Abstract
CeO2 nanoparticles (NPs) have shown promising approaches as therapeutic agents in biology and medical sciences. The physicochemical properties of CeO2-NPs, such as size, agglomeration status in liquid, and surface charge, play important roles in the ultimate interactions of the NP with target cells. Recently, CeO2-NPs have been synthesized through several bio-directed methods applying natural and organic matrices as stabilizing agents in order to prepare biocompatible CeO2-NPs, thereby solving the challenges regarding safety, and providing the appropriate situation for their effective use in biomedicine. This review discusses the different green strategies for CeO2-NPs synthesis, their advantages and challenges that are to be overcome. In addition, this review focuses on recent progress in the potential application of CeO2-NPs in biological and medical fields. Exploiting biocompatible CeO2-NPs may improve outcomes profoundly with the promise of effective neurodegenerative therapy and multiple applications in nanobiotechnology.
Introduction
CeO2 nanoparticles (NPs) have received much attention in nanotechnology due to their useful applications as catalysts, fuel cells and antioxidants in biological systems.Citation1–Citation5 In general, cerium can exist in two oxidation states: Ce3+ and Ce4+. Therefore, cerium dioxide can have two different oxide forms, CeO2 (Ce4+) or Ce2O3 (Ce3+), in bulk material.Citation4,Citation6 On the nanoscale, the cerium oxide lattice has a cubic fluorite structure, and both Ce3+ and Ce4+ can coexist on its surface. Charge deficiency due to the presence of Ce3+ is compensated by oxygen vacancy in the lattice; thus, CeO2-NPs contain intrinsic oxygen defects.Citation7 These oxygen defects are actually sites of catalytic reactions. The concentration of oxygen defects increases with reduction in particle size.Citation8 Therefore, CeO2-NPs have improved redox properties with respect to the bulk materials. Moreover, the presence of a mixed valance state plays an important role in scavenging reactive oxygen and nitrogen species. CeO2-NPs are found to be effective against pathologies associated with chronic oxidative stress and inflammation. Recently, CeO2-NPs have also been reported to have multienzyme, including superoxide oxidase, catalase and oxidase, and mimetic properties, and have emerged as a fascinating material in biological fields, such as in bioanalysis,Citation9–Citation14 biomedicineCitation15 and drug delivery.Citation16,Citation17 These applications are derived from quick transition of the oxidation state between Ce3+ and Ce4+.Citation6 The surface Ce3+:Ce4+ ratio is influenced by the microenvironment. Therefore, the microenvironment and synthesis method adopted also plays an important role in determining the biological activity and toxicity of CeO2-NPs. The CeO2-NPs have been prepared through the means of several routes and synthesis methods including solution precipitation,Citation18 sonochemical,Citation19 hydrothermal,Citation20 solvothermal,Citation21 ball milling,Citation22 thermal decomposition,Citation23 spray pyrolysis,Citation24 thermal hydrolysisCitation25 and sol–gel methods.Citation26–Citation28 However, applying the mentioned methods deals with several drawbacks, such as toxic solvents and reagents usage, high temperature and pressure, and the requirement of external additives as stabilizing or capping agents during the reaction. As the physiochemical properties of NPs mostly depend on the synthesis procedure, the synthesis method of NPs for biological applications is very important. The physical properties (size, surface charge, agglomeration status in liquid and coating or residual contamination of the surfactant on the surface) of NPs mainly influence interactions at the nano–bio interface.Citation29 Moreover, the surface Ce3+:Ce4+ ratio (chemical property) also influences the biocatalysis and the biological interactions. Manipulation of the surface Ce3+:Ce4+ ratio can be achieved by controlling their synthesis method.Citation30 However, coating the NPs with biocompatible/organic polymers increases dispersion/stability, decreases nonspecific interactions with cells and proteins, increases blood circulation time and reduces the toxicity of the NPs.Citation31
Biomaterials possess functional groups such as –COOH, –OH and –NH2, and have the potential to stabilize and/or cap metal ions for preparation of various NPs via green chemistry methods. Recently, CeO2-NPs have been synthesized through several bio-directed methods applying natural and organic matrices as stabilizing agents in order to prepare biocompatible CeO2-NPs and solve the challenges to safely and effectively use this metal oxide for biomedicinal purposes.Citation27,Citation28,Citation32 In the first part of the review, we discuss the literature on different green synthesis methods of CeO2-NPs (). Next, we discuss the effect of these CeO2-NPs on reducing their cytotoxicity in the biological environment. Finally, a brief review on the updates of the potential biological application of CeO2-NPs is presented.
Table 1 Green synthesis methods of CeO2-NPs
Green approaches for CeO2-NP synthesis
Plant-mediated synthesis of CeO2-NPs
Phytosynthesis of metal and metal oxide NPs is a new emerging issue in nanoscience and technology.Citation33 Recently, phytosynthesis of CeO2-NPs was reported using different plants, such as Gloriosa superba, Acalypha indica and even Aloe vera plant leaf extract ().Citation33–Citation35 The plant extracts acted as stabilizing and capping agents in the CeO2-NPs synthesis process. Investigating biological effects of the phytosynthesized NPs, antibacterial activity of them was examined. The results showed that smaller crystal sizes with a higher surface area led to higher antibacterial activity. These reports applied bio-directed methods of CeO2-NP synthesis. However, the synthesized nanoparticles were generally so large in size that, according to literature, they were not appropriate for biomedical applications.Citation1,Citation36 Recently, biosynthesis of NPs using yeast and fungi has also been noted. Munusamy et al had explained rapid and extracellular synthesis of cerium oxide NPs using fungus Curvularia lunata culture media.Citation37 The synthesized NPs had a cubic structure and exhibited antibacterial effects against different kinds of bacteria.Citation37 It is known that CeO2-NPs cannot enter bacterial and algal cells. Noninternalized CeO2-NPs seem to show toxic effects by direct attachment of CeO2-NPs to cell walls of algae and bacteria.Citation38–Citation41 Several mechanisms have been suggested to demonstrate how CeO2-NPs in contact with the membrane may exert cytotoxicity. CeO2-NPs could interfere with the nutrient transport functions of the membrane,Citation39 cause mechanical damage and membrane disruptionCitation42,Citation43 or generate reactive oxygen species (ROS) and induce oxidative stress.Citation38–Citation40 The generation of ROS, most probably hydrogen peroxide, by CeO2-NPs is in agreement with observations noted by Xia et alCitation44 and Zhao et al.Citation45 Hydrogen peroxide is capable of freely diffusing across cell walls and membranes, inducing cell damage.
Figure 1 Schematic representation of Gloriosa superba-based method of cerium oxide nanoparticle synthesis.
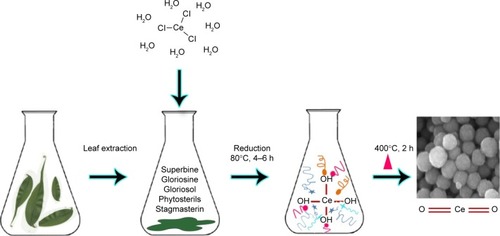
Consequently, myco-synthesis of CeO2-NPs showed advantages including manageability, cost-effectiveness, and used techniques that were less time-consuming and required less energy,Citation46 and therefore can be used as an economic and valuable alternative for the large-scale production of CeO2-NPs. Moreover, myco-synthesized CeO2-NPs had more stability, water dispersibility and high fluorescent properties. The fungal extracellular compounds, such as proteins (especially enzymes), and heterocyclic derivatives could act as reducing and capping agents. Other methods of plant-based CeO2-NPs synthesis were also easy, rapid and cost-effective, but the size of obtained NPs exhibited a wide distribution range, which demonstrates that the necessity of optimizing the biosynthesis methods mentioned earlier in order for application in biological systems.
Nutrient-mediated synthesis of CeO2-NPs
As mentioned, synthetic methods determine the size, charge, surface properties, solubility and morphology of NPs, therefore affecting response of CeO2-NPs in biological systems. That is why green synthesis of CeO2-NPs has received much attention recently. Several studies widely reported different nutrients and natural materials, such as egg white (EW) protein and honey for CeO2-NPs green synthesis.Citation47,Citation48 Kargar et alCitation47 proposed that the two major proteins of EW, ovalbumin and lysozyme, acted as a green binders/stabilizing agents for the preparation of CeO2-NPs. The general mechanism for synthesizing CeO2-NPs in EW media includes formation of the electrostatic interaction between cerium cations (Ce3+) and oppositely charged proteins which leads to controllable growth and subsequent isotropic formation of small and stable CeO2-NPs.Citation47,Citation49 Some of the green methods of CeO2-NP preparation mimic the common traditional approaches in NP synthesis in a safe and eco-friendly way.Citation48 For example, honey-based synthesis of CeO2-NPs mimics the sol–gel method. The extensive number of carbohydrates, enzymes and vitamins containing hydroxyl and amine groups in the honey matrix structure can facilitate the complexation of cerium cations (Ce3+) to an initial molecular matrix. Therefore, honey was capable of coating and stabilizing cerium species and CeO2-NPs while inhibiting their excessive aggregation or crystal growth.Citation48 However, advancement of the EW-based method for CeO2-NP green synthesis is obvious due to nontoxic effects of CeO2-NPs at concentrations up to 800 μg/mL, compared with the safe concentration of ~25 μg/mL for honey-based CeO2-NPs. Therefore, the synthesis of CeO2-NPs in EW was found to be an excellent alternative for the preparation of CeO2-NPs, using food and bio-derived materials.
Biopolymer-mediated synthesis of CeO2-NPs
Natural polymers in the form of macromolecules can also be used as templates for bio-directed synthesis of CeO2-NPs. As the surface of the NPs could be covered by hydroxyl groups, biopolymers that intrinsically possess hydroxyl moieties are capable of stabilizing CeO2-NPs. Applying the polymers as capping/stabilizing agents, the diameter of NPs can be logically controlled.Citation50 Kargar et al reported the green synthesis of small cerium oxide NPs, stabilized with agarose polymers via a sol–gel method.Citation51 While heating to >90°C, the agarose powder is normally dissolved in water, and when the temperature is reduced to 35°C–40°C, semisolid gel is formed that is stable over a wide pH range of (from 3 to 9). Interpenetrating H-binding between sugar moieties resulted in production of this sol–gel network and nanochannel containing pore sizes of 200 nm. CeO2-NPs were synthesized in these nanochannels. Similarly, Darroudi et al had synthesized CeO2-NPs using starch as a capping biopolymer.Citation27 The proposed mechanism, for starch-based synthesis of CeO2-NPs was that after dissolving starch in water, metal cations were attracted by oxygen of the OH branches. In vitro studies on Neuro2A cells demonstrated a dose-dependent toxicity with a nontoxic concentration of 175 μg/mL. Applying starch as a template for CeO2-NP synthesis by Darroudi et alCitation27 resulted in the formation of ultrafine CeO2-NP particles that were small in size and uniform in shape. Therefore, this method seems to be more appropriate for CeO2-NP synthesis for medical purposes. Furthermore, in line with the required characteristics, this method was found to be easy, economical and green for large-scale preparation of cerium oxide in nanoscale.
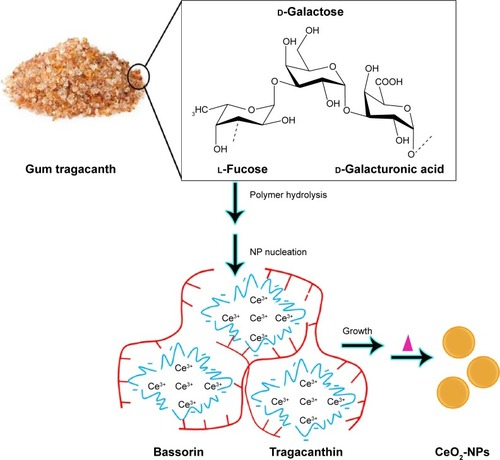
Regarding unique potential of biopolymers in the development of bio-directed methods of CeO2-NP synthesis, Darroudi et alCitation27 also used Gum tragacanth (GT) for the production of CeO2-NPs by both chemical and biological methods.Citation28 The soluble fraction (tragacanthin or tragacanthic acid) of GT gives a sol form in distilled water, whereas the insoluble fraction (bassorin) swells to a gel form ().Citation52,Citation53 While heating the sol–gel solution up to 40°C, the GT became soluble in water and the semicrystalline structures were lost. After adding the cerium nitrate to the solution, the metal cations were attracted by the oxygen of OH branches of GT polysaccharides. During the heating process, the amount of water was decreased and the nitrate decomposed to nitrogen dioxide and oxygen molecules, which were then removed from the compounds. Ce(OH)4 nuclei were converted into CeO2 nuclei via dehydration and, subsequently, highly crystallized CeO2-NPs particles grew. The required energy for the above reactions was provided by the subsequent sol–gel procedure and heat. The stabilizing effect of GT could be attributed to the steric repulsion force arising as the gum formed a layer around the cerium hydroxides and cerium oxide NPs. However, the ability of GT to stabilize CeO2-NPs might also be due to electrostatic interactions in addition to the enhancement of suspension viscosity.Citation54,Citation55 Although the formation of CeO2-NPs particles involved several complicated reactions,Citation56 controlling the nucleation of initial precipitate Ce(OH)3 would mainly determine the properties of the final CeO2-NPs. Furthermore, the CeO2-NPs exhibited very low cytotoxic effects on Neuro2A cell lines, making them suitable candidates for various biological applications. Dextran was also used for CeO2-NP stabilizing and coating, as it is a biocompatible, complex and highly water-soluble polysaccharide.Citation57 Accordingly, NPs as small as 5 nm were produced which were toxic to cancer cells at pH 6 and much less toxic to normal cells at the same pH value.Citation57 Moreover, the importance and versatility of polyethylene glycol (PEG) for the functionalization of rare earth cerium oxide NPs were also investigated.Citation58–Citation60 The suggested mechanism for PEG-mediated ceria synthesis was the presence of an electrostatic driving force for the complexation.Citation59 The branched structure of PEG is sufficient to solubilize the CeO2-NPs and create true dispersible nanopowders in aqueous solution and in certain organic solvents, providing a framework for designing a versatile hybrid metal oxide sol.Citation58 Furthermore, chitosan-based synthesis of CeO2-NPs was also reported due to specific properties, such as good film-forming ability, biocompatibility, nontoxicity, biodegradability and antibacterial activity ().Citation61,Citation62
Table 2 Advantages and challenges of different methods of CeO2-NPs green synthesis
The toxicologic effect of green synthesized CeO2-NPs
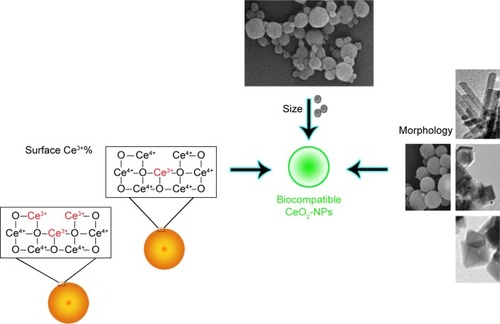
All cerium oxide NPs contain the same core elements, however, do not display similar biological effects. There are some studies that reported prooxidant toxicity of NPs in some cases and antioxidant protective effects in others that could be attributed to different physiochemical parameters of the various NPs that were used. Method of NP synthesis, type of stabilizing agent used, and the Ce3+/Ce4+ surface ratio have been demonstrated to play major roles in producing CeO2-NPs with different physicochemical properties.Citation63,Citation64 The most important parameters are discussed below ().
Particle size
Several green methods of CeO2-NPs synthesis have provided NPs as small as <10 nm. Previous results demonstrated that among different strategies reported for bio-directed synthesis of CeO2-NPs, biopolymer and nutrient-based methods provided the smallest NPs compared with plant-based processes. Reports indicated that plant-based CeO2-NP synthesis provided larger NP with antibacterial properties that exhibited high levels of cytotoxicity to bacterial cells.Citation35,Citation37 However, biopolymer- and nutrient-based methods have provided small NPs which show no cytotoxic effects to human cell lines at high concentrations of CeO2-NPs.Citation27,Citation28,Citation47,Citation48,Citation51
Morphology
Morphology is another physical property that is also required to be considered for biological applications. For example, NPs in polygonal, cube or rod shapes have sharp edges and could cause mechanical damage to cells.Citation7,Citation65,Citation66 Therefore, the effect of NP shape cannot be ignored for biological applications. As mentioned earlier, almost all the green methods of ceria synthesis that are mentioned herein have produced NPs with spherical morphology. However, starch-based synthesis of CeO2-NPs seems to be the most appropriate method to provide CeO2-NPs for biomedical purposes.Citation27
Percentage of surface Ce3+
In 2015, Pulido-Reyes et alCitation67 presented a report that differed from previous reports about CeO2-NPs synthesis. They demonstrated that neither concentration, surface charge nor size of CeO2-NPs plays any important role in their observed toxic properties. The report demonstrated that percentage of surface Ce3+ correlated with toxicity and was the main driver of CeO2-NPs toxic effects.Citation67 They proposed that CeO2-NPs with the highest percentage of surface Ce3+ (58%) exhibited the most toxic effect, and CeO2-NPs with lower percentage of surface Ce3+ values (between 26% and 36%) were evidently nontoxic for the model organism. In fact, CeO2-NPs with lower Ce3+ and, therefore, higher Ce4+ on their surface showed catalase mimetic activity,Citation68 which broke down H2O2 to molecular oxygen, protecting the cells against this toxic ROS. CeO2-NPs with higher Ce3+ on their surface could efficiently scavenge radicals of superoxide (superoxide dismutase [SOD] mimetic activity) and produce H2O2, which is toxic to the cells. They suggested that in a narrow range of surface Ce3+, there seemed to be a shift from SOD activity to catalase mimetic activity; however, the mechanisms and whether the observed biological effect reported at their study may also occur in other cellular systems, requires further investigation.Citation67 However, there is no report on the effect of applying green methods of CeO2-NPs synthesis on the percentage of surface Ce3+ of NPs and this should be investigated to clearly demonstrate the effect of green synthesis of CeO2-NPs on their cytotoxicity.
A CeO2-NP enters cells by energy-dependent, clathrin-mediated and caveolae-mediated endocytic pathways. Its localization in mitochondria, lysosomes and endoplasmic reticulum, as well as the cytoplasm and nucleus, were demonstrated by Singh et al.Citation69 Considering radical scavenging properties of cerium oxide and its widespread cellular disposition, a CeO2-NP likely acts as a cellular antioxidant in multiple compartments of the cell, presenting protection against a variety of oxidant injuries.Citation69
Biological applications of CeO2-NPs
Antibacterial effect
There are different studies that have reported antibacterial activity of CeO2-NPs and demonstrated their significant inhibition toward both gram-negative and gram-positive bacteria.Citation34–Citation37 It is suggested that CeO2-NPs with a particle size of over 20 nm possess antibacterial properties. Moreover, the most antibacterial effects due to the highest percentage of surface Ce3+ of NP are in agreement with Pulido-Reyes et al’s observations.Citation67
Neurodegenerative effect
The brain and central nervous system are the most active organ systems in the body; therefore, they are particularly sensitive to oxidative stress because of high oxygen utilization, high levels of polyunsaturated fatty acid peroxidation and low levels of endogenous antioxidant systems. Increased oxidative stress and free radical production could be attributed to several neurodegenerative diseases, such as Parkinson’s disease, trauma, ischemic stroke, Alzheimer’s disease (AD) and aging.Citation70 A beneficial therapy for neurodegenerative diseases is CeO2-NP utilization, which removes ROS or prevents their formation and affects different key points in the brain cells or central nervous tissue. Reducing ROS production, CeO2-NPs were demonstrated to affect (directly or indirectly) signal transduction pathways involved in neuronal death and neuroprotection. For example, it is reported that cerium oxide NPs could trigger neuronal survival in a human AD model through modulating the brain-derived neurotrophic factor (BDNF) pathway. BDNF is a factor involved in the signal transduction pathways of neuronal survival.Citation71 In a similar approach, Guo et al reported that ceria NPs protect neurons against oxidative stress induced injury by modulating transforming growth factor beta (TGF-β) signaling.Citation72 There are so many reports on the neuroprotective effect of engineered CeO2-NPs. Recently, Arya et alCitation3 reported that CeO2-NPs promoted neurogenesis and modulated hypoxia-induced memory impairment through the AMPK–PKC–CBP signaling cascade. Using PEG-coated 3 nm CeO2-NPs, they demonstrated that NPs were efficiently localized in the brain and significantly decreased oxidative stress. Therefore, associated damage during hypoxia exposure was also reduced by applying PEG/CeO2-NPs. They also provided evidence that PEG/CeO2-NPs enhanced hippocampus neuronal survival and promoted neurogenesis.Citation3
Regarding the reductive effect of CeO2-NPs on oxidative stress, which is known to play an important role in neurodegeneration, Fiorani et alCitation73 had investigated the role of CeO2-NPs on microglial activation and neurodegenerative processes in light damaged retina. They demonstrated the ability of CeO2-NPs to reduce microglial activation and their migration toward the outer nuclear layer,Citation73 raising the possibility of their use as therapeutic agents for neurodegenerative diseases.
Enzyme mimetic applications
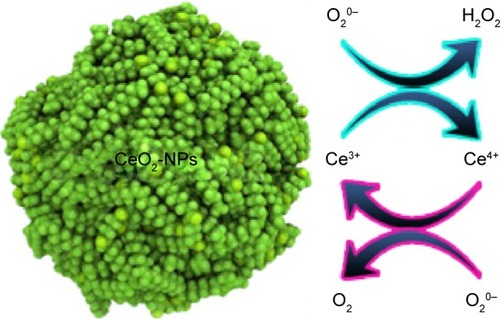
CeO2-NPs are forms of powerful artificial oxidase enzymes capable of mimicking catalase and SOD and peroxidase-like activities ().
Table 3 Different types of enzyme mimicking activities of cerium oxide nanoparticles
Oxidase-like activity of these NPs originated from surface Ce3+ atoms as the catalytic center.Citation74 CeO2-NPs with lower Ce3+ on their surface showed catalase or peroxidase mimetic activity,Citation68 which could break down H2O2 into water and oxygen. CeO2-NPs with higher Ce3+ on their surface could efficiently scavenge radicals of superoxide (SOD mimetic activity) and produce H2O2.
SOD mimicking activity
Comparing with natural enzymes, CeO2-NPs showed several advantages, such as high sensitivity, low cost, easy storage and catalytic stability under harsh conditions. Construction of efficient artificial enzymes, as a strong and cost-effective alternative to natural enzymes, has been an interesting subject in the field of biomimetic chemistry. In a new report on SOD-like activity of ceria, Bhushan and GopinathCitation75 developed a stable and biocompatible artificial enzymatic system based on CeO2-NPs that possessed high ROS scavenging activity over a period of time. They synthesized a CeO2-NP encapsulated biocompatible ceria-albumin nanoparticle (BCNP) capable of reducing intracellular ROS. The BCNPs preserved the antioxidant defense system of the cells and protected them from oxidant-mediated apoptosis.Citation75 Importantly, the enzyme mimicking activity of CeO2-NPs remained almost constant and stable over a wide range of pH and temperature. Therefore, the as-prepared BCNPs were promising as potential candidates against ROS-induced diseases and disorders utilizing SOD-like activity of ceria.Citation75 Moreover, the SOD ability of CeO2-NPs with sizes >5 nm and diversity in shape and a negligible Ce3+/Ce4+ ratio were also investigated by Li et al.Citation76 So far, inherent superoxide-scavenging ability has only been found in the CeO2-NPs with sizes of <5 nm, and these bioactive CeO2-NPs showed very limited diversity with respect to shape. Li et alCitation76 believed that without the coating of surface ligands to stabilize the oxygen vacancies, CeO2-NPs of >3 nm could not maintain a substantially higher Ce3+/Ce4+ ratio under ambient conditions when compared to their bulk counterpart.Citation77 Therefore, even CeO2-NPs of <5 nm would lose their inherent SOD mimetic activity because of Ce3+ oxidation, and the time required to regenerate that activity would usually take days and weeks.Citation78,Citation79 Li et alCitation76 proposed a strategy to significantly improve the superoxide-scavenging activity of CeO2-NPs of >5 nm. However, they activated the SOD mimetic activity of different sized CeO2-NPs within minutes by incubation with native CuZn-SOD in phosphate-buffered saline ().Citation76
Catalase mimicking activity
The first report on catalase mimicking activity of CeO2-NPs was presented by Pirmohamed et al.Citation68 Recently, the catalytic activity of CeO2-NPs was applied in different biomedical approaches.Citation80–Citation82 For example, Akhtar et al have demonstrated that the catalase activity of CeO2-NPs could increase the intracellular glutathione (GSH) in cells challenged with H2O2, protecting cells from oxidative damage.Citation80 Considering major roles of GSH in the regulation of cell growth and division, metabolism of carcinogens and protecting DNA from oxidative damage, the effect of CeO2-NPs on increasing the amount of intracellular GSH marks a revolution in medical biology. Moreover, Nicolini et al had introduced a kind of bioactive glass based on catalytic activity of CeO2-NPs, which was used for bone tissue engineering.Citation82 The design of bioactive glasses capable of preventing oxidative stress after implantation would reduce the convalescence and decrease the amount of anti-inflammatory responses in patients. Applying biomedical properties of CeO2-NPs requires more investigation of the NPs’ fate in vivo. For example, cerium atoms of CeO2-NPs have the potential to interact with peptides, sugar and small anion molecules, such as phosphate in vitro and in vivo. Singh et al investigated the role of phosphate on stability and catalase mimetic activity of cerium oxide NPs.Citation81,Citation83 Given the abundance of inorganic phosphate in biological systems, they demonstrated that catalase mimetic activity of CeO2-NPs (Ce4+) is resistant to the phosphate anions, pH changes and composition of cell culture media. Thus, Singh et al provided a promising approach to more practical and attractive biomedical applications for cerium oxide NPs.
Peroxidase mimicking activity
SOD and catalase mimetic activity of CeO2-NPs has been studied extensively; however, research regarding its peroxidase-like activity remains scant. As the newest research in this field, Tian et al exploited the peroxidase-like activity of CeO2-NPs for breast cancer cell detection using nanostructure-based enzyme-linked immunosorbent assay (ELISA).Citation2 In the designed system, the primary antibody against a biomarker of breast cancer (CA15-3) was coated on the ELISA plate and the second antibody was directly conjugated on the surface of CeO2-NPs through electrostatic forces. In the presence of cancer cells, the primary antibody could capture the cells and the secondary antibody-conjugated CeO2-NPs would attach to them, causing oxidation of H2O2 and color change. Comparing the CeO2-NPs-based sensor with the horse radish peroxidase (HRP)-based one, the high sensitivity of CeO2-NPs-based immunoassay, with a detection limit of 0.01 ng/mL, was approximately one order of magnitude higher than the HRP system.Citation2
Sensing applications
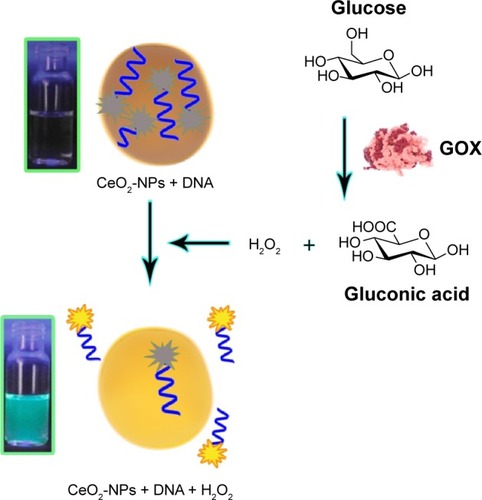
Different forms of biosensors were designed based on CeO2-NPs, including electrochemical, fluorometric and colorimetric sensors, which are briefly discussed here. In 2006, the catalytic activity of cerium oxide NP was exploited to develop a highly sensitive biosensor for the first time. A study has shown that synthesized electrochemical biosensors based on cerium oxide NPs were efficient tools for H2O2 detection in as low as 1 μM of water.Citation84 Currently, interfacing H2O2 with inorganic NPs has generated a number of nanozymes showing catalase or peroxidase-like activities. Recently, Liu et alCitation85 introduced a DNA/CeO2-NP-based fluorometric sensing system for highly sensitive detection of H2O2 (). Liu et al probed CeO2-NPs and H2O2 interaction, applying DNA. H2O2 often causes oxidative DNA damage in the presence of redox metals; however, the ability of H2O2 to displace adsorbed DNA without cleavage was used in this study. After adding CeO2-NPs to the solution of fluorescently labeled DNA, the fluorescence was completely quenched, demonstrating the adsorption of DNA on the NPs’ surface. Interestingly, fluorescence was completely and rapidly recovered after adding H2O2. Given the sensor performance for H2O2 with a detection limit of 130 nM, Liu et al then tested the presence of glucose. H2O2 is produced in situ using glucose oxidase (GOX) and glucose. When the glucose concentration varied, a linear response was observed with a detection limit of 8.9 μM in buffer and 4.37±0.32 mM in serum.Citation85
Figure 5 H2O2 could make displacement of adsorbed DNA from CeO2-NPs, resulting in fluorescence signal enhancement.
Abbreviation: CeO2-NPs, cerium oxide nanoparticles.
In other work, Sardesai et al developed a biosensor based on oxygen-rich platinum doped CeO2-NPs (Pt-ceria) and lactate oxidase for in vitro and in vivo monitoring of lactate during hypoxia.Citation86 Integration of the oxygen-rich CeO2-NPs in the enzyme-containing layer ensured operation of the biosensor in hypoxic conditions, and provided continuous, sensitive lactate monitoring. Measurements of lactate levels in blood and tissues are important indications of the state and progress of a variety of diseases. In vitro evaluation of the biosensor demonstrated a detection limit of 100 pM and high selectivity against physiological levels of coexisting interference species, as well as a quick response time of 6 seconds. In vivo studies have been performed by placing the designed biosensor in the hippocampus of anesthetized rats. The results provided the possibility of continuous lactate monitoring under 2 hours ischemia and reperfusion.Citation86 Moreover, all the mentioned reports have documented the ability of cerium oxide NPs to provide third-generation biosensors with high sensitivity and specificity of detection.
Angiogenesis induction
A unique property of CeO2-NPs could also induce angiogenesis in vivo. Angiogenesis is the physiological process through which new blood vessels form from pre-existing ones. In particular, CeO2-NPs trigger angiogenesis by modulating the intracellular oxygen environment and endogenously stabilizing hypoxia inducing factor 1α, which alters gene regulation. Furthermore, the high surface area, increased Ce3+/Ce4+ ratio and small size make CeO2-NPs more catalytically active toward regulating intracellular oxygen, which in turn leads to more robust induction of angiogenesis.Citation87
Conclusion
The unique property of CeO2-NPs that makes them distinct from other antioxidants is their ability to self-regenerate their surface. Thus, one small dose can work for a long time before being cleared from the body.Citation7 Accordingly, various kinds of CeO2-NPs have been synthesized in order to target the Achilles’ heel of any oxidative stress-associated diseases.Citation88,Citation89 Investigating previous literature on ceria NPs demonstrated that different synthesis methods could provide cerium oxide NPs with various catalytic and physiochemical properties that could contribute to antioxidant or prooxidant properties.Citation29 Considering CeO2-NPs as potential therapeutic agents, it is important to pay attention to their synthesis method. Among different strategies reported for the synthesis of CeO2-NPs, green synthesis methods have shown to be promising for CeO2-NP production and in their application in biological systems. Another consideration of CeO2-NPs is that the in vitro measured properties of the NP (eg, zeta potential, size and redox activity) could change under physiological conditions.Citation90 For example, Kumari et al has shown that the hydrodynamic diameter of CeO2-NPs increased dramatically in cell culture media due to the tendency of NPs to agglomerate in physiological conditions.Citation91 Furthermore, adsorption of proteins in biological fluids, such as blood, could also affect the size and distribution of metal oxide NPs. Generally, smaller sized particles that are free of contamination are suitable for bio-applications. Using bio-directed methods, synthesis of small CeO2-NPs is possible. For example, as mentioned earlier, applying starch-based methods resulted in the production of CeO2-NPs as small as 6 nm. Since bio-directed methods of CeO2-NP synthesis used biocompatible stabilizers and produced nontoxic NPs, of all the different methods of CeO2-NP synthesis, green synthesis is proposed to be applied for the production of CeO2-NPs for therapeutic purposes. Moreover, green synthesis of CeO2-NPs suggests several advantages, such as cost-effectiveness, large-scale commercial production and the potential for pharmaceutical applications.
Future perspectives
CeO2-NPs were recently shown to have regenerative antioxidant activity. Therefore, low levels of CeO2-NPs can work for extended time periods. However, these NPs provided some toxicologic concerns. Currently, the green synthesis of CeO2-NPs gets more attention in order to solve the challenges regarding safety and use of this metal oxide for biomedicine, but there are still some considerations. Previous reports suggested that the protein corona provides NPs with particular biological identity which subsequently play important roles in the ultimate interactions of NPs with target cells. Therefore, physiochemical characteristics of NPs after interaction with biological fluids should be investigated in order to achieve correct interpretations of the biocompatibility of green methods of CeO2-NPs synthesis. Moreover, regarding the effect of percentage of surface Ce3+ on the properties of CeO2-NPs in biological systems, the green synthesized CeO2-NPs should be investigated from this point of view. In addition, an important consideration in clinical usage of CeO2-NPs is how cerium oxide NPs behave in biological systems. Addressing this is not a simple endeavor and requires some in vivo-based research of the effect of CeO2-NPs produced by bio-directed methods.
Disclosure
The authors report no conflicts of interest in this work.
References
- GagnonJFrommKMToxicity and protective effects of cerium oxide nanoparticles (Nanoceria) depending on their preparation method, particle size, cell type, and exposure routeEur J Inorg Chem20152745104517
- TianZLiJZhangZGaoWZhouXQuYHighly sensitive and robust peroxidase-like activity of porous nanorods of ceria and their application for breast cancer detectionBiomaterials20155911612425968461
- AryaAGangwarASinghSKCerium oxide nanoparticles promote neurogenesis and abrogate hypoxia-induced memory impairment through AMPK–PKC–CBP signaling cascadeInt J Nanomedicine2016111159117327069362
- BeaudouxXVirotMChaveTDurandGLeturcqGNikitenkoSIVitamin C boosts ceria-based catalyst recyclingGreen Chem20161836563668
- GawandeMBBonifacioVDBVarmaRSMagnetically recyclable magnetite-ceria (Nanocat-Fe-Ce) nanocatalyst – applications in multicomponent reactions under benign conditionsGreen Chem201315512261231
- XuCQuXCerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applicationsNPG Asia Mater20146e90
- DasSDowdingJMKlumpKEMcGinnisJFSelfWSealSCerium oxide nanoparticles: applications and prospects in nanomedicineNanomedicine (Lond)2013891483150823987111
- DeshpandeSPatilSKuchibhatlaSVSealSSize dependency variation in lattice parameter and valency states in nanocrystalline cerium oxideAppl Phys Lett20058713133113
- AsatiASantraSKaittanisCNathSPerezJMOxidase-like activity of polymer-coated cerium oxide nanoparticlesAngew Chem Int Ed Engl200948132308231219130532
- AsatiAKaittanisCSantraSPerezJMThe pH-tunable oxidase-like activity of cerium oxide nanoparticles achieves sensitive fluorigenic detection of cancer biomarkers at neutral pHAnal Chem20118372547255321370817
- LiXSunLGeAGuoYEnhanced chemiluminescence detection of thrombin based on cerium oxide nanoparticlesChem Commun2011473947949
- KaittanisCSantraSAsatiAPerezJMA cerium oxide nanoparticle-based device for the detection of chronic inflammation via optical and magnetic resonance imagingNanoscale2012462117212322337314
- OrnatskaMSharpeEAndreescuDAndreescuSPaper bioassay based on ceria nanoparticles as colorimetric probesAnal Chem201183114273428021524141
- LinYXuCRenJQuXUsing thermally regenerable cerium oxide nanoparticles in biocomputing to perform label-free, resettable, and colorimetric logic operationsAngew Chem Int Ed Engl20125150125791258323136077
- CelardoIPedersenJZTraversaEGhibelliLPharmacological potential of cerium oxide nanoparticlesNanoscale2011341411142021369578
- LiMShiPXuCRenJQuXCerium oxide caged metal chelator: anti-aggregation and anti-oxidation integrated H2O2-responsive controlled drug release for potential Alzheimer’s disease treatmentChem Sci20134625362542
- XuCLinYWangJNanoceria-triggered synergetic drug release based on CeO2-capped mesoporous silica host–guest interactions and switchable enzymatic activity and cellular effects of CeO2Adv Healthc Mater20132121591159923630084
- ChenHIChangHYSynthesis of nanocrystalline cerium oxide particles by the precipitation methodCeramics Int2005316795802
- YuJCZhangLLinJDirect sonochemical preparation of high-surface-area nanoporous ceria and ceria–zirconia solid solutionsJ Colloid Interface Sci2003260124024312742056
- YanZWangJZouRLiuLZhangZWangXHydrothermal synthesis of CeO2 nanoparticles on activated carbon with enhanced desulfurization activityEnergy Fuels201226958795886
- ChunwenSHongLHuairuoZZhaoxiangWLiquanCControlled synthesis of CeO2 nanorods by a solvothermal methodNanotechnology20051691454
- YadavTPSrivastavaONSynthesis of nanocrystalline cerium oxide by high energy ball millingCeramics Int201238757835789
- WangYMoriTLiJGIkegamiTLow-temperature synthesis of praseodymium-doped ceria nanopowdersJ Am Ceramic Soc2002851231053107
- FengXSayleDCWangZLConverting ceria polyhedral nanoparticles into single-crystal nanospheresScience200631257791504150816763144
- HiranoMFukudaYIwataHHottaYInagakiMPreparation and spherical agglomeration of crystalline cerium(IV) oxide nanoparticles by thermal hydrolysisJ Am Ceramic Soc200083512871289
- HeHWWuXQRenWShiPYaoXSongZTSynthesis of crystalline cerium dioxide hydrosol by a sol–gel methodCeramics Int201238Suppl 1S501S504
- DarroudiMSaraniMKazemi OskueeRKhorsand ZakAHosseiniHAGholamiLGreen synthesis and evaluation of metabolic activity of starch mediated nanoceriaCeramics Int2014401, Part B20412045
- DarroudiMSaraniMKazemi OskueeRKhorsand ZakAAmiriMSNanoceria: gum mediated synthesis and in vitro viability assayCeramics Int201440228632868
- DowdingJMSealSSelfWTCerium oxide nanoparticles accelerate the decay of peroxynitrite (ONOO−)Drug Deliv Transl Res20133437537923936755
- DowdingJMDasSKumarACellular interaction and toxicity depend on physicochemical properties and surface modification of redox-active nanomaterialsACS Nano2013764855486823668322
- AdschiriTLeeYWGotoMTakamiSGreen materials synthesis with supercritical waterGreen Chem201113613801390
- KoJWLeeBIChungYJParkCBCarboxymethyl cellulose-templated synthesis of hierarchically structured metal oxidesGreen Chem201517841674172
- ArumugamAKarthikeyanCHaja HameedASGopinathKGowriSKarthikaVSynthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial propertiesMater Sci Eng C Mater Biol Appl20154940841525686966
- KannanSKSundrarajanMA green approach for the synthesis of a cerium oxide nanoparticle: characterization and antibacterial activityInt J Nanosci201413031450018
- PriyaGSKannegantiAKumarKARaoKVBykkamSBio synthesis of cerium oxide nanoparticles using Aloe arbadensis Miller GelInt J Sci Res Publications20144614
- KumarADasSMunusamyPBehavior of nanoceria in biologically-relevant environmentsEnviron Sci Nano201416516532
- MunusamySBhakyarajKVijayalakshmiLStephenANarayananVSynthesis and characterization of cerium oxide nanoparticles using Curvularia lunata and their antibacterial propertiesInt J Innovative Res Sci Eng201421318323
- ThillAZeyonsOSpallaOCytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanismEnviron Sci Technol200640196151615617051814
- ZeyonsOThillAChauvatFDirect and indirect CeO2 nanoparticles toxicity for Escherichia coli and SynechocystisNanotoxicology200934284295
- Rodea-PalomaresIGonzaloSSantiago-MoralesJAn insight into the mechanisms of nanoceria toxicity in aquatic photosynthetic organismsAquat Toxicol2012122–123133143
- HoeckeKVQuikJTKMankiewicz-BoczekJFate and effects of CeO2 nanoparticles in aquatic ecotoxicity testsEnviron Sci Technol200943124537454619603674
- RogersNJFranklinNMApteSCPhysico-chemical behaviour and algal toxicity of nanoparticulate CeO2 in freshwaterEnviron Chem2010715060
- Rodea-PalomaresIBoltesKFernández-PiñasFPhysico-chemical characterization and ecotoxicological assessment of CeO2 nanoparticles using two aquatic microorganismsToxicol Sci2011119113514520929986
- XiaTKovochichMLiongMComparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress propertiesACS Nano20082102121213419206459
- ZhaoLPengBHernandez-ViezcasJAStress response and tolerance of Zea mays to CeO2 nanoparticles: cross talk among H2O2, heat shock protein, and lipid peroxidationACS Nano20126119615962223050848
- MohanpuriaPRanaNKYadavSKBiosynthesis of nanoparticles: technological concepts and future applicationsJ Nanopart Res2007103507517
- KargarHGhazaviHDarroudiMSize-controlled and bio-directed synthesis of ceria nanopowders and their in vitro cytotoxicity effectsCeramics Int2015413, Part A41234128
- DarroudiMHoseiniSJKazemi OskueeRHosseiniHAGholamiLGerayliSFood-directed synthesis of cerium oxide nanoparticles and their neurotoxicity effectsCeramics Int201440574257430
- SinghAVBandgarBMKastureMPrasadBLVSastryMSynthesis of gold, silver and their alloy nanoparticles using bovine serum albumin as foaming and stabilizing agentJ Mater Chem2005154851155121
- DarroudiMAhmadMBAbdullahAHIbrahimNAGreen synthesis and characterization of gelatin-based and sugar-reduced silver nanoparticlesInt J Nanomedicine2011656957421674013
- KargarHGhasemiFDarroudiMBioorganic polymer-based synthesis of cerium oxide nanoparticles and their cell viability assaysCeramics Int2015411, Part B15891594
- LothFIndustrial Gums: Polysaccharides and Their Derivatives3rd editionWhistlerRoy LBeMillerJames N0-12-746253-8Academic Press, IncSan Diego/New York/Boston/London/Sidney/Tokyo/Toronto1993642 Acta Polymerica1993443172173
- DavidsonRLHandbook of Water-Soluble Gums and Resins/Robert L. Davidson, editor in chiefNew York, NYMcGraw-Hill1980
- RemaniKCGhoshSNanocrystalline ceria through homogeneous precipitation in alcohol-water mixed solventTrans Indian Ceramic Soc2009684185188
- YokoyamaASrinivasanKRFoglerHSStabilization mechanism of colloidal suspensions by gum tragacanth: the influence of pH on stabilityJ Colloid Interface Sci19881261141149
- Khorsand ZakAAbd MajidWHMahmoudianMRDarroudiMYousefiRStarch-stabilized synthesis of ZnO nanopowders at low temperature and optical properties studyAdv Powder Technol2013243618624
- AlpaslanEYaziciHGolshanNHZiemerKSWebsterTJpH-dependent activity of dextran-coated cerium oxide nanoparticles on prohibiting osteosarcoma cell proliferationACS Biomater Sci Eng201511110961103
- QiLFresnaisJMulleraPTheodolyOBerretbFChapelPInterfacial activity of phosphonated-polyethylene glycol functionalized cerium oxide nanoparticlesLangmuir20122831114481145622794100
- QiLSehgalACastaingJCRedispersible hybrid nanopowders: cerium oxide nanoparticle complexes with phosphonated-PEG oligomersACS Nano20082587988819206484
- SatapathySPEG-Assisted Synthesis and Characterization of Ceria NanoparticlesRourkela, IndiaNational Institute of Technology2011
- KaushikASolankiPRPandeyMKAhmadSMalhotraBDCerium oxide-chitosan based nanobiocomposite for food borne mycotoxin detectionAppl Phys Lett20099517173703
- HassannejadHNouriASynthesis and evaluation of self-healing cerium-doped chitosan nanocomposite coatings on AA5083-H321Int J Electrochem Sci20161121062118
- KarakotiASinghSDowdingJMSealSSelfWTRedox-active radical scavenging nanomaterialsChem Soc Rev201039114422443220717560
- AliliLSackMvon MontfortCDownregulation of tumor growth and invasion by redox-active nanoparticlesAntioxid Redox Signal201319876577823198807
- DahleJAraiYEnvironmental geochemistry of cerium: applications and toxicology of cerium oxide nanoparticlesInt J Environ Res Public Health20151221253127825625406
- PrabaharanDMDMSadaiyandiKMahendranMSagadevanSStructural, optical, morphological and dielectric properties of cerium oxide nanoparticlesMater Res2016192478482
- Pulido-ReyesGRodea-PalomaresIDasSUntangling the biological effects of cerium oxide nanoparticles: the role of surface valence statesSci Rep201551561326489858
- PirmohamedTDowdingJMSinghSNanoceria exhibit redox state-dependent catalase mimetic activityChem Commun2010461627362738
- SinghSKumarAKarakotiASealSSelfWTUnveiling the mechanism of uptake and sub-cellular distribution of cerium oxide nanoparticlesMol Biosyst20106101813182020697616
- UttaraBSinghAVZamboniPMahajanRTOxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic optionsCurr Neuropharmacol200971657419721819
- D’AngeloBSantucciSBenedettiECerium oxide nanoparticles trigger neuronal survival in a human Alzheimer disease model by modulating BDNF pathwayCurr Nanosci200952p167
- GuoCSmithRGantTWLeonardMOCerium dioxide nanoparticles protect against oxidative stress induced injury through modulation of TGF-β signallingToxicol Res201542464475
- FioraniLPassacantandoMSantucciSDi MarcoSBistiSMaccaroneRCerium oxide nanoparticles reduce microglial activation and neurodegenerative events in light damaged retinaPLoS One20151010e014038726469804
- JuarezRCormaAGarciaHGold nanoparticles promote the catalytic activity of ceria for the transalkylation of propylene carbonate to dimethyl carbonateGreen Chem2009117949952
- BhushanBGopinathPAntioxidant nanozyme: a facile synthesis and evaluation of the reactive oxygen species scavenging potential of nanoceria encapsulated albumin nanoparticlesJ Mater Chem B201532448434852
- LiYHeXYinJJAcquired superoxide-scavenging ability of ceria nanoparticlesAngew Chem Int Ed Engl20155461832183525515687
- ZhangDWenXShiLYanTZhangJEnhanced capacitive deionization of graphene/mesoporous carbon compositesNanoscale20124175440544622836788
- HeckertEGKarakotiASSealSSelfWTThe role of cerium redox state in the SOD mimetic activity of nanoceriaBiomaterials200829182705270918395249
- KarakotiASSinghSKumarAPEGylated nanoceria as radical scavenger with tunable redox chemistryJ Am Chem Soc200913140141441414519769392
- AkhtarMJAhamedMAlhadlaqHAKhanMAAlrokayanSAGlutathione replenishing potential of CeO2 nanoparticles in human breast and fibrosarcoma cellsJ Colloid Interface Sci2015453212725965428
- SinghRSinghSRole of phosphate on stability and catalase mimetic activity of cerium oxide nanoparticlesColloids Surf B Biointerfaces2015132788426011425
- NicoliniVGambuzziEMalavasiGEvidence of catalase mimetic activity in Ce3+/Ce4+ doped bioactive glassesJ Phys Chem B2015119104009401925710332
- SinghSDosaniTKarakotiASKumarASealSSelfWTA phosphate-dependent shift in redox state of cerium oxide nanoparticles and its effects on catalytic propertiesBiomaterials201132286745675321704369
- PatilSDFundamental Aspects of Regenerative Cerium Oxide Nano-particles and Their Applications in Nanobiotechnology [dissertation]Florida, USADepartment of Mechanical, Materials and Aerospace Engineering University of Central Florida2006
- LiuBSunZHuangPJJLiuJHydrogen peroxide displacing DNA from nanoceria: mechanism and detection of glucose in serumJ Am Chem Soc201513731290129525574932
- SardesaiNPGanesanaMKarimiALeiterJCAndreescuSPlatinum-doped ceria based biosensor for in vitro and in vivo monitoring of lactate during hypoxiaAnal Chem20158752996300325627400
- DasSSinghSDowdingJMThe induction of angiogenesis by cerium oxide nanoparticles through the modulation of oxygen in intracellular environmentsBiomaterials201233317746775522858004
- EstevezAYErlichmanJSCerium oxide nanoparticles for the treatment of neurological oxidative stress diseasesOxidative Stress: Diagnostics, Prevention, and Therapy1083New YorkAmerican Chemical Society2011255288
- AndreescuSHepelMOxidative Stress: Diagnostics, Prevention, and Therapy1083ACS Symposium SeriesNew YorkAmerican Chemical Society2011
- EstevezAYErlichmanJSThe potential of cerium oxide nanoparticles (nanoceria) for neurodegenerative disease therapyNanomedicine (Lond)20149101437144025253491
- KumariMSinghSPChindeSRahmanMFMahboobMGroverPToxicity study of cerium oxide nanoparticles in human neuroblastoma cellsInt J Toxicol2014332869724510415