Abstract
Cancer is a major public health problem, and is now the world’s leading cause of death. Traditional Chinese medicine (TCM)-combination therapy is a new treatment approach and a vital therapeutic strategy for cancer, as it exhibits promising antitumor potential. Nano-targeted drug-delivery systems have remarkable advantages and allow the development of TCM-combination therapies by systematically controlling drug release and delivering drugs to solid tumors. In this review, the anticancer activity of TCM compounds is introduced. The combined use of TCM for antitumor treatment is analyzed and summarized. These combination therapies, using a single nanocarrier system, namely codelivery, are analyzed, issues that require attention are determined, and future perspectives are identified. We carried out a systematic review of >280 studies published in PubMed since 1985 (no patents involved), in order to provide a few basic considerations in terms of the design principles and management of targeted nanotechnology-based TCM-combination therapies.
Introduction
Cancer is the leading cause of disease-associated death in China,Citation1 and is now the world’s leading cause of death.Citation2 According to the Global Cancer Report 2018 on the trend of 36 cancers in 185 countries worldwide by the WHO, the global burden of cancer is increasing at an alarming rate (one in eight deaths on average are due to cancer). The report also pointed out that the incidence and mortality rate of cancer continues to rise each year, with developing countries accounting for approximately 60% of the world’s new cases and 70% of annual deaths. In 2018, nearly half the world’s new cases of cancer occurred in Asia, most of which occurred in China.Citation3 Bray et alCitation2 provided a status report on the global burden of cancers using GLOBOCAN 2018. It is estimated that there will be 18 million new cases of cancer and 9.6 million cancer deaths in 2018. Lung cancer and breast cancer are the most frequent cancers in men and women, respectively, and the two leading causes of cancer death. Due to the high incidence and mortality rate of cancer, the global health care burden is also increasing rapidly.
Surgical treatment, chemotherapy, and radiotherapy are the primary treatment methods for cancer.Citation4 If cancer patients are diagnosed early and receive timely surgical treatment, the probability of surviving for 5 years after surgery is greatly improved. However, when cancers are diagnosed late, the vast majority of patients are already in the terminal stages, and thus may have lost the opportunity of surgical treatment. In addition, due to adverse reactions caused by radiotherapy, such as fatigue, gastrointestinal reactions, skin damage, bone-marrow suppression, and cardiotoxicity,Citation5 chemotherapy is still the main method of cancer treatment.
Nevertheless, due to lack of specificity and poor targeting, chemotherapy drugs not only kill tumor cells but also act on normal tissue, causing a reduction in immunity, significant side effects, and low drug efficacy. In addition, cancer patients can develop resistance to a single chemotherapy drug in clinical practice, resulting in a decrease in the subsequent curative effect. Multidrug resistance (MDR) was once considered the leading cause of chemotherapy failure, and may also promote tumor metastasis and recurrence.Citation6 Based on recent statistics from the American Cancer Society, >90% of cancer patients die from different levels of MDR.
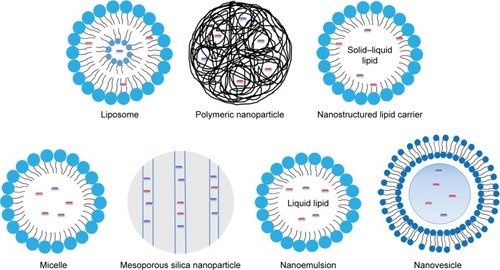
Therefore, the treatment of cancer should be changed from an initial single medication to combination therapy. The combination of two or more active antitumor ingredients plays a crucial role in complementarity and synergy, and has become the preferred scheme in cancer treatment. Notably, the combination of traditional Chinese medicines (TCMs) with chemotherapeutic drugs and the combination of various TCMs, which involves multiple targets and multiple signaling pathways, have improved efficacy compared with drugs with a single molecular target and become a new strategy for tumor therapy in recent years.Citation7 Due to a great deal of investment and rapid development, nanotechnology is already used in various fields of biomedical science.Citation8 Novel nanoformulation-based drug-delivery systems, such as liposomes, nanoparticles (NPs), vesicles, mesoporous silica NPs (MSNs), and micelles, provide promise in overcoming current limitations, including poor targeting, insufficient absorption, poor pharmacokinetics and bioavailability, and limited biodistribution.Citation9–Citation11
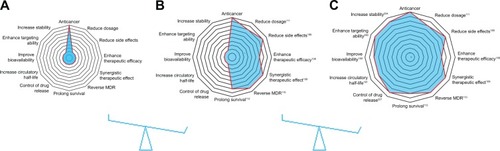
In this review, the anticancer activity of TCM compounds is introduced. The combined use of TCMs for antitumor therapy is analyzed and summarized. These combination therapies using a single nanocarrier system, namely code-livery, are analyzed to determine their potential in prolonging drug duration in vivo, targeting drug delivery, and reducing toxicity (). Matters requiring attention and future perspectives in this field are also reviewed, in order to accelerate the clinical application of combination antitumor therapy using targeted nanotechnology.
Figure 1 Advantages of targeted nanotechnology-based TCM-combination therapy.
Notes: (A) Antitumor effects of TCM; (B) antitumor effects of TCM-combination therapy; (C) antitumor effects of targeted nanotechnology-based TCM-combination therapy.
Abbreviations: TCM, traditional Chinese medicine; MDR, multidrug resistance.
Antitumor effects of TCMs
Herbs, animals, and minerals are used widely as health foods and medicines to remedy various diseases in Asia, and have been collected and recorded as effective and traditional therapies in the TCM literature. For example, artemisinin was isolated by Youyou Tu at the China Academy of Traditional Chinese Medicine in Beijing, and is now an effective medicine in the treatment of malaria. As a result, Tu won the Nobel Prize in Physiology or Medicine in 2015. In most developing countries, 80% of the population continue to use traditional medicines for primary health care.Citation12 From 2016 to 2017, the total amount of TCM herbal medicines and other related products exported to the Belt and Road Initiative countries reached US$295 million. In addition, the WHO also recognized traditional medicine in its influential global medical compendium.Citation13
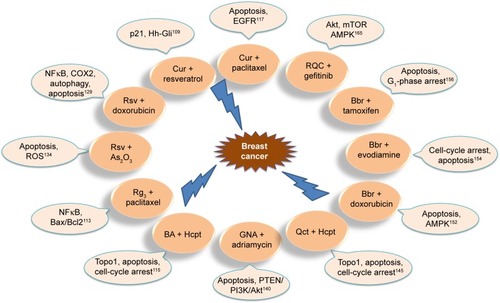
Worldwide, including in Western countries, TCM has been increasingly used in the past few decades, and is well known for its vital role in cancer prevention and treatment. A number of studies have confirmed that the active ingredients in TCM (curcumin [Cur], gambogic acid, and baicalein [BA], among others) are able to effectively induce apoptosis, interfere with tumor progression, inhibit tumor development, inhibit angiogenesis, cause cell-cycle arrest, and block metastasis. A summary of the antitumor effects of drugs isolated from TCMs is shown in . Structures of TCM compounds are shown in .
Table 1 Antitumor effects of traditional Chinese medicine
Antitumor effects of TCM-combination therapy
Initially, cancer therapy consisted of a single drug, which could involve a single target. However, malignant disease is caused by many complicated factors, and treatment with a single drug is not adequate. Patients are usually susceptible to drug resistance after sequential cycles of therapy with these chemotherapy drugs,Citation100 and a single medication frequently causes serious side effects. For instance, although cisplatin is clinically effective, it lacks selectivity for tumor tissue, resulting in serious side effects, such as kidney-function damage,Citation101 neurotoxicity,Citation102 ototoxicity,Citation103 and the emergence of MDR, resulting in the failure of chemotherapy.Citation104 In addition, long-term or high-dose cisplatin treatment can also cause severe anemia.Citation105 Therefore, the clinical application of single drugs, such as cisplatin, has been greatly restricted.
Cancer therapy urgently requires a new therapeutic approach to overcome these shortcomings. Combination-drug therapy is a new mode of treatment, and has gradually gained the attention of researchers.Citation106 Combination therapy involves the simultaneous or sequential use of two or more medicines for therapeutic purposes, and gradually plays a meaningful role in a complementary way, has synergistic action, and alleviates adverse reactions. It can not only produce a better therapeutic effect by regulating multiple signaling pathways in abnormal cells and act on multiple targets simultaneously but also reduce the occurrence of MDR and reduce both the dosage and side effects. The combination of two or more active antitumor ingredients is now a vital treatment method for tumors, and has received US Food and Drug Administration (FDA) approval.Citation107
Based on classic TCM theory, the combination of antitumor TCMs exhibits promising potential in cancer treatment such as: 1) enhancing the therapeutic efficacy of chemotherapeutic drugs – due to the combined effects of Cur and cisplatin determined in vitro and in vivo, experimental results demonstrate that Cur can enhance the antitumor effect of cisplatin in A549 cells in vitro, the combination markedly inhibiting tumor growth and promoting apoptosis in the A549-xenograft mouse model;Citation108 2) achieving synergistic therapeutic effects – resveratrol and Cur synergistically cause apoptosis in breast cancer cells by p2 (Waf/Cip1)-mediated inhibition of the Hedgehog–Gli cascade;Citation109 3) reversing drug resistance – the combination of cryptotanshinone and cisplatin leads to cell death and apoptosis, and cryptotanshinone reverses cisplatin resistance in human lung carcinoma A549 cells by downregulating the Nrf2 pathway;Citation110 4) reducing the dose of drugs – combination therapy with triptolide and cisplatin completely suppresses tumor growth, suggesting that lower concentrations of cisplatin and triptolide may produce a synergistic anticancer effect;Citation111 and 5) prolonging survival – As2O3 combined with ginsenoside Rg3 can significantly inhibit the proliferation of NCIH1299 cells and prolong survival of tumor-bearing nude mice, with a significant effect on lung cancer treatment.Citation112 In addition, TCM-combination therapy results in good prognosis, has fewer adverse reactions, has long-lasting curative effects, regulates the expression of intracellular marker proteins, and reduces the side effects of drugs.Citation112–Citation115 Further superior effects are shown in . The anti–lung cancer and anti–breast cancer mechanisms of TCM-combination therapy are shown in and . TCM-combination therapy achieves the effects that single chemotherapeutic drugs fail to achieve, and has become the main direction in clinical and experimental research on antitumor therapy.Citation116
Table 2 Antitumor effects of TCM-combination therapy
Figure 3 Anti–lung cancer mechanism of TCM-combination therapy.
Abbreviations: Cur, curcumin; Rsv, resveratrol; Tmp, tetramethylpyrazine; Cts, cryptotanshinone; Qct, quercetin; Bbr, berberine; Dhb, dihydroberberine; GA, gambogic acid.
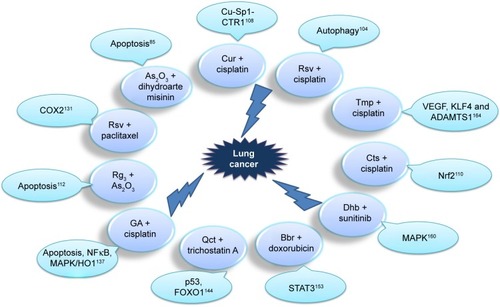
Figure 4 Anti–breast cancer mechanism of TCM-combination therapy.
Abbreviations: Cur, curcumin; Rsv, resveratrol; Bbr, berberine; Hcpt, hydroxycamptothecin; Qct, quercetin; GNA, gambogenic acid; BA, baicalein.
Nevertheless, there are three possible interactions in drug combinations: antagonistic, additive, and synergistic effects. Therefore, if we do not understand interactions among drugs, blindly combining drugs will not only fail to achieve the desired response but also lead to reduced efficacy and increased toxicity, and even produce drug-borne diseases. For instance, the combination of paclitaxel (Ptx) and BA shows antagonism in breast cancer MCF7 cells,Citation117 and Liu et alCitation118 suggested that the combination of gambogic acid and bortezomib should be avoided in patients. In addition, attention should be paid to the proportion and sequence of the two drugs in combination.
Applications of targeted nanotechnology in TCM-combination therapy
During the early 20th century, Paul Ehrlich proposed the concept of targeted drugs, which consisted of three components: the drug, targeting moiety, and drug carrier. The main aim was to deliver the drug to the specific target organ under the specific guiding mechanism.Citation168 Targeted preparations are characterized by increasing the intensity of pharmacological action in target tissue, controlling drug release, and decreasing systemic adverse reactions. Targeted drug-delivery systems have become one of the important high-profile topics in modern pharmacy. Nanotargeted drug-delivery systems have remarkable advantages in improving the bioavailability of drugs, enhancing the targeting ability of drugs, improving the distribution and pharmacokinetic properties of antitumor drugs in vivo and in vitro, increasing the stability of drugs, solubilizing poorly soluble drugs, protecting drugs from degradation in vivo, intelligently regulating the release of components, enhancing efficacy, and reducing toxicity.Citation169–Citation172 Moreover, metastasis of neoplastic cells is the major cause of death in cancer patients,Citation173,Citation174 and nanosize drug-delivery systems also provide an encouraging strategy for lymphatic metastases.Citation175 In 2004, the National Cancer Institute (NCI) launched the NCI Cancer Nanotechnology Alliance, which aims to use nanotechnology to combat cancer.Citation176
In recent years, many types of nanopreparations of TCMs, which involve the combination of nanotargeted drug-delivery systems and the advantages of TCM components in the treatment of tumors, have been reported.Citation177–Citation179 Simultaneously, nanotargeted drug-delivery systems are also promising multidrug carriers and allow the development of drug combinations by systematically controlling drug release and delivering drug to solid tumors.Citation180 Codelivery of multiple antitumor agents in a single well-designed nanocarrier has significant advantages over monotherapy.Citation181,Citation182 Generally, drug targeting can be classified into three categories: passive targeted preparations, active targeted preparations, and other physicochemical targeted drug-delivery systems.
Passive targeted drug-delivery systems
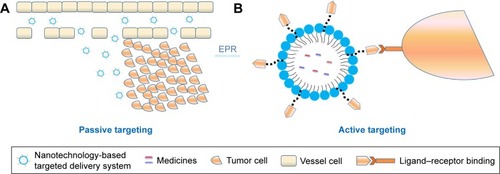
In a passive targeted drug-delivery system, lipids, adipoids, proteins, and biodegradable high-molecular-weight polymers are mainly used as carriers, and the drug is encapsulated or embedded into various colloidal systems, forming stable structures, such as polymeric NPs (PNPs), micelles, nanovesicles, and liposomes, to increase drug concentration in tumor cells, decrease drug distribution in blood and other organs, and prevent toxicity and adverse reactions. This spontaneous accumulation, or “passive” targeting, is particularly effective against tumors, due to leaky angiogenic vessels and poor lymphatic drainage of the tumor, which is currently referred to as the enhanced permeability and retention (EPR) effect. That is to say, the high permeability of tumor blood vessels allows nanosystems to enter the interstitial spaces of the tumor, while impaired lymphatic filtration allows these nanosystems to remain there. This phenomenon does not exist in normal tissue. Currently, EPR-mediated drug delivery is considered effective in delivering drugs into tumors, especially nanocarriers (). The size of the particles is also closely related to their distribution.Citation183 The different sizes of these nanosystems decide the in vivo distribution behavior. Nanopreparations of <100 nm can be slowly accumulated in bone marrow; nanocarriers of 100–200 nm are apt to become enriched in the solid-tumor site; nanosystems of 0.2–3 μm are taken by macrophagocytes in the liver and spleen and particles of >7 μm are often intercepted by pulmonary capillary beds and enter the pulmonary tissues or alveoli.Citation184
Figure 5 Passive (A) and active (B) targeting of tumors.
Abbreviation: EPR, enhanced permeability and retention.
Liposomes
Liposomes are microvesicles with one or more aqueous cavities formed by the encapsulation of one or two amphiphilic molecular double-layer membranes, and the drug is encapsulated or embedded into the liposomes to form the liposome drug. Due to the similarity between the structure and the biological membrane, the encapsulation of water-soluble and fat-soluble drugs can reduce the drug dose, lower drug toxicity, delay release, lower in vivo elimination speed, change in vivo distribution of the drug, and achieve targeted release. Due to these advantages, liposome drugs have attracted considerable attention, and many studies have been carried out on them.Citation185
Plumbagin is a quinonoid isolated from the roots of Plumbago zeylanica (bai hua dan in Chinese).Citation186 It has high antip-roliferative activity against a variety of tumor cell lines,Citation187,Citation188 and its anticancer properties have also been demonstrated in vivo.Citation186 Celecoxib and plumbagin are two antitumor drugs that synergistically kill melanoma cells instead of normal cells. The combined use of these two agents in traditional approaches was not possible, due to their poor bioavailability and toxicity concerns. In order to circumvent these challenges, Raghavendra et alCitation182 developed a nanoliposome containing celecoxib and plumbagin, named CelePlum 777, which has good stability and can release these two drugs at optimal proportion to achieve the maximum synergistic killing effect. Compared to nanoliposomes containing individual drugs, CelePlum 777 can enhance the inhibition of melanoma-cell proliferation in vitro and reduce the growth of melanoma tumors, with negligible systemic toxicity in nude mice. The goal of loading different individual drugs into a nanoliposome that releases the drugs at synergizing ratios was realized.
Previous studies have shown that Cur can reduce side effects caused by cisplatin (cis-diamminedichloroplatinum [CDDP]), including ototoxicity,Citation189 nephrotoxicity,Citation190 and neurotoxicity.Citation191 In addition, Cur can also overcome resistance to CDDP and improve the sensitivity of hepatocellular carcinoma cells to CDDP.Citation192 However, due to poor water-solubility and the different pharmacokinetics of CDDP and Cur,Citation193 the cocktail of both drugs controlling the drug proportions and dose regimen at target cancer cells would be challenging. Based on a previous approach and advantages of the liposome, Cheng et alCitation194 developed a liposomal delivery system using a reverse-microemulsion and film-dispersion method, which coencapsulated CDDP and Cur and transplanted them into hepatocellular carcinoma cells. The anti-tumor activity of CDDP-Cur liposomes against HepG2 cells was higher than that of free drug or encapsulated-monodrug therapy, and retention was prolonged (t½=2.38 hours). Therefore, coloaded liposomes can be used as an effective treatment for tumors, with great clinical application potential.
Polymeric nanoparticles
NPs are solid colloid particles 10–100 nm in size formed by drug dissolution, encapsulation, or adsorption on macromolecular materials. NPs within the particle-size range of 10–100 nm can hide the physicochemical characteristics of the drug, and the in vivo process of the drugs depends on the physicochemical characteristics of the carriers.Citation195 NPs are characterized by a relatively simple preparation process, a significant solubilization effect on active components, significantly improved drug targeting to tumors, and proneness to surface modification. They can improve drug stability, reduce digestive tract stimulation, achieve sustained release or controlled release,Citation196 effectively overcome multiple physiological barriers encountered in vivo, and achieve accurate, safe, efficient, and targeted therapeutic effects. Coating the surface of NPs with polyethylene glycol (PEG), or “PEGylation”, is a commonly used approach to improve the efficiency of drug delivery to target cells and tissue. PEGylation is capable of achieving prolonged blood circulation of nanocarriers, and can improve colloidal stability by providing steric surface hindrance. In addition, it has the ability to improve particle dispersion and decrease hemolysis.Citation197,Citation198 Polylactic-co-glycolic acid (PLGA), a biodegradable polymer, is atoxic to final degradation products and has been approved by the FDA.Citation181,Citation199 The antitumor effects of codelivered PNPs in TCM combinations are shown in .
Table 3 Polymeric nanoparticles (NPs) for codelivery of antitumor agents
Lipid–polymer hybrid nanoparticles
As indicated, it has been demonstrated by the increasing numbers of research reports that biodegradable PNPs and liposomes have become the two main types of active TCM nanocarriers. Lipid–polymer hybrid NPs (LPNs) are nuclear-shell NPs formed by polymer core–lipid/lipid–PEG shellsCitation206,Citation207 that have the advantages of biodegradable PNPs and bionic liposomes.
Li et alCitation208 prepared LPNs and PNPs loaded with cisplatin and Cur. Results indicated that LPNs had higher anticancer efficacy than PNPs and free drugs. Cytotoxicity was highest in vitro and antitumor effect best in vivo. Therefore, LPNs can be used as a targeted and synergistic nanodrug codelivery system for tumor chemotherapy. In a similar study, Zhu et alCitation209 developed vincristine–quercetin (Qct) dual-loaded LPNs. The experimental results proved that the LPNs loaded with both drugs exhibited better antitumor efficacy in vitro and in vivo.
Ruttala et alCitation210 developed nanocarriers loaded with Ptx and Cur using a method different from the previously mentioned studies. Ptx-loaded albumin NPs were prepared and encapsulated in PEGylated hybrid liposomes containing Cur by a thin-film hydration method. This combination guaranteed the release of Ptx and Cur in a sustained and sequential manner. Compared with a cocktail combination, the dual-drug-loaded nanocarrier had a better cytotoxic effect at a lower dose. Therefore, such coloading drug-delivery systems can be used as a promising treatment method to improve clinical efficacy in various malignant tumors. NPs containing genistein and Ptx have also showed similar experimental results.Citation211
Nanostructured lipid carriers
Nanostructured lipid carriers (NLCs) are novel lipid NPs and mixtures of solid and liquid lipids, which have the advantages of excellent drug-loading capacity and sustained-release properties. Jiang et alCitation212 prepared etoposide (Etp)- and Cur-loaded NLCs by the solvent-injection technique. Etp-Cur NLCs had the highest cytotoxicity in vitro and higher accumulation in tumor tissues in vivo compared with other preparations, including Etp NLCs, Etp + Cur solution, Etp solution, and NLCs. In addition, Etp-Cur NLCs displayed low cytotoxicity in normal tissue in vivo, suggesting that NLCs could serve as a promising therapeutic strategy in the treatment of tumors.
Mesoporous silica nanoparticles
MSNs have attracted much attention due to their potential biomedical applications. MSNs possess many attractive features for application in the delivery of TCMs, such as the size of tuning particles/pores, large surface, large pore volume, high loading capacity, mass producibility, biocompatibility, and chemical inertia.Citation213,Citation214 TCMs can be dissolved in surfactant micelles, simultaneously hydrolyzed, and concentrated with silica to form NPs.
Jia et alCitation215 prepared NPs using the self-assembly in situ drug-loading approach, in order to deliver the anticancer drug Ptx and the MDR-reversal agent tetrandrine (Tet) to increase the intracellular concentration of Ptx, enhance its antitumor effect, and minimize the exposure of normal cells to Ptx and Tet. The study demonstrated that Tet significantly increased the accumulation of NPs in cells. Furthermore, Ptx-Tet–cetyltrimethylammonium bromide MSNs suppressed tumor-cell growth more efficiently than the delivery of Ptx (Ptx–cetyltrimethylammonium bromide MSNs) or free Ptx alone. The prepared NPs released the drugs easily in the acidic environment of tumors, and thus, side effects and toxicity in normal tissue and organs were reduced. This nano-carrier may have important potential in clinical applications to avoid MDR by codelivering multiple TCMs. Solid self-nanoemulsifying drug-delivery systems containing tamoxifen and Qct have also shown similar experimental results.Citation216
PEGylated lipid bilayer–supported mesoporous silica nanoparticles
The anticancer drug axitinib (Axt) is a small-molecule tyrosine kinase–receptor inhibitor of VEGFR1, -2, and -3.Citation217,Citation218 Another anticancer drug, celastrol (Cst), can induce the suppression of angiogenesisCitation219 and enhance the antitumor activity of standard chemotherapeutic drugs.Citation220 Choi et alCitation221 loaded Cst into an MSN carrier and subsequently coated it with a lipid bilayer containing Axt, denoted by “ACML”, to increase the synergistic efficacy of the two agents. The difference in drug loading resulted in a sequential-release pattern where Axt was released first to exert its anticancer effect, and then Cst was released to further induce a synergistic effect. The experimental results showed that the synergistic apoptotic effect of ACML against cancer cells was stronger than the Axt-Cst cocktail. Moreover, ACML had a greater tumor-inhibitory effect than either drug administered alone in a tumor-xenograft mouse model. It has been proved that PEGylated lipid bilayer–supported MSNs have the potential to be used as an effective therapeutic strategy for malignant tumors.
Micellar systems
Self-assembled polymeric micelles have been studied widely, due to their excellent role in cancer treatment. Polymeric micelles have a core–shell structure, where hydrophobic drugs are soluble and remain stable in the hydrophobic core of the micelles, and the hydrophilic shell can prolong internal circulation and improve spatial stability by reducing opsonization during blood circulation. Furthermore, polymeric micelles can selectively and effectively accumulate in tumor tissues due to the EPR effect, thus enhancing the therapeutic effects of the loaded chemotherapeutic drugs. As such, codelivery micellar systems have attracted considerable attention.Citation222,Citation223
Doxorubicin (Dox) has extensive antitumor activity against various solid tumors, including lung cancers, melanoma, neurological cancers, sarcoma, leukemia, lymphoma, gastrointestinal cancers, genitourinary cancers, breast cancers, and ovarian cancers.Citation14 Due to the rapid elimination of drugs in vivo, the cocktail combination of free Dox and Cur often fails to provide enough antitumor efficacy or low systemic toxicity. Furthermore, the combination of Dox and Cur has not been realized clinically. In recent years, a few studies have proposed that codelivering Dox and Cur may result in less toxicity, good drug-release profiles, and improved drug distribution in tumor tissue.Citation224–Citation226 Zhang et alCitation223 prepared dual-loaded micelles with coencapsulated Dox and Cur. The experimental results showed that Dox delivered by this method prolonged systemic circulation and increased its accumulation in the tumor, resulting in a lower level of the toxic metabolite doxorubicinol in heart tissue than free Dox alone or the cocktail combination. In addition, Gu et alCitation225 assembled micelles loaded with Dox and Cur. The micelles prolonged the circulation of Dox or Cur when compared with the individual administration of either, and exhibited strong inhibition of tumor growth and reduced Dox side effects.
Ptx has a broad spectrum of activity against various tumors. It has been used clinically for more than two decades. However, it is poorly soluble and has considerable limitations in clinical applications. In addition, Ptx extravasation of cancer cells caused by Pgp activity is also a main factor limiting its clinical efficacy.Citation227,Citation228 Abouzeid et alCitation229 prepared micelles loaded with Ptx and Cur using the thin-film hydration method. Cur–Ptx-loaded micelles released the entrapped drugs with a slow pattern, and resulted in a threefold inhibition of tumors in vitro. The combination of Cur and Ptx was shown to reverse MDR in a resistant human ovarian adenocarcinoma model. Therefore, these combinations of micelles have significant advantages in vitro and in vivo over individual drug treatment, especially in drug-resistant tumors.
Microemulsions
A microemulsion is a transparent or semitransparent oil– water system with low viscosity, isotropism, and thermodynamic stability, and is spontaneously formed by an oil phase, water phase, emulsifier, and coemulsifier. As an ideal drug carrier, it has the advantages of solubilizing components with different solubility properties, good dispersibility, excellent absorbability, and increased bioavailability.Citation230
BA is one of the most commonly used traditional chemotherapeutic drugs for the treatment of various cancers, including HCT116 human colon cancer,Citation31 pancreatic cancer stem cells,Citation32 and bladder cancer.Citation33 It is known that BA has the ability to inhibit the function of Pgp.Citation231 Meng et alCitation230 developed nanoemulsions (NEs) coencapsulating Ptx and BA using rotary evaporation. The research showed that compared with other Ptx preparations, Ptx-BA NEs had a better antitumor effect on MCF7/Tax cells. Studies on cellular uptake have shown that Ptx-BA NEs accumulate effectively in cancer cells. More importantly, Ptx-BA NEs have a higher antitumor effect than other Ptx formulations used in antitumor in vivo studies. The combined delivery of Ptx and BA by NEs may provide a potential combination-treatment strategy to overcome MDR.
Nanovesicles
Nanovesicles are microvesicles with a quasiliposome duallayer structure formed by the self-assembly of synthesized or naturally modified amphiphilic polymers and cholesterol in hydrophilic media. In contrast to other micromolecular vesicles, the polymer vesicle is characterized by good molecular designability, high intensity of the vesicle, excellent stability, and strong permeability.Citation232,Citation233 As a TCM carrier, it can improve histocompatibility and cell permeability and encapsulate hydrophilic drugs or lipophilic drugs.
Alemi et alCitation234 loaded both Cur and Ptx into cationic PEGylated niosomal formulations using thin-film hydration method to enhance efficacy in MCF7 human breast adenocarcinoma cells. The combination of Ptx and Cur, particularly in the nanoniosome formulations, improved the effectiveness of cancer therapy. The novel cationic PEGylated niosome delivery of combined Ptx and Cur is an effective strategy in the treatment of breast cancer.
Schematic illustrations of these nanosystems for drugs are shown in . In addition, graphene oxide,Citation235 carbon nanotubes,Citation236,Citation237 nanorods,Citation238 nanosponges,Citation239 solid lipid NPs,Citation240 nanometal–organic frameworks,Citation241 metallosupra-molecular nanogels,Citation242 and microspheresCitation243 all provide new opportunities for the antitumor effects of TCM-combination therapy.
Despite the EPR effect, most nanosystems fail to find their way toward tumor sites.Citation244 Under most circumstances, 90% or more of the administered nanosystems end up in the liver or spleen, increasing adverse systemic reactions and causing low therapeutic efficacy.Citation245,Citation246
Active targeted drug-delivery systems
An active targeting preparation is a drug-delivery system which can utilize the modified drug carrier as a “missile” and deliver the drug selectively to the target area to allow the drug to accumulate and exert its efficacy. The mechanism of active targeting is that after surface modification with the specific targeted antibody or ligand via covalent or noncovalent binding, the nanodelivery system can avoid recognition and phagocytosis by macrophages and change natural in vivo distribution, so as to deliver the drug to the targeted tumor site and exert its active tumor-targeting effects.Citation247 For instance, due to the difference between tumor cells and normal cells in terms of receptor expression or other biological characteristics, the tumor-targeted drug-delivery system has been developed to ensure that the drug acts only on tumor cellsCitation248 and induces the off-target effect in normal tissue, which has become a high-profile topic in studies on drug-delivery systems.
Transferrin (Tf)-modified nanocarriers
The Tf receptor is commonly present in normal cells and tumor cells. However, expression of the Tf receptor is approximately four to five times that on the surface of tumor cells than on normal cells.Citation249 Transferrin can bind with the Tf receptor and be internalized into the cells under mediation by the receptors to reach the targeted site.
Cui et alCitation250 designed Tf-decorated NPs (Tf-PEG Cur–Dox NPs) to codeliver Cur and Dox for breast cancer therapy. Results showed that the combination of Tf-PEG-Cur and Dox NPs exerted higher cytotoxicity in MCF7 cells compared with Tf-PEG-Cur NPs alone. Higher accumulation of Tf-PEG Cur–Dox NPs was observed in tumors compared to the Cur-Dox injection. Therefore, Tf-PEG Cur–Dox NPs displayed higher efficiency in vitro and in vivo, and resulted in efficient tumor-targeted drug delivery, reduced cytotoxicity, and a stronger antitumor effect.
Folic acid–modified nanocarriers
Similarly to distribution of the Tf receptor on surfaces of tumor-cell membrane, folic acid receptors on tumor cells are overexpressed compared with normal cells, and their activity is also significantly higher than that on normal cells. In addition, folic acid is characterized by low immunogenicity, high modifiability, and high storability. Utilization of the difference in folic acid–receptor expression between tumor sites and normal tissue can achieve targeted delivery of a folic acid–modified drug to cancer cells.Citation251,Citation252
Prodrugs of Ptx and baicalein containing dual-targeted ligands of folate and hyaluronic acid have been synthesized. NPs loaded with these prodrugs (Ptx–baicalein) have also been prepared and the synergistic antitumor effect evaluated in vitro and in vivo. The results showed that the Ptx–baicalein NP drug-delivery system delivered Ptx–baicalein prodrugs to drug-resistant human lung cancer cells, and the delivery was proven to be effective. In addition, Ptx–baicalein NPs exerted an enhanced synergistic anticancer effect, which also overcame MDR to Ptx.Citation253
Low-density lipoprotein–modified nanocarriers
The low-density lipoprotein (LDL) receptor is widely present on the surface of various cells and tissue types, but is overexpressed in tumor cells. LDL is an endogenous NP with good biocompatibility, good biodegradability, and low immunogenicity, and can avoid being recognized and cleared by the in vivo endogenous reticuloendothelial system.Citation254,Citation255 Therefore, LDL is an ideal potential ligand for tumor targeting.
A novel nanocarrier containing Ptx-loaded micelles and siRNA-loaded LDL has been developed. Results showed that the delivery system delivered siRNA and Ptx directly to cancer cells, enhancing the intracellular release of drugs and genes, increasing intracellular drug concentration, decreasing drug efflux, prolonging circulation, and reversing MDR.Citation256,Citation257
Cell-penetrating/tumor-targeting peptide–modified nanocarriers
Nanocarriers using cell-penetrating and/or tumor-targeting peptides for functionalization are a promising strategy, and have attracted the attention of researchers. In our previous report, we reviewed the classification of polypeptide- and polypeptide-modified nanocarriers in detail.Citation258 In this report, recent research progress is summarized in the following paragraphs.
Epigallocatechin-3-gallate (EGCG), a major polyphenol in green tea, has been widely studied as a potential anticancer drug. Narayanan et alCitation259 prepared targeted drug-loaded core–shell NPs using anti-EGFR and anti-HER2 antibodies, and entrapped a combination of Ptx and EGCG at different doses in the core and shell, respectively, using emulsion precipitation. Cellular uptake in MDA-MB231 cells was higher for targeted NPs than untargeted NPs at 24 hours. The sequential release of EGCG followed by Ptx from this core–shell nanocarrier sensitized Ptx-resistant MDA-MB231 cells to Ptx, induced their apoptosis, and inhibited NFκB activation. In addition, EGFR-peptide (GE11)-targeted, pH-sensitive docetaxel Dtx–Cur NPsCitation260 and arginylglycylaspartic acid–modified lipid-coated PLGA NPs targeting delivery of both sorafenib and QctCitation261 achieved significant inhibition of tumor growth in vitro and in vivo.
In addition, galactosamine can recognize and bind to the asialoglycoprotein receptor on the surface of hepatocellular carcinoma cells, and a galactosamine-mediated drug-delivery carrier was significant for targeted liver cancer therapy.Citation262,Citation263 Glycyrrhizin, glycyrrhetinic acid, and mannose can serve as the guiding group in liver-targeted drug-delivery systems, with good potential.Citation264–Citation270 As natural endogenous ligands, bile acids have good biocompatibility and are ideal routes for targeting hepatocellular cancer.Citation271 In addition, sialic acid,Citation272 human Nanog,Citation273 and hyaluronic acidCitation274 are excellent targets in cancer therapy. Vapreotide is a somatostatin analogue and can be also used as a ligand for targeted drug delivery based on its high affinity to somatostatin receptors, which are overexpressed in many tumor cells.Citation275 Several studies have shown that double-modified nanocarriers have also attracted considerable attention in anticancer drug research.Citation276,Citation277 Dual or multiple targeting also provides a new approach for antitumor therapy.
Physicochemical targeted drug-delivery systems
Physicochemical targeting refers to the binding of magnetic, pH-sensitive, temperature-sensitive, or electromagnetic wave–responsive materials onto the surface of drug-delivery systems (such as NPs and liposomes) to make them respond to various stimuli in vitro and in vivo (such as pH, temperature, applied magnetic fields, ultrasonic waves, infrared rays, and electromagnetic radiation) to ensure that the drug acts directly on the target area, increases drug concentration at the lesion site, and reduces adverse reactions. Most studies have used magnetic NPs, temperature-sensitive NPs, and pH-sensitive NPs.
NPs that are pH-sensitive have been designed to promote uptake in tumor cellsCitation278 and accelerate drug release at tumor sites, as the extracellular pH (6.5–7.2) of the tumor is different to that of normal tissue.Citation216 Zhang et alCitation278 developed a codelivery system for Dox and Cur using pH-sensitive NPs. Enhanced release in the acidic environment of cancer cells and enhanced cellular internalization of the cargoes delivered from Dox–Cur NPs were observed in SMMC7721 cells and human umbilical vein endothelial cells compared to the free drugs. Therefore, pH-sensitive NPs can provide a promising strategy for the effective inhibition of cancer in a synergistic manner. Danafar et alCitation279 achieved codelivery of Cur and sulforaphane (SF) with PEGylated gold-coated Fe3O4 magnetic NPs as an effective and promising antitumor agent. Results showed that SF–Cur coloaded Fe3O4@Au NPs caused a decrease in cell viability and induced apoptosis by increasing the Bax:Bcl2 ratio. Moreover, photosensitizer NPs,Citation280 thermosensitive NPs,Citation281 and redox-sensitive NPsCitation282 also provide new opportunities for nanosystems with anti-tumor TCM combinations.
Conclusion
The significant challenge posed by cancers, as well as adverse reactions and drug resistance induced by long-term treatment of a single drug, compels us to change our focus from a single target to the regulation of networks in vivo. Many complex factors cause cancer; therefore, it is rational that treatment should involve multiple components, genes, systems, and target pathways. The combination of drugs has resulted in a new approach to cancer treatment. Rational combinations of drugs not only result in synergy but also reduce the occurrence of drug resistance and adverse reactions, which has resulted in combination therapy, thus becoming a significant antitumor treatment in the clinic and in research. As such, this significant research direction may allow medical researchers to identify a chemotherapeutic combination regimen with high efficacy and low toxicity.
The combination of TCMs for clinical therapy has increased. TCM combinations can exert improved synergistic antitumor effects by adjusting the multiple signaling pathways of tumor cells. Compared with single-drug therapy, combinations of TCMs can reduce the toxicity and side effects of chemotherapy drugs and increase the antitumor effect of drugs. Simultaneously, TCM combinations and chemical drugs can improve immunofunction, relieve clinical symptoms, improve patient survival and quality of life, and improve the efficacy of chemotherapy drugs.
It should be noted that there are usually three approaches in targeted nanotechnology-based TCM-combination-therapy: nanodrugs combined with conventional preparations, coloading of two or more anticancer drugs in a single nanocarrier system (recorded as codelivery), and combined administration of different nanosystems. The loading of two drugs into a single well-designed nanocarrier synchronizes the pharmacokinetic and biological distribution of different drugs to achieve a synergistic effect, which has distinct advantages. This method has been summarized in detail. An increasing number of studies have shown that dual nanosystems have distinct advantages in antitumor treatment and can provide drugs for different targets or sites of action, as they are administered flexibly using different dose/time schedules. Consequently, the remaining two methods urgently require further investigation. In addition, with further comparative analyses of these three research methods, the most suitable form of drug use for cancers can be identified to provide basic considerations in terms of design principles and management progress.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (81673605).
Disclosure
The authors report no conflicts of interest in this work.
References
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- BrayFFerlayJSoerjomataramIGLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countriesCA Cancer J Clin2018201868394424
- FerlayJColombetMIGlobal and regional estimates of the incidence and mortality for 38 cancers: GLOBOCAN 2018LyonInternational Agency for Research on Cancer/World Health Organization2018
- LiFMWangXQIdentifying anticancer peptides by using improved hybrid compositionsSci Rep201663391027670968
- ZhengPPLiJKrosJMBreakthroughs in modern cancer therapy and elusive cardiotoxicity: critical research-practice gaps, challenges, and insightsMed Res Rev201838132537628862319
- WuQYangZNieYShiYFanDMulti-drug resistance in cancer chemotherapeutics: mechanisms and lab approachesCancer Lett2014347215916624657660
- LiSZhangBJiangDWeiYZhangNHerb network construction and co-module analysis for uncovering the combination rule of traditional Chinese herbal formulaeBMC Bioinformatics201011S11S6
- HeathJRNanotechnologies for biomedical Science and translational medicineProc Natl Acad Sci U S A201511247144361444326598663
- HoBNPfefferCMSinghATUpdate on Nanotechnology-based drug delivery systems in cancer treatmentAnticancer Res201737115975598129061776
- WangKKievitFMZhangMNanoparticles for cancer gene therapy: recent advances, challenges, and strategiesPharmacol Res2016114566627771464
- YangYYuCAdvances in silica based nanoparticles for targeted cancer therapyNanomedicine201612231733226706409
- GroverJKYadavSPPharmacological actions and potential uses of Momordica charantia: a reviewJ Ethnopharmacol200493112313215182917
- CyranoskiDWhy Chinese medicine is heading for clinics around the worldNature2018561772444845030258149
- AnandPSundaramCJhuraniSKunnumakkaraABAggarwalBBCurcumin and cancer: an “old-age” disease with an “age-old” solutionCancer Lett2008267113316418462866
- DuvoixABlasiusRDelhalleSChemopreventive and therapeutic effects of curcuminCancer Lett2005223218119015896452
- ZhangTDChenGQWangZGWangZYChenSJChenZArsenic trioxide, a therapeutic agent for APLOncogene200120497146715311704843
- DograSBandiSViswanathanPGuptaSArsenic trioxide amplifies cisplatin toxicity in human tubular cells transformed by HPV-16 E6/E7 for further therapeutic directions in renal cell carcinomaCancer Lett20153562 Pt B95396125444910
- JiHLiYJiangFInhibition of transforming growth factor beta/ SMAD signal by MiR-155 is involved in arsenic trioxide-induced anti-angiogenesis in prostate cancerCancer Sci2014105121541154925283513
- WangXJiangFMuJArsenic trioxide attenuates the invasion potential of human liver cancer cells through the demethylation-activated microRNA-491Toxicol Lett20142272758324680928
- JiangXChenCZhaoWZhangZSodium arsenite and arsenic tri-oxide differently affect the oxidative stress, genotoxicity and apoptosis in A549 cells: an implication for the paradoxical mechanismEnviron Toxicol Pharmacol201336389190224004876
- WalkerAMStevensJJNdebeleKTchounwouPBEvaluation of arsenic trioxide potential for lung cancer treatment: assessment of apoptotic mechanisms and oxidative damageJ Cancer Sci Ther2016080119
- AlkhalafMJaffalSPotent antiproliferative effects of resveratrol on human osteosarcoma SJSA1 cells: novel cellular mechanisms involving the ERKs/p53 cascadeFree Radic Biol Med200641231832516814113
- ZhuWQinWZhangKTrans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancerNutr Cancer201264339340022332908
- PatelKRBrownVAJonesDJClinical pharmacology of resveratrol and its metabolites in colorectal cancer patientsCancer Res201070197392739920841478
- RaufAImranMButtMSNadeemMPetersDGMubarakMSResveratrol as an anti-cancer agent: a reviewCrit Rev Food Sci Nutr20185891428144728001084
- CaiHScottEKholghiACancer chemoprevention: evidence of a nonlinear dose response for the protective effects of resveratrol in humans and miceSci Transl Med20157298298ra117
- OiNYuanJMalakhovaMResveratrol induces apoptosis by directly targeting Ras-GTPase-activating protein SH3 domain-binding protein 1Oncogene201534202660267124998844
- ZouMWangJGaoJHanHFangYPhosphoproteomic analysis of the antitumor effects of ginsenoside Rg3 in human breast cancer cellsOncol Lett20181532889289829435015
- YangXZouJCaiHGinsenoside Rg3 inhibits colorectal tumor growth via down-regulation of C/EBPβ/NF-κB signalingBiomed Pharmacother2017961240124529169725
- LiuTZhaoLHouHDingLChenWLiXGinsenoside 20(S)-Rg3 suppresses ovarian cancer migration via hypoxia-inducible factor 1 alpha and nuclear factor-kappa B signalsTumour Biol2017395101042831769222
- SuMQZhouYRRaoXBaicalein induces the apoptosis of HCT116 human colon cancer cells via the upregulation of DEPP/ Gadd45a and activation of MAPKsInt J Oncol201853275076029749481
- SongLChenXWangPGaoSQuCLiuLEffects of baicalein on pancreatic cancer stem cells via modulation of sonic hedgehog pathwayActa Biochim Biophys Sin201850658659629697746
- ChaoJISuWCLiuH-FBaicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AktMol Cancer Ther20076113039304818025287
- ZhouQWangSZhangHThe combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cellsActa Pharmacol Sin200930121648165819960010
- YanXIRuiXZhangKAIBaicalein inhibits the invasion of gastric cancer cells by suppressing the activity of the p38 signaling pathwayOncol Rep201533273774325502212
- WangYFLiTTangZHBaicalein triggers autophagy and inhibits the protein kinase B/Mammalian target of rapamycin pathway in hepatocellular carcinoma HepG2 cellsPhytother Res201529567467925641124
- WuJYTsaiKWLiYZAnti-Bladder-Tumor effect of baicalein from Scutellaria baicalensis Georgi and its application in vivoEvid Based Complement Alternat Med2013201357975123573134
- GuoZHuXXingZBaicalein inhibits prostate cancer cell growth and metastasis via the caveolin-1/AKT/mTOR pathwayMol Cell Biochem20154061–211111925957503
- PengYGuoCYangYBaicalein induces apoptosis of human cervical cancer HeLa cells in vitroMol Med Rep20151132129213425373554
- ChandrashekarNSelvamaniASubramanianRPandiAThiruvengadamDBaicalein inhibits pulmonary carcinogenesis-associated inflammation and interferes with COX-2, MMP-2 and MMP-9 expressions in-vivoToxicol Appl Pharmacol20122611102122369883
- YuJGuoQLYouQDGambogic acid-induced G2/M phase cell-cycle arrest via disturbing CDK7-mediated phosphorylation of CDC2/p34 in human gastric carcinoma BGC-823 cellsCarcinogenesis200728363263817012222
- WangXDengRLuYGambogic acid as a non-competitive inhibitor of ATP-binding cassette transporter B1 reverses the multidrug resistance of human epithelial cancers by promoting ATP-binding cassette transporter B1 protein degradationBasic Clin Pharmacol Toxicol20131121253322759348
- WuZQGuoQLYouQDZhaoLGuH-YGambogic acid inhibits proliferation of human lung carcinoma SPC-A1 cells in vivo and in vitro and represses telomerase activity and telomerase reverse transcriptase mRNA expression in the cellsBiol Pharm Bull200427111769177415516720
- WangCZhangHChenYShiFChenBGambogic acid-loaded magnetic Fe(3)O(4) nanoparticles inhibit Panc-1 pancreatic cancer cell proliferation and migration by inactivating transcription factor ETS1Int J Nanomedicine2012778178722393285
- GuoQLYouQDWuZQYuanSTZhaoLGeneral gambogic acids inhibited growth of human hepatoma SMMC-7721 cells in vitro and in nude miceActa Pharmacol Sin200425676977415169630
- ZhuXZhangHLinYMechanisms of gambogic acid-induced apoptosis in non-small cell lung cancer cells in relation to transferrin receptorsJ Chemother200921666667220071291
- NieFZhangXQiQReactive oxygen species accumulation contributes to gambogic acid-induced apoptosis in human hepatoma SMMC-7721 cellsToxicology20092601–3606719464570
- YanFWangMChenHGambogenic acid mediated apoptosis through the mitochondrial oxidative stress and inactivation of Akt signaling pathway in human nasopharyngeal carcinoma CNE-1 cellsEur J Pharmacol20116521–3233221118682
- ChenHBZhouLZMeiLGambogenic acid-induced time- and dose-dependent growth inhibition and apoptosis involving Akt pathway inactivation in U251 glioblastoma cellsJ Nat Med2012661626921879332
- WangKTangYSunMThe mechanism of neogambogic acid-induced apoptosis in human MCF-7 cellsActa Biochim Biophys Sin201143969870221785112
- MeiWDongCHuiCGambogenic acid kills lung cancer cells through aberrant autophagyPLoS One201491e8360424427275
- LiQChengHZhuGGambogenic acid inhibits proliferation of A549 cells through apoptosis-inducing and cell cycle arrestingBiol Pharm Bull201033341542020190402
- ChengHSuJJPengJYGambogenic acid inhibits proliferation of A549 cells through apoptosis inducing through up-regulation of the p38 MAPK cascadeJ Asian Nat Prod Res20111311993100222007630
- ZhangXXuQSaikiIQuercetin inhibits the invasion and mobility of murine melanoma B16-BL6 cells through inducing apoptosis via decreasing Bcl-2 expressionClin Exp Metastasis200018541542111467774
- TsaiPHChengCHLinC-YDietary flavonoids luteolin and quercetin suppressed cancer stem cell properties and metastatic potential of isolated prostate cancer cellsAnticancer Res201636126367638027919958
- JiaLHuangSYinXZanYGuoYHanLQuercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy inductionLife Sci201820812313030025823
- CalgarottoAKMasoVJuniorGCAntitumor activities of quercetin and green tea in xenografts of human leukemia HL60 cellsSci Rep201881345929472583
- Anand DavidAVArulmoliRParasuramanSOverviews of biological importance of quercetin: a bioactive flavonoidPharmacogn Rev20161020848928082789
- LugliEFerraresiRRoatEQuercetin inhibits lymphocyte activation and proliferation without inducing apoptosis in peripheral mononuclear cellsLeuk Res200933114015018774171
- ZhangHLiHLiuZTriptolide inhibits the proliferation and migration of medulloblastoma DAOY cells by upregulation of microRNA-138J Cell Biochem2018119129866987730156009
- MaoXTongJWangYZhuZYinYWangYTriptolide exhibits antitumor effects by reversing hypermethylation of WIF1 in lung cancer cellsMol Med Rep20181833041304930015908
- ZhouZLYangYXDingJLiYCMiaoZ-HTriptolide: structural modifications, structure-activity relationships, bioactivities, clinical development and mechanismsNat Prod Rep201229445747522270059
- LiSGShiQWYuanLYC-Myc-dependent repression of two oncogenic miRNA clusters contributes to triptolide-induced cell death in hepatocellular carcinoma cellsJ Exp Clin Cancer Res2018375129523159
- WangYLiuTLiHEnhancement of triptolide-loaded micelles on tumorigenicity inhibition of human ovarian cancerJ Biomater Sci Polym Ed201627754555626786618
- HuGGongXWangLTriptolide promotes the clearance of α-synuclein by enhancing autophagy in neuronal cellsMol Neurobiol20175432361237226957304
- KimSHKangJGKimCSSynergistic cytotoxicity of BIIB021 with triptolide through suppression of PI3K/AKT/mTOR and NF-κB signal pathways in thyroid carcinoma cellsBiomed Pharmacother201683223227470546
- LiWYangYHuZLingSFangMNeuroprotective effects of DAHP and triptolide in focal cerebral ischemia via apoptosis inhibition and PI3K/Akt/mTOR pathway activationFront Neuroanat201594825954164
- YaoZWanYLiBBerberine induces mitochondrialmediated apoptosis and protective autophagy in human malignant pleural mesothelioma NCIH2452 cellsOncol Rep20184063603361030272347
- FukudaKHibiyaYMutohMKoshijiMAkaoSFujiwaraHInhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cellsJ Ethnopharmacol199966222723310433483
- WangZWangY-SChangZ-MBerberine-loaded Janus nanocarriers for magnetic field-enhanced therapy against hepatocellular carcinomaChem Biol Drug Des201789346446927618577
- KuoHPChuangTCYehMHGrowth suppression of HER2-overexpressing breast cancer cells by berberine via modulation of the HER2/PI3K/AKT signaling pathwayJ Agric Food Chem201159158216822421699261
- KimSHanJLeeSKBerberine suppresses the TPA-induced MMP-1 and MMP-9 expressions through the inhibition of PKC-α in breast cancer cellsJ Surg Res20121761e21e2922381172
- LinCCYangJSChenJTBerberine induces apoptosis in human HSC-3 oral cancer cells via simultaneous activation of the death receptor-mediated and mitochondrial pathwayAnticancer Res2007275A3371337817970083
- MantenaSKSharmaSDKatiyarSKBerberine inhibits growth, induces G1 arrest and apoptosis in human epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin cascade, disruption of mitochondrial membrane potential and cleavage of caspase 3 and PARPCarcinogenesis200627102018202716621886
- ShinDSKimHNShinKDCryptotanshinone inhibits constitutive signal transducer and activator of transcription 3 function through blocking the dimerization in DU145 prostate cancer cellsCancer Res200969119320219118003
- ChenWLuoYLiuLCryptotanshinone inhibits cancer cell proliferation by suppressing mammalian target of rapamycin-mediated cyclin D1 expression and RB phosphorylationCancer Prev Res20103810151025
- ChenLWangHJXieWYaoYZhangYSWangHCryptotanshinone inhibits lung tumorigenesis and induces apoptosis in cancer cells in vitro and in vivoMol Med Rep2014962447245224682389
- XiaoWZhuMXZhuMXPanXJYangZHZhouS-YInhibition of cyclooxygenase-2 by tetramethylpyrazine and its effects on A549 cell invasion and metastasisInt J Oncol20124062029203722344367
- WangYFuQZhaoWTetramethylpyrazine inhibits osteosarcoma cell proliferation via downregulation of NF-κB in vitro and in vivoMol Med Rep20138498498823912183
- WangSJGaoYChenHDihydroartemisinin inactivates NF-kappaB and potentiates the anti-tumor effect of gemcitabine on pancreatic cancer both in vitro and in vivoCancer Lett201029319910820137856
- JiYZhangYCPeiLBShiLLYanJLMaXHAnti-tumor effects of dihydroartemisinin on human osteosarcomaMol Cell Biochem20113511–29910821234653
- GaoXLuoZXiangTWangKLiJWangPDihydroartemisinin induces endoplasmic reticulum stress-mediated apoptosis in HepG2 human hepatoma cellsTumori201197677178022322845
- LaiHSinghNPSelective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrinCancer Lett199591141467750093
- LaiHSasakiTSinghNPMessayAEffects of artemisinin-tagged holotransferrin on cancer cellsLife Sci200576111267127915642597
- ChenHGuSDaiHLiXZhangZDihydroartemisinin sensitizes human lung adenocarcinoma A549 cells to arsenic trioxide via apoptosisBiol Trace Elem Res2017179220321228261759
- OhMChoiYHChoiSAnti-proliferating effects of ginsenoside Rh2 on MCF-7 human breast cancer cellsInt J Oncol199914586987510200336
- NakataHKikuchiYTodeTInhibitory effects of ginsenoside Rh2 on tumor growth in nude mice bearing human ovarian cancer cellsJpn J Cancer Res19988977337409738980
- XieXEberdingAMaderaCRh2 synergistically enhances paclitaxel or mitoxantrone in prostate cancer modelsJ Urol200617551926193116600800
- XiaTWangYNZhouCXGinsenoside Rh2 and Rg3 inhibit cell proliferation and induce apoptosis by increasing mitochondrial reactive oxygen species in human leukemia Jurkat cellsMol Med Rep20171563591359828440403
- YangJYuanDXingTGinsenoside Rh2 inhibiting HCT116 colon cancer cell proliferation through blocking PDZ-binding kinase/T-LAK cell-originated protein kinaseJ Ginseng Res201640440040827746693
- YangZZhaoTLiuHZhangLGinsenoside Rh2 inhibits hepatocellular carcinoma through β-catenin and autophagySci Rep2016611938326783250
- HuangJPengKWangLGinsenoside Rh2 inhibits proliferation and induces apoptosis in human leukemia cells via TNF-α signaling pathwayActa Biochim Biophys Sin201648875075527177748
- LiSGaoYMaWEGFR signaling-dependent inhibition of glioblastoma growth by ginsenoside Rh2Tumor Biol201435655935598
- HanSJeongAJYangHGinsenoside 20(S)-Rh2 exerts anticancer activity through targeting IL-6-induced JAK2/STAT3 pathway in human colorectal cancer cellsJ Ethnopharmacol2016194839027566200
- GuanNHuoXZhangZZhangSLuoJGuoWGinsenoside Rh2 inhibits metastasis of glioblastoma multiforme through Akt-regulated MMP13Tumor Biol201536967896795
- AbedinpourPBaronVTChrastinaAPlumbagin improves the efficacy of androgen deprivation therapy in prostate cancer: a pre-clinical studyProstate201777161550156228971491
- KawiakADomachowskaAPlumbagin suppresses the invasion of HER2-overexpressing breast cancer cells through inhibition of IKKα-Mediated NF-κB activationPLoS One20161110e016406427727280
- OhTIYunJMParkEJPlumbagin suppresses α-MSH-Induced melanogenesis in B16F10 mouse melanoma cells by inhibiting tyrosinase activityInt J Mol Sci2017182320
- LiuYCaiYHeCChenMLiHAnticancer properties and pharmaceutical applications of plumbagin: a reviewAm J Chin Med201745342344128359198
- PanSTLiZLHeZXQiuJXZhouS-FMolecular mechanisms for tumour resistance to chemotherapyClin Exp Pharmacol Physiol201643872373727097837
- CronaDJFasoANishijimaTFMcgrawKAGalskyMDMilowskyMIA systematic review of strategies to prevent cisplatin-induced nephrotoxicityOncologist201722560961928438887
- ZhuJCarozziVAReedNEthoxyquin provides neuroprotection against cisplatin-induced neurotoxicitySci Rep2016612886127350330
- KarasawaTSteygerPSAn integrated view of cisplatin-induced nephrotoxicity and ototoxicityToxicol Lett2015237321922726101797
- HuSLiXXuRThe synergistic effect of resveratrol in combination with cisplatin on apoptosis via modulating autophagy in A549 cellsActa Biochim Biophys Sin201648652853527084520
- GaoLPLiZGuoZYZhaoY-MThe effects of vitamin C on DDP-induced anemia in ratsToxicol Mech Methods201323638338823343350
- LuDYChenEHWuHYAnticancer drug combinations, how far we can go through?Anticancer Agents Med Chem2017171212827039923
- WebsterRMCombination therapies in oncologyNat Rev Drug Discov2016152818226837588
- ZhangWShiHChenCCurcumin enhances cisplatin sensitivity of human NSCLC cell lines through influencing Cu-Sp1-CTR1 regulatory loopPhytomedicine201848516130195880
- MohapatraPSatapathySRSiddharthSDasDNayakAKunduCNResveratrol and curcumin synergistically induces apoptosis in cigarette smoke condensate transformed breast epithelial cells through a p21(Waf1/Cip1) mediated inhibition of Hh-Gli signalingInt J Biochem Cell Biol201566758426212257
- XiaCBaiXHouXCryptotanshinone reverses cisplatin resistance of human lung carcinoma A549 cells through down-regulating Nrf2 pathwayCell Physiol Biochem201537281682426356271
- LiCJChuCYHuangLHSynergistic anticancer activity of triptolide combined with cisplatin enhances apoptosis in gastric cancer in vitro and in vivoCancer Lett2012319220321322306340
- CheJBLiuZHMaHBInfluence of As2O3 combined with ginsenosides Rg3 on inhibition of lung cancer NCI-H1299 cells and on subsistence of nude mice bearing hepatomaAsian Pac J Trop Med201471077277525129458
- YuanZJiangHZhuXLiuXLiJGinsenoside Rg3 promotes cytotoxicity of paclitaxel through inhibiting NF-κB signaling and regulating Bax/Bcl-2 expression on triple-negative breast cancerBiomed Pharmacother20178922723228231544
- ChangLHuoBLvYWangYLiuWGinsenoside Rg3 enhances the inhibitory effects of chemotherapy on esophageal squamous cell carcinoma in miceMol Clin Oncol2014261043104625279195
- TangQJiFSunWCombination of baicalein and 10-hydroxy camptothecin exerts remarkable synergetic anti-cancer effectsPhytomedicine201623141778178627912880
- ZhenYSAnticancer Drug research and developmentBeijing, ChinaChemical Industry Press2004
- ZhanYChenYLiuRZhangHZhangYPotentiation of paclitaxel activity by curcumin in human breast cancer cell by modulating apoptosis and inhibiting EGFR signalingArch Pharm Res20143781086109524318305
- LiuNHuangHXuLThe combination of proteasome inhibitors bortezomib and gambogic acid triggers synergistic cytotoxicity in vitro but not in vivoToxicol Lett2014224333334024291039
- MajumdarAPBanerjeeSNautiyalJCurcumin synergizes with resveratrol to inhibit colon cancerNutr Cancer200961454455319838927
- MasuelliLMarzocchellaLFocaccettiCResveratrol and diallyl disulfide enhance curcumin-induced sarcoma cell apoptosisFront Biosci2012171498508
- SánchezYSimónGPCalviñoEde BlasEAllerPCurcumin stimulates reactive oxygen species production and potentiates apoptosis induction by the antitumor drugs arsenic trioxide and lonidamine in human myeloid leukemia cell linesJ Pharmacol Exp Ther2010335111412320605902
- FehlDJAhmedMCurcumin promotes the oncoltyic capacity of vesicular stomatitis virus for the treatment of prostate cancersVirus Res2017228142327865863
- ZhangJYLinMTZhouMJCombinational treatment of cur-cumin and quercetin against gastric cancer MGC-803 cells in vitroMolecules2015206115241153426111180
- LiYFengLLiYJiangWShanNWangXArtesunate possesses anti-leukemia properties that can be enhanced by arsenic trioxideLeuk Lymphoma20145561366137223906016
- TomuleasaCSoritauOFischer-FodorEArsenic trioxide plus cisplatin/interferon α-2b/doxorubicin/capecitabine combination chemotherapy for unresectable hepatocellular carcinomaHematol Oncol Stem Cell Ther201142606621727766
- WangWQinSKChenBAChenHYExperimental study on antitumor effect of arsenic trioxide in combination with cisplatin or doxorubicin on hepatocellular carcinomaWorld J Gastroenterol20017570270511819858
- ZhaoYYYuLLiuBLHeXJZhangB-YDownregulation of P-gp, Ras and p-ERK1/2 contributes to the arsenic trioxide-induced reduction in drug resistance towards doxorubicin in gastric cancer cell linesMol Med Rep20151257335734326459009
- YuQChenBZhangXQianWYeBZhouYArsenic trioxide-enhanced, matrine-induced apoptosis in multiple myeloma cell linesPlanta Med2013790977578123700110
- RaiGMishraSSumanSShuklaYResveratrol improves the anticancer effects of doxorubicin in vitro and in vivo models: a mechanistic insightPhytomedicine201623323324226969377
- JiangQYangMQuZZhouJZhangQResveratrol enhances anticancer effects of paclitaxel in HepG2 human liver cancer cellsBMC Complement Altern Med201717147728978315
- KongFZhangRZhaoXZhengGWangZWangPResveratrol raises in vitro anticancer effects of paclitaxel in NSCLC cell line A549 through COX-2 expressionKorean J Physiol Pharmacol201721546547428883751
- YuanYXueXGuoRBSunXLHuGResveratrol enhances the antitumor effects of temozolomide in glioblastoma via ROS-dependent AMPK-TSC-mTOR signaling pathwayCNS Neurosci Ther201218753654622530672
- Tomas-HernándezSBlancoJRojasCResveratrol potently counteracts quercetin starvation-induced autophagy and sensitizes HepG2 cancer cells to apoptosisMol Nutr Food Res20186251700610
- ZhaoXYYangSChenYRLiPCDouMMZhangJResveratrol and arsenic trioxide act synergistically to kill tumor cells in vitro and in vivoPLoS One201496e9892524901647
- KimSMLeeSYYukDYInhibition of NF-kappaB by ginsenoside Rg3 enhances the susceptibility of colon cancer cells to docetaxelArch Pharm Res200932575576519471891
- PanQXueMXiaoS-SWanY-JXuD-BA combination therapy with baicalein and taxol promotes mitochondria-mediated cell apoptosis: involving in Akt/β-Catenin signaling pathwayDNA Cell Biol2016351164665627414207
- WangLHLiYYangSNGambogic acid synergistically potentiates cisplatin-induced apoptosis in non-small-cell lung cancer through suppressing NF-κB and MAPK/HO-1 signallingBr J Cancer2014110234135224300974
- XiaGWangHSongZMengQHuangXHuangXGambogic acid sensitizes gemcitabine efficacy in pancreatic cancer by reducing the expression of ribonucleotide reductase subunit-M2 (RRM2)J Exp Clin Cancer Res201736110728797284
- JiangXLZhangYLuoCLWuXHTargeting renal cell carcinoma with gambogic acid in combination with sunitinib in vitro and in vivoAsian Pac J Cancer Prev201213126463646823464475
- HeYDingJLinYGambogenic acid alters chemosensitivity of breast cancer cells to adriamycinBMC Complement Altern Med201515118126066793
- ChenRZhangHLiuPWuXChenBGambogenic acid synergistically potentiates bortezomib-induced apoptosis in multiple myelomaJ Cancer20178583985128382147
- MaZSHuynhTHNgCPDoPTNguyenTHHuynhHReduction of CWR22 prostate tumor xenograft growth by combined tamoxifen-quercetin treatment is associated with inhibition of angiogenesis and cellular proliferationInt J Oncol20042451297130415067354
- SunSGongFLiuPMiaoQMetformin combined with quercetin synergistically repressed prostate cancer cells via inhibition of VEGF/ PI3K/Akt signaling pathwayGene2018664505729678660
- ChanSTChuangCHLinYCLiaoJWLiiCKYehS-LQuercetin enhances the antitumor effect of trichostatin A and suppresses muscle wasting in tumor-bearing miceFood Funct20189287187929292417
- TangQJiFWangJGuoLLiYBaoYQuercetin exerts synergetic anti-cancer activity with 10-hydroxy camptothecinEur J Pharm Sci201710922323228822757
- LeiCSHouYCPaiMHLinMTYehS-LEffects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: in vitro and in vivo studiesJ Nutr Biochem20185110511329125991
- AlsaiedOASangwanVBanerjeeSSorafenib and triptolide as combination therapy for hepatocellular carcinomaSurgery2014156227027924953273
- MatsuiYWatanabeJIkegawaMKamotoTOgawaONishiyamaHCancer-specific enhancement of cisplatin-induced cytotoxicity with triptolide through an interaction of inactivated glycogen synthase kinase-3beta with p53Oncogene200827334603461418391982
- LiuYXiaoEYuanLLiGTriptolide Synergistically Enhances Antitumor Activity of Oxaliplatin in Colon Carcinoma In Vitro and In VivoDNA Cell Biol201433741842524720675
- TangXYZhuYQTaoWHWeiBLinXLSynergistic effect of triptolide combined with 5-fluorouracil on colon carcinomaPostgrad Med J20078397933834317488865
- QiaoZHeMHeMUSynergistic antitumor activity of gemcitabine combined with triptolide in pancreatic cancer cellsOncol Lett20161153527353327123146
- PanYZhangFZhaoYBerberine enhances chemosensitivity and induces apoptosis through Dose-orchestrated AMPK signaling in breast cancerJ Cancer2017891679168928775788
- ZhuTLiLLXiaoGFBerberine increases doxorubicin sensitivity by suppressing STAT3 in lung cancerAm J Chin Med20154371487150226503561
- DuJSunYLuYYBerberine and evodiamine act synergistically against human breast cancer MCF-7 cells by inducing cell cycle arrest and apoptosisAnticancer Res201737116141615129061795
- RenKZhangWWuGSynergistic anti-cancer effects of galangin and berberine through apoptosis induction and proliferation inhibition in oesophageal carcinoma cellsBiomed Pharmacother2016841748175927876206
- WenCWuLFuLZhangXZhouHBerberine enhances the anti-tumor activity of tamoxifen in drugsensitive MCF7 and drugresistant MCF7/TAM cellsMol Med Rep20161432250225627432642
- HuangYWangKGuCBerberine, a natural plant alkaloid, synergistically sensitizes human liver cancer cells to sorafenibOncol Rep20184031525153230015938
- WangCJWangCHanJEffect of combined treatment with recombinant interleukin-2 and allicin on pancreatic cancerMol Biol Rep201340126579658524135803
- ZouXLiangJSunJAllicin sensitizes hepatocellular cancer cells to anti-tumor activity of 5-fluorouracil through ROS-mediated mitochondrial pathwayJ Pharmacol Sci2016131423324027177453
- DaiBMaYWangWDihydroberberine exhibits synergistic effects with sunitinib on NSCLC NCI-H460 cells by repressing MAP kinase pathways and inflammatory mediatorsJ Cell Mol Med201721102573258528444871
- LiuYBiTWangZOxymatrine synergistically enhances antitumor activity of oxaliplatin in colon carcinoma through PI3K/ Akt/mTOR pathwayApoptosis201621121398140727671687
- SongMQZhuJSChenJLSynergistic effect of oxymatrine and angiogenesis inhibitor NM-3 on modulating apoptosis in human gastric cancer cellsWorld J Gastroenterol200713121788179317465467
- ByunJMJeongDHLeeDSTetraarsenic oxide and cisplatin induce apoptotic synergism in cervical cancerOncol Rep20132941540154623338680
- TangJHZhangHMZhangZHZhangX-LEffect of tetramethylpyrazine combined with cisplatin on VEGF, KLF4 and ADAMTS1 in Lewis lung cancer miceAsian Pac J Trop Med201710881381828942831
- Castillo-PichardoLDharmawardhaneSFGrape polyphenols inhibit Akt/mammalian target of rapamycin signaling and potentiate the effects of gefitinib in breast cancerNutr Cancer20126471058106923061908
- StearnsMEAmatangeloMDVarmaDSellCGoodyearSMCombination therapy with epigallocatechin-3-gallate and doxorubicin in human prostate tumor modeling studies: inhibition of metastatic tumor growth in severe combined immunodeficiency miceAm J Pathol201017763169317920971741
- LiangGTangALinXGreen tea catechins augment the antitumor activity of doxorubicin in an in vivo mouse model for chemoresistant liver cancerInt J Oncol201037111112320514403
- BrodskyFMMonoclonal antibodies as magic bulletsPharm Res198805119
- YeBLRuanXJZhengZHCaiHJChitosan-coated doxorubicin nanoparticles drug delivery system inhibits cell growth of liver cancer via p53/PRC1 pathwayBiochem Biophys Res Commun2018495141442029097204
- GaoGHLiYLeeDSEnvironmental pH-sensitive polymeric micelles for cancer diagnosis and targeted therapyJ Control Release2013169318018423195533
- ZhangXAchaziKSteinhilberDKratzFDerneddeJHaagRA facile approach for dual-responsive prodrug nanogels based on den-dritic polyglycerols with minimal leachingJ Control Release201417420921624225227
- ZhangHWangKZhangPHeWSongALuanYRedox-sensitive micelles assembled from amphiphilic mPEG-PCL-SS-DTX conjugates for the delivery of docetaxelColloids Surf B Biointerfaces2016142899726938324
- SunYWXuJZhouJLiuW-JTargeted drugs for systemic therapy of lung cancer with brain metastasesOncotarget2018945459547229435193
- WickMRMetastases of malignant neoplasms: Historical, biological, & clinical considerationsSemin Diagn Pathol201835211212229198586
- ObinuAGaviniERassuGMaestriMBonferoniMCGiunchediPLymph node metastases: importance of detection and treatment strategiesExpert Opin Drug Deliv201815545946729504430
- HullLCFarrellDGrodzinskiPHighlights of recent developments and trends in cancer nanotechnology research – View from NCI Alliance for nanotechnology in cancerBiotechnol Adv201432466667823948249
- YallapuMMKhanSMaherDMAnti-cancer activity of cur-cumin loaded nanoparticles in prostate cancerBiomaterials201435308635864825028336
- ZhangZQianHYangMGambogic acid-loaded biomimetic nanoparticles in colorectal cancer treatmentInt J Nanomedicine2017121593160528280328
- ZhangYQShenYLiaoMMGalactosylated chitosan triptolide nanoparticles for overcoming hepatocellular carcinoma: enhanced therapeutic efficacy, low toxicity, and validated network regulatory mechanismsNanomedicine2019151869730244085
- Jabr-MilaneLSvan VlerkenLEYadavSAmijiMMMultifunctional nanocarriers to overcome tumor drug resistanceCancer Treat Rev200834759260218538481
- NarayananSPavithranMViswanathASequentially releasing dual-drug-loaded PLGA-casein core/shell nanomedicine: design, synthesis, biocompatibility and pharmacokineticsActa Biomater20141052112212424389318
- GowdaRKardosGSharmaASinghSRobertsonGPNanoparticle-based celecoxib and plumbagin for the synergistic treatment of melanomaMol Cancer Ther201716344045228003325
- TorchilinVTumor delivery of macromolecular drugs based on the EPR effectAdv Drug Deliv Rev201163313113520304019
- GaumetMVargasAGurnyRDelieFNanoparticles for drug delivery: the need for precision in reporting particle size parametersEur J Pharm Biopharm20086911917826969
- YuCZhouQXiaoFEnhancing doxorubicin delivery toward tumor by hydroxyethyl Starch-g-Polylactide partner nanocarriersACS Appl Mater Interfaces2017912104811049328266842
- HafeezBBZhongWFischerJWPlumbagin, a medicinal plant (Plumbago zeylanica)-derived 1,4-naphthoquinone, inhibits growth and metastasis of human prostate cancer PC-3M-luciferase cells in an orthotopic xenograft mouse modelMol Oncol20137342843923273564
- LiYCHeSMHeZXPlumbagin induces apoptotic and autophagic cell death through inhibition of the PI3K/Akt/mTOR pathway in human non-small cell lung cancer cellsCancer Lett2014344223925924280585
- LeeJHYeonJHKimHThe natural anticancer agent plumbagin induces potent cytotoxicity in MCF-7 human breast cancer cells by inhibiting a PI-5 kinase for ROS generationPLoS One201279e4502323028742
- YoumIWestMBLiWDuXEwertDLKopkeRDsiRNA-loaded biodegradable nanocarriers for therapeutic MAPK1 silencing against cisplatin-induced ototoxicityInt J Pharm20175281–261162328627458
- KuhadAPilkhwalSSharmaSTirkeyNChopraKEffect of curcumin on inflammation and oxidative stress in cisplatin-induced experimental nephrotoxicityJ Agric Food Chem20075525101501015518001039
- Al MoundhriMSAl-SalamSAl MahrouqeeABeegamSAliBHThe effect of curcumin on oxaliplatin and cisplatin neurotoxicity in rats: Some behavioral, biochemical, and histopathological studiesJ Med Toxicol201391253322648527
- ZhangJLiuJXuXLiLCurcumin suppresses cisplatin resistance development partly via modulating extracellular vesicle-mediated transfer of MEG3 and miR-214 in ovarian cancerCancer Chemother Pharmacol201779347948728175963
- HuQSunWWangCGuZRecent advances of cocktail chemotherapy by combination drug delivery systemsAdv Drug Deliv Rev201698193426546751
- ChengYZhaoPWuSCisplatin and curcumin co-loaded nano-liposomes for the treatment of hepatocellular carcinomaInt J Pharm20185451–226127329730175
- ZhangYDNanobiotechnologyScience PressBeijing, China2005
- MoghimiSMHunterACMurrayJCLongcirculating and target specific nanoparticles: theory to practicePharmacol Rev20015328331811356986
- PasutGVeroneseFMState of the art in pegylation: the great versatility achieved after forty years of researchJ Control Release2012161246147222094104
- SukJSXuQKimNHanesJEnsignLMPEGylation as a strategy for improving nanoparticle-based drug and gene deliveryAdv Drug Deliv Rev201699285126456916
- FatmaSTalegaonkarSIqbalZNovel flavonoid-based biodegradable nanoparticles for effective oral delivery of etoposide by P-glycoprotein modulation: an in vitro, ex vivo and in vivo investigationsDrug Deliv201623250051124937381
- SchmidDJarvisGEFayFNanoencapsulation of ABT-737 and camptothecin enhances their clinical potential through synergistic antitumor effects and reduction of systemic toxicityCell Death Dis2014510e145425299779
- XuYWangCDingYNanoparticles with optimal ratiometric co-delivery of docetaxel with gambogic acid for treatment of multidrug-resistant breast cancerJ Biomed Nanotechnol20161291774178129345888
- JainAKThankiKJainSCo-encapsulation of tamoxifen and quercetin in polymeric nanoparticles: implications on oral bioavailability, antitumor efficacy, and drug-induced toxicityMol Pharm20131093459347423927416
- LiuQZhaoDZhuXColoaded nanoparticles of paclitaxel and Piperlongumine for enhancing synergistic antitumor activities and reducing toxicityJ Pharm Sci2017106103066307528552690
- KatiyarSSMuntimaduguERafeeqiTADombAJKhanWCo-delivery of rapamycin- and piperine-loaded polymeric nanoparticles for breast cancer treatmentDrug Deliv2016232608261626036652
- ChenYZhengXLFangDLDual agent loaded PLGA nanoparticles enhanced antitumor activity in a multidrug-resistant breast tumor eenograft modelInt J Mol Sci20141522761277224552875
- HadinotoKSundaresanACheowWSLipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a reviewEur J Pharm Biopharm2013853 Pt A42744323872180
- MandalBBhattacharjeeHMittalNCore–shell-type lipid– polymer hybrid nanoparticles as a drug delivery platformNanomedicine20139447449123261500
- LiCGeXWangLConstruction and comparison of different nanocarriers for co-delivery of cisplatin and curcumin: a synergistic combination nanotherapy for cervical cancerBiomed Pharmacother20178662863628027539
- ZhuBYuLYueQCo-delivery of vincristine and quercetin by nanocarriers for lymphoma combination chemotherapyBiomed Pharmacother20179128729428463792
- RuttalaHBKoYTLiposomal co-delivery of curcumin and albumin/ paclitaxel nanoparticle for enhanced synergistic antitumor efficacyColloids Surf B Biointerfaces201512841942625797481
- MendesLPGaetiMPde ÁvilaPHMulticompartimental nanoparticles for co-encapsulation and multimodal drug delivery to tumor cells and neovasculaturePharm Res20143151106111924170281
- JiangHGengDLiuHLiZCaoJCo-delivery of etoposide and curcumin by lipid nanoparticulate drug delivery system for the treatment of gastric tumorsDrug Deliv20162393665367327749102
- TangFLiLChenDMesoporous silica nanoparticles: synthesis, bio-compatibility and drug deliveryAdv Mater201224121504153422378538
- ZhangXGMiaoJDaiYQDuYZYuanHHuF-QReversal activity of nanostructured lipid carriers loading cytotoxic drug in multi-drug resistant cancer cellsInt J Pharm20083611–223924418586075
- JiaLLiZShenJMultifunctional mesoporous silica nanoparticles mediated co-delivery of paclitaxel and tetrandrine for overcoming multidrug resistanceInt J Pharm20154891–231833025956050
- JainAKThankiKJainSSolidified self-nanoemulsifying formulation for oral delivery of combinatorial therapeutic regimen: Part II in vivo pharmacokinetics, antitumor efficacy and hepatotoxicityPharm Res201431494695824135934
- PemovskaTJohnsonEKontroMAxitinib effectively inhibits BCR-ABL1(T315I) with a distinct binding conformationNature2015519754110210525686603
- EscudierBGoreMAxitinib for the management of metastatic renal cell carcinomaDrugs R D201111211312621679004
- PangXYiZZhangJCelastrol suppresses angiogenesis-mediated tumor growth through inhibition of Akt/mammalian target of rapamycin pathwayCancer Res20107051951195920160026
- SethiGAhnKSPandeyMKAggarwalBBA novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB activationBlood20071092727273517110449
- ChoiJYRamasamyTKimSYPEGylated lipid bilayer-supported mesoporous silica nanoparticle composite for synergistic co-delivery of axitinib and celastrol in multi-targeted cancer therapyActa Biomater2016399410527163403
- RamasamyTRuttalaHBChitrapriyaNEngineering of cell microenvironment-responsive polypeptide nanovehicle co-encapsulating a synergistic combination of small molecules for effective chemotherapy in solid tumorsActa Biomater20174813114327794477
- ZhangDXuQWangNA complex micellar system co-delivering curcumin with doxorubicin against cardiotoxicity and tumor growthInt J Nanomedicine2018134549456130127606
- MaWGuoQLiYWangXWangJTuPCo-assembly of doxorubicin and curcumin targeted micelles for synergistic delivery and improving anti-tumor efficacyEur J Pharm Biopharm201711220922327913127
- GuYLiJLiYNanomicelles loaded with doxorubicin and curcumin for alleviating multidrug resistance in lung cancerInt J Nanomedicine2016115757577027843316
- WangJMaWTuPSynergistically improved anti-tumor efficacy by co-delivery doxorubicin and curcumin polymeric micellesMacromol Biosci20151591252126125981672
- ShapiraALivneyYDBroxtermanHJAssarafYGNanomedicine for targeted cancer therapy: towards the overcoming of drug resistanceDrug Resist Updat201114315016321330184
- SreekanthCNBavaSVSreekumarEAntoRJMolecular evidences for the chemosensitizing efficacy of liposomal curcumin in paclitaxel chemotherapy in mouse models of cervical cancerOncogene201130283139315221317920
- AbouzeidAHPatelNRTorchilinVPPolyethylene glycol-phosphatidylethanolamine (PEG-PE)/vitamin E micelles for co-delivery of paclitaxel and curcumin to overcome multi-drug resistance in ovarian cancerInt J Pharm20144641–217818424440402
- MengLXiaXYangYCo-encapsulation of paclitaxel and baicalein in nanoemulsions to overcome multidrug resistance via oxidative stress augmentation and P-glycoprotein inhibitionInt J Pharm20165131–281627596118
- IshiiKTanakaSKagamiKEffects of naturally occurring polymethyoxyflavonoids on cell growth, P-glycoprotein function, cell cycle, and apoptosis of daunorubicin-resistant T lymphoblastoid leukemia cellsCancer Invest201028322022919863351
- LetchfordKBurtHA review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomesEur J Pharm Biopharm200765325926917196803
- SinghRLillardJWNanoparticle-based targeted drug deliveryExp Mol Pathol200986321522319186176
- AlemiAZavar RezaJHaghiralsadatFPaclitaxel and curcumin coadministration in novel cationic PEGylated niosomal formulations exhibit enhanced synergistic antitumor efficacyJ Nanobiotechnol201816128
- HanCZhangCMaTHypericin-functionalized graphene oxide for enhanced mitochondria-targeting and synergistic anticancer effectActa Biomater20187726828130006311
- PereiraSLeeJRubioNCationic Liposome- multi-walled carbon nanotubes hybrids for dual siPLK1 and doxorubicin delivery in vitroPharm Res201532103293330826085038
- MartincicMTobiasGFilled carbon nanotubes in biomedical imaging and drug deliveryExpert Opin Drug Deliv201512456358125430876
- ZhuFTanGJiangYYuZRenFRational design of multi-stimuli-responsive gold nanorod–curcumin conjugates for chemo-photothermal synergistic cancer therapyBiomater Sci20186112905291730209445
- LockhartJNStevensDMBeezerDBKravitzAHarthEDual drug delivery of tamoxifen and quercetin: regulated metabolism for anticancer treatment with nanospongesJ Control Release2015220Pt B75175726344396
- LiuBHanLLiuJHanSChenZJiangLCo-delivery of paclitaxel and TOS-cisplatin via TAT-targeted solid lipid nanoparticles with synergistic antitumor activity against cervical cancerInt J Nanomedicine20171295596828203075
- HeCLuKLiuDLinWNanoscale Metal–Organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cellsJ Am Chem Soc2014136145181518424669930
- YaoXChenLChenXpH-responsive metallo-supramolecular nanogel for synergistic chemo-photodynamic therapyActa Biomater20152516217126190797
- GasparVMMoreiraAFCostaECGas-generating TPGS-PLGA microspheres loaded with nanoparticles (NIMPS) for co-delivery of minicircle DNA and anti-tumoral drugsColloids Surf B Biointerfaces201513428729426209779
- WilhelmSTavaresAJDaiQAnalysis of nanoparticle delivery to tumoursNat Rev Mat201615
- AlbaneseATangPSChanWCWThe effect of nanoparticle size, shape, and surface chemistry on biological systemsAnnu Rev Biomed Eng201214111622524388
- ZhangXQXuXBertrandNPridgenESwamiAFarokhzadOCInteractions of nanomaterials and biological systems: implications to personalized nanomedicineAdv Drug Deliv Rev201264131363138422917779
- BertrandNWuJXuXKamalyNFarokhzadOCCancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biologyAdv Drug Deliv Rev20146622524270007
- GabizonAHorowitzATGorenDTzemachDShmeedaHZalipskySIn vivo fate of folate-targeted polyethylene-glycol liposomes in tumor-bearing miceClin Cancer Res200396551655914695160
- CalzolariAOlivieroIDeaglioSTransferrin receptor 2 is frequently expressed in human cancer cell linesBlood Cells Mol Dis2007391829117428703
- CuiTZhangSSunHCo-delivery of doxorubicin and pH-sensitive curcumin prodrug by transferrin-targeted nanoparticles for breast cancer treatmentOncol Rep20173721253126028075466
- LowPSAntonyACFolate receptor-targeted drugs for cancer and inflammatory diseasesAdv Drug Deliv Rev20045681055105815094205
- WangYWangYXiangJYaoKTarget-specific cellular uptake of taxol-loaded heparin-PEG-folate nanoparticlesBiomacromolecules201011123531353821086982
- WangWXiMDuanXWangYKongFDelivery of baicalein and paclitaxel using self-assembled nanoparticles: synergistic antitumor effect in vitro and in vivoInt J Nanomedicine2015103737375026045664
- FirestoneRALow-density lipoprotein as a vehicle for targeting antitumor compounds to cancer cellsBioconjug Chem1994521051138031872
- SudhofTGoldsteinJBrownMRussellDThe LDL receptor gene: a mosaic of exons shared with different proteinsScience198522847018158222988123
- ZhuWJYangSDQuCXLow-density lipoprotein-coupled micelles with reduction and pH dual sensitivity for intelligent co-delivery of paclitaxel and siRNA to breast tumorInt J Nanomedicine2017123375339328490877
- YangSDZhuWJZhuQLBinary-copolymer system base on low-density lipoprotein-coupled N-succinyl chitosan lipoic acid micelles for co-delivery MDR1 siRNA and paclitaxel, enhances anti-tumor effects via reducing drugJ Biomed Mater Res2017105511141125
- KebebeDLiuYWuYVilakhamxayMLiuZLiJTumor-targeting delivery of herb-based drugs with cell-penetrating/tumor-targeting peptide-modified nanocarriersInt J Nanomedicine2018131425144229563797
- NarayananSMonyUVijaykumarDKKoyakuttyMPaul-PrasanthBMenonDSequential release of epigallocatechin gallate and paclitaxel from PLGA-casein core/shell nanoparticles sensitizes drug-resistant breast cancer cellsNanomedicine20151161399140625888278
- YanJWangYJiaYCo-delivery of docetaxel and curcumin pro-drug via dual-targeted nanoparticles with synergistic antitumor activity against prostate cancerBiomed Pharmacother20178837438328122302
- WangCSuLWuCWuJZhuCYuanGRGD peptide targeted lipid-coated nanoparticles for combinatorial delivery of sorafenib and quercetin against hepatocellular carcinomaDrug Dev Ind Pharm201642121938194427142812
- ZhaoRLiTZhengGJiangKFanLShaoJSimultaneous inhibition of growth and metastasis of hepatocellular carcinoma by co-delivery of ursolic acid and sorafenib using lactobionic acid modified and pH-sensitive chitosan-conjugated mesoporous silica nanocomplexBiomaterials201714311628755539
- ShenZWeiWTanakaHA galactosamine-mediated drug delivery carrier for targeted liver cancer therapyPharmacol Res201164441041921723392
- YanTLiDLiJEffective co-delivery of doxorubicin and curcumin using a glycyrrhetinic acid-modified chitosan-cystamine-poly(ε-caprolactone) copolymer micelle for combination cancer chemotherapyColloids Surf Biointerfaces201614552653827281238
- TsujiHOsakaSKiwadaHTargeting of liposomes surface-modified with glycyrrhizin to the liver. I. Preparation and biological disopositionChem Pharm Bull1991394100410081893485
- IshidaSSakiyaYIchikawaTTairaZUptake of glycyrrhizin by isolated rat hepatocytesBiol Pharm Bull19931632932978364475
- NegishiMIrieANagataNIchikawaASpecific binding of glycyrrhetinic acid to the rat liver membraneBiochim Biophys Acta19911066177822065071
- LinALiuYHuangYGlycyrrhizin surface-modified chitosan nanoparticles for hepatocyte-targeted deliveryInt J Pharm20083591–224725318457928
- LeeSJZhengNYClavijoMNussenzweigMCNormal host defense during systemic candidiasis in mannose receptor-deficient miceInfect Immun200371143744512496194
- HirataKMaruyamaTWatanabeHGenetically engineered mannosylated-human serum albumin as a versatile carrier for liver-selective therapeuticsJ Control Release2010145191620304018
- ZhangJYuCJiangGSynthesis of cholic-acid-carrying polymer and in-vitro evaluation of hepatoma-targeting nanoparticles decorated with the polymerJ Biomater Sci Polym Ed201627986587927045998
- ElgoharyMMHelmyMWAbdelfattahE-ZATargeting sialic acid residues on lung cancer cells by inhalable boronic acid-decorated albumin nanocomposites for combined chemo/herbal therapyJ Control Release201828523024330009892
- XiaYXuTWangCNovel functionalized nanoparticles for tumor-targeting co-delivery of doxorubicin and siRNA to enhance cancer therapyInt J Nanomedicine20181314315929317822
- WanLJiaoJCuiYHyaluronic acid modified mesoporous carbon nanoparticles for targeted drug delivery to CD44-overexpressing cancer cellsNanotechnology2016271313510226901756
- FengQYuMZWangJCSynergistic inhibition of breast cancer by co-delivery of VEGF siRNA and paclitaxel via vapreotide-modified core–shell nanoparticlesBiomaterials201435185028503824680191
- ChenJLiSShenQFolic acid and cell-penetrating peptide conjugated PLGA–PEG bifunctional nanoparticles for vincristine sulfate deliveryEur J Pharm Sci201247243044322796217
- GaoZYouCWuHWangMZhangXSunBFA and cRGD dual modified lipid-polymer nanoparticles encapsulating polyaniline and cisplatin for highly effective chemo-photothermal combination therapyJ Biomater Sci Polym Ed201829439741129271285
- ZhangJLiJShiZpH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activitiesActa Biomater20175834936428455219
- DanafarHSharafiAKheiriSKheiri ManjiliHCo-delivery of sulforaphane and curcumin with pegylated iron Oxide-Gold core shell nanoparticles for delivery to breast cancer cell lineIran J Pharm Res201817248049429881406
- ChangJEChoHJYiEKimDDJheonSHypocrellin B and paclitaxel-encapsulated hyaluronic acid-ceramide nanoparticles for targeted photodynamic therapy in lung cancerJ Photochem Photobiol B201615811312126967521
- YinYHuQXuCCo-delivery of doxorubicin and interferon-γ by thermosensitive nanoparticles for cancer immunochemotherapyMol Pharm20181594161417230011369
- YinTWangLYinLZhouJHuoMCo-delivery of hydrophobic paclitaxel and hydrophilic AURKA specific siRNA by redox-sensitive micelles for effective treatment of breast cancerBiomaterials201561102525996409