT typically involve initial hospital or community-based ambulatory care with subsequent follow-up in a secondary care setting. An increasing number of patients with low-risk PE are also being discharged early from hospital or treated entirely as outpatients. For many years, traditional anticoagulant treatment for acute VTE was limited to the use of initial parenteral heparin, overlapping with and followed by a vitamin K antagonist (VKA). This regimen is cumbersome for outpatients; VKA therapy necessitates routine coagulation monitoring of the international normalized ratio and frequent dose adjustment owing to a narrow therapeutic window and multiple drug and food interactions.Citation2 Although prolonged VKA treatment further reduces the incidence of recurrent VTE compared with shorter treatment durations, it is also associated with increased risk of major bleeding.Citation3 Consequently, the balance between the benefits and risks of continued anticoagulation remains a subject of debate, and many patients with VTE do not receive extended-duration anticoagulant therapy, despite the high long-term risk of recurrence and guideline recommendations supporting extended treatment.Citation4–Citation7
The non-VKA oral anticoagulants (NOACs; also known as novel oral anticoagulants) rivaroxaban, apixaban, dabigatran, and edoxaban have become available as alternative options for the management of several thromboembolic disorders, including the treatment of DVT/PE, secondary prevention of VTE, and stroke prevention in patients with non-valvular atrial fibrillation.Citation8–Citation11 Unlike VKAs, NOACs offer fixed dosing regimens without the need for routine coagulation monitoring, which makes the initial management of patients with DVT feasible in primary care settings, as well as facilitating easy transition from in-hospital to community care.
Primary care physicians play an increasingly important role in the long-term management of patients with VTE, but they may be far less familiar with newer treatment options (ie, NOACs) compared with traditional therapy. To assist primary care physicians in further understanding the role of the NOACs, this article outlines the main differences between NOACs and traditional anticoagulant therapy for the treatment and secondary prevention of recurrent VTE and discusses key considerations for their use in the primary care setting.
Differences between NOACs and traditional standard therapy for the treatment of VTE
Traditional anticoagulant therapy for patients with DVT (or PE in hemodynamically stable patients) utilizes a dual-drug approach consisting of a parenteral agent (most commonly low molecular weight heparin [LMWH] or fondaparinux) for ≥5 days, overlapping with a VKA until the international normalized ratio of VKA therapy is ≥2.0 for at least 24 hours, at which point VKA therapy alone is continued.Citation12,Citation13 This initial “bridging therapy” with a parenteral anticoagulant is required because of the slow onset of action of VKAs. In addition, VKAs require frequent coagulation monitoring and dose adjustment. By contrast, the predictable pharmacokinetic and pharmacodynamic properties of the NOACs allow for fixed dosing regimens without the need for routine coagulation monitoring. Furthermore, the NOACs have a fast onset of action, reaching their maximum plasma concentrations within a few hours of oral tablet intake ().Citation14,Citation15
Table 1 Key pharmacological properties of NOACs and VKAs (eg, warfarin)
Favorable benefit–risk profile of NOACs: evidence from Phase III clinical trials
In all the Phase III clinical trials for the treatment of acute VTE, the NOACs showed non-inferior efficacy compared with standard therapy, with significantly reduced rates of major bleeding in EINSTEIN PE (rivaroxaban) and AMPLIFY (apixaban; ).Citation16–Citation21 The trials included patients with symptomatic VTE, but there is no reason to suspect that the NOACs would not be equally effective and safe in patients with asymptomatic VTE. It should be noted that the study design and patient populations differed between these studies, and that there are no head-to-head comparisons between the different NOACs.Citation16–Citation21 A pooled analysis of the EINSTEIN DVT and EINSTEIN PE studies showed a significantly more favorable net clinical benefit for rivaroxaban in key subgroups vs standard therapy, eg, in fragile patients (defined as one or more of the following criteria: age >75 years; creatinine clearance [CrCl] <50 mL/min; or low body weight ≤50 kg), those with active cancer, and those with a previous VTE.Citation22 Although these data are promising, it should be acknowledged that certain subgroups of patients, eg, those aged >75 years, with a body weight of <60 kg, with active cancer or thrombophilia, or taking dual antiplatelet therapy, were underrepresented in the Phase III clinical trials. Therefore, further research is needed to establish the benefit–risk profile of the NOACs in these specific patient groups.
Table 2 Efficacy and safety outcomes of NOACs vs standard therapy in Phase III clinical trials for the treatment of acute VTE
Long-term secondary prevention of recurrent VTE: NOACs or acetylsalicylic acid
The duration of anticoagulant treatment in patients with VTE has been a subject of debate. Although the extended VKA therapy is highly effective in preventing recurrent VTE, it is also associated with an increased risk of major bleeding.Citation3 This, together with other limitations, discourages the extended use of VKA therapy in patients with VTE. In Phase III clinical trials, rivaroxaban (EINSTEIN EXT), apixaban (AMPLIFY EXT), and dabigatran (RE-SONATE) demonstrated superior efficacy compared with placebo for extended anticoagulation (6 months or 12 months) in patients who had already received anticoagulation therapy for 6–18 months.Citation16–Citation18,Citation23 Compared with placebo, rivaroxaban (20 mg once daily) led to an 82% relative risk reduction (RRR) in the incidence of recurrent VTE, although there was a low incidence of nonfatal major bleeding events (0.7%).Citation16 The RRR for recurrent VTE associated with apixaban was 81% for both doses (2.5 mg and 5 mg twice daily), without a significant increase in major bleeding events. Dabigatran significantly reduced the risk of recurrent VTE (RRR 92%) compared with placebo, but it was also associated with a significant increase in major and clinically relevant bleeding (principal safety end point).Citation23
Acetylsalicylic acid (ASA) has also been investigated for long-term secondary prevention of recurrent VTE in patients with a first unprovoked VTE who have already had VKA therapy for 6–18 months. In the WARFASA study, which compared ASA 100 mg daily with placebo for 2 years, ASA treatment was associated with a 42% RRR in VTE recurrence, with no significant increase in the risk of bleeding.Citation24 Using the same study design, the ASPIRE trial investigated continued treatment with ASA for up to 4 years. In this study, a significant decrease in VTE recurrence with ASA was not found compared with placebo, raising further doubts over the long-term efficacy of ASA in secondary prevention of recurrent VTE.Citation25
Owing to concerns about bleeding, some physicians may prefer to prescribe ASA rather than oral anticoagulants for the secondary prevention of VTE. It should be noted that in the BAFTA study in an elderly (≥75 years) community population with atrial fibrillation, the risk of extracranial hemorrhage was similar for ASA and warfarin, but the incidence of primary end point events (fatal or disabling stroke, intracranial hemorrhage, or clinically significant arterial embolism) was significantly higher with ASA compared with warfarin.Citation26 Similarly, although ASA treatment can reduce the rates of VTE recurrence, it is less effective than the NOACs (or VKAs). Current European Society of Cardiology guidelines specify that ASA should be considered for extended secondary prevention of VTE only if patients refuse or are unable to tolerate any form of oral anticoagulant.Citation27
NOACs for the treatment of VTE: what do you need to know?
Contraindications for use
The NOACs are contraindicated in patients with active clinically significant bleeding, or in patients who have a lesion or a condition considered to be a significant risk for major bleeding. Concomitant treatment with any other anticoagulant is contraindicated, except under specific circumstances of switching anticoagulant therapy, or when unfractionated heparin is given at doses necessary to maintain an open central venous or arterial catheter.Citation8–Citation11 Apixaban, edoxaban, and rivaroxaban are contraindicated in patients with hepatic disease associated with coagulopathy and clinically relevant bleeding risk, and dabigatran is contraindicated in patients with hepatic impairment or liver disease that is expected to have any impact on survival.Citation8–Citation11 Dabigatran is also contraindicated in patients with severe renal impairment (CrCl <30 mL/min), and the other NOACs are not recommended in patients with CrCl <15 mL/min.Citation8–Citation11 Edoxaban and rivaroxaban are contraindicated during pregnancy, and all NOACs should be avoided during breastfeeding (edoxaban and rivaroxaban are contraindicated).Citation8–Citation11 Edoxaban is contraindicated in patients with uncontrolled severe hypertension, and rivaroxaban is not recommended in these patientsCitation9,Citation10 Additional dabigatran contraindications are concomitant treatment with systemic ketoconazole, cyclosporin, itraconazole, and dronedarone, and patients with prosthetic valves who require anticoagulant treatment.Citation8 Precautions for comedication use with other NOACs are discussed in the “Continued risk assessment and patient follow-up” section.
Dose regimens and managing dose transition
Of the NOACs currently licensed for VTE treatment, only rivaroxaban and apixaban offer the advantage of a single-drug approach for the total treatment duration, comprising an initial intensified treatment period without parenteral bridging. The approved NOAC dosing regimens for the treatment of VTE are shown in .
Figure 1 Approved dose regimens of the NOACs (based on the EU labels) for the treatment of VTE.
Abbreviations: bid, twice daily; CrCl, creatinine clearance; DVT, deep-vein thrombosis; EU, European Union; od, once daily; NOAC, non-vitamin K antagonist oral anticoagulant; PE, pulmonary embolism; P-gp, P-glycoprotein; VTE, venous thromboembolism.
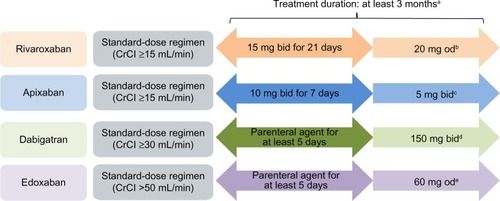
In routine clinical practice, many patients with DVT (and/or low risk PE) may have been treated solely as outpatients, or transferred to outpatients or primary care after a short hospital stay (early discharge). Therefore, it is likely that some primary care physicians will manage dose transitions. Dose reductions may be considered in certain patient populations who have an increased risk of bleeding that outweighs the risk of recurrent DVT (eg, patients with renal impairment; ). Using the correct dosing regimen is essential to provide optimal protection for patients, and this is particularly important during the acute phase when patients are at higher risk of thrombus extension and embolization. After the initial acute treatment period, rivaroxaban is given as a once-daily regimen for long-term VTE treatment and secondary prevention of recurrent VTE, whereas both apixaban and dabigatran are administered twice daily. Once-daily dosing regimens (particularly as long-term therapy) have been shown to be associated with improved patient adherence,Citation28 which may have positive effects on clinical outcomes. In the Phase III EINSTEIN DVT and EINSTEIN PE studies, patients receiving rivaroxaban reported higher treatment satisfaction than those receiving standard therapy with enoxaparin/VKA.Citation29,Citation30
Continued risk assessment and patient follow-up
Although routine coagulation or drug monitoring is not required for patients treated with NOACs, regular clinical follow-up is important for all patients receiving anticoagulants. This allows for ongoing assessment of the risks of recurrent VTE vs bleeding, as well as providing continued guidance and support to patients, particularly around drug adherence. Regular follow-up also offers opportunities to advise on anticoagulation management before invasive procedures (such as dental treatment). Risk factors for bleeding and VTE recurrence are listed in . Apart from recurrent DVT/PE, other consequences of VTE include post-thrombotic syndrome, a relatively frequent complication of DVT that may lead to long-term morbidity, and chronic thromboembolic pulmonary hypertension, a relatively rare but serious complication of PE.Citation13
Table 3 Risk factors associated with recurrent VTE and anticoagulant-related bleeding
The timing and frequency of follow-up visits are influenced by a number of factors (such as the initial diagnosis and the individual patient’s bleeding risk) and are guided by local protocols. At each visit, patient medication compliance and use of comedications (including over-the-counter medications) should be reviewed. Moreover, patients should be assessed to ensure that VTE symptoms are resolving on anticoagulation therapy and asked whether they have experienced any bleeding events. Laboratory testing is not usually required in the early phase of follow-up, but assessment of renal function should be performed at least annually in patients treated with NOACs, using CrCl (Cockcroft–Gault method) rather than glomerular filtration rate. In patients with declining renal function or those receiving dabigatran (which is primarily eliminated renally), more frequent monitoring of CrCl is recommended. If potential drug accumulation or poor absorption is suspected, or if patients have impaired renal function and extreme body weight or require urgent surgery, there are assays available to measure NOAC plasma concentrations.Citation31,Citation32 A full blood count may be useful in patients with suspected bleeding.
Elderly patients often present with renal impairment, which is associated with increased risks of both thromboembolic and bleeding events. The NOACs are eliminated renally to differing extents (∼30%–80%; ), and patients with severe renal impairment were excluded from the Phase III studies. A prespecified subgroup analysis of the EINSTEIN DVT and EINSTEIN PE studies showed that patients with symptomatic VTE and renal impairment are at increased risk of recurrent VTE, and that the rate of major bleeding increased in patients with renal impairment in the enoxaparin/VKA group but not in the rivaroxaban group.Citation33 The Summary of Product Characteristics for rivaroxaban and apixaban states that these agents are not recommended for patients with CrCl <15 mL/min, and that caution should be taken when prescribing to patients with CrCl 15–29 mL/min.Citation10,Citation11 Dabigatran is contraindicated in patients with severe renal impairment (CrCl <30 mL/min).Citation8 VKAs are currently the oral anticoagulants of choice in patients with the most severe renal dysfunction (CrCl <15 mL/min).Citation34
How to minimize bleeding risks and manage bleeding events?
In all cases, the balance between the risk of bleeding and risk of recurrent VTE should be the primary consideration when prescribing anticoagulant therapy. There are many factors that are associated with increased bleeding risks, such as comorbidities and previous bleeding events (). Routine concomitant use of nonsteroidal anti-inflammatory drugs (including over-the-counter agents) and antiplatelet drugs (such as ASA or clopidogrel), which can lead to an increased risk of bleeding,Citation35 should be avoided or used with caution. Although not commonly used in daily practice, coadministration of strong inhibitors of both cytochrome P450 (CYP) 3A4 and P-glycoprotein (P-gp) with rivaroxaban or apixaban is not recommended, because these agents (mainly azole antimycotics and HIV protease inhibitors) could lead to increased drug exposure and bleeding risk ().Citation10,Citation11 In addition, coadministration of rivaroxaban or apixaban with rifampicin (a strong CYP3A4/P-gp inducer) may lead to an ∼50% decrease in rivaroxaban or apixaban exposure; therefore, coadministration of strong CYP3A4 inducers with rivaroxaban should be avoided unless the patient is closely observed for signs and symptoms of thrombosis.Citation10 The product label for apixaban states that, for the treatment of DVT and PE in patients receiving concomitant systemic treatment with strong inducers of both CYP3A4 and P-gp, apixaban should not be used because the efficacy may be compromised.Citation11 Rivaroxaban has no clinically relevant drug interactions with digoxin, atorvastatin, or omeprazole.Citation10
Dabigatran is a substrate of P-gp; therefore, concomitant administration with strong P-gp inhibitors (ketoconazole, cyclosporin, itraconazole, and dronedarone) can lead to clinically relevant increases in dabigatran concentrations; thus, these agents are contraindicated.Citation8 Edoxaban clearance is also affected by concomitant use of strong P-gp inhibitors ().Citation9
If minor bleeding occurs in a patient taking an NOAC, it is often sufficient to delay or omit the next dose and to apply local hemostatic measures. For moderate–severe bleeding episodes, the NOAC should be temporarily stopped, and the patient should be directed to the hospital emergency department, where bleeding should be managed using established hemorrhage protocols, including mechanical compression, surgical hemostasis with bleeding control procedures, fluid replacement and hemodynamic support, and blood products or platelets. If bleeding cannot be controlled by these measures (or for life-threatening hemorrhage), administration of a specific procoagulant reversal agent can be considered.Citation8–Citation11
Long-term anticoagulation: when to review and when to stop
Current guidelines recommend 3 months of anticoagulation for provoked VTE and a minimum of 3-month anticoagulation for patients with unprovoked (or idiopathic) VTE.Citation12,Citation13,Citation27 Previously anticoagulated patients remain at risk of recurrent VTE, and studies have shown that the benefits of extended anticoagulation treatment were not maintained after anticoagulation discontinuation, suggesting that indefinite treatment may be required for patients at a very high risk of recurrent VTE, such as those with more than one episode of unprovoked thrombosis, or those with active cancer.Citation36,Citation37
In general, the duration of therapy should be individualized following careful assessment of the treatment benefit (reduction in recurrent VTE) vs the risk of bleeding. A short duration of therapy (ie, 3 months) should be given if there are transient risk factors (eg, recent surgery, trauma, or immobilization), and longer durations (>3 months to indefinite) in the ase of permanent risk factors or unprovoked proximal DVT or unprovoked PE.Citation8,Citation10,Citation11 Treatment should be reviewed at 3 months, and the decision on whether to stop or continue anticoagulant therapy should be based on a formal risk assessment and patient preference, preferably involving a practitioner with a sound knowledge of the evidence base of VTE management and anticoagulation therapy.
In patients with VTE and active cancer, the current guidelines recommend LMWH for the initial 3–6 months.Citation13,Citation27 It should be noted that a sub-analysis of the pooled EINSTEIN DVT and EINSTEIN PE data showed that, in patients with active cancer and VTE, rivaroxaban had similar efficacy and reduced the number of major bleeding events compared with enoxaparin/VKA.Citation38 NOACs are contraindicated or not recommended during pregnancy, whereas LMWH can be used in pregnant women.Citation8,Citation10,Citation11,Citation39 As mentioned earlier, all of the NOACs should strictly be avoided during breastfeeding.Citation8,Citation11
Peri-procedural management
According to the relevant Summary of Product Characteristics, if a patient requires an invasive procedure or surgical intervention, edoxaban and rivaroxaban should be stopped at least 24 hours before; apixaban should be stopped at least 24 hours before a low-bleeding-risk procedure and at least 48 hours before a moderate- or high-bleeding-risk procedure.Citation9–Citation11 The European Heart Rhythm Association guidelines on the practical use of NOACs in patients with non-valvular atrial fibrillation also recommend that edoxaban and rivaroxaban are stopped at least 48 hours prior to a high-bleeding-risk procedure.Citation40 For dabigatran, discontinuation timings are dependent on the renal function of the patient. In a patient with CrCl ≥80 mL/min, dabigatran should be stopped 48 hours (high risk of bleeding or major surgery) or 24 hours (standard risk) before the procedure. In patients with CrCl ≥50 mL/min to <80 mL/min, these timings are 2–3 days and 1–2 days, respectively, and in patients with CrCl ≥30 mL/min to <50 mL/min, they are 4 days and 2–3 days, respectively.Citation8 For all of the NOACs, the increased risk of bleeding should be weighed against the urgency of the intervention. Anticoagulation with the NOACs should only be restarted once adequate hemostasis has been established.Citation8–Citation11
Patient education
NOACs offer convenient, fixed-dose regimens without the need for routine coagulation monitoring; however, strict adherence to the correct dosing regimen is critical for optimal outcomes given the relatively short half-lives of these drugs. Patients should be reminded about the increased risk of recurrent DVT/PE when stopping anticoagulant treatment. There is evidence suggesting that in patients with atrial fibrillation who require long-term anticoagulant therapy, patients’ fear of stroke outweighs their fear of bleeding.Citation41 Similar evidence for patients with VTE is lacking, but patient education should further emphasize that noncompliance may lead to adverse outcomes. Missing a dose of their prescribed NOAC means that a patient is without adequate anticoagulation until the next dose (or until the missing dose is replaced). Patients who discontinue anticoagulant treatment should receive advice from their physician to increase their awareness of signs and symptoms of recurrent VTE. Patient counseling should include advice on contraception, invasive procedures, and long-distance travel, and when to seek advice on thromboprophylaxis at times of future increased risk.
Patients need to alert other health care professionals about their use of anticoagulants by carrying an anticoagulation alert card, eg, when undergoing dental procedures, seeing other doctors about other illnesses and visiting pharmacists for over-the-counter drugs, and especially if admitted to hospital via the emergency department. They should also be informed when to seek medical attention in case of adverse events or concerns regarding falls, minor trauma, or bleeding complications while receiving NOACs, particularly after head injury (even if it is minor). Clear guidance should be provided to patients on when or whether to stop their NOAC treatment before any elective surgery.Citation8–Citation11 In general, no bridging with a preoperative parenteral anticoagulant is needed, and in patients with normal renal function, stopping NOAC treatment 24 hours before minor surgical procedures and 48 hours before major procedures is sufficient.
Conclusion
With the introduction of NOACs into routine clinical practice, physicians and patients are no longer limited to VKAs for the treatment of VTE. All NOACs have proven to be at least as effective as the conventional standard therapy (eg, LMWH/VKA) in Phase III clinical trials, with favorable safety profiles. These newer agents offer more convenient treatment options without the need for routine anticoagulation monitoring. In addition, rivaroxaban and apixaban are given without the use of initial parenteral anticoagulation, which simplifies patient management. Primary care physicians play an important role in the treatment of patients with VTE. Continued risk assessment, regular follow-up, good adherence, and patient education are all essential for optimal clinical outcomes.
Acknowledgments
The author would like to acknowledge Yong-Ling Liu, who provided editorial support with funding from Bayer HealthCare Pharmaceuticals and Janssen Scientific Affairs, LLC.
Disclosure
The author has received honoraria as a guest speaker at educational programs and advisory boards for Bayer. The author reports no other conflicts of interest in this work.
References
- CohenATAgnelliGAndersonFAVTE Impact Assessment Group in Europe (VITAE)Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortalityThromb Haemost200798475676417938798
- AgenoWGallusASWittkowskyAAmerican College of Chest PhysiciansOral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelinesChest20121412 supple44Se88S22315269
- HuttenBAPrinsMHDuration of treatment with vitamin K antagonists in symptomatic venous thromboembolismCochrane Database Syst Rev20003CD00136710908494
- CapriniJAHyersTMCompliance with antithrombotic guidelines: current practice, barriers, and strategies for improvementManag Care2006159496617036939
- MerliGImproving venous thromboembolism performance: a comprehensive guide for physicians and hospitalistsHosp Pract (1995)201038371620499768
- VatsVNutescuEATheobaldJCWojtynekJESchumockGTSurvey of hospitals for guidelines, policies, and protocols for anticoagulantsAm J Health Syst Pharm200764111203120817519463
- PrandoniPVillaltaSBagatellaPThe clinical course of deep-vein thrombosis. Prospective long-term follow-up of 528 symptomatic patientsHaematologica19978244234289299855
- Pradaxa® (dabigatran etexilate) summary of product characteristicsIngelheim am RheinBoehringer Ingelheim International GmbH2015
- Lixiana® (edoxaban) summary of product characteristicsMunichDaiichi Sankyo Europe GmbH2015
- Xarelto® (rivaroxaban) summary of product characteristicsBerlinBayer Pharma AG2015
- Eliquis® (apixaban) summary of product characteristicsUxbridgeBristol-Myers Squibb, Pfizer2015
- National Institute for Health and Care Excellence [webpage on the Internet]Venous thromboembolic diseases: the management of venous thromboembolic diseases and the role of thrombophilia testing. Clinical guidelines, CG1442012 Available from: http://guidance.nice.org.uk/CG144Accessed September 25, 2015
- KearonCAklEAComerotaAJAmerican College of Chest PhysiciansAntithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelinesChest20121412 supple419Se494S22315268
- EikelboomJWWeitzJINew anticoagulantsCirculation2010121131523153220368532
- ErikssonBIQuinlanDJEikelboomJWNovel oral factor Xa and thrombin inhibitors in the management of thromboembolismAnnu Rev Med201162415721226611
- The EINSTEIN InvestigatorsBauersachsRBerkowitzSDOral rivaroxaban for symptomatic venous thromboembolismN Engl J Med2010363262499251021128814
- The EINSTEIN–PE InvestigatorsBüllerHRPrinsMHOral rivaroxaban for the treatment of symptomatic pulmonary embolismN Engl J Med2012366141287129722449293
- AgnelliGBullerHRCohenAAMPLIFY InvestigatorsOral apixaban for the treatment of acute venous thromboembolismN Engl J Med2013369979980823808982
- The Hokusai-VTE InvestigatorsBüllerHRDécoususHEdoxaban versus warfarin for the treatment of symptomatic venous thromboembolismN Engl J Med2013369151406141523991658
- SchulmanSKearonCKakkarAKRE-COVER Study GroupDabigatran versus warfarin in the treatment of acute venous thromboembolismN Engl J Med2009361242342235219966341
- SchulmanSKakkarAKGoldhaberSZRE-COVER II Trial InvestigatorsTreatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysisCirculation2014129776477224344086
- PrinsMHLensingAWABauersachsREINSTEIN InvestigatorsOral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studiesThromb J20131112124053656
- SchulmanSKearonCKakkarAKRE-MEDY Trial InvestigatorsRE-SONATE Trial InvestigatorsExtended use of dabigatran, warfarin, or placebo in venous thromboembolismN Engl J Med2013368870971823425163
- BecattiniCAgnelliGSchenoneAWARFASA InvestigatorsAspirin for preventing the recurrence of venous thromboembolismN Engl J Med2012366211959196722621626
- BrightonTAEikelboomJWMannKASPIRE InvestigatorsLow-dose aspirin for preventing recurrent venous thromboembolismN Engl J Med2012367211979198723121403
- MantJHobbsFDRFletcherKBAFTA investigatorsMidland Research Practices Network (MidReC)Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged study, BAFTA): a randomised controlled trialLancet2007370958649350317693178
- KonstantinidesSVTorbickiAAgnelliG2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolismEur Heart J201435433033306925173341
- ColemanCIRobertsMSSobierajDMLeeSAlamTKaurREffect of dosing frequency on chronic cardiovascular disease medication adherenceCurr Med Res Opin201228566968022429067
- BamberLWangMYPrinsMHPatient-reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of acute symptomatic deep-vein thrombosisThromb Haemost2013110473274123846019
- PrinsMHBamberLCanoSJPatient-reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of pulmonary embolism; results from the EINSTEIN PE trialThromb Res2015135228128825483215
- BaglinTKeelingDKitchenSBritish Committee for Standards in HaematologyEffects on routine coagulation screens and assessment of anticoagulant intensity in patients taking oral dabigatran or rivaroxaban: guidance from the British Committee for Standards in HaematologyBr J Haematol2012159442742922970737
- HeidbuchelHVerhammePAlingsMEuropean Heart Rhythm AssociationEuropean Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillationEuropace201315562565123625942
- BauersachsRMLensingAWAPrinsMHRivaroxaban versus enoxaparin/vitamin K antagonist therapy in patients with venous thromboembolism and renal impairmentThromb J2014122525750589
- CapodannoDAngiolilloDJAntithrombotic therapy in patients with chronic kidney diseaseCirculation2012125212649266122644369
- DavidsonBLVerheijenSLensingAWABleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti- inflammatory drugs or aspirinJAMA Intern Med2014174694795324733305
- BounameauxHPerrierADuration of anticoagulation therapy for venous thromboembolismHematology Am Soc Hematol Educ Program2008125225819074092
- EikelboomJWGinsbergJSHirshJAnticoagulation for venous thromboembolismBMJ2007334759564517395904
- PrinsMHLensingAWABrightonTAOral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trialsLancet Haematol201411e37e4627030066
- BatesSMGreerIAMiddeldorpSAmerican College of Chest PhysiciansVTE, thrombophilia, antithrombotic therapy, and pregnancy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelinesChest20121412 supple691Se736S22315276
- HeidbuchelHVerhammePAlingsMUpdated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillationEuropace201517101467150726324838
- DevereauxPJAndersonDRGardnerMJDifferences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational studyBr Med J200132373231218122211719412
- TorbickiAPerrierAKonstantinidesSESC Committee for Practice Guidelines (CPG)Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)Eur Heart J200829182276231518757870
- BoutitieFPinedeLSchulmanSInfluence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trialsBr Med J2011342d303621610040
- EichingerSHeinzeGJandeckLMKyrlePARisk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction modelCirculation2010121141630163620351233
- TosettoAIorioAMarcucciMPredicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH)J Thromb Haemost20121061019102522489957
- NietoJASolanoRRuiz-RibóMDRiete InvestigatorsFatal bleeding in patients receiving anticoagulant therapy for venous thromboembolism: findings from the RIETE registryJ Thromb Haemost2010861216122220345727
- NijkeuterMSöhneMTickLWChristopher Study InvestigatorsThe natural course of hemodynamically stable pulmonary embolism: clinical outcome and risk factors in a large prospective cohort studyChest2007131251752317296656

