Abstract
Background
Silver nanoparticles (AgNPs) are widely used in industrial and household applications, arousing concern regarding their safety in humans. The risks posed by stabilizer-coated AgNPs continue to be unclear, and assessing their toxicity is for an understanding of the safety issues involved in their use in various applications.
Purpose
We aimed to investigated the long-term toxicity of citrate-coated silver nanoparticles (cAgNPs) in liver tissue using several toxicity tests and transcriptomic analysis at 7 and 28 days after a single intravenous injection into rabbit ear veins (n=4).
Materials and methods
The cAgNPs used in this study were in the form of a 20% (w/v) aqueous solution, and their size was 7.9±0.95 nm, measured using transmission electron microscopy. The animal experiments were performed based on the principles of good laboratory practice.
Results
Our results showed that the structure and function of liver tissue were disrupted due to a single exposure to cAgNPs. In addition, in vivo comet assay showed unrepaired genotoxicity in liver tissue until 4 weeks after a single injection, suggesting a potential carcinogenic effect of cAgNPs. In our transcriptomic analysis, a total of 244 genes were found to have differential expression at 28 days after a single cAgNP injection. Carefully curated pathway analysis of these genes using Pathway Studio and Ingenuity Pathway Analysis tools revealed major molecular networks responding to cAgNP exposure and indicated a high correlation of the genes with inflammation, hepatotoxicity, and cancer. Molecular validation suggested potential biomarkers for assessing the toxicity of accumulated cAgNPs.
Conclusion
Our investigation highlights the risk associated with a single cAgNP exposure with unrepaired damage persisting for at least a month.
Introduction
Engineered nanoparticles (NPs) have unique physiochemical properties. Silver NPs (AgNPs) are frequently used in the production of a variety of commercial products including creams and ointments, cosmetics, and paint.Citation1–Citation3 The antimicrobial nature of AgNPs has been exploited in different industries in bactericidal and fungicidal coatings, and in medical devices, water purification, and air quality management.Citation4,Citation5 All of these applications have increased the environmental exposure of AgNPs, which has raised concern regarding potential toxicity hazards to human health.Citation1,Citation6 Indeed, AgNP toxicity on growth and reproduction has been described in invertebrates such as the waterfleaCitation7 and vertebrates such as fish.Citation8 Because NPs are easily agglomerated in the molecular environment, AgNPs are commonly used as coatings to stabilize nanosized objects. Citrate is the most common stabilizer in the synthesis process, and it imparts a negative surface charge.Citation9,Citation10 Recent studies have reported the effects of stabilizer-coated AgNPs in vitro, indicating cyto- and genotoxicity of such coated AgNPs.Citation11–Citation13 Recent studies have also reported the potential toxicity of AgNPs to humans;Citation3,Citation14 however, the mechanisms involved have not yet been clearly identified.
AgNPs that are absorbed in mammals may be circulated and distributed to various soft tissues including liver, spleen, blood, kidney, and brain.Citation3,Citation15,Citation16 Liver has been proposed as a major target organ for the evaluation of AgNP toxicity in rodents.Citation17,Citation18 A significant quantity of AgNPs is detected in the liver of rats following a 90-day oral administration.Citation17 In addition, several toxicological studies have reported that AgNPs might cause hepatotoxicity due to the generation of reactive oxygen species (ROS).Citation19–Citation21 However, mechanisms of AgNP toxicity in different tissues are still unclear. Thus, the usage of AgNPs in various applications remains widespread, potentially threatening human health.
Toxicogenomic approaches have been used in recent assessment of AgNP toxicity.Citation22,Citation23 Toxicogenomics has been emerged in toxicological research, following the development of genomics technology. It is also used in bioinformatics to better understand the molecular mechanisms of toxicants in biosystems.Citation24,Citation25 Microarray technology permits the examination of the transcriptome, which potentially includes the global genomic profile of cellular responses at the mRNA level. The majority of microarray analyses to date have focused on disease-related mechanisms.Citation26 Recently, a meaningful interpretation of transcriptomic data has been emphasized to provide a comprehensive view of biological responses to chemicals.Citation24 To gain insights of interest from complex and dynamic biological systems, a thorough review of published reports and integration of information is necessary. Currently, various companies and researchers have developed pathway analysis software which functions via computational text-mining and analysis of vast amounts of literature. Toxicogenomics enables the prediction of mode of action and deeper knowledge of mechanism of action, even when little is known regarding the compounds of interest.Citation25
Only a few studies have addressed alterations in the transcriptome due to in vivo AgNP exposure.Citation22,Citation27 In the present study, we investigated the in vivo toxicity of stabilized AgNps in a rabbit model following the organization for economic cooperation and development (OECD) principles of good laboratory practice (GLP). Rabbit is one of the most commonly used animal models for standardized developmental toxicity testing of pharmaceuticals and chemicals including that of the human teratogen thalidomide.Citation28 We used citrate-coated, stabilized AgNPs (cAgNps) in the present study. In our previous study, we investigated time-dependent kinetics of cAgNPs after a single intravenous injection and found that liver was the main site for the accumulation and pigmentation of cAgNPs with harmful effects was observed over a prolonged period.Citation29 Hepatotoxicity was one of the effects observed, which was evaluated by detecting transcriptional alterations, cytotoxicity, generation of oxidative stress, and genotoxicity. Two different doses of cAgNPs were intravenously injected into rabbits, and toxicology analyses were conducted at different time points. The purpose of the present study was to assess unrepaired damage in rabbit liver tissue in terms of genotoxicity, oxidative stress, and differential gene expression, after a single injection of cAgNPs.
Materials and methods
Chemicals
Purified (99.98% pure) and colloidal AgNPs coated with citrate (cAgNPs; SARPU 200 KW) were obtained from ABC NANOTECH (Daejeon, Republic of Korea). We have previously described their characteristics.Citation29 The cAgNPs were provided as a 20% (w/v) aqueous stock solution. Particle size was 7.9±0.95 nm as determined using transmission electron microscopy. The average±SD zeta potential of 10 ppm aqueous cAgNP suspensions was −17.55±4.16 mV. The suspension of cAgNPs for intravenous injection was prepared by diluting the stock cAgNPs with isotonic 5% glucose solution to final concentrations of 0.25 and 2.5 mg/mL, which corresponded to 0.5 mg/kg (low dose) and 5 mg/kg (high dose) when injected into rabbits at 2 mL/kg body weight.
Animals and treatment
The animals were handled following the principles of GLP. Specific pathogen-free New Zealand White rabbits were purchased from Samtako Bio Korea Company (Osan, Gyeonggi-do, Republic of Korea). They were allowed to adapt to the animal room conditions for 1 week prior to the initiation of the study. Further details have been provided previously.Citation29 Briefly, four male rabbits received a single injection of the low- or high-dose cAgNps through an ear vein. The doses were chosen per preliminary experiments based on OECD test guideline 417. No rabbit died and no significant adverse effects that might affect the kinetics study were observed during the study period. Following the single injection of cAgNPs, liver tissues of rabbits were isolated for toxicity tests and microarray analysis at 7 and 28 days after the injection.
Animal ethics
All animal procedures were reviewed and approved by the animal ethics committee of the Institutional Animal Care and Use Committee of ChemOn Inc., a contract research organization for non-clinical area (Reference number: 11-B020; Gyeonggi-do, Republic of Korea). Animal care procedures were performed in accordance with the principles outlined in the “Guide for the Care and Use of Laboratory Animals” issued by the Animal Care and Committee of National Veterinary Research and Quarantine Service and with the guidelines of the Institutional Animal Care and Use Committee of ChemOn Inc.
ALP and lactate dehydrogenase (LDH) assays
The ALP assay was performed using a tartrate-resistant acid phosphatase and ALP Assay kit (cat. no MK301; Takara Bio, Shiga, Japan). The LDH assay kit was purchased from Sigma-Aldrich Co. (cat. no tox7; St Louis, MO, USA). Liver tissues and lysis solution (cat. no L2152; Sigma-Aldrich Co.) were added at a 1:10 ratio. The tissues were homogenized and incubated for 45 minutes.
TUNEL assay
The TUNEL assay was performed using a DeadEnd™ Fluorometric TUNEL System kit (cat. no G3250; Promega Corporation, Fitchburg, WI, USA). After removing the specimens and rehydrating the paraffin, the slides were washed in 0.85% NaCl in PBS for 5 minutes. The slides were fixed with 4% formaldehyde in PBS followed by washing with PBS. A 100 µL aliquot of a 20 µg/mL Proteinase K solution was added and incubated at room temperature for 8–10 minutes. Then, the slides were sequentially immersed in PBS for 5 minutes, 4% formaldehyde in PBS for 5 minutes, and PBS for 5 minutes. A 100 µL aliquot of equilibration buffer was added and incubated at room temperature for 5–10 minutes. A 50 µL aliquot of the terminal deoxynucleotidyl transferase reaction mix was added to the tissue and incubated for 60 minutes at 37°C in a humidified chamber. This and the subsequent steps were done in the dark. The coverslips were removed, and the slides were immersed in 2× saline-sodium citrate buffer for 15 minutes followed by three washes of 5 minutes each in PBS. Specimens were mounted and visualized with Vectashield® (Vector Laboratories, Burlingame, CA, USA) and DAPI. Localized green fluorescence of the apoptotic tissue was observed using confocal fluorescence microscopy. DAPI-stained nuclei were blue.
Malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) ELISA
The MDA assay was performed by using an OxiSelect™ MDA Adduct ELISA kit (cat. no STA-332–5; Cell Biolabs, Inc., San Diego, CA, USA). The HNE assay was done using an OxiSelect™ HNE Adduct ELISA kit (cat. no STA-338–5; Cell Biolabs, Inc.). BSA standards or protein samples (10 µg/mL) were adsorbed in wells of a 96-well plate for 2 hours at 37°C. The MDA- or HNE-protein adducts present in the sample or standard were probed with anti-MDA or anti-HNE antibody, followed by horseradish peroxidase-conjugated secondary antibody. Content of each protein adduct in the unknown sample was determined by comparing the OD values with those of a standard curve prepared using predetermined target protein-BSA standards.
Comet assay
The alkaline (pH.13) comet assay for quantifying DNA lesions was conducted following the protocol used in the international validation of the in vivo rodent alkaline comet assay for detecting genotoxic carcinogens. Rabbit livers were collected and minced using scissors in cold mincing buffer. The mincing buffer consisted of Mg++ and Ca++ free Hank’s Balanced Salt Solution (Thermo Fisher Scientific, Waltham, MA, USA) with 20 mM Na EDTA (Sigma-Aldrich Co.) and 10% v/v dimethyl-sulfoxide (Sigma-Aldrich Co.). The comet assay protocol has been previously described.Citation30
Microarray
After sacrificing the rabbits, liver tissue was obtained and preserved in liquid nitrogen. RNA was extracted immediately to avoid any deterioration and was isolated using an RNeasy Mini kit 250 (cat. no 74106; Qiagen NV, Venlo, the Netherlands) following the manufacturer’s recommendations. RNA quality was determined using the Bioanalyzer Nano Chip 2100 (Agilent Technologies, Santa Clara, CA, USA). Total RNA (1 µg) was used for amplification and labeling using the Low Input Quick Amp Labeling Kit (Agilent Technologies) as described by the manufacturer. Yields of cRNA and the dye-incorporation rate were measured with an ND-1000 spectrophotometer (NanoDrop Technologies, Winooski, VT, USA). The hybridization procedure was performed per the Two-Color Microarray-Based Gene Expression Analysis protocol using a Gene Expression Hybridization Kit (Agilent Technologies). Briefly, 825 ng of the corresponding Cy3- and Cy5-labeled cRNA was combined and hybridized overnight in a 65°C hybridization chamber with a Rabbit Gene Expression Microarray 4×44K (ID G2519F-020908; Agilent Technologies). The array was scanned using a DNA microArray scanner (Agilent Technologies), and probe signals were quantified using Feature Extraction software (version 10.2.1.3; Agilent Technologies). Normalized data were analyzed using platform v1.16.4376 (Subio Inc., Kagoshima, Japan).
Functional interaction and gene pathway analysis
Pathway Studio web-based edition 11.0.6 software (Ariadne Genomics, Rockville, MD, USA) and Ingenuity Pathway Analysis (Ingenuity Systems; Qiagen NV) were used to investigate the functional interactions and possible pathways among the genes identified from the microarray experiment. These software help in assessing the biological significance of genes by analyzing their interactions with related cell processes and diseases. Pathway Studio uses an inbuilt RESNET database of molecular interactions based on text-mining and processing of scientific abstracts in PubMed.Citation31 It also utilizes all the existing pathway database and identifies connections among all proteins in a new specific pathway. Ingenuity Pathway Analysis is based on the comprehensive, manually curated content of the Ingenuity Knowledge Base.Citation32 It helps predict upstream regulators and downstream effects in biological and disease processes.
Reverse transcription PCR (RT-PCR) and quantitative real-time PCR
Conventional RT-PCR for the rabbit genes identified from the microarray experiments was performed. Quantitative real-time PCR was performed using SYBR premix Ex Taq (Takara) on an Applied Biosystems 7500 Real-Time PCR system (Thermo Fisher Scientific). The PCR primers were synthesized by Integrated DNA technology (Coralville, IA, USA). All samples were run in duplicate, and the mRNA levels were normalized to those of glyceraldehyde-3-phosphate dehydrogenase.
Results
Damage to liver function and tissue structure due to cAgNPs
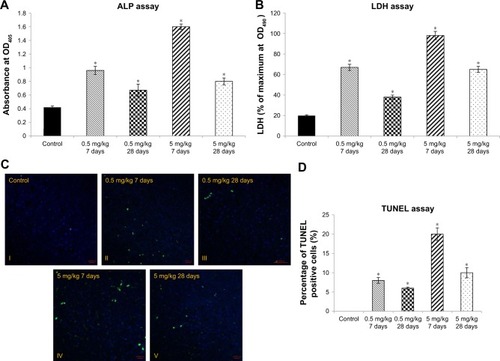
To evaluate liver toxicity of cAgNPs, we initially examined the level of common liver function markers, ALP and LDH. Increased ALP, which is an enzyme related to bile ducts, is associated with drug-induced cholestasis and liver damage in humans.Citation33 The ALP level increased in a dose-dependent manner and decreased in a time-dependent manner in response to cAgNP treatment (). LDH catalyzes the interconversion of lactate and pyruvate as a key step in aerobic glycolysis.Citation34 The level of LDH showed a pattern of expression that was similar to ALP (). In addition, the TUNEL assay data showed increased apoptosis in the cAgNP-exposed rabbit liver tissue in a dose-dependent manner, with a subsequent time-dependent decline (). Apoptosis increased significantly with the high dose (5 mg/kg) cAgNP treatment compared to the low dose (0.5 mg/kg) treatment at day 7, with a decline at day 28 in both the groups. The results indicated that the high dose of cAgNPs was more toxic and that tissue damage persisted in the rabbit liver tissue.
Figure 1 Liver tissue damage caused by cAgNP treatment.
Notes: ALP and LDH levels were used as enzymatic indication of cAgNP liver toxicity (A and B, respectively). The high dose (5 mg/kg) resulted in greater levels of both the enzymes than the low dose (0.5 mg/kg), and the enzyme levels decreased but persisted till the end of the experiment at both the treatment doses. (C and D) show results of the TUNEL assay and statistical analysis of the apoptosis-positive cells scoring in cAgNP-treated liver tissue, respectively, where the high dose (5 mg/kg) resulted in more TUNEL-positive cells than the low dose (0.5 mg/kg), and TUNEL-positive cells decreased at the end. The asterisk indicates a significant difference compared to the control group (P-value <0.05).
Abbreviations: cAgNP, citrate-coated silver nanoparticle; LDH, lactate dehydrogenase.
Oxidative stress and DNA damage induced in cAgNP-treated rabbit liver tissue
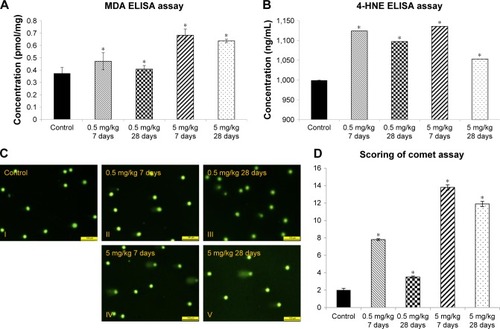
Lipid peroxidation is a well-defined mechanism of response to cellular damage. Peroxidation is an indicator of unstable oxidative stress that involves decomposition to form more complex and reactive compounds such as MDA and 4-HNE.Citation35 MDA and HNE expression in the rabbit liver was higher in the 5 mg/kg group than in the 0.5 mg/kg group at day 7 and subsequently decreased with time (). To assess the genotoxicity of cAgNPs in liver tissue, the in vivo alkaline comet assay was performed with dose-dependent or time-dependent exposure. Assessment of in vivo genotoxic potency is a major step to predict in vivo carcinogenicity. DNA strand breaks in liver tissue dose-dependently increased after cAgNP treatment; interestingly, the DNA damage had slightly declined in the high-dose treated group after 28 days compared to the damage at day 7 (). These results demonstrated dose- and time-dependent changes in oxidative stress and DNA damage after a single injection of cAgNPs.
Figure 2 Increased oxidative stress in cAgNP-treated rabbit liver tissue.
Notes: In the malondialdehyde (MDA) assay (A) and the 4-hydroxynonenal (4-HNE) assay (B), levels of oxidative stress markers were increased significantly, and the levels were not recovered after 28 days of cAgNPs treatment. (C) The oxidative DNA damage was increased in cAgNP-treated liver tissue. The high dose (5 mg/kg) resulted in more DNA damage than the low dose (0.5 mg/kg) in the comet assay analysis. (D) Statistical analysis showed that the DNA damage at the high dose was repaired slightly after 28 days. The scale bar is 100 µm. The asterisk indicates a significant difference compared to the control group (P-value <0.05).
Abbreviation: cAgNP, citrate-coated silver nanoparticle.
Global gene expression profiling under cAgNP exposure in liver tissue
Microarrays are widely used for transcriptome analysis. To investigate vestigial damages in liver tissue, microarray analysis using GeneString was done to identify differentially expressed genes 28 days after exposure to 5 mg/kg cAgNPs. Genes showing a change of 1.5-fold with P<0.05 were selected for further analysis to identify potential biomarkers of cAgNP exposure. Overall, the expression of 244 genes was altered with 134 being upregulated and 110 being downregulated. Biological interactions between the genes with significantly altered expression were analyzed using Pathway Studio (Elsevier, Amsterdam, the Netherlands) and Ingenuity Pathway Analysis (Qiagen NV).
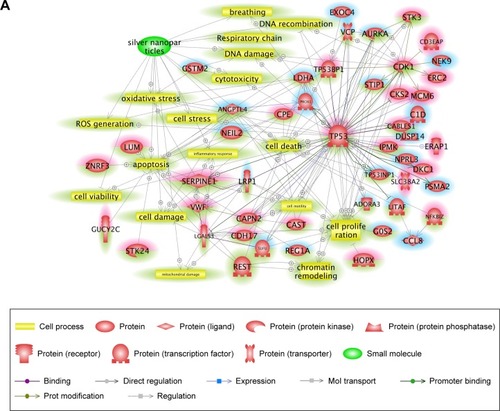
Pathway Studio is based on performing text-mining from published reports. By using Pathway Studio, direct interactions among the differentially expressed genes were identified, and functional relations with the genes were analyzed in terms of cellular processes () and diseases (). Up- and downregulated genes were highlighted in red and blue, respectively. In the network analysis, ROS generation, cellular stress, and oxidative stress among the cellular process-related pathways were highly connected with our input genes. In disease-related pathways, inflammation and hepatotoxicity were mostly connected with the differentially expressed genes. Among the genes, TP53, CDK1, SERPINE1, AURKA, and VWF had several connections with other elements. The identified cell processes and diseases and the significantly associated genes were considered potential key factors involved in cAgNP toxicity.
Figure 3 Signaling pathways associated with specifically regulated genes 28 days after cAgNP exposure.
Notes: Pathway Studio was used to explore the interactions among differentially expressed genes. (A) Cell process-related pathways with the genes identified 28 days after exposure were analyzed in the context of AgNPs. (B) Disease-related pathways with the genes are indicated in a purple box. Up- and downregulated genes 28 days after cAgNP exposure are highlighted in red and blue, respectively. The green highlight indicates AgNP-associated cell processes, diseases, or related genes.
Abbreviation: cAgNP, citrate-coated silver nanoparticle.
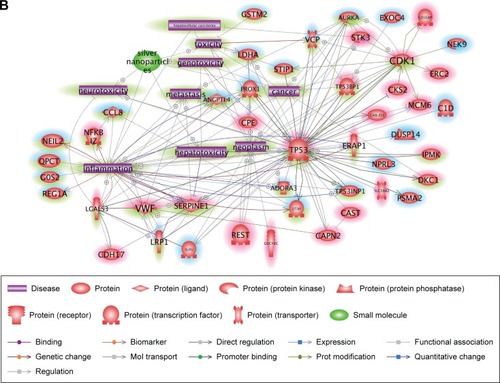
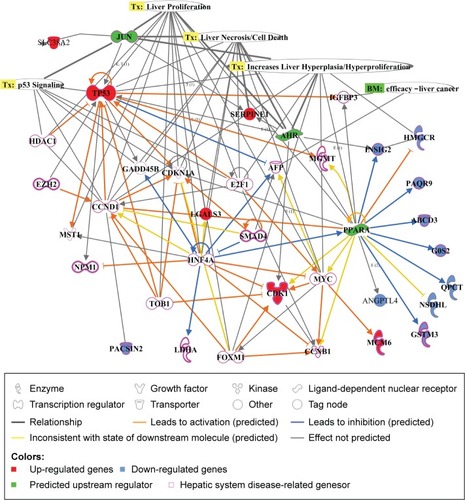
Ingenuity Pathway Analysis explores gene expression data sets through algorithms integrating literature-supported relationships between a variety of contexts in the Ingenuity Knowledge Base.Citation32 Differentially expressed genes were analyzed 28 days after cAgNP treatment, and the top-ranked network among the genes and association with liver proliferation, liver necrosis, increase of liver hyperplasia, and p53 signaling were identified (). The upstream regulators AHR, JUN, and PPARA were predicted by this tool, indicating their relevance to liver toxicity and their predicted role in gene modulators affected by cAgNPs, even though their expression levels were not significantly altered. The analysis found that CDK1, SERPINE1, TP53, and INSIG2 were modulated by at least two of the predicted upstream regulators and were associated with hepatic system disease-related genes. These findings suggest potential biomarkers of cAgNP toxicity after 28 days of treatment.
Figure 4 Molecular relationships among the cAgNP-affected genes 28 days after exposure.
Notes: Ingenuity Pathway Analysis was used for understanding the biological interactions between up- and downregulated genes induced 28 days after cAgNP exposure (highlighted in red and blue, respectively). The green highlight represents the upstream regulators predicted by Ingenuity Pathway Analysis. The pink-bordered elements are related to hepatic system diseases.
Abbreviation: cAgNP, citrate-coated silver nanoparticle.
Identification of biological networks in response to cAgNP exposure
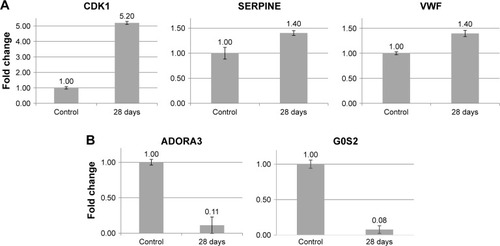
To obtain insight into more specific mechanisms of cAgNP toxicity, the pathways were analyzed in greater depth using Pathway Studio considering the number of references and connectivity, to identify major associations. Results of the network analysis indicated reliable correlations. Meticulous curation of pathways revealed major networks of genes related to the persistent toxicity of cAgNPs (). There were eight upregulated and five downregulated genes, considering key elements in the cAgNP toxicity pathway. Quantitative RT-PCR validation identified serpin family E member 1 (SERPINE1), cyclin-dependent kinase 1 (CDK1), von Willebrand factor (VWF), Adenosine A3 Receptor (ADORA3), and G0/G1 switch 2 (G0S2) consistent with the results of the microarray analysis (). These data provide important evidence for the discovery of molecular biomarkers to assess cAgNP exposure risk.
Figure 5 Core networks of significantly AgNP-associated genes.
Notes: The simplified pathway shows major associations among the genes related to the toxicity of retained cAgNPs and was carefully curated based on the number of references and connectivity. The pathway is derived from previous complex interactions analyzed by Pathway Studio or Ingenuity Pathway Analysis. Red and blue highlights indicate up- and downregulated genes, respectively. The green highlight indicates AgNP-associated cell processes, diseases, or related genes.
Abbreviation: cAgNP, citrate-coated silver nanoparticle.
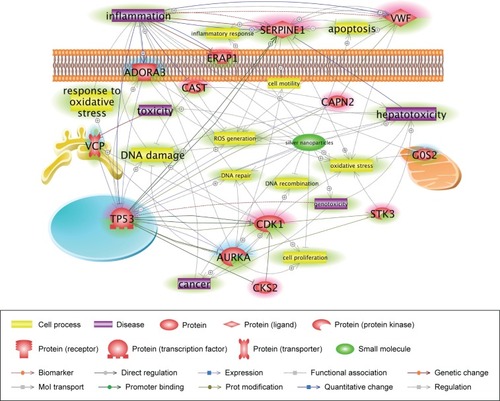
Figure 6 Validation of potential biomarkers of cAgNP exposure in rabbit liver tissue using qRT-PCR.
Notes: (A and B) Illustrate expression of up- and downregulated genes, respectively. Bars indicate the gene expression levels measured using qRT-PCR. The Y-axis denotes the fold-expression change of the respective genes.
Abbreviation: cAgNP, citrate-coated silver nanoparticle.
Discussion
The widespread use of AgNPs in industrial and household applications has raised concern regarding their safety. Although various studies have demonstrated the risk of AgNP toxicity upon human exposure, the evidence remains unclear due to variability in the studies including those in particle size, shape, and dispersion medium.Citation3,Citation6,Citation14,Citation36 A recent study showed synergistic toxicity of AgNPs in terms of oxidative stress under coexistence condition with silver ion in Escherichia coli.Citation37 Some studies have reported that the shape of surfactant-coated AgNPs could affect the level of toxicity in bacteria or invertebrates;Citation38–Citation40 however, there is insufficient molecular studies of true toxicity of AgNPs in mammalian. AgNP toxicity might depend on stabilizer. In present study, AgNPs were coated with the most common stabilizer, citrate, and the cAgNP size was estimated to be 7.9±0.95 nm in our previous study.Citation29 In particular, our research focused on dose- and time-dependent alterations in cAgNP-induced toxicity after a single injection of the particles and the transcriptional changes due to retained AgNPs at 28 days after the injection.
Though the toxicity of AgNPs is still unclear, increasing evidence does indicate a human safety concern.Citation14,Citation41 Coated AgNPs can enter the systemic circulation through damaged skin or other pathways, subsequently being distributed to other organs including liver, kidney, and brain.Citation3,Citation36,Citation42 The absorbed AgNPs are likely to pass through liver tissue first, resulting in liver accumulation.Citation4,Citation43 Two different studies on AgNP toxicity using a rat model but different routes of exposure (intravenous injection or inhalation) showed contrasting results; one study reported that treatment with AgNPs induced clinical markers of liver damage,Citation41 whereas the other study reported no adverse toxicological effects.Citation44 In our previous study, serum concentration of silver, histopathological damage, and inflammatory response in liver tissue were found to be higher compared with other tissue parameters.Citation29 To clarify the toxic effects on liver tissue in depth, the present study investigated in vivo toxicity of stabilized cAgNPs using rabbit liver tissue by conducting several toxicity tests and transcriptomic analyses.
NP deposition in liver tissue is followed by the penetration of the particles into the cells and altered enzyme activityCitation45 and may cause changes in enzymatic composition.Citation46 In the present study, we evaluated toxicity on liver tissue after cAgNP exposure by detecting enzymatic markers of liver function and level of apoptosis. Significant changes in both ALP and LDH levels after cAgNP treatment were evident (). LDH and ALP are indicators of hepatocellular damage, with increased levels indicating liver damage.Citation46 The results of the TUNEL assay revealed increased apoptosis after cAgNP injection. cAgNPs induced liver tissue damage up to 28 days after exposure. These data reveal that cAgNPs can provoke continued and prolonged damage of liver tissue structure and function after a single exposure to the particles.
NPs induce oxidative stress by generating ROS, suggesting a common mechanism for cytotoxicity and genotoxicity.Citation36,Citation47,Citation48 Highly reactive ROS are generated by accumulated NPs in mitochondria and lysosomes, leading to major structural damage to proteins and DNA.Citation44,Citation48,Citation49 Our observations led us to check the oxidative stress level of lipid peroxidation in rabbit liver tissue in response to cAgNP exposure (). The results supported previous observations that a high dose of cAgNPs might result in higher amounts of MDA and 4-HNE, which slightly decrease over time. Detecting these end-products of lipid peroxidation is a very reliable assay of oxidative damage, and they are also accepted as oxidative stress markers. Moreover, intracellular ROS production results in DNA damage, lipid peroxidation, and protein oxidation,Citation50 which leads to cellular component damage to tissues. Likewise, our results showed that a high dose of cAgNPs produced more DNA strand breaks than a low dose. Interestingly, recovery from DNA damage after 28 days was unclear in the high-dose treated groups, with a similar pattern in the MDA level. The in vivo comet assay results revealed that oxidative stress is a major mechanism for cAgNP-mediated toxicity in rabbit liver tissue, suggesting continuous genomic instability due to a single cAgNP exposure that persists (28 days in this study). Indeed, a wealth of evidence indicates that accumulation of DNA damage leads to loss of genomic integrity, which can induce carcinogenesis. Thus, DNA damage is considered as an initial hallmark of cancer.Citation51–Citation53 It is conceivable that the consequences of cAgNP toxicity include increased risk of cancer.
Results of transcriptomic analysis in the assessment of toxicogenomic responses can help explain molecular mechanisms and signaling pathways and help discover toxicant-related biomarkers valuable in chemical risk assessment.Citation54 To date, there have been only a few gene expression studies implicating AgNPs as a toxic substance in mammalian cell lines or animal models including mouse, rat, and daphnia.Citation55,Citation56 The present findings reveal that cAgNP-induced damages can persist. The gene expression profile was explored using cDNA microarray to investigate the toxic effects of AgNPs 28 days after a single injection of 5 µg/mL AgNPs. A total of 244 genes were differentially expressed with biological significance (fold change >1.5 and P-value <0.05). Knowledge-based network analysis using Pathway Studio and Ingenuity Pathway analysis tools revealed that similar to the toxicity test results, genes involved in cell death, apoptosis, and oxidative stress were affected by the presence of cAgNPs. In disease-related pathway analysis, the genes identified were involved in inflammation, toxicity, and cancer. These data are consistent with other reports and indicate potential responses involving differentially expressed genes at different time periods after cAgNP exposure.
However, the analyzed pathways were too complicated to identify specific important genes among the cAgNP-responsive genes. Accordingly, the pathways were carefully curated with various considerations including the number of references, connectivity with other data, and association with liver tissue. Major networks were sorted and key proteins were identified as potential biomarker candidates of cAgNP toxicity. After validation, CDK1, SERPINE1, VWF, ADORA3, and G0S2 were determined as key proteins. G0S2 also has a protective function as a proapoptotic factor in human cancer cells,Citation57 although it is known to be a master regulator of lipolysis in adipose and liver tissues.Citation58,Citation59 ADORA3 is one of the G-protein-coupled receptors with a crucial role in intracellular signaling pathways.Citation60 Various studies have demonstrated its protective role in inflammation-related diseasesCitation61,Citation62 and that downregulation of ADORA3 induces abnormal signal transduction causing tissue damage. Also, SERPINE1 (also known as plasminogen activator inhibitor-1) and VWF, which were upregulated in the presence of cAgNPs, have been reported as diagnostic biomarkers of inflammation,Citation63 and both genes induce oxidative stress under toxic conditions.Citation64,Citation65 Indeed, the correlation between AgNPs and the induction of inflammation and oxidative stress was reported in a human liver cell line.Citation66 CDK1 has a recognized role in cell cycle checkpoints at the G1/S and G2/M phase transitions.Citation67 It was upregulated by cAgNP treatment. Interestingly, its overexpression is strongly associated with carcinogenesis.Citation68,Citation69 The present data from toxicity tests also suggest the potential carcinogenicity of cAgNPs in terms of continuous genomic instability. Our results suggest that induced gene expression up to 28 days after a single injection of cAgNPs leads to toxic dysfunction of liver tissue. These genes could be considered as potential biomarkers for assessing the toxicity of accumulated cAgNPs.
Our results showed that cAgNPs were toxic to rabbit liver. Rabbit is a proven toxicity model for several human conditions. To our knowledge, no previous information regarding cAgNP-induced genotoxicity or its carcinogenic risk in a rabbit model has been reported. We chose liver as the target organ because of its central role in metabolism and it is vital in the clearance of toxic compounds from the body. We analyzed apoptosis, oxidative stress, and DNA damage, which can be used to assess NP toxicity in vivo. We successfully evaluated cAgNP toxicity in rabbit liver and discovered potential biomarkers for prolonged cAgNP toxicity using microarray analysis, bioinformatics network analysis, and qRT-PCR. Taken together, the results suggest that toxicogenomic analysis in the rabbit in vivo model is suitable for assessing the risks of accumulated AgNPs to human health. Furthermore, the present study provides new evidence for understanding prolonged hepatotoxicity of cAgNPs, particularly DNA damage and oxidative stress.
Acknowledgments
This research was supported by a grant (14CTAP-C086804-01) from the Technology Advancement Research Program funded by the Ministry of Land, Infrastructure and Transport of the Korean government. This work was also supported by a grant (NIER-SP2014-053-P10) from the National Institute of Environmental Research (NIER) and by a grant (2016001360009) funded by the Ministry of Environment of the Republic of Korea.
Disclosure
The authors report no conflicts of interest in this work.
References
- ParkH-GYeoM-KNanomaterial regulatory policy for human health and environmentMol Cell Toxicol2016123223236
- RajSJoseSSumodUSSabithaMNanotechnology in cosmetics: opportunities and challengesJ Pharm Bioallied Sci20124318619322923959
- SkalskaJDąbrowska-BoutaBStrużyńskaLOxidative stress in rat brain but not in liver following oral administration of a low dose of nanoparticulate silverFood Chem Toxicol20169730731527658324
- AroraSJainJRajwadeJMPaknikarKMInteractions of silver nanoparticles with primary mouse fibroblasts and liver cellsToxicol Appl Pharmacol2009236331031819269301
- PelletierDASureshAKHoltonGAEffects of engineered cerium oxide nanoparticles on bacterial growth and viabilityAppl Environ Microbiol201076247981798920952651
- MassarskyATrudeauVLMoonTWPredicting the environmental impact of nanosilverEnviron Toxicol Pharmacol201438386187325461546
- ZhaoCMWangWXBiokinetic uptake and efflux of silver nanoparticles in Daphnia magnaEnviron Sci Technol201044197699770420831153
- FarkasJChristianPUrreaJAEffects of silver and gold nanoparticles on rainbow trout (Oncorhynchus mykiss) hepatocytesAquat Toxicol2010961445219853932
- GutierrezLAubryCCornejoMCroueJPCitrate-coated silver nanoparticles interactions with effluent organic matter: influence of capping agent and solution conditionsLangmuir201531328865887226230840
- SharmaVKYngardRALinYSilver nanoparticles: green synthesis and their antimicrobial activitiesAdv Colloid Interface Sci20091451–2839618945421
- BastosVDuarteIFSantosCOliveiraHGenotoxicity of citrate-coated silver nanoparticles to human keratinocytes assessed by the comet assay and cytokinesis blocked micronucleus assayEnviron Sci Pollut Res Int20172455039504828000072
- BastosVFerreira-de-OliveiraJMPCarrolaJCoating independent cytotoxicity of citrate- and PEG-coated silver nanoparticles on a human hepatoma cell lineJ Environ Sci201751191201
- GligaARSkoglundSWallinderIOFadeelBKarlssonHLSize-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag releasePart Fibre Toxicol2014111124529161
- KimJSSongKSSungJHGenotoxicity, acute oral and dermal toxicity, eye and dermal irritation and corrosion and skin sensitisation evaluation of silver nanoparticlesNanotoxicology20137595396022417112
- GenterMBNewmanNCShertzerHGAliSFBolonBDistribution and systemic effects of intranasally administered 25 nm silver nanoparticles in adult miceToxicol Pathol20124071004101322549977
- ParkKParkEJChunIKBioavailability and toxicokinetics of citrate-coated silver nanoparticles in ratsArch Pharm Res201134115315821468927
- KimYSSongMYParkJDSubchronic oral toxicity of silver nanoparticlesPart Fibre Toxicol201072020691052
- SungJHJiJHParkJDSubchronic inhalation toxicity of silver nanoparticlesToxicol Sci2009108245246119033393
- Ebabe ElleRGailletSVidéJDietary exposure to silver nanoparticles in Sprague-Dawley rats: effects on oxidative stress and inflammationFood Chem Toxicol20136029730123933361
- El MahdyMMEldinTAAlyHSMohammedFFShaalanMIEvaluation of hepatotoxic and genotoxic potential of silver nanoparticles in albino ratsExp Toxicol Pathol2015671212925446800
- HeydrnejadMSSamaniRJAghaeivandaSToxic effects of silver nanoparticles on liver and some hematological parameters in male and female mice (Mus musculus)Biol Trace Elem Res2015165215315825637567
- DongMSChoiJYSungJHGene expression profiling of kidneys from Sprague-Dawley rats following 12-week inhalation exposure to silver nanoparticlesToxicol Mech Methods201323643744823517440
- GaoXToppingVDKeltnerZSprandoRLYourickJJToxicity of nano- and ionic silver to embryonic stem cells: a comparative toxicogenomic studyJ Nanobiotechnology20171513128399865
- YrAKimJYKimYSConstruction of a predictive model for evaluating multiple organ toxicityMol Cell Toxicol201612116
- KoedrithPKimHWeonJISeoYRToxicogenomic approaches for understanding molecular mechanisms of heavy metal mutagenicity and carcinogenicityInt J Hyg Environ Health2013216558759823540489
- ShinWCEunJWShenQIdentification of aberrant overexpression of long non-coding RNA MALAT1 and role as a regulatory microRNA in liver cancerMol Cell Toxicol2017134443451
- GagnéFAndréCSkirrowRToxicity of silver nanoparticles to rainbow trout: a toxicogenomic approachChemosphere201289561562222727896
- FabroSSmithRLThe teratogenic activity of thalidomide in the rabbitJ Pathol Bacteriol19669125115194958643
- LeeYKimPYoonJSerum kinetics, distribution and excretion of silver in rabbits following 28 days after a single intravenous injection of silver nanoparticlesNanotoxicology2013761120113022770226
- KimHLSeoYRMolecular and genomic approach for understanding the gene-environment interaction between Nrf2 deficiency and carcinogenic nickel-induced DNA damageOncol Rep20122861959196723023193
- NikitinAEgorovSDaraseliaNMazoIPathway studio – the analysis and navigation of molecular networksBioinformatics200319162155215714594725
- KrämerAGreenJPollardJTugendreichSCausal analysis approaches in Ingenuity Pathway AnalysisBioinformatics201430452353024336805
- RamaiahSKA toxicologist guide to the diagnostic interpretation of hepatic biochemical parametersFood Chem Toxicol20074591551155717658209
- YuYLiaoMLiuRChenJFengHFuZOverexpression of lactate dehydrogenase-A in human intrahepatic cholangiocarcinoma: its implication for treatmentWorld J Surg Oncol2014127824679073
- KimDHKwackSJYoonKSChoiJSLeeBM4-Hydroxynonenal: a superior oxidative biomarker compared to malondialdehyde and carbonyl content induced by carbon tetrachloride in ratsJ Toxicol Environ Health A201578161051106226252470
- VrčekIVŽuntarIPetlevskiRComparison of in vitro toxicity of silver ions and silver nanoparticles on human hepatoma cellsEnviron Toxicol201631667969225448069
- ChoiYKimHAKimKWLeeBTComparative toxicity of silver nanoparticles and silver ions to Escherichia coliJ Environ Sci2018665060
- ChakrabortyIFeliuNRoySDawsonKParakWJProtein-mediated shape control of silver nanoparticlesBioconjug Chem20182941261126529461809
- TejamayaMRömerIMerrifieldRCLeadJRStability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology mediaEnviron Sci Technol201246137011701722432856
- El BadawyAMSilvaRGMorrisBScheckelKGSuidanMTTolaymatTMSurface charge-dependent toxicity of silver nanoparticlesEnviron Sci Technol201145128328721133412
- CasselCKMeierDEMorals and moralism in the debate over euthanasia and assisted suicideN Engl J Med1990323117507522388673
- WalkerMParsonsDThe biological fate of silver ions following the use of silver-containing wound care products – a reviewInt Wound J201411549650423173975
- TakenakaSKargERothCPulmonary and systemic distribution of inhaled ultrafine silver particles in ratsEnviron Health Perspect2001109Suppl 4547551
- HyunJSLeeBSRyuHYSungJHChungKHYuIJIjYEffects of repeated silver nanoparticles exposure on the histological structure and mucins of nasal respiratory mucosa in ratsToxicol Lett20081821–3242818782608
- KimSChoiJEChoiJOxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cellsToxicol In Vitro20092361076108419508889
- TiwariDKJinTBehariJDose-dependent in-vivo toxicity assessment of silver nanoparticle in Wistar ratsToxicol Mech Methods2011211132421080782
- M-RJChungH-EKimH-JEffects of zinc oxide nanoparticle dispersants on cytotoxicity and cellular uptakeMol Cell Toxicol2016123281288
- WeiLLuJXuHPatelAChenZSChenGSilver nanoparticles: synthesis, properties, and therapeutic applicationsDrug Discov Today201520559560125543008
- WangEHuangYduQSunYSilver nanoparticle induced toxicity to human sperm by increasing ROS (reactive oxygen species) production and DNA damageEnviron Toxicol Pharmacol20175219319928433807
- PiaoMJKangKALeeIKSilver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosisToxicol Lett201120119210021182908
- KangSHKwonJYLeeJKSeoYRRecent advances in in vivo genotoxicity testing: prediction of carcinogenic potential using comet and micronucleus assay in animal modelsJ Cancer Prev201318427728825337557
- O’ConnorMJTargeting the DNA damage response in cancerMol Cell201560454756026590714
- WangHZhangXTengLLegerskiRJDNA damage checkpoint recovery and cancer developmentExp Cell Res2015334235035825842165
- LiHHHydukeDRChenRDevelopment of a toxicogenomics signature for genotoxicity using a dose-optimization and informatics strategy in human cellsEnviron Mol Mutagen201556650551925733355
- HuangCLHsiaoILLinHCWangCFHuangYJChuangCYSilver nanoparticles affect on gene expression of inflammatory and neurodegenerative responses in mouse brain neural cellsEnviron Res201513625326325460644
- McquillanJSShawAMDifferential gene regulation in the Ag nanoparticle and Ag(+)-induced silver stress response in Escherichia coli: a full transcriptomic profileNanotoxicology20148Suppl 117718424392705
- YamadaTParkCSShenYRabinKRLacorazzaHDG0S2 inhibits the proliferation of K562 cells by interacting with nucleolin in the cytosolLeuk Res201438221021724183236
- SunJYangZShiXCJiHduZYChenLQG0S2a1 (G0/G1 switch gene 2a1) is downregulated by TNF-α in grass carp (Ctenopharyngodon idellus) hepatocytes through PPARα inhibitionGene20186411729038001
- WangYZhangYQianHThe g0/g1 switch gene 2 is an important regulator of hepatic triglyceride metabolismPLoS One201388e7231523951308
- PoulsenSAQuinnRJAdenosine receptors: new opportunities for future drugsBioorg Med Chem1998666196419681130
- Bar-YehudaSSilvermanMHKernsWDOchaionACohenSFishmanPThe anti-inflammatory effect of A3 adenosine receptor agonists: a novel targeted therapy for rheumatoid arthritisExpert Opin Investig Drugs2007161016011613
- RenTQiuYWuWActivation of adenosine A3 receptor alleviates TNF-α-induced inflammation through inhibition of the NF-κB signaling pathway in human colonic epithelial cellsMediators Inflamm Epub2014422
- KaweckiCLentingPJDenisCVvon Willebrand factor and inflammationJ Thromb Haemost20171571285129428671350
- AgostiniSLionettiVNew insights into the non-hemostatic role of von Willebrand factor in endothelial protectionCan J Physiol Pharmacol201795101183118928715643
- OggianuLLancellottiSPitoccoDThe oxidative modification of von Willebrand factor is associated with thrombotic angiopathies in diabetes mellitusPLoS One201381e5539623383177
- BraakhuisHMGosensIKrystekPParticle size dependent deposition and pulmonary inflammation after short-term inhalation of silver nanoparticlesPart Fibre Toxicol2014114925227272
- BrownNRKorolchukSMartinMPCDK1 structures reveal conserved and unique features of the essential cell cycle CDKNat Commun20156676925864384
- YangWChoHShinHYAccumulation of cytoplasmic Cdk1 is associated with cancer growth and survival rate in epithelial ovarian cancerOncotarget2016731494814949727385216
- ZhaoJHanSXMaJLJlMThe role of CDK1 in apoptin-induced apoptosis in hepatocellular carcinoma cellsOncol Rep201330125325923619525

