Abstract
Irritable bowel syndrome (IBS) is a complex functional gastrointestinal disorder that is exceedingly common in clinical practice. IBS with predominant constipation (IBS-C) is a subtype of IBS that accounts for more than a third of the IBS diagnosed. Diagnosis of IBS requires a careful personalized approach, a comprehensive clinical history, limited but relevant investigations, and continued follow-up. Major IBS societies and guidelines recommend offering a positive diagnosis of IBS based on presenting symptomatology. Abdominal pain that may or may not be relieved by defecation is the cardinal symptom of IBS; distension and bloating are other common symptoms. Careful attention should be paid to alarm symptoms before a diagnosis of IBS is made. Pharmacotherapy with linaclotide is recommended for moderate–severe IBS-C, based on high-quality evidence from randomized controlled trials. Diarrhea is the major side effect of linaclotide, and limited cost-effectiveness data currently exist.
Introduction
What is irritable bowel syndrome?
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder that is commonly seen in clinical practice. Specifically, it is a functional bowel disorder that is thought to result from a disorder of gut–brain interaction.Citation1 Though patients with IBS often have a heterogeneous symptom profile, the predominant theme is the presence of abdominal pain or discomfort that is usually relieved by defecation. Over the last few decades, numerous clinical practice guidelines, consensus statements, and position papers have attempted to define and clarify the diagnostic criteria for IBS.Citation2–Citation4 This review aims to summarize the epidemiology, diagnosis, and treatment of IBS in primary care, with particular emphasis on the constipation-predominant subtype of IBS and its pharmacotherapy using a first-in-class guanylate cyclase inhibitor, linaclotide.
The best-known and most widely accepted criteria for diagnosing IBS are the Rome criteria, which have undergone substantive revisions since their introduction,Citation4,Citation5 with the most recent update being Rome IV, introduced in 2016.Citation6 Rome IV classifies IBS as a functional bowel disorder that is associated with recurrent abdominal pain for at least 1 day/week in the 3 months preceding diagnosis with a concomitant association with two or more of the following: pain related to defecation, pain associated with change in frequency of stool, and pain associated with a change in form (appearance) of stool. These changes need to be present for at least 3 months, with symptom onset at least 6 months prior to the diagnosis of IBS.Citation7 Based on predominant bowel habit, Rome IV classifies IBS into four main subtypes: IBS with predominant constipation (IBS-C), IBS with predominant diarrhea (IBS-D), IBS with mixed bowel habits (IBS-M), and unclassified IBS (IBS-U). Predominant bowel habit is based on quantification of stool consistency (appearance), typically determined using the Bristol Stool Form Scale (BSFS)Citation8 on days with at least one abnormal bowel movement.Citation7
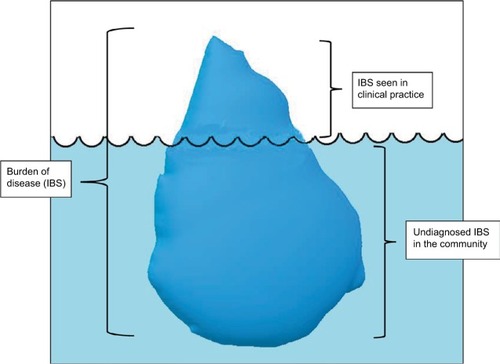
Irritable bowel syndrome: an iceberg disease?
Globally, IBS is one of the commonest gastrointestinal diseases diagnosed, though prevalence estimates vary widely in different parts of the world. According to a meta-analysis of 80 separate study populations comprising 260,960 subjects, the global prevalence of IBS is estimated to be 11.2% (95% CI 9.8%–12.8%) with different regions of the world differing in their prevalence rates (2.6%–32%).Citation9 When IBS is broken down into subtypes, the major subtype is IBS-C, comprising more than a third of all IBS (35%).Citation9 In a large meta-analysis of 56 studies with 188,229 eligible subjects, the prevalence of IBS in women was 67% higher when compared to men (95% CI 1.53–1.82).Citation10 This increased prevalence in women was mostly limited to countries in the Western hemisphere. Women with IBS were also more likely to exhibit constipation predominant IBS (OR 2.38, 95% CI 1.45–3.92), as opposed to the diarrhea-predominant subtype.Citation10
IBS is predominantly seen in ages <50 years, with a peak prevalence between ages 20 and 30 years. It has been estimated that only about 25% of patients with IBS consult a physician.Citation11 Between 10% and 70% of individuals with IBS symptoms consult a primary-care physician, but these estimates vary widely among countries.Citation12 In the US, it is reported that around 30% of people with IBS symptoms consult a primary-care physician.Citation12 As a substantial number of people with IBS symptoms do not seek clinical care, the exact prevalence of IBS is unclear, and it is assumed that the proportion of people with IBS symptoms seen in clinical practice only represents the tip of the iceberg ().
Burden of irritable bowel syndrome
The burden of IBS both on the patient and the health-care system is significant. IBS is known to be associated with severe morbidity and disability-adjusted life-years lost. Patients with mild–moderate IBS miss approximately 73 days per year from work.Citation11 Another study found that having a diagnosis of IBS-C is associated with 4.9 disrupted-productivity days per month on average.Citation13 The individual financial burden of IBS is estimated to be US$1,562–$7,547 per year.Citation14 The treatment of IBS in the US is estimated to cost between $1.7 billion and $10 billion in direct medical costs and $20 billion in indirect costs annually.Citation15 Annual health-care costs have been found to be significantly higher for IBS-C patients when compared to controls ($8,621 vs $4,765).Citation16
Diagnosis of irritable bowel syndrome in primary care
Primary care differs from secondary and tertiary care because of the familiarity of the primary-care physician with his patient, and offers the advantage of an established patient–physician rapport, which enables the viewing of IBS in context rather than in isolation. This is particularly important in the management of IBS, given its symptom chronicity,Citation12 thereby necessitating continuity of care.
IBS presents an interesting conundrum for the primary care physician, given its diagnostic ambiguity and overlap with other functional gastrointestinal disorders.Citation12 In primary care, the diagnosis of IBS is generally based on an empirical approach, and is often a diagnosis of exclusion.Citation17,Citation18 This approach, while arguably pragmatic and thorough, can be expensive, time-consuming, and cost-prohibitive in resource-limited settings. Diagnostic criteria like the Rome criteria and National Institute for Health and Care Excellence (NICE) guidelines recommend offering patients a positive diagnosis of IBS based on the symptom profile. At the same time, these formal diagnostic criteria have been criticized as being too narrow and ill suited for the diagnosis of IBS in primary care.Citation19 It has been shown that knowledge of formal diagnostic criteria among primary-care physicians is often lacking.Citation17 Nevertheless, studies have shown that formal diagnostic criteria can serve as valuable tools for diagnosing IBS in primary care. For instance, in one study, applying Rome III criteria led to successful identification of 75% of patients who already had a diagnosis of IBS in primary care, thus endorsing its applicability in primary-care settings.Citation20 The latest revision of Rome also incorporates biopsychosocial aspects, multicultural aspects, gender-based provisions, and the role of the brain–gut axis, and is likely more applicable to primary care, though validation studies are awaited.Citation1
Both the Rome foundation and NICE guidelines recommend offering patients a positive diagnosis of IBS based on four guiding principles: comprehensive and effective clinical history, physical examination, limited but pertinent laboratory investigations, and when clinically indicated, other relevant investigations and procedures, such as colonoscopies to rule out organic causes of abdominal pain.
Clinical history
A detailed clinical history forms the cornerstone of IBS diagnosis. The history taking should focus on the following key aspects:Citation7
Abdominal pain – this is required for the diagnosis of IBS; the absence of abdominal pain effectively rules out the diagnosis of IBS. Pain is usually vague and unlocalized, but is sometimes localized to the lower abdomen and is relieved on passing stools. However, this is not universally true, as abdominal pain can sometimes worsen after defecation.Citation7
Disordered defecation – a history of disordered bowel habit relating to abdominal pain is almost always identifiable in IBS patients. The BSFS should be used to record stool consistency. An increasing number of consecutive days without a bowel movement suggests a diagnosis of IBS-C.Citation7 Specifically, IBS-C signifies more than 25% of bowel movements with BSFS types 1 and 2 and less than 25% of bowel movements with BSFS types 6 and 7.Citation7 An alternate way of diagnosing IBS-C is when the patient reports a predominance of constipation in bowel movements, ie, resembles stool types 1 and 2 in the BSFS.Citation7 It is recommended that patients with IBS symptoms fill out a 2- to 3-week bowel diary based on the BSFS prior to their clinical visit to aid the primary-care physician to arrive at a positive diagnosis of IBS. In order to classify a patient as having IBS-C, the patient should not be on any medications that are used for treating bowel-habit abnormalities.Citation7
Bloating – this may be present in a large percentage of IBS patients, but it is neither specific nor required for the diagnosis of IBS.
Abdominal distension – this is also present in a majority of IBS patients, but is not required for the diagnosis of IBS.
Psychosocial history
Primary-care clinicians must recognize the role of psychological aspects and improve the assessment of psychological comorbidities in IBS. It is recommended that clinicians approach psychosocial assessment from a screening perspective with the objective of identifying patients who are at risk of refractory IBS, those who might respond poorly to treatment, and those with lower quality of life.Citation21 Eliciting a psychosocial history requires subtlety, particularly when inquiring about sensitive topics, such as history of abuse, depression, suicidal ideation, and the nature of intimate relationships.Citation21
Patients should be asked about their perceived availability of social-support systems. It has been shown that patients who experience stressful life events are more likely to have IBS-symptom exacerbations and tend to seek health care more frequently.Citation21 Primary-care practitioners should also be aware of the independent associations of anxiety and depression with IBS. Anxiety has been shown to be present in as many as 30%–50% of IBS patients.Citation21 Patients with IBS also frequently tend to have extraintestinal symptoms, such as headaches, fibromyalgia, and chronic pelvic pain: this is likely a somatization of their IBS symptoms.Citation21 lists the common comorbidities associated with IBS.
Table 1 Frequent comorbidities in IBS seen in primary care
Questionnaires may be used to enhance the clinical examination. It is necessary to use questionnaires that are valid, reliable, and relatively free of potential biases. Instruments like the Trauma History Questionnaire, Perceived Stress Questionnaire, Hospital Anxiety and Depression Scale, depression and anxiety-disorder modules of the Patient Health Questionnaire 9 and Generalized Anxiety Disorder 7 (GAD-7), Short Form (SF)-36, or the shorter SF-12 and SF-8 are some examples of helpful questionnaires that can assist in obtaining a psychosocial history.Citation21
Dietary history
A detailed dietary history could be paramount in aiding the diagnosis of IBS. For instance, certain foods can worsen IBS: diets high in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) can worsen symptoms of IBS, such as bloating and excessive flatus.Citation22 Patients should be encouraged to keep a detailed food diary that can be used to assess the relationship between their IBS symptoms and food intake during the clinical encounter.
Menstrual, gynecologic, and sexual history
The clinical history is incomplete without a proper gynecologic, sexual, and menstrual history. Gender differences in IBS, while sometimes nuanced, must be recognized and investigated. Abdominal pain might be related to the menstrual cycle causing cyclical changes in pain thresholds.Citation23 Women appear to have more frequent and severe IBS symptoms during menstruation, and worsening of abdominal pain is likely related to changes in the menstrual cycle.Citation24 Similarly, menopause seems to have an association with exacerbation of IBS complaints.Citation25 Women with dysmenorrhea are also twice as likely to have increased IBS symptoms when compared to those without dysmenorrhea.Citation25 Gynecologic problems in women with IBS are more widely reported; hysterectomy rates have been reported to be three times higher in women with IBS.Citation24 Therefore, in females with IBS symptoms, particular emphasis should be paid to menstrual irregularities, menopausal status, use of contraceptives and hormone-replacement therapy, and history of gynecologic surgery.
Diagnostic testing for IBS-C in primary care
A positive diagnosis of IBS-C should be made after limited relevant investigations. When warranted, diagnostic tests should be based on the age of the patient, duration and severity of symptoms, psychosocial factors, presence of alarm symptoms, and family history of colon cancer. A complete blood count should be the first investigation ordered by the primary-care physician. Erythrocyte-sedimentation rate (ESR), C-reactive protein (CRP), or fecal calprotectin might be necessary to rule out inflammatory bowel disease (IBD), although these patients have predominant diarrhea rather than constipation symptoms. Similarly, there is a high prevalence of gluten sensitivity in diarrhea-predominant IBS, and thus testing for tissue transglutaminase antibodies (tTG-IgA) may be necessary, though the evidence to recommend routine antibody testing is not clear.Citation26 Thyroid-function tests could be obtained if thyroid abnormalities are suspected.Citation7,Citation27 Testing for hypo/hypercalcemia might be necessary if thyroid and/or parathyroid abnormalities are suspected. A recent study has shown that the prevalence of vitamin D deficiency is significant in IBS sufferers, though evidence to support routine screening or treatment of vitamin D deficiency is currently lacking.Citation28
Such tests as ultrasound, rigid/flexible sigmoidoscopy and colonoscopy, barium enema, thyroid function, stool ova and parasites, fecal occult blood, and hydrogen breath (for lactose intolerance and bacterial overgrowth) are not necessary for patients who conform to the IBS criteria.Citation27 Screening colonoscopy is indicated for those patients aged >50 years without warning signs or when a patient presents with alarm symptoms.Citation7
Physical examination
The physical examination in patients with IBS is almost always unremarkable. Nevertheless, while of limited use, a physical examination can be helpful in ruling out organic causes of IBS and systemic disease while simultaneously providing reassurance to the patient. Particular attention should be paid to examination of the abdomen: one has to be on the lookout for abdominal masses and organomegaly. An anorectal examination is a quintessential component of the abdominal examination, and helps not only to identify anorectal causes of bleeding and dyssynergic defecation but also to assess anorectal tone and squeeze pressure.Citation29 Anorectal manometry, however, is a more suitable test for patients with diarrhea-predominant IBS rather than constipation-predominant IBS.Citation30 In females, a bimanual pelvic examination may be warranted.
Primary-care physicians must be diligent to recognize alarm symptoms, such as unintentional or unexplained weight loss, anemia, occult blood in stool, abdominal or rectal masses, new IBS-symptom onset after age 50 years, family history of colon or ovarian cancer, positive markers for IBD, celiac disease, arthritis or skin findings on physical examination, signs and symptoms of malabsorption, and signs and symptoms of thyroid dysfunction.Citation27,Citation31 When such “red flags” are encountered, patients must be referred to secondary care for further investigation and management.
Treatment of IBS-C in primary care
Treatment of IBS-C should focus on expressing empathy, providing reassurance, suggesting dietary and lifestyle modifications, brief counseling, and limited but evidence based pharmacologic therapy.Citation21 Therapy for mild disease should be focused on education, reassurance, and dietary modifications.Citation32 Moderate and severe IBS needs pharmacotherapy and behavioral interventions, such as cognitive behavior therapy and mindfulness-based therapy.Citation32,Citation33
A multidisciplinary approach to treating IBS is increasingly becoming the norm. Such an integrated approach involves a team comprised of the primary-care physician, a psychologist or psychiatrist, a dietician, and a nurse practitioner.Citation34 The roles of the primary-care physician and psychologist are particularly important, given the persistence of symptoms in IBS and numerous psychological comorbidities associated with this illness.
Dietary and lifestyle advice
The importance of self-help in managing their condition should be explained to people with IBS-C. Patient education should be individualized to their specific needs.Citation21 They should be encouraged to learn relaxation techniques and counseled on increasing physical activity levels if deemed inadequate. The adoption of healthy eating habits like having regular meals and avoiding long gaps between meals should be emphasized.Citation27 Patients should also be encouraged to drink plenty of water. Fiber intake should be reviewed at each visit, and when increase in dietary fiber is recommended, soluble fiber such as psyllium powder or foods high in soluble fiber such as oats are preferred.Citation27 Limiting dietary FODMAPs may help with some improvement in IBS-C symptoms, particularly bloating.Citation35 Gluten reduction might be helpful, but excluding gluten from the diet when already on a low-FODMAP diet may not offer additional benefit.Citation7
A variety of other treatments, including pharmacological and behavioral interventions, are available for IBS-C patients, with varying levels of evidence quality. A list of prominent therapies for IBS-C is provided in . The following section focuses on linaclotide, a novel drug with pro-secretory and anti-nociceptive properties that has been shown to be highly effective for reducing symptoms of IBS-C.
Table 2 Commonly used therapies for IBS-C
Pharmacotherapy for IBS-C: role of linaclotide
It has been shown that more than 80% of IBS patients receive some form of treatment during their initial consultation, with the vast majority of IBS patients (74%) prescribed some form of medication for their GI symptoms.Citation36 Patients who have IBS report using at least three medications, with only a third of them noting any significant improvement with these medications or satisfaction with their current therapy.Citation37 Clearly, this indicates a role for an effective medication that can be easily prescribed in primary care, the use of which is informed by high-quality evidence and is relatively free of major side effects. Linaclotide is a first-in-class synthetic guanylate cyclase C (GCC) agonist that has been shown to improve IBS-C symptoms and has been given a strong recommendation by the American Gastroenterological Association for the treatment of IBS-C.Citation38
Linaclotide (Linzess; Allergan) is an intestinal GCC-receptor activator that causes an increase in both intracellular and extracellular cyclic guanosine monophosphate (cGMP) levels by mimicking endogenous intestinal peptides, guanylin, and uroguanylin.Citation39,Citation40 Activation of GCC results in stimulation of chloride and bicarbonate secretion through activation of CFTR, and it in turn results in inhibition of Na+ absorption through blockade of an apical Na+–H+ exchanger.Citation40 It causes accelerated colonic transit in IBS-C in a dose-dependent fashion.Citation41 Linaclotide’s pain-modulatory effects are likely related to its increased expression of extracellular cGMP, which inhibits visceral nociception.Citation42 It is both acid-stable and pepsin-stable and is minimally absorbed. In human colonic mucosa, linaclotide binds GCC receptors with high affinity and is pH-agnostic.Citation39
Three randomized controlled trials inform us about the evidence for the use of linaclotide for IBS-C.Citation43–Citation45 All three trials used Rome III diagnostic criteria to recruit patients with a female predominance (>90% in each of the three trials). Linaclotide also reduced symptoms of constipation and abdominal pain in IBS-C patients in Phase IIB and Phase III trials. It has secured approval for the treatment of IBS-C (290 μg once daily) by various global drug-regulatory bodies, such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). In comparison to placebo, more patients on linaclotide achieved important end points, such as adequate relief response and global relief response.Citation46 In addition, in these randomized trials, linaclotide was significantly better than placebo in meeting more conservative outcome end points instituted by the FDA and the EMA for evaluation of IBS-C drugs.Citation46 The FDA end point has been shown to be reasonably sensitive (60.7%) and highly specific (93.5%) for detecting a clinically meaningful improvement in IBS-C symptoms.Citation47
Diarrhea was the most commonly reported side effect of linaclotide in pooled IBS-C placebo-controlled trials. In these trials, 20% of linaclotide-treated patients reported diarrhea compared to 3% of placebo-treated patients.Citation48 Additionally, severe adverse events leading to treatment discontinuation were significantly higher in the linaclotide group in comparison with those on placebo (RR 14.75, 95% CI 4–53.8). In the open-label, long-term trials, 2,147 patients with IBS-C received 290 μg of linaclotide daily for up to 18 months. In these trials, 29% of patients had their dose reduced or suspended due to adverse reactions, the major one being diarrhea.Citation48
An expert consensus report on optimizing the use of linaclotide for IBS-C has provided the following recommendations about the drug: linaclotide is indicated for the treatment of moderate–severe IBS-C in adults; it is recommended that patients take linaclotide continuously and not sporadically; patients should be warned about the risk of diarrhea, and provided with choices concerning how to deal with this possible side effect; and the absence of tachyphylaxis or potential risks implies that linaclotide treatment can be continued for extended periods.Citation49
There exist some limitations that might preclude the use of linaclotide in primary-care settings. First, diarrhea can be a serious side effect and might be troublesome to some patients, leading to treatment discontinuation. Second, though rare, severe dehydration resulting from diarrhea cannot always be managed in primary-care facilities, and might need referral to more expensive secondary or tertiary clinics. Third, only limited cost-effectiveness data are available on linaclotide.Citation50,Citation51 Lastly, linaclotide should be avoided in pediatric patients (aged 6–18 years), as its safety has not been established in clinical trials for this population.Citation48
Conclusion
Diagnosis of IBS requires a careful personalized approach, limited but relevant investigations, and continuity of care. While many primary-care physicians correctly arrive at a preliminary diagnosis of IBS, they still fall back on expensive and superfluous investigations to confirm the diagnosis of IBS.Citation17 Nevertheless, the role of the primary-care physician in reducing the burden of IBS in the community cannot be overstated.Citation17
Primary care is often resource-limited, and hence this calls for providing a positive diagnosis of IBS based on formal diagnostic criteria with emphasis on a sound clinical history aided by limited, but relevant investigations. Legitimizing the complaints of IBS sufferers, providing clear explanations of the disorder, showing empathy, and building strong rapport with the patient can help in effective management of this condition.Citation11 As primary-care physicians are expected to see a variety of patients from different cultural backgrounds, recognizing these cross-cultural nuances in symptom manifestation and management is crucial.Citation52
In the ever-expanding repertoire of IBS drugs available to the primary-care physician, linaclotide assumes a powerful role. Clinical trials have provided ample evidence regarding its effectiveness in improving major IBS-C symptoms, including the patient’s quality of life. However, despite its apparent benefits, it is of note that linaclotide can produce diarrhea as a side effect, which can be bothersome to many patients, leading to premature discontinuation of treatment. As treatment compliance or the lack thereof is an important aspect of primary care, linaclotide may not be suitable for every patient with IBS-C. Cost considerations should also be factored in before prescribing linaclotide in primary care. The American Gastroenterological Association provides a strong recommendation for the use of linaclotide for IBS-C, while noting that it might become necessary for patients who place a high value on avoiding diarrhea and minimizing out-of-pocket expenses to opt for alternate treatments.Citation38
While the prevalence of IBS decreases with age in both men and women, the prevalence of constipation increases with age. However, there exists few to no data on the effectiveness of IBS-C drugs like linaclotide in the elderly. Older patients might have a different safety profile than younger populations. Trials of IBS-C drugs have often oversampled younger white women with symptoms, and hence generalizability to patient populations beyond the archetypal IBS female patient becomes difficult. Such patients are also generally recruited from tertiary clinics, where women are more likely to be referred than men, resulting in potential selection bias. Future trials with adequate sample sizes of each gender and comprising more heterogeneous age-groups would inform us better of the effectiveness of newer IBS-C drugs.
Disclosure
The author reports no conflicts of interest in this work.
References
- DrossmanDAFunctional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IVGastroenterology2016150612621279.e2
- ManningAPThompsonWGHeatonKWMorrisAFTowards positive diagnosis of the irritable bowelBr Med J197826138653654698649
- RubinGDe WitNMeineche-SchmidtVSeifertBHallNHunginPThe diagnosis of IBS in primary care: consensus development using nominal group techniqueFam Pract200623668769217062586
- ThompsonWGThe road to RomeGastroenterology200613051552155616678568
- LongstrethGFThompsonWGCheyWDHoughtonLAMearinFSpillerRCFunctional bowel disordersGastroenterology200613051480149116678561
- DrossmanDAHaslerWLRome IV – functional GI disorders: disorders of gut-brain interactionGastroenterology201615061257126127147121
- LacyBEMearinFChangLBowel disordersGastroenterology2016150613931407.e5
- LewisSJHeatonKWStool form scale as a useful guide to intestinal transit timeScand J Gastroenterol19973299209249299672
- LovellRMFordACGlobal prevalence of and risk factors for irritable bowel syndrome: a meta-analysisClin Gastroenterol Hepatol2012107712721.e422426087
- LovellRMFordACEffect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysisAm J Gastroenterol20121077991100022613905
- HoughtonLAHeitkemperMCrowellMAge, gender and women’s health and the patientGastroenterology Epub2016215
- CanavanCWestJCardTThe epidemiology of irritable bowel syndromeClin Epidemiol20146718024523597
- HeidelbaughJJStelwagonMMillerSASheaEPCheyWDThe spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burdenAm J Gastroenterol2015110458058725781368
- NellesenDYeeKChawlaALewisBECarsonRTA systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipationJ Manag Care Pharm201319975576424156644
- HuliszDThe burden of illness of irritable bowel syndrome: current challenges and hope for the futureJ Manag Care Pharm200410429930915298528
- DoshiJACaiQBuonoJLEconomic burden of irritable bowel syndrome with constipation: a retrospective analysis of health care costs in a commercially insured populationJ Manag Care Spec Pharm201420438239024684643
- HunginAPMolloy-BlandMClaesRSystematic review: the perceptions, diagnosis and management of irritable bowel syndrome in primary care: a Rome Foundation working team reportAliment Pharmacol Ther201440101133114525230281
- HarknessEFHarringtonVHinderSGP perspectives of irritable bowel syndrome: an accepted illness, but management deviates from guidelines: a qualitative studyBMC Fam Pract2013149223805998
- KolarGJLockeGRWhat is the best way to identify and quantify irritable bowel syndrome?EmmanuelAQuigleyEMIrritable Bowel Syndrome: Diagnosis and Clinical ManagementHoboken (NJ)Blackwell20135167
- EngsbroALBegtrupLMKjeldsenJPatients suspected of irritable bowel syndrome: cross-sectional study exploring the sensitivity of Rome III criteria in primary careAm J Gastroenterol2013108697298023419383
- Van OudenhoveLLevyRLCrowellMDBiopsychosocial aspects of functional gastrointestinal disordersGastroenterology2016150613551367.e2
- BarbaraGFeinle-BissetCGhoshalUCThe intestinal microenvironment and functional gastrointestinal disordersGastroenterology2016150613051318.e8
- VannerSGreenwood-Van MeerveldBMaweGFundamentals of neurogastroenterology: basic scienceGastroenterology2016150612801291
- AdeyemoMASpiegelBMChangLMeta-analysis: do irritable bowel syndrome symptoms vary between men and women?Aliment Pharmacol Ther201032673875520662786
- OlafsdottirLBGudjonssonHJonsdottirHHBjörnssonEThjodleifssonBNatural history of irritable bowel syndrome in women and dysmenorrhea: a 10-year follow-up studyGastroenterol Res Pract2012201253420422474441
- IrvineAJCheyWDFordACScreening for celiac disease in irritable bowel syndrome: an updated systematic review and meta-analysisAm J Gastroenterol20171121657627753436
- HookwayCBucknerSCroslandPLongsonDIrritable bowel syndrome in adults in primary care: summary of updated NICE guidanceBMJ2015350h70125716701
- TazzymanSRichardsNTruemanARVitamin D associates with improved quality of life in participants with irritable bowel syndrome: outcomes from a pilot trialBMJ Open Gastroenterol201521e000052
- QuigleyEMFriedMGweeKAWorld Gastroenterology Organisation global guidelines: irritable bowel syndrome – a global perspectiveJ Clin Gastroenterol201650970471327623513
- PriorAMaxtonDGWhorwellPJAnorectal manometry in irritable bowel syndrome: differences between diarrhoea and constipation predominant subjectsGut19903144584622338274
- VannerSJDepewWTPatersonWGPredictive value of the Rome criteria for diagnosing the irritable bowel syndromeAm J Gastroenterol199994102912291710520844
- DrossmanDAThompsonWGThe irritable bowel syndrome: review and a graduated multicomponent treatment approachAnn Intern Med199211612 Pt 1100910161586090
- BallouSKeeferLPsychological Interventions for irritable bowel syndrome and inflammatory bowel diseasesClin Transl Gastroenterol201781e21428102860
- ChangFYIrritable bowel syndrome: the evolution of multi-dimensional looking and multidisciplinary treatmentsWorld J Gastroenterol201420102499251424627587
- LonshteynMChandarAFalck-YtterYLarge effects of a low FODMAPs diet in patients with irritable bowel syndrome: a systematic review and meta-analysisGastroenterology20141465 Suppl 1S535S536
- FaresjoAGrodzinskyEFoldeviMJohanssonSWallanderMAPatients with irritable bowel syndrome in primary care appear not to be heavy healthcare utilizersAliment Pharmacol Ther200623680781416556183
- DrossmanDAChangLKellowJCheyWDTackJWhiteheadWERome IV Functional Gastrointestinal Disorders for Primary Care and non-GI CliniciansRaleigh (NC)Rome Foundation2016
- WeinbergDSSmalleyWHeidelbaughJJSultanSAmerican Gastroenterological Association institute guideline on the pharmacological management of irritable bowel syndromeGastroenterology201414751146114825224526
- LacyBELevenickJMCrowellMDLinaclotide: a novel therapy for chronic constipation and constipation-predominant irritable bowel syndromeGastroenterol Hepatol (N Y)201281065366024683372
- BryantAPBusbyRWBartoliniWPLinaclotide is a potent and selective guanylate cyclase C agonist that elicits pharmacological effects locally in the gastrointestinal tractLife Sci20108619–2076076520307554
- CamilleriMBuenoLAndresenVDe PontiFChoiMGLemboAPharmacological, pharmacokinetic, and pharmacogenomic aspects of functional gastrointestinal disordersGastroenterology2016150613191331.e20
- CastroJHarringtonAMHughesPALinaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3’,5’-monophosphateGastroenterology2013145613341346e11123958540
- JohnstonJMKurtzCBMacdougallJELinaclotide improves abdominal pain and bowel habits in a phase IIB study of patients with irritable bowel syndrome with constipationGastroenterology2010139618771886.e220801122
- CheyWDLemboAJLavinsBJLinaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safetyAm J Gastroenterol2012107111702171222986437
- RaoSLemboAJShiffSJA 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipationAm J Gastroenterol2012107111714172522986440
- AtluriDKChandarAKBharuchaAEFalck-YtterYEffect of linaclotide in irritable bowel syndrome with constipation (IBS-C): a systematic review and meta-analysisNeurogastroenterol Motil201426449950924351035
- MacdougallJEJohnstonJMLavinsBJAn evaluation of the FDA responder endpoint for IBS-C clinical trials: analysis of data from linaclotide phase 3 clinical trialsNeurogastroenterol Motil201325648148623384406
- US Food and Drug AdministrationLinaclotide [prescribing information]2012 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202811s000lbl.pdfAccessed September 16, 2017
- ReyEMearinFAlcedoJOptimizing the use of linaclotide in patients with constipation-predominant irritable bowel syndrome: an expert consensus reportAdv Ther201734358759828083815
- FisherMWalkerAFalquesMCost-effectiveness of linaclotide compared to antidepressants in the treatment of irritable bowel syndrome with constipation in ScotlandEur J Health Econ20161791091110026728984
- HuangHTaylorDCCarsonRTEconomic evaluation of linaclotide for the treatment of adult patients with irritable bowel syndrome with constipation in the United StatesJ Med Econ201518428329425333331
- FrancisconiCFSperberADFangXMulticultural aspects in functional gastrointestinal disorders (FGIDs)Gastroenterology2016150613441354.e2
- LadabaumUBoydEZhaoWKDiagnosis, comorbidities, and management of irritable bowel syndrome in patients in a large health maintenance organizationClin Gastroenterol Hepatol2012101374521871250
- WhiteheadWEPalssonOSLevyRRFeldADTurnerMVon KorffMComorbidity in irritable bowel syndromeAm J Gastroenterol2007102122767277617900326
- WhiteheadWEPalssonOJonesKRSystematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications?Gastroenterology200212241140115611910364
- KabraNNadkarniAPrevalence of depression and anxiety in irritable bowel syndrome: a clinic based study from IndiaIndian J Psychiatry2013551778023439939
- Fadgyas-StanculeteMBugaAMPopa-WagnerADumitrascuDLThe relationship between irritable bowel syndrome and psychiatric disorders: from molecular changes to clinical manifestationsJ Mol Psychiatry201421425408914
- ChapmanRWStanghelliniVGeraintMHalphenMRandomized clinical trial: macrogol/PEG 3350 plus electrolytes for treatment of patients with constipation associated with irritable bowel syndromeAm J Gastroenterol201310891508151523835436
- CheyWDKurlanderJEswaranSIrritable bowel syndrome: a clinical reviewJAMA2015313994995825734736