Abstract
Background
This study explored the pharmacokinetic parameters and tissue distribution of magnetic iron oxide nanoparticles (Fe3O4 MNPs) in imprinting control region (ICR) mice.
Methods
The Fe3O4 MNPs were synthesized by chemical coprecipitation, and their morphology and appearance were observed by transmission electron microscopy. ICR mice were divided into a control group and a Fe3O4 MNP-treated group. Probable target organs in ICR mice were observed, and the pharmacokinetic parameters and biodistribution of Fe3O4 MNPs in tissues were identified using atomic absorption spectrophotometry.
Results
Fe3O4 MNPs were spherical with a well distributed particle diameter, and were distributed widely in various target organs and tissues including the heart, liver, spleen, lungs, kidneys, brain, stomach, small intestine, and bone marrow. The majority of Fe3O4 MNPs were distributed to the liver and the spleen. Fe3O4 MNP levels in brain tissue were higher in the Fe3O4 MNP-treated group than in the control group, indicating that Fe3O4 MNPs can penetrate the blood–brain barrier.
Conclusion
These results suggest that the distribution of Fe3O4 MNPs was mostly in the liver and spleen, so the curative effect of these compounds could be more pronounced for liver tumors. Furthermore, Fe3O4 MNPs might be used as drug carriers to overcome physiologic barriers.
Introduction
With the development of nanotechnology, magnetic nanoparticles have shown application prospects since the 1970s.Citation1 Much attention has been paid over the past few years to the study of magnetic nanoparticles due to their particularly large surface- to- volume ratio, quantum size effect, and magnetic character, as well as their potential application in the areas of bioscience and medicine. Recently, magnetic nanoparticles have been used more and more frequently in biomedical and biotechnology studies, including targeted drug delivery, tumor magnetic hyperthermia therapy, contrast enhancement of magnetic resonance imaging, biosensors, rapid separation in environmental biology, and concentration tracing of specific targets, such as bacteria, leukocytes, and proteins.Citation2 So far, the magnetic nanoparticles with clinical applications are mainly composed of magnetic iron oxide, which has good chemical stability, magnetic responsiveness, and biocompatibility, in addition to possessing the general characteristics of nanoparticles. Moreover, iron oxide nanoparticles are the only magnetic nanomaterials approved for clinical use by the US Food and Drug Administration (FDA),Citation3,Citation4 and the preparation method is relatively simple.
Table 1 Concentration of magnetic Fe3O4 nanoparticles in bone marrow of mice (n = 6 ± standard deviation)
In our previous studies,Citation5,Citation6 we have demonstrated that Fe3O4 MNPs combined with chemotherapeutic drugs can inhibit tumor proliferation and induce apoptosis of tumor cells in a dose- and time-dependent manner, which may be related to changes in drug formulation caused by the magnetic nanomaterials. However, the in vivo metabolic processes of Fe3O4 MNPs within the organism, as well as their distribution in the important organ tissues, are as yet not completely understood.Citation7 Using chemical coprecipitation to synthesis Fe3O4 MNPs, we selected imprinting control region (ICR) mice as an investigative model. The mice were given the Fe3O4 NMPs by the intragastric route in view of the application perspective of Fe3O4 NMPs, and the concentrations of Fe3O4 MNPs in different organs and tissues were measured at particular time points using atomic absorption spectrophotometry. The pharmacokinetic parameters and biodistribution of Fe3O4 MNPs in tissues and the probable target organs in mice were determined to provide theoretic evidence for follow-up research and clinical application.
Materials and methods
Animals
ICR mice (6–8 weeks of age) weighing 18–22 g were used for the pharmacokinetic and tissue distribution studies, and were purchased from the Experimental Animal Center at Nantong University (Animal Certificate of Conformity SCXK 2008-0010). All animal experiments were evaluated and approved by the Animal and Ethics Review Committee of the Southeast University. The mice were kept at a room temperature controlled at 22° with relative humidity controlled at about 45% ± 5%, fed with standard solid composite feedstuff, and received tap water ad libitum as previously described.Citation8
Main apparatus and reagents
The main apparatus and reagents used in this experiment were an atomic absorption spectrophotometer (HG-9602A, Shenyang Huaguang Precise Instrument Co., Ltd), a transmission electron microscope (TEM; Sujing Group Antai Co., Ltd), and an iron normostock solution (500 mg/L, Institute for Reference Materials of SEPA).
Preparation of Fe3O4 NMPs
As described previously,Citation9 we dissolved a quantity of ferric chloride hexahydrate (0.02 mol/L) and ferrous sulfate heptahydrate (0.012 mol/L) in 200 mL of deionized water under nitrogen protection. Ammonium hydroxide 25% was then added slowly with vigorous magnetic force stirring until pH was 9. The procedure took 30 minutes to generate a dark precipitate. Next, we separated the resultant with a permanent magnet and washed this repeatedly with deionized water 5–7 times until the pH of the supernatant was 7. Finally, the resultant was lyophilized and observed under TEM.
Experimental group
Forty-two ICR mice were divided into two groups. Thirty-six mice were divided (n = 6) into A, B, C, D, E, and F experimental groups, given 600 mg/kg Fe3O4 MNPs by intragastric administration, and fed a normal diet. Another six control mice were given 0.5 mL of sterile physiologic saline by intragastric administration and also fed a normal diet.
Preparation of sample
After intragastric administration, 0.2–0.5 mL of blood from one mouse in each group were collected into an ethylene-diaminetetraacetic acid tube by enucleating eyeballs at the time points of zero minutes, hours 1, 3, 5, 6, and 7, and days 1, 3, 5, 7, and 10. The mice were then sacrificed by cervical dislocation, and samples of heart, liver, spleen, lungs, kidneys, bone marrow, brain, stomach, and small intestine were collected. After removing the fatty and connective tissue on the joint, the remaining tissues were washed with physiologic saline, dried with filter paper, and weighed to determine Fe3O4 MNP content.
Distribution and concentration of Fe3O4 NMPs
Tissue samples of heart, liver, spleen, lungs, kidney, brain, stomach, and small intestine were freeze-dried and digested using microwave digestion with a HNO3-HClO4(V:V = 5:1) acid mixture. Each digested sample (0.2 g) was diluted to 50 mL with deionized water, while the heparinized blood was diluted in 0.02% Triton X-100 and 0.02% HNO3. The iron elements of the spectrum were absorbed by iron atoms of the sample steam, and iron content in the sample was determined using atomic absorption spectrophotometry.
Results
Morphology and appearances
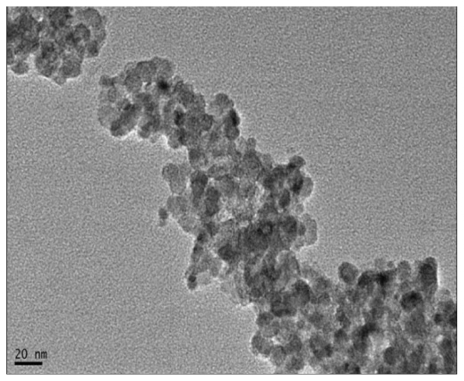
The size and appearance of the Fe3O4 MNPs were examined by TEM. shows representative images of the spinel ferrite compounds, which were spherical with a narrow diameter distribution, and the average particle size observed under TEM was 20 nm after being lyophilized ().
General condition of mice
Mice in the control group were all in good condition and were agile, responsive, and covered with soft and glossy hair. Their consumption of water and food was normal, and their stool was formed and yellowish-brown. Those treated with Fe3O4 MNPs showed less activity and gathered together with enhanced resistance, and had slightly disordered hair. They ate and drank a little less, but all survived for the duration of monitoring.
Blood concentration of Fe3O4 MNPs at different time points
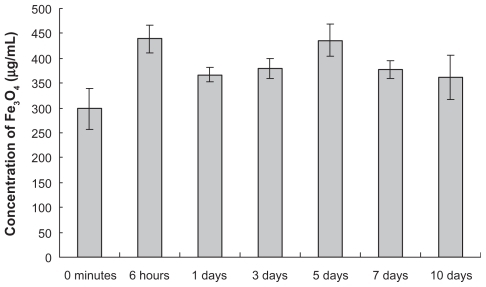
The results of atomic absorption spectrophotometry showed that the highest concentration of Fe3O4 MNPs in the peripheral blood of mice was 439 ± 28 μg/mL at six hours in the experimental group after intragastric dosing, and decreased slowly but remained at a higher level until it reached another distribution peak of 436 ± 32 μg/mL on day 5 and then gradually declined again. During the entire observation period, the concentration of Fe3O4 MNPs in the experimental group at particular time points was higher than that of the control group, and there were significant differences between the groups (P < 0.05, ).
Distribution of Fe3O4 MNPs in various organs and tissues
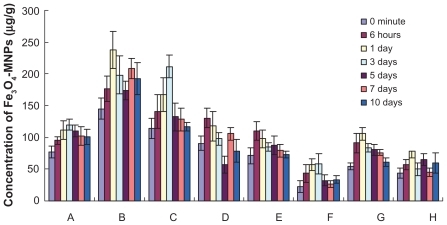
The distribution of Fe3O4 MNPs in the various organs and tissues is shown in and 4. By comparing the concentrations of Fe3O4 MNPs at all time points between the experimental and control groups, there were statistically significant differences in heart and bone marrow tissue distribution (P < 0.05) in the liver and small intestine on days 1, 3, and 7; in the spleen and brain on days 1 and 3; in the lungs at six hours; in the kidneys at six hours and on day 1; and in the stomach from six hours to day 7 after administration, suggesting that Fe3O4 MNPs are distributed widely in various organs in vivo, including the heart, liver, spleen, lungs, kidneys, bone marrow, brain, stomach, and small intestine. Furthermore, we also found that the peak distribution of Fe3O4 MNPs in various organs and tissues was different. There were two distribution peaks in the liver. The first distribution peak was on day 1 and then decreased gradually until it reached a second peak on day 7 after administration. The second peak concentration of Fe3O4 MNPs was 208 ± 16 μg/g and was slightly lower compared with day 1, but there was no significant difference between the two peak values (P > 0.05). While the concentration of Fe3O4 MNPs in the heart or spleen increased gradually with the passage of time, the peak values were 119 ± 9 μg/g and 211 ± 18 μg/g, respectively, on day 3 after dosing. The value in the lung was 131 ± 15 μg/g at six hours after dosing, and then decreased steadily. Levels in both kidneys also peaked six hours after dosing, and decreased gradually to a small degree. Brain levels rose rapidly after dosing, achieved a peak value of 58 ± 16 μg/g on day 3, and thereafter decreased gradually. Meanwhile, peak stomach, small intestine, and bone marrow levels were reached on day 1, with respective peak values of 106 ± 10 μg/g, 79 ± 11 μg/g, and 1.97 ± 0.12 μg/g. Interestingly, concentrations of Fe3O4 MNPs were higher in the liver and spleen than those in other organs. Maximum uptake in the liver was 237 ± 29 μg/g, reached on day 1, and that in the spleen was 211 ± 18 μg/g, reached on day 3 after dosing, indicating that the liver and spleen were the main distribution tissues and are the probable target organs for Fe3O4 MNPs.
Discussion
Iron is an essential element, widely distributed in the body, and primarily involved in oxygen transport and utilization. Almost all organs contain iron in vivo, but the most highly ferruginous organs are the liver and spleen, and the lungs also contain considerable amounts of iron.Citation10 Iron metabolism in the body is a relatively closed systemCitation11,Citation12 and there is very little loss of body iron in normal circumstances compared with metabolism of other materials.Citation13 Human cells lack an excretion mechanism for iron under physiologic conditions and, therefore, iron balance in the body is regulated by absorption of dietary iron through the small intestine. Fe3O4 MNPs have a diameter of less than 100 nm. The physical and chemical properties of iron when being made into nanoparticles and the iron metabolism model in vivo would also be different from that of normal ferric iron.
From the results of our experiment, we can see that there is a close relationship between the blood concentration-time changes for Fe3O4 MNPs and changes in their tissue concentrations in the liver and spleen. A large number of Fe3O4 MNPs were ingested by mononuclear macrophages in the liver or spleen after being absorbed into the blood, with a distribution peak in blood of 439 ± 28 μg/mL at six hours. As a result, the concentration of Fe3O4 MNPs in blood declined rapidly, while that in the liver and spleen reached highest levels on days 1 and 3, respectively. Thereafter, the liver and spleen released ferric iron which participated in the cycle of iron metabolism in vivo, resulting in a decreased concentration of Fe3O4 MNPs in the liver and spleen (mainly spleen). An increased concentration of Fe3O4 MNPs in peripheral blood was observed up to the distribution peak at day 5 after administration. With the gradual senescence of red blood cells and impaired absorption in the liver and spleen (mainly the liver), the concentration of Fe3O4 MNPs in the liver increased again and reached a second distribution peak of 208 ± 16 μg/g on day 7, while that in peripheral blood decreased gradually. Under normal circumstances, about 80% of iron is combined with plasma transferrin, and enters the bone marrow and participates in the synthesis of reticulocytes.Citation15 About four to six days later, this iron goes into the circulation again, leading to increased iron content in the blood.Citation11 In this experiment, the changes in concentration of Fe3O4 MNPs in the peripheral blood of mice were consistent with those reported by Moore et al.Citation15 The concentration of Fe3O4 MNPs in the kidney remained slightly above the background level during the entire observation period, indicating that renal excretion of iron is modest, which is consistent with a report by Pouliquen et al.Citation16
In this study, there was a significant difference in brain tissue concentrations of Fe3O4 MNPs between the experimental and control groups, indicating that Fe3O4 MNPs can penetrate the blood–brain barrier. The blood–brain barrier is composed of a layer of endothelial capillary cells between the blood and the brain, where these cells connect tightly on the one hand to prevent invasion by exogenous pathogens and, on the other hand, form an obstacle which is hard to cross in terms of drug and gene delivery. It is likely that utilization of Fe3O4 MNPs as drug carriers may overcome the physiologic limitations of drug transport by capillary barriers.Citation17 Currently, the mechanism by which nanoparticles traverse the barrier is unclear.Citation18 Although iron oxide nanoparticles are known to be well tolerated by the body and degrade with time,Citation19 it is worthwhile verifying whether the structure of Fe3O4 MNPs changes after digestion and absorption through the intestine. Hence, we need to investigate further their potential to cause chronic long-term harm in the human body.
Conclusions
This study of the pharmacokinetics and tissue distribution of magnetic Fe3O4 nanoparticles in ICR mice showed that these nanoparticles were mostly taken up in the liver and spleen, so the curative effect could be more pronounced for tumors in the liver. Furthermore, Fe3O4 MNPs can penetrate the blood–brain barrier, so they might be used as drug carriers to overcome the limitations of physiologic barriers.
Acknowledgments
This work was supported by the 973 National Key Fundamental Research Project of China (Number 2006CB933205), 863 Project of the People’s Republic of China (Number 2007AA022007), National Nature Science Foundation of the People’s Republic of China (Numbers 30740062, 30872970), and the Special Purpose Science Research Foundation for High Schools (Number 20070286042).
Disclosure
The authors report no conflicts of interest relevant to this study.
References
- LiZQChenZMPreparation characterization and application of nano magnetic polymer materialsChem Ind Times20072165760
- SinghRLillardJWNanoparticle-based targeted drug deliveryExp Mol Pathol200986321522319186176
- BourrinetPBengeleHHBonnemainBDencausseAIdecJMJacobsPMPreclinical safety and pharmacokinetic profile of Ferumoxtran-10, an ultrasmall superparamagnetic iron oxide magnetic resonance contrast agentInvest Radiol200641331332416481915
- NeubergerTSchöpfBHofmannHHofmannMvon RechenbergBSuperparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery systemJ Magn Magn Mater20052931483496
- ChenBAChengJWuYNReversal of multidrug resistance by magnetic Fe3O4 nanoparticle copolymerizating daunorubicin and 5-bromotetrandrine in xenograft nude-miceInt J Nanomedicine200941737819421372
- ChenBAChengJShenMFMagnetic nanoparticle of Fe3O4 and 5-bromotetrandrin interact synergistically to induce apoptosis by daunorubicin in leukemia cellsInt J Nanomedicine200941657119421371
- PowersKWPalazuelosMMoudgilBMCharacterization of the size, shape, and state of dispersion of nanoparticles for toxicological studiesNanotoxicology200711142151
- ChenBALaiBBChengJDaunorubicin-loaded magnetic nanoparticles of Fe3O4 overcome multidrug resistance and induce apoptosis of K562-n/VCR cells in vivoInt J Nanomedicine2009420120819918366
- JainPKMoralesMASahooSKLeslie-PeleckyDLabhasetwarVIron oxide nanoparticles for sustained delivery of anticancer agentsMol Pharm20052319420515934780
- KaufmanCLWilliamsMRyleLMSmithTLTannerMHoCSuperparamagnetic iron oxide particles transactivator protein-fluorescein isothiocyanate particle labeling for in vivo magnetic resonance imaging detection of cell migration: Uptake and durabilityTransplantation20037671043104614557750
- SunSZengHRobinsonDBGenoveGMorelPAMonodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticlesJ Am Chem Soc2004126127327914709092
- AhrensETFeili-HaririMXuHReceptor-mediated endocytosis of iron-oxide particles provides efficient labeling of dendritic cells for in vivo MR imagingMagn Reson Med20034961006101312768577
- JingMLiuXQLiangPLabeling neural stem cells with superparamagnetic iron oxide in vitro and tracking after implantation with MRI in vivoChin Med J2004841613861389
- DoddCHHsuHCChuWJNormal T-cell response and in vivo magnetic resonance imaging of T cells loaded with HIV transactivator-peptide-derived superparamagnetic nanoparticlesJ Immunol Methods2001256128910511516750
- MooreAGrimmJHanBSantamariaPTracking the recruitment of diabetogenic CD8(+)T-cells to the pancreas in real timeDiabetes20045361459146615161749
- PouliquenDGalloisYPhysicochemical properties of structured water in human album in and gammaglobulin solutionsBiochimie200183989189811698111
- LeonardFKulkarniRKBrandesGSynthesis and degradation of poly(alkyl α-cyanoacrylate)J Appl Polym Sci1966102259272
- LockmanPRKoziaraJRoderKEIn vivo and in vitro assessment of baseline blood-brain barrier parameters in the presence of novel nanoparticlesPharm Res200320570571312751624
- OkonEPouliquenDOkonPBiodegradation of magnetite dextran nanoparticles in the rat. A histologic and biophysical studyLab Invest1994718959037807971