 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Resveratrol (Res) is a common phytoalexin present in a few edible materials, such as grape skin, peanuts, and red wine. Evidence has shown the beneficial effects of Res on human health, which may be attributed to its anti-inflammatory activity. However, the poor aqueous solubility of Res limits its therapeutic effectiveness. Therefore, the use of nanostructured delivery systems for Res, such as liquid-crystalline systems, could be beneficial. In this study, we aimed to develop, characterize, and determine the in vivo effectiveness of Res-loaded liquid-crystalline systems. Systems containing copaiba balsam oil, polyethylene glycol-40 hydrogenated castor oil, and water were designed. Results of polarized light microscopy, small-angle X-ray scattering, texture-profile analysis, and flow-rheology analysis showed that the Res-loaded liquid-crystalline system had a lamellar structure, textural and mechanical (hardness, compressibility, and adhesiveness) properties, and behaved as a non-Newtonian fluid, showing pseudoplastic behavior upon skin application. Furthermore, all liquid-crystalline systems presented bioadhesive properties that may have assisted in maintaining the anti-inflammatory activity of Res, since the topical application of the Res-loaded lamellar mesophase liquid crystals resulted in edema inhibition in a carrageenan-induced paw-inflammation mouse model. Therefore, Res-loaded lamellar mesophases represent a promising new therapeutic approach for inhibition of skin inflammation.
Introduction
Skin diseases affect millions of people every day. Inflammatory conditions are known to be the major cause of skin diseases.Citation1 Topical drug delivery has many advantages over other conventional routes of drug administration,Citation2,Citation3 because it can provide a noninvasive alternative to the parenteral route.Citation4 The large surface area of skin and ease of access allows transdermal absorption of drugs.Citation5
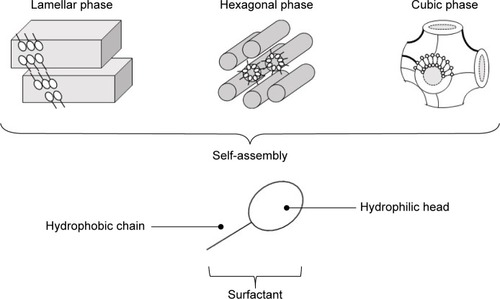
Several strategies are available to overcome the skin barrier, including the use of penetration enhancers, electroporation, iontophoresis, and nanocarrier systems.Citation6–Citation11 In this aspect, liquid crystals have been developed for cutaneous delivery of drugs.Citation12–Citation19 Liquid crystals are systems that can be formed using lipids and amphiphilic molecules, which spontaneously reorganize into three-dimensional structures, such as emulsions, microemulsions, or liquid-crystalline (LC) mesophases (lamellar, hexagonal, and cubic), upon contact with water, and these unique internal structures can be loaded with drugsCitation20 (). The lamellar phase is formed from bilayers separated by layers of surfactants and solvents, which form a one- or two-dimensional network. In the hexagonal phase, aggregates are formed by the arrangement of long cylinders that form two- or three-dimensional structures. Lyotropic cubic phases have more complicated structures consisting of a curved, bicontinuous lipid bilayer that extends in three dimensions to generate two interpenetrating but noncontacting aqueous nanochannels.Citation20 LC systems (LCSs) have been shown to provide sustained release of drug molecules.Citation21–Citation25
Figure 1 Schematic representation of lamellar, hexagonal, and cubic liquid-crystal mesophases formed by surfactant-molecule self-assembly.
Resveratrol (Res; trans-3,4′,5-trihydroxystilbene), a phytoalexin found in grapes, red wine, and fruit, is a potent antioxidant and anti-inflammatory agent.Citation26–Citation30 However, its poor aqueous solubility limits its therapeutic effectiveness. In addition, oral administration of Res is challenging, owing to its low bioavailability in vivo because of its poor solubility, and thus peak plasma levels decrease rapidly.Citation31–Citation33 Therefore, topical application of Res may be convenient for cutaneous local delivery. However, limited aqueous solubility decreases its topical therapeutic effectiveness, because it decreases its skin penetration.Citation34
The use of nanostructured delivery for Res, such as LCSs, could be advantageous, because these systems can be administered easily. In addition, they possess good textural, sensory, and bioadhesive properties. Moreover, they can solubilize both lipophilic and hydrophilic drugs and increase the skin permeation of the drug.Citation19,Citation25,Citation35 In this study, we aimed to develop, characterize the physicochemical properties, and evaluate the in vivo effectiveness of Res-loaded lamellar LCSs.
Materials and methods
Chemicals and reagents
Copaiba balsam oil, polyethylene glycol (PEG)-40 hydrogenated castor oil, trans-Res with 99.9% purity, and λ-carrageenan were purchased from Sigma-Aldrich (St Louis, MO, USA), PharmaSpecial, (Itapevi, SP, Brazil), Galena (Portland, OR, USA), and Sigma-Aldrich, respectively. Water was purified and deionized using a Milli-Q system obtained from Merck Millipore (Billerica, MA, USA). All other reagents were commercially available and used without further purification.
Ternary-phase diagram
A ternary-phase diagram was constructed point to point using copaiba oil as the oily phase (O) and hydrogenated castor oil as the surfactant (S), in proportions of each component generating 100% of a total formulation. Mixtures of O and S were titrated with deionized water to reach a final amount of 2 g. Then, all vials were heated in a water bath at 45°C with vigorous stirring using a glass rod for 5 minutes. The vials were closed and allowed to stand in the dark for 24 hours at 25°C±0.5°C. Then, they were visually examined, and classified as phase separation (PS), transparent viscous system (TVS), or transparent liquid system (TLS).
Polarized light microscopy
A small amount of the formulations was placed on a glass slide, covered with a coverslip, and examined by polarized light microscopy (PLM) to evaluate the homogeneity of the dispersion and detect the presence of anisotropy. The test was performed at 25°C±0.5°C, and photomicrographs were obtained at magnification of 40×.
Small-angle X-ray scattering
This test was performed at the National Synchrotron Light Laboratory (LNLS, Campinas, Brazil), using a small-angle X-ray scattering (SAXS)-1 beamline. This beamline was equipped with a monochromator (λ=1.488 Å), Pilatus 300K vertical detector (Dectris, Baden, Switzerland) located 1.5 m from the sample, and a multichannel analyzer to collect SAXS data in a range of q=0.1–5 nm. All measurements were performed at room temperature (20°C–25°C) under the same conditions to calibrate the sample-to-detector distance. Transmission of Kapton tape and mica-sheet corrections was carried out. Parasitic scattering, produced by slits, was subtracted from the total scattering intensity. Analysis time was 30–45 seconds.
Continuous shear (flow) rheology
Flow measurements were carried out using a controlled-stress AR2000 rheometer (TA Instruments, New Castle, DE, USA) with cone–plate geometry (diameter 40 mm, truncation angle 2°, gap, 52 μm) or plate geometry (diameter 40 mm, gap 200 μm) according to the consistency of each formulation. All measurements were carried out in triplicate at 32°C±0.1°C. Samples of the formulations were carefully applied to the lower plate to minimize the shear. Then, they were incubated to equilibrate for 2 minutes prior to analysis. The shear rate ranged from 0 to 100 reciprocal second for the upward curve and from 100–0 reciprocal second for the downward curve for a duration of 120 seconds for each stage, separated by an interval of 10 seconds. Consistency and flow indices were determined from the power law in EquationEquation 1(1) for quantitative analysis of flow behavior:
where τ is the shear stress, γ the shear rate, k the consistency index, and n the flow index.
Texture-profile analysis
Texture-profile analysis (TPA) was carried out to determine the mechanical properties of the formulations, such as hardness, compressibility, adhesion, and cohesion. Samples (50 g) were weighed and placed into 50 mL conical centrifuge tubes (Falcon). Then, they were centrifuged in a Sorvall TC6 centrifuge (Thermo Fisher Scientific, Waltham, MA, USA) at 2,665× g for 4 minutes to ensure uniformity of surface and remove air bubbles. These tubes were then transferred to a thermostatic bath set at 32°C to mimic skin temperature. A TA.XT Plus texture analyzer (Stable Micro Systems, Surrey, UK) was programmed to compress the sample uniaxially at 1 mm/s until a predefined depth (10 mm), and then return to the surface at a speed of 0.5 mm/second. After 5 seconds, a second compression was applied under the same conditions. All samples were analyzed in triplicate.
In vitro evaluation of bioadhesion
Dermatomed pig-ear skin (300 μm) was incubated for approximately 30 minutes in a petri dish containing 0.9% saline solution. The formulations were placed in conical centrifuge tubes, which were maintained in a thermostatic bath at 32°C. Pig-ear skin was fixed with elastic rubber to the cylindrical probe. The cylindrical probe was lowered to allow the skin to be in contact with the sample surface. Contact time was 60 seconds, then the probe was removed. The force required to detach the skin from the sample was determined from the force versus time curve. This experiment was performed in triplicate using texture-analysis equipment.
In vivo evaluation of anti-inflammatory activity
In vivo evaluation was performed in male Swiss mice weighing 25–35 g. The mice were kept in a temperature-controlled environment (22°C) under 12-hour light–dark cycles, and provided with free access to food and water, except during the experiments. The experimental protocol was performed in accordance with the Guide for the Care and Use of Laboratory AnimalsCitation36 and the ethical principles for animal experimentation established by the Brazilian Committee for Animal Experimentation. This investigation was approved by the animal experimentation ethics committee of the School of Pharmaceutical Sciences, São Paulo State University (protocol CEUA number 71/2015), and complied with international laws.
Mice were subdivided into seven groups (five per group): group I mice were not treated (negative control), group II received topical dexamethasone (positive control), group III received CB-23 formulation without drug, group IV received CB-23R formulation, group V received CB-24 formulation without drug, group VI received CB-24R formulation loaded with Res, and group VII received free Res dissolved in avocado oil.
Paw edema was induced by intraplantar injection of 100 μL of 1% (w:v) λ-carrageenan into the paws of the mice. After 30 minutes, 100 mg of dexamethasone cream or formulation was applied to the paw. After 6 hours, paw diameters were measured using a digital caliper. Data were plotted using GraphPad Prism version 6.0, and one-way analysis of variance was performed followed by Dunnett’s test (α=0.05). Inhibition of edema was calculated:
Results and discussion
Ternary-phase diagram
Surfactants are amphiphilic molecules that form aggregates in solution. Supramolecular interactions can determine the size and shape of the self-assembled aggregates. Various mesophases, such as micelles, lamellar, bicontinuous, and reverse micelles, can be generated.Citation37,Citation38 Several studies have shown the ability of amphiphilic molecules, water, and oil to form LC mesophases.Citation35,Citation39,Citation40
shows the ternary-phase diagram of water (W), oil (O), and surfactant (S) mixtures. A large region at the upper vertex showed transparent liquid systems (TLSs) with high concentrations of S up to 65%, independent of the O:W ratio. The decrease in the concentration of S, with W and O proportions up to 10 and 70%, respectively, led to the formation of TLSs.
Figure 2 Ternary-phase diagram of copaiba oil as the oily phase (O), hydrogenated castor oil as the surfactant (S), and water (W).
Note: Red points indicate the selected formulations.
Abbreviations: PS, phase separation; VSs, viscous systems; TLSs, transparent liquid systems.
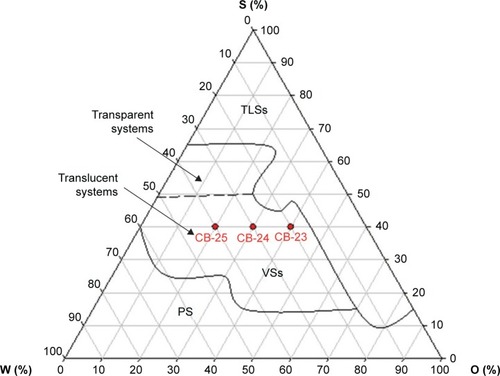
The dilution of the TLS region led to the formation of a viscous system, like a gel, with a proportion of W of 35%–60% and O ratio up to 60%. When S concentration was below 15%, phase separation occurred. Moreover, with the decrease in O and increase in W ratio up to 60%, these regions increased.
Phase behavior showed that we were able to obtain a readily flowing system by combining S, O, and W. In addition, the degree of organization of the system increased when W was added, resulting in a rigid and viscous matrix. This transition could be attributed to the increase in packing and hydration of the hydrophilic heads of S, which reduced the curvature of the interface droplets of the microemulsion.Citation39 Moreover, the subsequent hydration of S generated a large repulsive force between the head groups, increasing the distances between the lamellar mesophase until a hexagonal mesophase was formed.Citation41–Citation43
The different geometries of the amphiphilic molecules and their resultant self-assembled structures that were formed in the presence of a solvent can be understood using the critical packing parameter (CPP) concept.Citation44 CPP is often defined using EquationEquation 3(3) :
where v is the volume of the hydrophobic tail, a the polar head-group area, and l the length of the hydrophobic chain of the surfactant.
Hydrocarbon chains tend to associate with each other upon contact with water to minimize their contact with the aqueous phase; therefore, micelles are formed because of both the increased curvature and hydrocarbon chain-packing density.Citation45 A change in CPP values can roughly predict the order of the surfactant transition associated with the change in the curvature of the water or oil interface.Citation46 Increasing the number of water molecules increases the CPP value, owing to an increase in the volume of the lipophilic moiety and a reduction in the chain length and head-group area.Citation47
Selection of formulations
Transparent or translucent viscous system formations and low-viscosity transparent or translucent system formations were observed over a wide range (). These features are important in the design of nanostructured systems for topical application. Certain flow resistance of the formulations is required to facilitate skin-product application. Compositions of the studied formulations are shown in . Res (0.1%, w:w) was loaded into the oil phase of the formulations. Then, these Res-loaded (CB-23R, CB-24R, and CB-25R) and unloaded (CB-23, CB-24, and CB-25) formulations were characterized by PLM, SAXS, rheological techniques, and bioadhesion studies.
Table 1 Composition of the studied formulations
Visual inspection, PLM, and SAXS
Both the translucent visual aspect and viscosity of the formulations were dependent on the water content (). PLM showed that formulations were anisotropic and composed of lamellar LC mesophases, as Malta crosses were observed in all formulationsCitation48 (). Anisotropic materials have optical properties that change with the orientation of the incident light in nonequivalent directions, like the lamellar mesophase. The lamellar phase consists of bilayers that are separated by layers of surfactants and solvents, forming a one- or two-dimensional network.Citation43 The photomicrographs also showed that the structure of the LC mesophases was not altered by Res loading.
Figure 3 Characterization of the liquid-crystalline dispersions.
Notes: (A) Macroscopic appearance, (B) photomicrographs obtained by polarized light microscopy (figures were obtained at 40× magnification and the enlarged area at 250× magnification), and (C) small-angle X-ray scattering patterns of the samples.
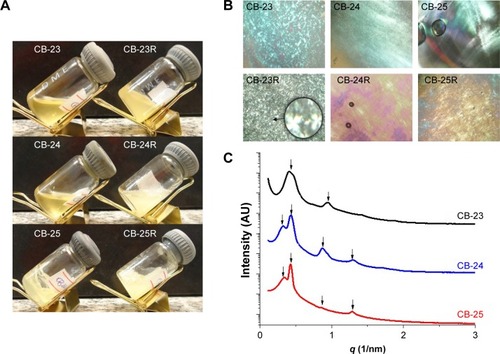
shows the intensity of the scattering patterns and scattering vector modulus (q 1/nm). Curves of SAXS data are shown in . shows peak position (q) values, interplanar distances, and their relationships. For a lamellar LC structure, the relationship between the calculated correlation distances for each Bragg peak follows the ratio 1:2:3.Citation49–Citation51 Although a peak was found in the SAXS data, no correlation was found with the Bragg distances. We suggest that the formed systems were in transition, suggestive of mixing formation with hexagonal mesophase, for samples CB-24 and CB-25. Similar studies have shown that Res loading into lamellar structures does not affect the structural organization of the mesophase of the system.Citation51,Citation52 The parameters of the microstructure lattice are represented by the distance between planes (d, lamellar structures)Citation53 and d-values were 14–19 nm.
Table 2 Peak positions (q) of the SAXS curves, interplanar distances (d), and classification of formulations
Continuous shear (flow) rheology
Flow data are shown in , and mathematical parameters in . These data showed that all formulations exhibited non-Newtonian flow, because there was no linear relationship between the shear stress and shear rate. Moreover, the flow index showed that all formulations had pseudoplastic flow (n<1).Citation54
Table 3 Flow index (n) and consistency index (k) of all formulations obtained from the power law (n=3)
Figure 4 Flow rheograms of unloaded and Res-loaded formulations.
Notes: (A) Flow rheograms of unloaded formulations; (B) flow rheograms of Res-loaded formulations. The closed symbols represent up curves, and open symbols represent down curves. The standard deviations were omitted for clarity; however, in all cases, coefficients of variation of triplicate analyses were less than 10%. Analysis was carried out at 32°C±0.5°C.
Abbreviation: Res, resveratrol.
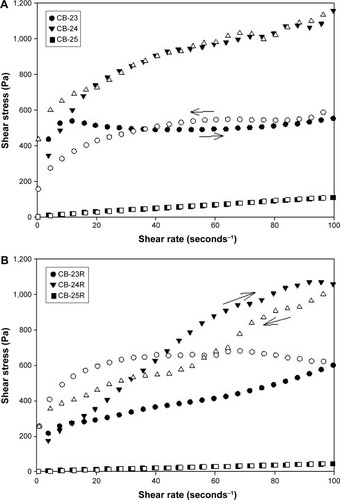
These features are preferable for topical application, because when a force is not applied upon the formulation, ie, when the formulation is kept at rest in the package, it has high viscosity. However, when a force is applied, eg, at the time of application of the formulation on the skin, the formulation viscosity decreases, because the molecules align toward the flow; therefore, the formulation is better spread at the site of action. In addition, after formulation application, ie, when the force application ceases, the formulation has the ability to recover its initial high viscosity, and thus it remains at the site of action for a longer time.Citation55 As such, the addition of cosurfactants, salts, or other components, such as drugs, may influence the characteristics of LC mesophases, such as viscosity, via interference with the electrostatic interactions or chemical bonds between components of the formulation.Citation56–Citation58
It is noteworthy that the incorporation of Res decreased the consistency index (k) of all formulations, which demonstrated that this drug affected molecular bonds between formulation components, resulting in a decrease in viscosity. Matos et alCitation59 reported that emulsions containing Res exhibited viscosities slightly lower than that of the Res-unloaded emulsion. Fujimura et alCitation52 also observed that Res incorporation decreased the viscosity of an LCS containing silicone glycol copolymer as a surfactant, polyether-functional siloxane as an oily phase, and Carbopol 974P dispersion as an aqueous phase.
Texture-profile analysis
TPA was approved only for the formulations CB-23 and CB-24, because these formulations exhibited mechanical resistance to flow. The CB-25 formulation did not show mechanical resistance to compression; therefore, it was impossible to analyze. TPA results are shown in .
Table 4 Mechanical and bioadhesion properties of the loaded and unloaded formulations (n=3)
TPA showed that drug incorporation decreased the mechanical properties of the formulations, including hardness, compressibility, adhesiveness, and cohesiveness. Hardness, compressibility, and adhesiveness showed significant differences (P<0.05) between mean values of the different formulations and those of the drug-loaded and unloaded formulations. However, no difference was observed for mean values of cohesiveness (P>0.05).
The hardness of materials expresses their resistance to deformation, ie, the maximum force required to cause deformation of a sample.Citation60 Compressibility is defined as the work required to deform the formulation during the first compression of the probe.Citation61 The increase in water content and drug loading into the formulation led to the formation of a less packed network, owing to the interpenetration and entanglement into the lamellar mesophase. This phenomenon may have decreased hardness and compressibility values.
Adhesiveness is the work required to overcome the attractive forces between the surface of the sample and the surface of the probe.Citation61,Citation62 High adhesiveness and cohesiveness of the gel formulations ensure prolonged adhesion of the formulation to the biological surfaces and complete structural recovery following application.Citation61–Citation63 Certain characteristics are desirable for topical products, including patient acceptability, spreadability, adhesiveness, resistance to rubbing off, capacity to enhance drug release, and (when needed) ability to facilitate drug permeation into the skin.Citation64–Citation66 Moreover, these mechanical characteristics provide information about the interactions among system componentsCitation67 that is important in developing bioadhesive topical formulations.Citation18
Bioadhesion studies
Bioadhesive systems are advantageous, because they can prolong the residence time of the drug at the site of application – the skin. This prolonged contact decreases the frequency of application of the product, increases the bioavailability of the drug, and improve the consumer’s adherence to product application.Citation68–Citation70
Bioadhesive force values are shown in . Bioadhesive force showed significant differences (P<0.05) between mean values of the different formulations and between those of the loaded and unloaded formulations. No difference was observed between CB-25 and CB-25R (P>0.05). Drug loading slightly decreased mechanical and bioadhesive properties. As previously reported, addition of water or drug may alter the molecular structure and arrangement of lamellar mesophases.
Polymer dispersions, such as hydrogels, have been studied intensively for skin bioadhesion in topical cutaneous drug delivery,Citation70–Citation77 and showed good results for bioadhesion.Citation78–Citation80 The developed LCSs showed similar values, and thus these LCSs are good candidates as topical cutaneous drug-delivery systems, because these amphiphilic systems have shown bioadhesive ability, biocompatibility, and controlled release of drugs.Citation13,Citation15–Citation19,Citation35,Citation51,Citation52,Citation81–Citation87
In vivo anti-inflammatory effects
Guest molecules reside in an interconnected network and become part of the nanostructured architecture of the LC matrix.Citation20,Citation88–Citation90 Biological effects of the nanostructured systems were assessed using biological assays. Res has received considerable attention in several in vitro and in vivo studies, owing to its biological activities, particularly in skin disorders.Citation29 Furthermore, Res should be delivered to the site of action to attain an ideal response, intensify its therapeutic effects, and reduce its side effects.Citation30
The anti-inflammatory effects of the vehicles (CB-23 and CB-24) and loaded formulations (CB-23R and CB-24R) were evaluated in vivo. shows the anti-inflammatory activity of Res incorporated in the lamellar mesophase. The incorporation of Res into LC mesophases affected its intrinsic anti-inflammatory activity, as evidenced by edema inhibition in mouse paws.
Figure 5 Anti-inflammatory activity of Res-loaded formulations (CB-23R and CB-24R).
Notes: Res-unloaded formulations served as the vehicle, an ointment containing dexamethasone 0.5% (w:w) was used as the positive control, and untreated mice were considered the negative control. Data represent means ± standard deviation of five mice. The statistical significance of the differences in paw thickness between the groups was analyzed using analysis of variance followed by Dunnett’s multiple-comparison test. **P<0.01; #no significance.
Abbreviation: Res, resveratrol.
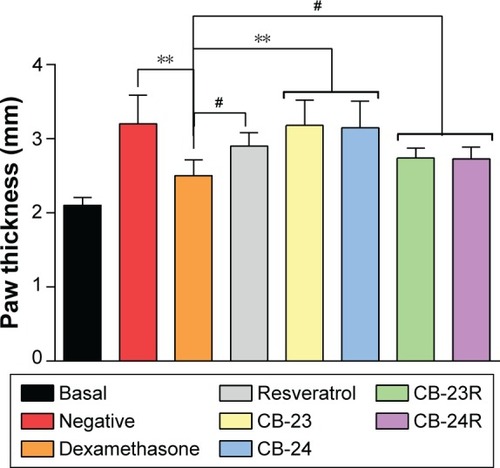
The negative control and unloaded formulations showed a statistically significant difference in activity compared to the dexamethasone group (P<0.01). No significant difference was observed between free-Res and Res-loaded LCSs (P>0.05). The maximal inhibition of inflammation was 63.4%, 27.4%, 42.2%, and 43.1% for dexamethasone, free Res, CB-23R, and CB-24R, respectively. The anti-inflammatory activity of Res-loaded systems was less than that of dexamethasone (0.5%, w:w) as a positive control.
Furthermore, lamellar phases formed by lipids are comparable to the structure of the cell membrane; therefore, they are exploited as model cell membranes.Citation91 As such, lamellar phases have been used as simple model systems for cell membranes to study the process of membrane fusion.Citation92,Citation93
Several assumptions can be made about the mechanism by which the lamellar mesophase affects drug penetration into the skin. First, the structural similarity between this system and skin cells may be responsible for the increase in drug penetration into deep layers of the skin after topical application.Citation12 Second, the surfactant or oil molecules can diffuse on the skin surface and act as permeation enhancers of Res, because they disrupt the lipid structure of the stratum corneum.Citation94 This facilitates diffusion across the barrier, which normally limits the penetration of substances. Moreover, this system may increase the solubility of the drug in the skin, which increases the partition coefficient of the drug between the skin and the vehicle.Citation95,Citation96
Conclusion
It was possible to develop Res-loaded lamellar LCSs containing copaiba balsam oil (20%–40% w:w), PEG-40 hydrogenated castor oil (40% w:w), and purified water (20%–40% w:w). Rheological and TPA data showed that both Res-loaded and unloaded LCSs had proper characteristics for skin administration, such as pseudoplasticity and adhesiveness. Moreover, all LCSs were as bioadhesive as conceptualized bioadhesive formulations, and in particular these LCSs were able to maintain the anti-inflammatory activity of Res. Therefore, it is feasible to conclude that these systems can be used for optimization of drug delivery into the skin for treatment of inflammatory skin diseases.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This work was financially supported by the National Council of Technological and Scientific Development (CNPq) in the form of an Initiation Scholarship in Innovation and Technological Development (PIBIT/CNPq) to grantee CYS and regular research grants by the São Paulo Research Foundation (FAPESP) under grant number 14/24180-0. We acknowledge the Brazilian Synchrotron Light Laboratory – LNLS (Campinas, SP, Brazil) staff for the use of their SAXS facilities and Programa de Apoio ao Desenvolvimento Científico (PADC) for their financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
- SigmundsdottirHImproving topical treatments for skin diseasesTrends Pharmacol Sci201031623924520413166
- Tuan-MahmoodTMMcCruddenMTTorrisiBMMicroneedles for intradermal and transdermal drug deliveryEur J Pharm Sci201350562363723680534
- MalikDSMitalNKaurGTopical drug delivery systems: a patent reviewExpert Opin Ther Pat201626221322826651499
- MooreLChienYWTransdermal drug delivery: a review of pharmaceutics, pharmacokinetics, and pharmacodynamicsCrit Rev Ther Drug Carrier Syst1988442853493133121
- SchoellhammerCMBlankschteinDLangerRSkin permeabilization for transdermal drug delivery: recent advances and future prospectsExpert Opin Drug Deliv201411339340724392787
- HadgraftJPassive enhancement strategies in topical and transdermal drug deliveryInt J Pharm199918411610425346
- PrausnitzMRLangerRTransdermal drug deliveryNat Biotechnol200826111261126818997767
- HuiZYingjieZXiaoyeYGuangxiZBreaking the skin barrier: achievements and future directionsCurr Pharm Des201521202713272425925124
- dos SantosFKOyafusoMHKiillCPDaflon-GremiãoMPChorilliMNanotechnology-based drug delivery systems for treatment of hyperproliferative skin diseases: a reviewCurr Nanosci201391159167
- DenetARVanbeverRPréatVSkin electroporation for transdermal and topical deliveryAdv Drug Deliv Rev200456565967415019751
- RigonRBOyafusoMFujimuraATNanotechnology-based drug delivery systems for melanoma antitumoral therapy: a reviewBiomed Res Int2015201584181726078967
- EstracanholliEAPraçaFSCintraABPierreMBLaraMGLiquid crystalline systems for transdermal delivery of celecoxib: in vitro drug release and skin permeation studiesAAPS PharmSciTech20141561468147524980082
- Borgheti-CardosoLNDepieriLVDinizHSelf-assembling gelling formulation based on a crystalline-phase liquid as a non-viral vector for siRNA deliveryEur J Pharm Sci201458728224726985
- Borgheti-CardosoLNDepieriLVKooijmansSAAn in situ gelling liquid crystalline system based on monoglycerides and polyethylenimine for local delivery of siRNAsEur J Pharm Sci20157410311725917525
- Borgheti-CardosoLNVicentiniFTGratieriTBentleyMVLiquid crystalline systems containing vitamin E TPGS for the controlled transdermal nicotine deliveryBraz J Pharm Sci2016521191200
- LopesLBLopesJLCOliveiraDCRLiquid crystalline phases of monoolein and water for topical delivery of cyclosporin A: characterization and study of in vitro and in vivo deliveryEur J Pharm Biopharm200663214615516621488
- PetrilliREloyJOPraçaFSLiquid crystalline nanodispersions functionalized with cell-penetrating peptides for topical delivery of short-interfering RNAs: a proposal for silencing a pro-inflammatory cytokine in cutaneous diseasesJ Biomed Nanotechnol20161251063107527305826
- da SilvaPBCalixtoGMJúniorJAStructural features and the anti-inflammatory effect of green tea extract-loaded liquid crystalline systems intended for skin deliveryPolymers20179130
- Fonseca-SantosBdos SantosAMRoderoCFDaflon GremiãoMPChorilliMDesign, characterization, and biological evaluation of curcumin-loaded surfactant-based systems for topical drug deliveryInt J Nanomedicine2016114553456227660447
- GuoCWangJCaoFLeeRJZhaiGLyotropic liquid crystal systems in drug deliveryDrug Discov Today20101523–241032104020934534
- DrummondCJFongCSurfactant self-assembly objects as novel drug delivery vehiclesCurr Opin Colloid Interface Sci199946449456
- ShahJCSadhaleYChilukuriDMCubic phase gels as drug delivery systemsAdv Drug Deliv Rev2001472–322925011311994
- BurrowsRCollettJHAttwoodDThe release of drugs from monoglyceride-water liquid crystalline phasesInt J Pharm19941113283293
- FongWKHanleyTBoydBJStimuli responsive liquid crystals provide ‘on-demand’ drug delivery in vitro and in vivoJ Control Release2009135321822619331865
- de SouzaALKiillCPdos SantosFKNanotechnology-based drug delivery systems for dermatomycosis treatmentCurr Nanosci201284512519
- SengottuvelanMDeepthaKNaliniNResveratrol ameliorates DNA damage, prooxidant and antioxidant imbalance in 1,2-dimethylhydrazine induced rat colon carcinogenesisChem Biol Interact2009181219320119523937
- KaruppagounderVArumugamSThandavarayanRAResveratrol attenuates HMGB1 signaling and inflammation in house dust mite-induced atopic dermatitis in miceInt Immunopharmacol201423261762325466270
- KjærTNThorsenKJessenNStenderupKPedersenSBResveratrol ameliorates imiquimod-induced psoriasis-like skin inflammation in micePLoS One2015105e012659925965695
- PangeniRSahniJKAliJSharmaSBabootaSResveratrol: review on therapeutic potential and recent advances in drug deliveryExpert Opin Drug Deliv20141181285129824830814
- SantosACVeigaFRibeiroAJNew delivery systems to improve the bioavailability of resveratrolExpert Opin Drug Deliv20118897399021668403
- CottartCHNivet-AntoineVLaguillier-MorizotCBeaudeuxJLResveratrol bioavailability and toxicity in humansMol Nutr Food Res201054171620013887
- WenzelESomozaVMetabolism and bioavailability of trans-resveratrolMol Nutr Food Res200549547248115779070
- WalleTBioavailability of resveratrolAnn N Y Acad Sci20111215191521261636
- YutaniRMoritaSYTeraokaRKitagawaSDistribution of polyphenols and a surfactant component in skin during aerosol OT microemulsion-enhanced intradermal deliveryChem Pharm Bull201260898999422863702
- OyafusoMHCarvalhoFCChiavacciLAGremiãoMPChorilliMDesign and characterization of silicone and surfactant based systems for topical drug deliveryJ Nanosci Nanotechnol201515181782626328446
- National Research CouncilGuide for the Care and Use of Laboratory Animals8th edWashingtonNational Academies Press2010
- TadrosTLiquid crystalline phaseEncyclopedia of Colloid and Interface ScienceHeidelbergSpringer2013682683
- RomstedLSIntroduction to surfactant self-assemblySteedJWGalePASupramolecular Chemistry: From Molecules to NanomaterialsHoboken (NJ)Wiley2012
- CarvalhoFCCamposMLPeccininiRGGremiãoMPNasal administration of liquid crystal precursor mucoadhesive vehicle as an alternative antiretroviral therapyEur J Pharm Biopharm201384121922723207328
- CarvalhoFCSarmentoVHChiavacciLABarbiMSGremiãoMPDevelopment and in vitro evaluation of surfactant systems for controlled release of zidovudineJ Pharm Sci20109952367237419967779
- MezzengaRPhysics of self-assembly of lyotropic liquid crystalsGartiNSomasundaranPMezzengaRSelf-Assembled Supramolecular Architectures: Lyotropic Liquid CrystalsHoboken (NJ)Wiley2012120
- ChongJYMuletXBoydBJDrummondCJSteric stabilizers for cubic phase lyotropic liquid crystal nanodispersions (cubosomes)IgličAKulkarniCVRappoltMAdvances in Planar Lipid Bilayers and Liposomes21Cambridge (MA)Academic Press2015131187
- GartiNLibsterDAserinALipid polymorphism in lyotropic liquid crystals for triggered release of bioactivesFood Funct20123770071322592749
- IsraelachviliJNMitchellDJNinhamBWTheory of self-assembly of lipid bilayers and vesiclesBiochim Biophys Acta19774702185201911827
- WardMDHornerMJStructure and order in soft matter: symmetry transcending length scaleCrystEngComm2004667401407
- MalmstenMSurfactants and Polymers in Drug DeliveryBoca Raton (FL)CRC Press2002
- LiQNanoscience with Liquid Crystals: From Self-Organized Nanostructures to ApplicationsHeidelbergSpringer2014
- HydeSTIdentification of lyotropic liquid crystalline mesophasesHolmbergKHandbook of Applied Surface and Colloid ChemistryHoboken (NJ)Wiley2002299332
- RamanIASuhaimiHTiddyGJLiquid crystals and microemulsions formed by mixtures of a non-ionic surfactant with palm oil and its derivativesAdv Colloid Interface Sci20031061–310912714672844
- HolmqvistPAlexandridisPLindmanBModification of the microstructure in poloxamer block copolymer-water-“oil” systems by varying the “oil” typeMacromolecules1997302267886797
- ChorilliMPrestesPSRigonRBStructural characterization and in vivo evaluation of retinyl palmitate in non-ionic lamellar liquid crystalline systemColloids Surf B Biointerfaces201185218218821411295
- FujimuraATMartinezRMPinho-RibeiroFAResveratrol-loaded liquid-crystalline system inhibits UVB-induced skin inflammation and oxidative stress in miceJ Nat Prod20167951329133827191910
- SoniSSBrotonsGBellourMNarayananTGibaudAQuantitative SAXS analysis of the P123/water/ethanol ternary phase diagramJ Phys Chem B200611031151571516516884230
- ChhabraRPNon-Newtonian fluids: an introductionKrishnanJMDeshpandeAPKumarPBRheology of Complex FluidsHeidelbergSpringer2010334
- JunqueiraMVBorghi-PangoniFBFerreiraSBRabelloBRHiokaNBruschiMLFunctional polymeric systems as delivery vehicles for methylene blue in photodynamic therapyLangmuir2016321192726673856
- NakanoMTeshigawaraTSugitaADispersions of liquid crystalline phases of the monoolein/oleic acid/Pluronic F127 systemLangmuir2002182492839288
- YaghmurAde CampoLSagalowiczLLeserMEGlatterOEmulsified microemulsions and oil-containing liquid crystalline phasesLangmuir200521256957715641825
- YaghmurAKriechbaumMAmenitschHSteinhartMLaggnerPRappoltMEffects of pressure and temperature on the self-assembled fully hydrated nanostructures of monoolein-oil systemsLangmuir20102621177118519681634
- MatosMGutiérrezGIglesiasOCocaJPazosCEnhancing encapsulation efficiency of food-grade double emulsions containing resveratrol or vitamin B12 by membrane emulsificationJ Food Eng2015166212220
- JonesDSWoolfsonADBrownAFTextural analysis and flow rheometry of novel, bioadhesive antimicrobial oral gelsPharm Res19971444504579144730
- JonesDSWoolfsonADBrownAFTextural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymersInt J Pharm19971512223233
- JonesDSWoolfsonADBrownAFO’NeillMJMucoadhesive, syringeable drug delivery systems for controlled application of metronidazole to the periodontal pocket: In vitro release kinetics, syringeability, mechanical and mucoadhesive propertiesJ Control Release19974917179
- BansalKRawatMKJainARajputAChaturvediTPSinghSDevelopment of satranidazole mucoadhesive gel for the treatment of periodontitisAAPS PharmSciTech200910371672319479385
- JonesDSLawlorMSWoolfsonADExamination of the flow rheological and textural properties of polymer gels composed of poly(methylvinylether-co-maleic anhydride) and poly(vinylpyrrolidone): rheological and mathematical interpretation of textural parametersJ Pharm Sci20029192090210112210055
- OzcanIAbacıOUztanAHEnhanced topical delivery of terbinafine hydrochloride with chitosan hydrogelsAAPS Pharm Sci Tech200910310241031
- HurlerJEngeslandAKermanyBPSkalko-BasnetNImproved texture analysis for hydrogel characterization: gel cohesiveness, adhesiveness, and hardnessJ Applied Polym Sci20121251180188
- FerreiraSBMoçoTDBorghi-PangoniFBJunqueiraMVBruschiMLRheological, mucoadhesive and textural properties of thermoresponsive polymer blends for biomedical applicationsJ Mech Behav Biomed Mater20155516417826590909
- ParenteMEAndradeAOAresGRussoFJiménez-KairuzÁBioadhesive hydrogels for cosmetic applicationsInt J Cosmet Sci201537551151825854849
- BonacucinaGCespiMMisici-FalziMPalmieriGFRheological, adhesive and release characterisation of semisolid Carbopol/tetraglycol systemsInt J Pharm2006307212914016297581
- CarvalhoFCCalixtoGHatakeyamaINLuzGMGremiãoMPChorilliMRheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systemsDrug Dev Ind Pharm201339111750175723216218
- Subheet KumarJRichaPDevelopment, characterization and in vivo localization study of topical 5-fluorouracil gels: a comparative study with conventional formulationCurr Drug Deliv201411340141424328603
- KhanMAPanditJSultanaYNovel Carbopol-based transfersomal gel of 5-fluorouracil for skin cancer treatment: in vitro characterization and in vivo studyDrug Deliv201522679580224735246
- ChoCWKimDBShinSCDevelopment of bioadhesive transdermal bupivacaine gels for enhanced local anesthetic actionIran J Pharm Res201211242343124250466
- GuoRDuXZhangRDengLDongAZhangJBioadhesive film formed from a novel organic-inorganic hybrid gel for transdermal drug delivery systemEur J Pharm Biopharm201179357458321723945
- PeppasNABuresPLeobandungWIchikawaHHydrogels in pharmaceutical formulationsEur J Pharm Biopharm2000501274610840191
- AnNMKimDDShinYHLeeCHDevelopment of a novel soft hydrogel for the transdermal delivery of testosteroneDrug Dev Ind Pharm20032919910512602497
- ShinSCKimHJOhIJChoCWYangKHDevelopment of tretinoin gels for enhanced transdermal deliveryEur J Pharm Biopharm2005601677115848058
- CarvalhoFCBruschiMLEvangelistaRCGremiãoMPMucoadhesive drug delivery systemsBraz J Pharm Sci2010461117
- MachidaYNagaiTBioadhesive preparations as topical dosage formsBioadhesive Drug Delivery SystemsBoca Raton (FL)CRC Press1999641658
- HorstmannMMüllerWAsmussenBPrinciples of skin adhesion and methods for measuring adhesion of transdermal systemsBioadhesive Drug Delivery SystemsBoca Raton (FL)CRC Press1999175195
- de SilvaHRSurfactant-based transdermal system for fluconazole skin deliveryJ Nanomed Nanotechnol2014551000231
- OliveiraMBdo PradoAHBernegossiJTopical application of retinyl palmitate-loaded nanotechnology-based drug delivery systems for the treatment of skin agingBiomed Res Int2014201463257024772430
- CintraGPintoLCalixtoGBioadhesive surfactant systems for methotrexate skin deliveryMolecules2016212E23126901183
- LiangXChenYLJiangXJWangSMZhangJWGuiSYHII mesophase as a drug delivery system for topical application of methyl salicylateEur J Pharm Sci201710015516228063969
- CarvalhoALSilvaJALiraAAEvaluation of microemulsion and lamellar liquid crystalline systems for transdermal zidovudine deliveryJ Pharm Sci201610572188219327220471
- KadhumWRTodoHSugibayashiKSkin permeation: enhancing ability of liquid crystal formulationsDragicevicNMaibachHIPercutaneous Penetration Enhancers: Chemical Methods in Penetration Enhancement – Drug Manipulation, Strategies, and Vehicle Effects2015243253
- UchinoTMurataAMiyazakiYOkTKagawaYGlyceryl monooleyl ether-based liquid crystalline nanoparticles as a transdermal delivery system of flurbiprofen: characterization and in vitro transportChem Pharm Bull201563533434025948327
- SagalowiczLMezzengaRLeserMEInvestigating reversed liquid crystalline mesophasesCurr Opin Colloid Interface Sci2006114224229
- Amar-YuliILibsterDAserinAGartiNSolubilization of food bioactives within lyotropic liquid crystalline mesophasesCurr Opin Colloid Interface Sci20091412132
- NegriniRMezzengaRpH-responsive lyotropic liquid crystals for controlled drug deliveryLangmuir20112795296530321452814
- SimonsKVazWLModel systems, lipid rafts, and cell membranesAnnu Rev Biophys Biomol Struct20043326929515139814
- SiegelDPThe modified stalk mechanism of lamellar/inverted phase transitions and its implications for membrane fusionBiophys J19997612913139876142
- SiegelDPInverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal lipid phases – II: implications for membrane-membrane interactions and membrane fusionBiophys J1986496117111833719075
- PhamQDBjörklundSEngblomJTopgaardDSparrEChemical penetration enhancers in stratum corneum: relation between molecular effects and barrier functionJ Control Release201623217518727108613
- BarelAOPayeMMaibachHIHandbook of Cosmetic Science and Technology2nd edBoca Raton (FL)CRC Press2005
- WilliamsACBarryBWPenetration enhancersAdv Drug Deliv Rev201264Suppl128137

