Abstract
Ultrasmall superparamagnetic iron oxide (USPIO) particles are maghemite or magnetite nanoparticles currently used as contrast agent in magnetic resonance imaging. The coatings surrounding the USPIO inorganic core play a major role in both the in vitro stability and, over all, USPIO’s in vivo fate. Different physicochemical properties such as final size, surface charge and coating density are key factors in this respect. Up to now no precise structure – activity relationship has been described to predict entirely the USPIOs stability, as well as their pharmacokinetics and their safety. This review is focused on both the classical and the latest available techniques allowing a better insight in the magnetic core structure and the organic surface of these particles. Concurrently, this work clearly shows the difficulty to obtain a complete physicochemical characterization of USPIOs particles owing to their small dimensions, reaching the analytical resolution limits of many commercial instruments. An extended characterization is therefore necessary to improve the understanding of the properties of USPIOs when dispersed in an aqueous environment and to set the specifications and limits for their conception.
Introduction
Superparamagnetic nanoparticles are currently used as contrast agent in magnetic resonance imaging (MRI) (CitationBonnemain 1998; CitationSonvico, Dubernet et al 2005). They are originally ferromagnetic substances which have lost their permanent magnetism due to their small size. The magnetization of such nanoparticles follows an external magnetic field without any hysteresis and they are better known as “superparamagnetic” due to their large magnetic susceptibility (CitationLawaczeck et al 2004; CitationCorot et al 2006).
These nanoparticles consist of a coated iron oxide core (magnetite, maghemite or other insoluble ferrites) characterized by a large magnetic moment in the presence of a static external magnetic field. They are classified into two main groups according to their size (CitationBowen et al 2002; CitationMornet et al 2005; CitationRoch et al 2005; CitationCorot et al 2006).
SPIOs (superparamagnetic iron oxides), whose nanoparticles have a hydrodynamic size greater than 50 nm (coating included). These nanoparticles have in common their specific uptake by the mononuclear phagocyte system (MPS). For example, Endorem® and Resovist® are European commercial names of SPIOs available on the market for intravenous use. Their clinical targets are liver tumor and metastasis (CitationReimer and Tombach 1998).
USPIOs (ultrasmall superparamagnetic iron oxides) (CitationClement et al 1998), whose nanoparticles are smaller than 50 nm (hydrodynamic size coating included). After intravenous administration, these devices can be related to long-circulating “stealth” nanoparticles and will be the subject of this review.
USPIOs are composed of an iron oxide core with a crystal size measuring generally less than 10 nm. The size will control the T2/T1 relaxivity (CitationWeissleder et al 1990) and therefore the signal in MRI which is the key factor for the quality of the diagnosis. It should be noted that the relaxation mechanisms of USPIOs have been widely treated in the literature (CitationGillis and Koening 1987; CitationMuller et al 1992; CitationRoch et al 1999) even though the relaxation process in the presence of some magnetic compounds is still on debate (CitationGossuin et al 2002).
The coatings, surrounding the USPIO inorganic core, play a major role in both the in vitro stability and, over all, USPIO’s in vivo fate. According to the polymer(s) or small organic molecule(s) chosen and to its (their) interaction with the core, the system will feature different physicochemical properties such as final size, surface charge, density of covering, etc (CitationCorot et al 2006).
Owing to their small final size and their hydrophilic coating, USPIOs are generally able to avoid the early and massive uptake by the macrophages from the MPS (especially spleen and liver macrophages). This confers to them long circulating properties in the bloodstream after intravenous administration, as well as the possibility of targeting macrophages in the deep compartments (CitationRaynal et al 2004). Hence, these systems can be used for blood pool imaging where their effects on the T1 relaxation time will be exploited. They will also be useful for detecting inflammatory or degenerative disorders associated with the spread of macrophages in which they depict negative enhancement properties on T2-weighted sequences. For example, USPIOs will allow the detection of lymph node metastasis (CitationLeenders 2003) or vulnerable atherosclerotic plaques (CitationCorot et al 2004). Important clinical indications in CNS pathologies such as stroke, brain tumor proliferation, multiple sclerosis, spinal cord injury are also currently developed with USPIOs (CitationCorot et al 2004). Furthermore, ex vivo labeling of progenitor and stem cells which can be subsequently tracked in vivo with MRI will also be a field of application of iron oxide USPIOs nanoparticles (and also SPIO). Hence, given the broad possible clinical indications of these systems, it is expected that USPIOs should be launched on the market in a near future, as depicted by Sinerem® whose phase 3 clinical trials have been recently achieved.
With regard to the synthesis of magnetic nanoparticles, numerous methods have been reported (CitationPascal et al 1999; CitationJolivet 2000; CitationTartaj et al 2003; CitationCushing et al 2004; CitationGupta and Gupta 2005; CitationTartaj et al 2005). The synthesis most commonly used involves an alkaline co-precipitation of ferrous and ferric ions in aqueous solution (CitationBabes et al 1999), in the presence of a stabilizing agent (for example, dextran). Noteworthy, stabilization may also be achieved after the synthesis by the single surface adsorption of these agents (CitationOhgushi et al 1978; CitationMolday and Mackenzie 1982; CitationKim et al 2005). However, in terms of chemical synthesis, it is still challenging to obtain magnetic particles with a narrow monodisperse population for large scale clinical uses.
Moreover and quite importantly, there is no precise structure – activity relationship to make it possible to predict entirely their stability, as well as their pharmacokinetics, biodistribution, metabolism, clearance from the vascular system in vivo and their safety. The multiple components which govern the properties of the USPIOs should hence be characterized as accurately and as broadly as possible, in order to better understand their future particular behavior.
Complete physicochemical characterization of USPIOs particles is difficult, due to their small dimensions. Commercially available instruments are sometimes unsuitable to study the complex composition of the USPIOs and problems associated with the analytical resolution limits of the apparatus can often occur too.
Hence, this paper will focus on the composition of USPIOs, which exhibit very different properties from those of the bulk material. This review will also detail the classical and the latest available techniques which can be applied to have a better insight in their magnetic core structure and their organic surface. This will allow to improve the understanding of the physicochemical properties of these small magnetic particles and to set the specifications and limits for the conception of USPIOs. This will also greatly help to define and develop models of structure-activity relationship.
Morphology and structural characterization of the magnetic core
The morphological characteristics and size distribution of nanoparticles samples are generally observed by TEM. Since high resolution transmission electron microscopy (HRTEM) has the ability to resolve the atomic arrangement in nano size area, it has been employed to investigate interfacial local microstructures of the nanoparticles. This technique has demonstrated the capability of characterizing lattice deformation in a very thin layer (CitationPeng et al 2004). HRTEM images were successfully employed to describe the crystallography (ie, ideal and real structure: lattice fringes characteristic, glide plane, screw axis, lattice vacancies and defects, as well as shape) of crystalline nanoparticles (CitationMorales et al 1999; CitationHyeon et al 2001; CitationSerna et al 2001; CitationMiser et al 2004; CitationBrice-Profeta et al 2005).
One of the most discussed (and a controversial) aspect of the structural characterization of an USPIO sample is to define the real limit that exists between an ordered (crystalline) phase and a disordered (amorphous) phase. From the physical point of view, a crystal is a solid having a highly regular atomic structure in which the atoms are packed in a regular order by three-dimensional pattern repeating of atoms. The surface of the crystal has to be considered as a boundary region where there is a layer constituted by incomplete cells due to the breakdown of the crystalline growth.
The limit between a crystal and an amorphous sample could be positioned when the surface layer became prevalent in respect to the whole sample volume. Nevertheless the small particles, measuring less than 10 nm, exhibit high surface/volume ratio and are commonly named nanocrystals. Up to now this definition is attributed without clear physical evidences since the properties of these materials are very different from those of the bulk materials ().
Figure 1 The figure shows how the number of atoms in the sphere volume and the one in a superficial layer differently increase growing the nanoparticle dimension. The ratio, in the inset, shows that the number of atoms in the surface layer is prevalent in the smallest nanoparticles.
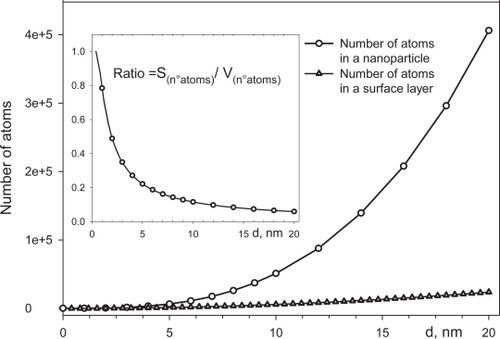
In the smallest particles it is possible to recognize the cationic disorder, disordered surface layer and short-range order inside the whole volume. The USPIO particles contain a limited number of cubic cell units and, for the above reasons, the crystal definition is not completely correct. These nanosystems may be considered to be a less-ordered system which is neither completely crystalline nor completely amorphous and their core characterization is difficult; the combination of various analysis techniques is needed (CitationDi Marco et al 2006).
The most important methodology for nanoparticles structure characterization is the X-ray diffraction using both conventional and synchrotron radiation sources. Thermal analysis, Mossbauer (CitationSerna et al 2001) and Infra Red spectroscopy (CitationMorales et al 1999) provide additional useful information.
Most of these techniques require the drying of the samples whereas for pharmaceutical application, the nanoparticles need to be dispersed in a liquid. Drying the sample and placing it on/or near a surface might induce some significant changes concerning the physicochemical characteristics of USPIO which can be displayed by the occurrence of irreversible particles aggregation, for example. Thus, the results obtained may not accurately reflect the nature of the species in the liquid dispersion. Interestingly, nanoparticles can be characterized as a liquid suspension mainly by small angle X-ray and neutron scattering (CitationShen et al 2001; CitationMoeser et al 2004). Energy Dispersive X-ray Diffraction (EDXD), suitable for the systems with a low degree of crystallinity, (CitationCaminiti et al 1999; CitationAtzei et al 2001; CitationSadun et al 2002) is also relevant for the structural analysis of nanoparticles even in suspension (CitationDi Marco et al 2006).
The above cited difficulty in determining the real nature of nanoparticles, crystal or amorphous phase is reflected in a series of discordant data when various methodologies are used. For example, the mean diameter of the particles obtained from electron microscopy is often compared to the mean diameter from X-ray diffraction and to the magnetic size obtained by the Langevin function treatment of the magnetization curve. While there is quite satisfying concordance between the dimensions obtained with TEM and traditional x-ray analysis, often the values obtained for the same sample between these two methods and magnetic data are fairly different. The magnetic diameter is generally smaller than the diameter obtained by TEM. Despite the fact that several reasons could be outlined to justify this discrepancy, as the presence of particles interactions and particle size distribution that can bring deviation from the Langevin function, a structural disorder contribution seems to be more likely, especially for the smallest particles (CitationMorales et al 1997; CitationSerna et al 2001; CitationBatlle and Labarta 2002; CitationChatterjee et al 2003; CitationIglesias and Labarta 2004). Magnetization measurements have shown that the saturation magnetization of γ-Fe2O3 nanocrystals, small enough to show superparamagnetic properties, decreases with decreasing particle size. However, such reduction is difficult to be interpreted by considering only the finite size and surface effects (CitationMorales et al 1999). To explain this phenomenon several hypotheses, even those concerning the cationic disorder in the entire volume of the crystal structure, have been proposed. There is, however, no unequivocal way for clearly differentiating the individual contributions arising from finite-size, surface effects and structure of the particles. Different magnetic properties have been observed in materials with similar nominal grain-size but produced by different synthetic routes thus making the study of the interrelation between microstructure and magnetism very interesting. A possible explanation is that various syntheses may lead to particles having the same size magnitude order but different structural coherence in the whole particle volume (CitationSerna et al 2001). There is no abrupt breakdown in atomic order, but it is present in a three-dimensional lattice distortion due to the defects and finite size resulting in different magnetization values (CitationDi Marco et al 2006). Some authors have reported that even the stabilizing agent could influence the structure of the particles and, thus, the magnetizations value (CitationYee et al 1999; CitationTartaj et al 2006).
Functionalization of metal oxide surfaces
The medical applications of ferrofluids require stable formulated suspensions. Because of the high specific surface area of these fine particles, the contact between the surface and the aqueous dispersion medium is very extensive, and besides, the interface is very reactive.
In the absence of an efficient surface coating, the formation of agglomerates and aggregates result from the attraction forces between the magnetic nanoparticles (mainly van der Waals) which can destabilize the suspension.
After their intravenous administration, particles with hydrophobic surfaces are efficiently covered with plasma components especially proteins (opsonisation) and are rapidly removed from the circulation by the reticulum endothelial system (RES), whereas particles that display hydrophilic surfaces can resist to the opsonisation process, being cleared more slowly from the blood compartment (CitationGaur et al 2002). In order to prevent particles aggregation and to improve their hydrophilicity as well as to address them to certain cells in a specific manner, it is possible to modify the iron oxide surface using biocompatible ligands and/or polymers which are attached at the particle surface by physical or chemical adsorption (Sonvico, CitationMornet et al 2005). The coating of the inorganic cores by polymers or small organic molecules also reduces the aging effects observed at their surfaces (as oxidation processes or formation of complexes with ions in solution) that may turn the surface of a material into a different one (CitationPlaza et al 2002).
Moreover, the physicochemical surface properties of USPIOs strongly interfere with their capacity to be internalized by the macrophages or other phagocytic cells following their intravenous administration. Consequently, the presence of the coating is fundamental to modulate the USPIOs fate by masking and controlling their electrical surface properties (CitationArias et al 2001, Citation2006).
Ferumoxtran-10 which has a small hydrodynamic diameter (15–30 nm) shows a prolonged blood residence time which allows those USPIO to access macrophages located in deep and pathologic tissues (such as lymph nodes, kidney, brain, osteoarticular tissues, etc) (CitationCorot et al 2006). Other USPIOs such as ferucarbotran or VSOP (very small superparamagnetic iron oxide particles) have a more important liver uptake associated with a faster blood clearance and, consequently, a more limited access to the deep compartments (CitationTaupiz et al 2004). Conversely, feruglose (Clariscan®), because of the pegylation of the coating starch, can be regarded as true “stealth nanoparticles” which are hardly recognized by the macrophage-monocytic system and probably not suitable for macrophage imaging (CitationBjornerud et al 2001).
Generally, coating agents which are physically adsorbed (by electrostatic interactions or hydrogen bounding) show limited stability in comparison to coating agents which are chemically adsorbed. Indeed, physical adsorption is sensitive to the surrounding medium since competition with other macromolecules generally occurs. However, the problem of distinguishing between chemical and physical adsorption is basically the same as that of distinguishing between chemical and physical interaction in general. No absolutely sharp distinction can be made and intermediate cases exist, for instance, adsorptions involving strong hydrogen bonds or weak charge transfer. The stability of the coating grafting also depends on the quantity of the chemical interaction that each individual molecule or macromolecule can establish with the surface of the nanoparticle. As a result, each interaction between coating and metal oxide surface has to be analyzed and discussed in an individual basis.
Different types of coating can be investigated and the choice of the appropriate one depends on many factors and principally on the clinical purposes of the functionalized particle. The most common coatings for biocompatible iron oxide suspensions are polymers such as derivatives of dextran, carboxymethylated dextran, carboxydextran or polyethylene-glycol but also starch, arabinogalactan, glycosaminoglycan, organic siloxane, sulphonated styrene-divinylbenzene, poly(lactic acid), poly(e-caprolactone) and polyalkylcyanoacrylate (CitationZhang et al 2002; CitationArias et al 2005; CitationFlesch et al 2005; CitationCorot et al 2006; CitationGomez-Lopera et al 2006).
The effect of some chemical modifications of dextran on formation and stability of dextran-coated ultrasmall superparamagnetic iron oxides (USPIO) has been explored, and it has especially been demonstrated that reduction of the terminal reducing sugar can have a significant effect on particle size, coating stability, and magnetic properties. For low molecular weight dextrans (MW < 10 kDa), reduction resulted in a 10 fold or greater decrease in the carbohydrate-to-iron ratio necessary during particle formation to produce the desired particle size (<20 nm) in the coprecipitation process (CitationPaul et al 2004). Particles prepared with carboxyalkyl ether of a polysaccharide (CitationMaruno and Hasegawa 1993) especially reduced dextran yielded a more stable coating as evidenced by stability on autoclaving (CitationGroman et al 2003).
Considering the wide use of dextran-coated particles in biological applications, it is surprising that the nature of the interaction between this coating and iron oxide surface has not been more extensively investigated (CitationJung 1995; CitationBautista et al 2005). No evidence of strong chemical adsorption was observed by FTIR (Fourier transform infrared spectroscopy) and SSIMS (statistic secondary ion mass spectra) spectra analysis (CitationJung 1995). Both analyses suggest that the dextran is not covalently bonded to the iron oxide surface. Moreover, the spectra obtained by SSIMS, which is a surface selective analysis, show the presence of Fe+ ions indicating an incomplete surface coverage of the iron oxide particles by the dextran layer. It is suggested that the interaction occurs through multiple hydrogen bonds between the polymer hydroxyl groups and the surface of iron oxide particles.
The nature of the interactions between the dextran and the iron oxide nanoparticle surface and its evolution with temperature has been investigated by thermogravimetric and differential thermal analyses and by coupling these data with FTIR spectra analysis (CitationBautista et al 2005). Just after heating, it was observed that carboxylate bonds between dextran and iron oxide surface could form by oxidation and partial water elimination, a chemical process that probably reinforce the stability of the coating. However, even in this study, the suggested dominant mechanism was the formation of collective hydrogen bonding between dextran hydroxyl groups and iron oxide particle surface. Noteworthy, these dextran-coated iron oxide particles do not show any long-term toxicity (CitationAnzai et al 1994; CitationBellin et al 1998; CitationClément et al 1998; CitationBellin et al 2000; CitationBourrinet et al 2006). However, it was also observed that dextran-coated USPIO, should not always present sufficient cellular uptake to enable cell tracking due to their small sizes leading to relatively inefficient fluid phase endocytosis pathway (CitationBerry and Curtis 2003). As a result, other surface-modifying agents have been explored to increase stability of magnetic nanoparticle.
In order to obtain a strong conjugation of dextran to the maghemite surface, CitationMornet et al (2005) have developed an original synthetic route. Versatile ultrasmall superpara-magnetic iron oxide particles (VUSPIO) were obtained in a multistep procedure consisting of colloidal maghemite synthesis, surface modification by silanation of the iron core with aminopropylsilane groups and covalent conjugation with partially oxidized dextran and subsequent reduction of the shiff base (CitationZhang et al 2002; CitationMornet et al 2005). The bonding nature of organosilanes to iron surfaces is generally analyzed by FTIR-reflection absorption spectroscopy and secondary ion mass spectroscopy (ToF-SIMS). The Si–O–Fe bond is commonly described as covalent even though the discussion on this topic is still open (CitationWapner and Grundmeier 2005). Silanes are an example of a powerful and highly flexible approach to design functional metal surfaces by self-assembled monolayers (SAMs).
Such systems are ordered molecular assemblies formed by the adsorption of an active molecule (siloxane, carboxylates, thiolates, phosphate etc) on a solid surface (as iron oxide) with different terminal groups (usually −OH, −COOH, and −NH) (CitationUlman 1996; CitationChen et al 2001; CitationLove et al 2005). shows a SAMs model where the active part is a carboxylate with generic terminal groups R. These R functional groups are not always necessary as in the case of long-chain fatty acids self-assembled monolayers () (R=CH3).
Figure 2 (a) A model of SAMs grafted onto nanoparticle surface, the active part being a carboxylate carrying a generic terminal groups R. (b) A detail of the nanoparticle showing an interaction schema between carboxylate SAMS and the first layer atoms of the iron oxide surface.
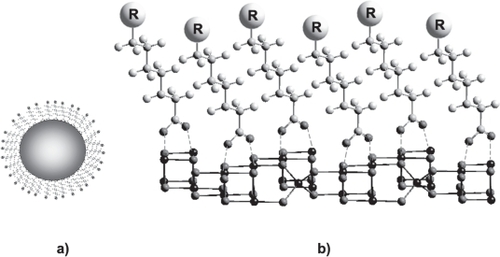
Figure 3 (a) A nanoparticle grafted with long-chain fatty acids self-assembled monolayers without R functional groups. (b) A scheme of possible fatty acids (alpha linoleic acid) to graft into iron oxide surface particles.
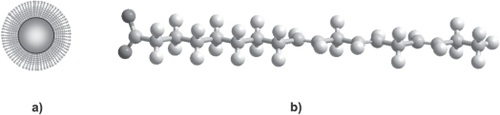
Self-assembled monolayers enable to control surface properties, the terminal groups allowing further functionalization by chemical reactions. These functionalities are also frequently incorporated in various polymers where attachment of species on the surface is desired (as the dextran grafted with siloxanes described above). SAMs can be prepared simply by adding a solution of the desired molecule onto the substrate suspension and washing off the excess not adsorbed onto particles surfaces. Their stability depends basically on the affinity of the active molecule for the substrate (solid surface), pH and ionic strength of the environment.
SAMs are currently the subject of intensive studies due to their potential applications as particles coatings. However the basic mechanisms of adhesion of this kind of molecules onto metal surface are not yet satisfactorily understood, mainly due to limited understanding of the adhesive-surface interactions. Of course, the study of these phenomena requires a detailed knowledge of the surfaces and of the molecules to be studied (CitationTirrell et al 2002).
Carboxylic acids may be adsorbed on many metal oxides but their interactions are weak, except for long-chain fatty acids which form a dense monolayer and are widely used in metal oxide nanocrystal syntheses. The mechanism of adsorption of dimercaptosuccinic acid (DMSA) has been studied by conductimetric measurements and adsorption isotherms curves. DMSA is oxidized during the coating process in tetrameric polysulfide chains [DMSAox]4 which are absorbed by the carboxylate moiety on the particles after alkalisation and neutralisation. The obtained particles are stable particles at pH = 7 (CitationFauconnier et al 1997; CitationRoger et al 1999).
The interaction between various anions and hydrated oxide particles has been investigated in many studies; the reactive groups including phosphates and phosphonates were used to form monolayers on a wide range of transition metal oxide surfaces having high affinity especially for those containing aluminium and iron oxide (CitationBrovelli and Hähner 1999; CitationTextor et al 2000; CitationKreller et al 2002; CitationBorggaard et al 2005). Several studies are reported in the literature which demonstrated that phosphonates and phosphates bind efficiently to iron oxide particle surfaces and can serve, in general, as potential alternatives to fatty acids as coating agents for metal oxide surface (CitationChen et al 2001; CitationSahoo et al 2001; CitationWhite et al 2006). Moreover, functionalized phosphonate and phosphate seems to have an acceptable biocompatibility (CitationAuernheimer et al 2005) and it is possible to suggest their utilization as coating agents of magnetic nanoparticles in medical applications.
Phosphonates are molecules that contain one or more R−PO(OH)2 Lewis acid groups. The P− C bond is generally very stable toward oxidation or hydrolysis so that many reactions can be carried out on the rest of the organic part of the molecule. These compounds not only possess a very high ability to form strong complexes with transition metals in aqueous solution with stability constant (log K values between 14 and 23) but also show a large affinity for the metal oxide surfaces (CitationMartell et al 1997; CitationBarja et al 2001). As a result, there has been an increasing interest in monolayers of long-chain phosphonic acids or alkylphosphoric acids. Another advantage is that the preparation of alkylphosphonic acids or alkylphosphoric acids is quite easy. The adsorption interactions of phosphate on iron oxide surfaces are complex and still discussed even if a variety of analytical methods have been employed for their investigation. The most widely used are methodologies in which removal of species from a solution following their adsorption onto the iron oxide particles is monitored through the determination of the species concentration prior to and after equilibrium with suspended solids has been reached (CitationZeng et al 2004). Infrared spectroscopy (IR) has been employed extensively to study phosphate adsorption on oxide surfaces (CitationTejedor-Tejedor and Anderson 1990; CitationPersson et al 1996; CitationNowack and Stone 1999). This technique has provided important insight into the identity of active surface sites involved in phosph(on)ate adsorption as well as the structures of the adsorbed species. Furthermore, the infrared features of phosphate are in a very characteristic way dependent on the symmetry of the ion, and it is therefore often possible to deduce the coordination geometry from the IR spectrum. However, certain errors in band assignment are possible and the bridging bidentate complexes of orthophosphate with adjacent atoms of iron on the surface are not the only possible explanation for the spectra of phosphate species. The surface bonding of the alkylphosphonates could be investigated by Solid State 31P NMR, this technique being useful for revealing the nature of interaction of the phosphonic acid headgroup with the different metal oxides (CitationGao et al 1996).
In addition to the above reported methods, the nature of the surface interactions could also be examined by supplementary techniques including atomic and chemical force microscopy (AFM and CFM). The first technique is generally used to study the morphological changes observed for metal oxide surfaces after exposure to phosphate (or another coating) solution (CitationNooney et al 1998). The second one can be used to probe the interaction between solution phase species (as orthophosphate or carboxylate) and hydrous iron oxide colloids (CitationKreller et al 2002, Citation2003).
Electrophoretic mobility is used in this context to compare the effect on zeta potential values of various coatings while interfacial hydrophilicity of the covered iron oxide particles can be investigated by interfacial tension measurements (contact angles techniques) (CitationArias et al 2001, Citation2006; CitationButkus and Grasso 2001). Thermal gravimetric analysis (TGA) and differential scanning calorimetry (DSC) can also be used to investigate the formation of strong chemical bonds between the substrates and various molecules (CitationYee et al 1999). Modern ultra high vacuum surface analytical methods including X ray photoelectron spectroscopy and thermally programmed desorption have also been used to examine phosphate adsorbed on an iron oxide surface (CitationNooney et al 1996).
The physicochemical processes behind iron oxide surfaces interaction with phosph(on)ates have presented many perplexing questions for well over a century being widely used for water treatment purposes. Answering all these questions could be of great interest but, unfortunately, until now there is no univocal answer. The main mechanism of ligand adsorption is ligand exchange: the surface hydroxyl, coming from the water to complete the coordination sphere of the iron, is exchanged by another ligand. Ligands are generally bonded to a metal ion on the surface by a coordinate covalent bond (donating electrons from a lone pair into an empty metal orbital), and are thus said to be coordinated to the ion. The extent of surface coordination and its pH dependence can be explained by considering the affinity of the iron surface sites for ligands and the pH dependence of the activity of surface sites and ligands. Since the adsorption of anions is coupled with a release of OH− ions, adsorption is favored by lower pH values (CitationStumm 1992). Some metal complexes are formed by bonds that are quite strong and can be considered irreversible. The type and magnitude of the interactions between these ligands and particles surface can also affect the magnetic properties of the USPIOs (CitationVestal and Zhang 2003). In fact it is known that the surface chemistry of the USPIO particles is responsible for their magnetic properties because of the exceedingly high ratio of atoms at the surface to those within the particle. Thus, the nature of the interaction between the nanoparticle surface Fe3+ sites and the adsorbing group is an important concern. Several studies and hypotheses have been made taking into consideration various ligands as reported by CitationTartaj et al (2006). For example, phosphonate coatings result in magnetization values of the iron oxide one order of magnitude lower than those obtained by coating with carboxylic acid or alcohol. The extra negative charge on the phosphonate groups cause the formation of stronger interaction to the surface of Fe3+ than carboxylate or alcohol groups. It is also suggested that the spin state of surface Fe3+ ions is affected by the bonded surfactant, through a mechanism of pπ-dπ O−P, and dπ-dπ Fe-P interactions and that the phosphonate empty d orbitals increase magnetic interactions between neighboring Fe3+ spins. In the case of carboxylic acid and alcohols, where the adsorbates have no empty d orbitals and where the oxygen atoms are less negatively charged, the iron is in a high spin state (CitationYee et al 1999). CitationNowack and Stone (1999) reported that the stability of metal-phosphonate complexes increases with increasing number of orthophosphate. Thus, the phosphonate complexes formed are known to be highly stable, and the retained coating does not readily desorb or exchange with competing ions in solution even at neutral pH.
A model for the binding and the structural organization of the alkane-phosph(on)ate molecules on the metal oxide surface has been proposed. It involves direct coordination of the terminal phosphate headgroup to metal cations forming a strong complexation bond, differentiating two types of bonding of the alkane-phosph(on)ate with both monodentate and bidentate phosphate-metal cation coordinative interactions (CitationBrovelli and Hähner 1999; CitationBarja and Dos Santos Afonso 2005). For monophosphate coating both possibilities, mono-dentate and bidentate, seem possible but the dominating binding is that of phosphonate groups bridging two Fe3+ ions (CitationYee et al 1999). Zeta potential and absorption measurements as well as IR spectroscopy (CitationTejedor-Tejedor and Anderson 1990; CitationPersson et al 1996) have suggested that the phosphate ions form bidentate complexes with adjacent sites on the iron oxide surface.
Nanoparticles in solution: Properties
When USPIO are dispersed in an aqueous medium, the electric double layer (a diffuse layer) surrounds the particle carrying surface charge (CitationJoly et al 2004). It has properties similar to a capacitor, with an electric potential whose absolute value decays with distance from the surface. Different models were proposed to describe the diffuse layer (CitationWestall and Hohl 1980). In general, they are based on the adsorption and redistribution of the ions from the bulk solution near the particle surface. The characteristic thickness of the double layer (also named the reciprocal Debye-Hückel length, κ−1) is related to the ionic strength of the suspension (CitationXu 1998). The existence of the shear layer has great influence on the stability of the colloidal systems and, specifically, the hydrodynamic motion of the suspended particles.
It has long been noticed that the hydrodynamic size values obtained by laser light scattering are often larger than diameters obtained from transmission electron microscopic (TEM) measurements and, for the same sample, the hydrodynamic size can change with the suspension conditions (CitationPrescott et al 1993; CitationPrescott et al 1997). There are many theories regarding the nature of this discrepancy. One is the “hairy layer” model that attributes the larger apparent size to a hairy layer formed by surface molecular chains (CitationSeebergh and Berg 1995). Another is the hydration model that uses surface hydration and the electric double layer to explain the observed difference (CitationJohnson 1993; CitationFischer and Kenndler 1997). Probably both phenomena give rise to this discrepancy because the water and coating electronic density are not surveying by TEM and X-ray diffractions techniques. The discrepancy between the hydrodynamic size and the solid dimension of the particles poses a challenge regarding the reliability of applying only one technique as dynamic light scattering which is commonly used for stability studies (CitationXu 1998). This effect is particularly relevant for USPIOs because there is a great difference between their crystal and their hydrodynamic size. Finally the question remains how the double layer plane location is related to the determination of both hydrodynamic diameter and zeta potential.
Zeta potential
Zeta-potential plays an important role in the electrokinetic characterization of solid–liquid interfaces; it is defined as the electrical potential at the shear plane (also known as slipping plane). The shear plane is a common concept in colloid sciences, nevertheless it is difficult to find in the literature clear information concerning the determination of the shear plane location that remains unknown. The shear plane is commonly considered smaller than the double layer, despite no exact relationship has been formulated yet. The zeta potential is a function of surface charge density, shear plane location, and surface structure and it is a very important parameter with respect to many features of the dispersed materials.
One fundamental property of metal oxide surfaces is their tendency to build up a surface charge when in contact with water. That will induce electrostatic effects in the neighborhood of the charged particle. Indeed, in solution, the presence of a net charge on a particle affects the distribution of surrounding ions, resulting in an increase in the concentration of counter-ions (ions of opposite charge to the particle) in the vicinity of the particle. This implies that the double layer is determined by the ionic strength of the solution (CitationHunter 2001). When the electrolyte concentration is modified, the changes in the shear plane location may be caused either by changes in the double layer thickness and polarization or by modification of the surface morphology. Thus, it is difficult to differentiate the shift in the shear plane from zeta potential measurements in different electrolyte concentration conditions. As a consequence, ζ-potential cannot be measured directly, but it has to be calculated from experimental techniques (streaming current or potential, electrophoretic mobility and electric conductivity) with the help of theoretical approaches (CitationEl-Gholabzouri et al 2006). There is a new alternative method based on ultrasound which is rapidly becoming important. The ultrasound method has a large advantage over traditional light based techniques because it is able to characterize concentrated systems without dilution (CitationDukhin et al 1999). Indeed, light based methods require, in general, extreme dilution suspensions in order to make the sample sufficiently transparent for measurement (especially for USPIOs samples that are mat and dark brown).
Usually, the zeta potential of colloidal suspensions is measured from electrokinetic techniques. An electrokinetic phenomenon occurs when an external field acts on a colloidal suspension. When a particle moves in an arbitrary electrolyte solution, a thin liquid layer will move with the particle, too. The layer between the moving and stationary liquid defines the slipping plane and the potential in that plane is the electrokinetic potential also called the ζ-potential. Inside the slip plane the particles are considered to be solid spheres with no bulk conductivity. The value of the ζ-potential for a determined charged interface should be independent of the electrokinetic technique used. However, there are a lot of reports (CitationRussel et al 1995; CitationMidmore et al 1996; CitationGusev and Horvath 2002) where significant differences are found between the values of ζ-potential obtained for the same charged interface using different electrokinetic techniques or theoretical approaches. Zeta-potential values obtained from electric conductivity experiments are generally much higher than those obtained from electrophoretic mobility or streaming potential. Also, ζ-potentials provided from electrophoretic mobility measurements usually show higher values than those obtained from streaming potential (CitationEl-Gholabzouri et al 2006). As a consequence, the ζ-potentials values obtained with different methodologies are hardly compared and the standardization of these measures is far to be achieved.
It is worth to be mentioned that if the zeta potential of colloidal suspensions used for medical purpose are usually characterized by electrophoretic methodologies, the expressions relating the mobility to the ζ-potential are quite complicated and it is out of question that approximate analytical or numerical solutions may only be obtained by properly defining appropriate boundary conditions. This has led to different simplified expressions for relating measured mobility to ζ-potential, but it’s very important to keep in mind that many of them have restrictions on the ζ-potentials values and/or κa (the ratio of the particle radius to electrical double layer thickness) (CitationLyklema 1995; CitationHunter 2001).
Commonly used expressions in commercial instruments to convert the electrokinetic mobility into ζ-potential derive from approximations of the Henry Equation by a separate analytical theory of the two following cases. The first case is represented by the most commonly used Smoluchowski equation (CitationThode et al 2000) which is valid for relatively conducting aqueous solutions (polar solvents) where the double layer is usually much thinner than the particle radius a (κa > > 1). The second opposite case (κa < < 1) is represented by the Huckel equation which is valid for low conducting liquids (non polar solvent). Both, deriving from Henry equation, give appropriate zeta potentials only for rigid spheres at quite low mobility values (low values of zeta potential).
Despite it is possible to obtain more accurate numerical solutions for rigid spheres by the O’Brien and White theory the algorithms used are quite complicated (CitationO’Brien and White 1978).
Most of the colloidal nanoparticles used for medical purpose are coated with polymer or polyelectrolytes and they cannot be really considered as rigid spheres. To take into account the influence of the coating on the zeta potential values, a soft particle model has been proposed (CitationOhshima 1995) by combining the theory of rigid spherical colloids with the theory of completely permeable polyelectrolytes or polymers. Nevertheless, even in this case, the definition of appropriate boundary conditions is needed to solve the complex algorithms.
It is important to point out that when using the electrophoretic technique the zeta potential is not measured directly, but calculated from the electrophoretic mobility by application of model equations (usually the Henry Equation or the most widely employed Smoluchowski one).Thus, the reliability of the zeta potential data depends upon the applicability of the equation used to the system under investigation.
However, none of the preceding theories fits with USPIOs (very small particles with an important double layer thickness, strongly negative charges and coated). The definition of appropriate boundary conditions is indeed very difficult since they don’t really fit to this kind of system, and artefacts can result from the zeta potential measurements (CitationDi Marco et al 2007). An illustration of this point can be found in CitationDi Marco et al 2007 where the zeta potential of different coated USPIOs was determined in NaCl 1 mM: a difference of about 20 mV was reported depending on whether the Smoluchowski or Hückel formula were used.
Moreover, USPIOs tend to denaturate, especially at the high voltage required in the case of such tiny particles to have a suitable sensitivity. The optimal frequency and voltage have then to be precisely determined to minimize this phenomenon.
Stability
Colloidal stability of iron oxide nanoparticles in aqueous suspensions is certainly one of the key points for pharmaceutical application.
Undoubtedly, the DLVO (CitationDerjaguin and Landau 1941; CitationVervey and Overbeek 1948) theory extended to account for hydration, steric, and magnetic interactions between particles has been and is still very useful for investigating the stability of colloidal dispersions (CitationOrtega-Vinuesa et al 1996). According to this theory and in the absence of an external applied magnetic field (CitationJanssen et al 1990), the stability of the magnetic colloid principally depends on the balance between attractive (dipole-dipole van der Waals interactions), VA, and repulsive forces (steric VS and electrostatic VE interactions) acting between the particles. This balance is commonly named total interaction potential (or energy barrier) VT between colloidal particles (CitationValle-Delgado et al 2003). The VT value depends, among others, on the surface electric potential of the particles, the electrolyte concentration in the medium, the valence of the counterions, the particle size and the Hamaker constant. Since the electrostatic interaction energy is sensitive to the electrolyte concentration while attractive forces (VA) just depend on the particle nature, the stability of colloidal dispersions can be monitored by changing the ionic strength of the solution. In the absence of a sufficient steric stabilization of the particles, an increase in the electrolyte concentration causes a significant decrease of the thickness of the double layer and consequently of VT. Thus, an electrolyte concentration (the so-called critical coagulation concentration, c.c.c.) exists, at which the energy barrier vanishes because the repulsion forces are completely cancelled. Then, the colloidal dispersion becomes unstable above the c.c.c. (CitationRomero-Cano et al 2001; CitationValle-Delgado et al 2003).
Since charge stabilization shows high ionic strength sensitivity besides the poor electrolyte resistance, steric stabilization prevents the flocculation of a colloid by attaching an efficient coating onto the particle surface. Steric stabilization is, therefore, a very useful method which provides strong stabilization even at high salt conditions and in a wide range of pH. In this situation, the suspension is then found to be stable despite zeta potential values close to zero (CitationThode et al 2000).
The efficient coating of magnetic particles by a large variety of agents may provide enhanced stability by combining electrostatic and steric (electrosteric) stabilization (CitationOrtega-Vinuesa et al 1996). These coatings modify the surface properties of the magnetic nanoparticles to a certain degree, depending on the amount adsorbed. If sufficient stabilizing agent is present, the coating layer will stabilize the particles in a way of combined steric and electrostatic effects; therefore, the colloidal stability is significantly improved. The stability factor W of the suspension has been extensively used in the literature to characterize the colloidal stability of particles having a larger size than the USPIOs, but rarely for so tiny particles (CitationPuertas and de las Nieves 1999; CitationRomero-Cano et al 2001; CitationIshikawa et al 2005; CitationDi Marco et al 2007). W is given as the ratio of the rate constants for rapid and slow coagulation kinetics, respectively. The experimental W-values can be used to calculate both the Hamaker constant (A), which characterizes the attraction between two particles, and the diffuse potential (ψd), which is related to the electrostatic repulsion. These calculations can be carried out following different methods, which are described in the literature (CitationHolthoff et al 1996; CitationOrtega-Vinuesa et al 1996; CitationSchudel et al 1997; CitationPuertas and de las Nieves 1999; CitationRomero-Cano et al 2001), nevertheless the mathematical treatment taking into account the steric contribution is quite difficult. A typical experimental method developed to investigate the colloidal stability of the particles is based on the study of the time evolution of the hydrodynamic particle size by Dynamic light scattering (DLS) as a function of the ionic strength (CitationHolthoff et al 1996; CitationSchudel et al 1997). In this approach, the lower limit of the temporal resolution for a given sample is determined by the time needed to obtain an autocorrelation function with sufficient statistical accuracy. Noteworthy, with USPIOs, the coagulation phenomenon starts rapidly (CitationDi Marco et al 2007). It is also possible to investigate colloidal stability as a function of the salt concentration by measuring the aggregation kinetics via turbidity measurements (CitationOrtega-Vinuesa et al 1996; CitationViota et al 2005). Due to the tendancy of DLS to overweight large particles however, one will have to pay attention to the experimental settings such as the angle of scattered light (generally 90° but can be varied) and the delay time especially when different batches have to be compared (CitationThode et al 2000).
Conclusion
Although USPIOs are commonly considered for medical purposes, their physicochemical properties still remain insufficiently understood. The influence of the coating layer on their structural and magnetic properties deserves further clarification whereas the nature of the grafting is still sometimes under debate. Even in the case where a general agreement exists, as for Si−O−Fe bond, the experimental evidences are not sufficient to assert it with certainty and some authors bring these theories into question.
Systematic studies, reporting the influence of polymer modifications and concentration on particle size, coating efficiency, and on USPIOs stability are quite rare whereas zeta potential measurements are far to be standardized.
For these reasons there is an urgent need to perform further investigations on the physicochemical characterization of USPIOs at the molecular and supramolecular level.
These further investigations should help to define rationnal models in order to optimize the physicochemical and biological properties of USPIO as proposed in a recent work (CitationMarinescu et al 2006) where a relationship between the saturation magnetization, the size of the nanoparticles and some simple electronic descriptors of the coating was established using a Quantitative Structure Property Relationship analysis.
References
- AnzaiYMcLachlanSMorrisM1994Dextran-coated superpara-magnetic iron oxide, an MR contrast agent for assessing lymph nodes in the head and neckAm J Neuroradiol158797511324
- AriasJLGallardoVGomez-LoperaSA2001Synthesis and characterization of poly(ethyl-2-cyanoacrylate) nanoparticles with a magnetic coreJ Control Release773092111733098
- AriasJLGallardoVGomez-Lopera2005Loading of 5-fluorouracil to poly(ethyl-2-cyanoacrylate) nanoparticles with a magnetic coreJ Biomed Nanotech121423
- AriasJLGallardoVLinares-MolineroF2006Preparation and characterization of carbonyl iron/poly(butylcyanoacrylate) core/shell nanoparticlesJ Colloid Inter Sci299599607
- AtzeiDFerriTSadunC2001Structural characterization of complexes between iminodiacetate blocked onstyrene-divinylbenzene matrix (Chelex 100 resin) and Fe(III), Cr(III), and Zn(II) in solid phase by energy-dispersive X-ray diffractionJ Am Chem Soc1232552811456924
- AuernheimerJZukowskiDDahmenC2005Titanium implant materials with improved biocompatibility through coating with phosphonate-anchored cyclic RGD peptidesChem Bio Chem6203440
- BabesLDenizotBTanguyG1999Synthesis of iron oxide nanoparticles used as MRI contrast agents: A parametric studyJ Colloid Inter Sci21247482
- BarjaBCHerszajeJDos Santos AfonsoM2001Iron(III)-phosphonates complexesPolyhedron20182130
- BarjaBCDos Santos AfonsoM2005Aminomethylphosphonic acid and glyphosate adsorption onto goethite: A comparative studyEnviron Sci Technol395859215707059
- BatlleXLabartaA2002Finite-size effects in fine particles: magnetic and transport propertiesJ Phys D: Appl Phys35R1542
- BautistaMCBomati-MiguelOMoralesMD2005Surface characterisation of dextran-coated iron oxide nanoparticles prepared by laser pyrolysis and coprecipitationJ Magn Magn Mater293207
- BellinMFRoyCKinkelK1998Lymph node metastases: safety and effectiveness of MR imaging with ultrasmall superparamagnetic iron oxide particles. Initial clinical experienceRadiology207799089609907
- BellinMFBeigelmanCPrecetti-MorelC2000Iron oxide-enhanced MR lymphography: initial experienceEur J Radiol342576410927166
- BerryCCCurtisSG2003Functionalisation of magnetic nanoparticles for applications in biomedicineJ Phys D: Appl Phys36R198206
- BjornerudAJohanssonLOAhlstromHK2001Pre-clinical results with Clariscan (NC100150 Injection); experience from different disease modelsMAGMA129910311390264
- BonnemainB1998Superparamagnetic agents in magnetic resonance imaging: physicochemical characteristics and clinical applicationsJ Drug Target6167749888302
- BorggaardOKRaben-LangeBGimsingAL2005Influence of humic substances on phosphate adsorption by aluminium and iron oxidesGeoderma1272709
- BourrinetPBengeleHHBonnemainB2006Preclinical safety and pharmacokinetic profile of ferumoxtran-10, an ultrasmall super-paramagnetic iron oxide magnetic resonance contrast agentInvest Radiol413132416481915
- BowenCVZhangXSaabG2002Application of the static dephasing regime theory to superparamagnetic iron-oxide loaded cellsMagn reson med48526112111931
- Brice-ProfetaSArrioMATroncE2005Magnetic order in g-Fe2O3 nanoparticles:a XMCD studyJ Magn Magn Mat28835465
- BrovelliDHähnerG1999Highly oriented, self-assembled alkanephosphate monolayers on tantalum(V) Oxide SurfacesLangmuir1543247
- ButkusMAGrassoD2001The nature of surface complexation: a continuum approachEnviron Geol4044653
- CaminitiRCarboneMPaneroS1999Conductivity and structure of poly(ethylene glycol) complexes using energydispersive X-ray diffractionJ Phys Chem B1031034855
- ChatterjeeJHaikYChenCJ2003Size dependent magnetic properties of iron oxide nanoparticlesJ. Magn Magn Mater2571138
- ChenYLiuWYeC2001Preparation and characterization of self-assembled alkanephosphate monolayers on glass substrate coated with nano-TiO2 thin filmMater Res Bulletin36260512
- ClémentOSiauveNCuénodCA1998Liver imaging with Ferumoxides – Feridex, fundamentals, controversies and practical aspectsTopics in Magnetic Reson Imaging916782
- CorotCPetryKGTrivediR2004Macrophage imaging in central nervous system and in carotid atheroscleroticplaque using ultrasmall superparamagnetic iron oxide in magnetic resonance imagingInvest Radiol396192515377941
- CorotCRobertPIdéeJM2006Recent advances in iron oxide nanocrystal technology for medical imagingAdv Drug Deliv Rev58147150417116343
- CushingBLKolesnichenkoVLO’ConnorCJ2004Recent Advances in the liquid-phase syntheses of inorganic nanoparticlesChem Rev104389394615352782
- DerjaguinBVLandauLD1941Theory of the stability of strongly charged lyophobic sols and the adhesion of strongly charged particles in solutions of electrolytesActa Physicochim1463362
- Di MarcoMPortMCouvreurP2006Structural characterization of ultrasmall superparamagnetic iron oxide (USPIO) particles in aqueous suspension by energy dispersive X-ray diffraction (EDXD)J Am Chem Soc12810054916881633
- Di MarcoMGuilbertIPortM2007Colloidal stability of ultrasmall superparamagnetic iron oxide (USPIO) particles with different coatingsInter J Phar331197203
- DukhinASOhshimaHShilovVN1999Electroacoustics for concentrated dispersionsLangmuir15344551
- El-GholabzouriOCabrerizo-VilchezMAHidalgo-AlvarezR2006Zeta-potential of polystyrene latex determined using different electrokinetic techniques in binary liquid mixturesColloids Surf A291307
- FauconnierNPonsJNRogerJ1997Thiolation of maghemite nanoparticles by dimercaptosuccinic acidJ Colloid Interf Sci19442733
- FischerCHKenndlerEJ1997Analysis of colloids IX. Investigation of the electrical double layer of colloidal inorganic nanometer-particles by size-exclusion chromatographyJ Chromatogr A77317987
- FleschCBourgeaut-LamiEMornetS2005Synthesis of colloidal superparamagnetic nanocomposites by grafting poly(e-caprolactone) from the surface of organosilane-modified maghemite nanoparticlesJ Polym Sci Part A: Polym Chem43322131
- GaoWDickinsonLGrozingerC1996Self-assembled monolayers of alkylphosphonic acids on metal oxidesLangmuir12642935
- GaurUSahooSKDeTK2002Biodistribution of fluoresceinated dextran using novel nanoparticles evading reticuloendothelial systemInt J Pharm20211010915921
- GillisPKoenigSH1987Transverse relaxation of solvent protons induced by magnetized spheres : application to ferritin, erythrocytes and magnetiteMagn Reson Med5323452824967
- Gomez-LoperaSAAriasJLGallardoV2006Colloidal stability of magnetite/poly(lactic acid) core/shell nanoparticlesLangmuir2228162116519488
- GossuinYRochAMullerRN2002An evaluation of the contributions of diffusion and exchange in relaxation enhancement by MRI contrast agentsJ Magn Res1583642
- GromanEVPaulKGFrigoTB2003Heat stable colloidal iron oxides coated with reduced carbohydrates and carbohydrate derivativesUnited States Patent6599498
- GuptaAKGuptaM2005Synthesis and surface engineering of iron oxide nanoparticles for biomedical applicationsBiomaterials263995402115626447
- GusevIHorvathC2002Streaming potential in open and packed fused-silica capillariesJ Chromatogr A9482032312831198
- HolthoffHEgelhaafSUBorkovecM1996Coagulation rate measurements of colloidal particles by simultaneous static and dynamic light scatteringLangmuir12554149
- HunterRJ2001Foundations of colloid science2nd edOxfordClarendon Press
- HyeonTLeeSSParkJ2001Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection processJ Am Chem Soc1231279880111749537
- KimTReisLRajanK2005Magnetic behavior of iron oxide nanoparticle–biomolecule assemblyJ Magn Magn Mater2951328
- KrellerDIGibsonGNovakW2002Chemical force microscopy investigation of phosphate adsorption on the surfaces of iron(III) Oxyhydroxide ParticlesJ Colloid Interf Sci25420513
- KrellerDIGibsonGNovakW2003Competitive adsorption of phosphate and carboxylate with natural organic matter on hydrous iron oxides as investigated by chemical force microscopyColloids Surf A21224964
- IglesiasOLabartaA2004Role of surface disorder on the magnetic properties and hysteresis of nanoparticlesPhysica B34328692
- IshikawaYKatohYOhshimaH2005Colloidal stability of aqueous polymeric dispersions: Effect of pH and salt concentrationColloid and Surf B42538
- JanssenJJMBaltussenJJMvan GelderAP1990Kinetics of magnetic flocculation. II: Flocculation of coarse particlesJ Phys D: Appl Phys23145560
- JohnsonP1993Dilute solution behavior of polystyrene latex particles and their interaction with Triton X-100Langmuir9231825
- JolivetJP2000Metal oxide chemistry and Synthesis: From solutions to solid StateMarcel DekkerNew York
- JolyLYbertCTrizacE2004Hydrodynamics within the electric double layer on slipping surfacesPhys Rev Lett9325780514
- JungCW1995Surface properties. of superparamagnetic iron oxide MR. contrast agents: ferumoxides, ferumoxtran, ferumoxsilMagn Reson Imaging13675918569442
- LawaczeckRMenzelMPietschH2004Superparamagnetic iron oxide particles: contrast media for magnetic resonance imagingAppl Organometal Chem1850613
- LeendersW2003Ferumoxtran-10Drugs698793
- LoveJCEstroffLAKriebelJK2005Self-assembled monolayers of thiolates on metals as a form of nanotechnologyChem Rev10511036915826011
- LyklemaJ1995Fundamentals of interface and colloid science: solid-liquid interfacesNew YorkAcademic Press
- MarinescuGPatronLCulitaDC2006Synthesis of magnetite in the presence of aminoacidsJ Nanoparticules Research8104551
- MartellAESmithRMMotekaitisRJ1997Critically selected stability constants of metals complexes database, NIST standard
- MarunoSHasegawaM4201993Organic magnetic complexUnited States Patent5204457
- MidmoreBRPrattGVHerringtonTM1996Evidence for the Validity of electrokinetic theory in the thin double layer regionJ Colloid Interf Sci1841704
- MiserDEShinEJHajaligolMR2004HRTEM characterization of phase changes and the occurrence of maghemite during catalysis by an iron oxideApp Cat A: Gen258716
- MoeserGDGreenWHLaibinisPE2004Structure of polymer-stabilized magnetic fluids: small-angle neutron scattering and mean-field lattice modelingLangmuir2052233415986656
- MoldayRSMackenzieD1982Immunospecific ferromagnetic iron-dextran reagent for the labeling and magnetic separation of cellsJ Immunol Methods52353677130710
- MoralesMPSernaCJBødkerF1997Spin canting due to structural disorder in maghemiteJ Phys: Condens Matter954617
- MoralesMPVeintemillas-VerdaguerSMonteroMI1999Surface and internal spin canting in -Fe2O3 nanoparticlesChem Mater11305864
- MornetSPortierJDuguetE2005A method for synthesis and functionalization of ultrasmall superparamagnetic covalent carriers based on maghemite and dextranJ Magn Magn Mater29312734
- MullerRNRochA199211th Annual Scientific Meeting; SMRM; Works in ProgressBerlin8141447
- NooneyMGMurrellTSCorneilleJS1996A spectroscopic investigation of phosphate adsorption onto iron oxidesJ Vac Sci Technol A14135761
- NooneyMGCorneilleJSMurrellTS1998Nucleation and growth of phosphate on metal oxide thin filmsLangmuir14275055
- NowackBStoneAT1999Adsorption of phosphonates onto the goethite–water interfaceJ Colloid Interf Sci2142030
- O′BrienRWWhiteLR1978Electrophoretic mobility of a spherical colloidal particleJ Chem Soc Faraday Trans 274160726
- OhgushiMNagayamaKWadaA1978Dextran-magnetite: a new relaxation reagent and its application to T2 measurements in gel systemsJ magn Reson29599601
- OhshimaH1995Electrophoresis of soft particlesAdv Colloid Interf Sci62189235
- Ortega-VinuesaJLMartin-RodriguezAHidalgo-AlvarezR1996Colloidal stability of polymer colloids with different interfacial properties: mechanismsJ Colloid Interf Sci18425967
- PascalCPascalJLFavierF1999Electrochemical synthesis for the control of γ-Fe2O3 nanoparticle sizeChem Mater111417
- PaulGKFrigoTBGromanJY2004Synthesis of ultrasmall super-paramagnetic iron oxides using reduced polysaccharidesBioconjugate Chemistry1539440115025537
- PengYParkCZhuJG2004Characterization of interfacial reactions in magnetite tunnel junctions with transmission electron microscopyJ Appl Phys956798800
- PerssonPNilssonNSjöbergS1996structure and bonding of orthophosphate ions at the iron oxide–aqueous interfaceJ Colloid Interf Sci17726375
- PlazaRCAriasJLEspinM2002Aging effects in the electrokinetics of colloidal iron oxidesJ Colloid Interf Sci2458690
- PrescottJHShiauSJRowellRL1993Characterization of polystyrene latexes by hydrodynamic and electrophoretic finger printingLangmuir9207176
- PrescottJHRowellRLBassettDR1997Dependence of particle size on pH, electrolyte, and time for expandable copolymer latexes by hydrodynamic fingerprintingLangmuir13197886
- PuertasAMde las NievesFJ1999Colloidal stability of polymer colloids with variable surface chargeJ Colloid Interf Sci2162219
- RaynalIPrigentPPeyramaureS2004Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10Invest Radiol391566314701989
- ReimerPTombachB1998Hepatic MRI with SPIO, detection and characterization of focal liver lesionsEur Radiol811982049724439
- RochAMullerRNGillisP1999Theory of proton relaxation Induced by superparamagnetic particlesJ Chem Phys110540311
- RochAGossuinYMullerRN2005Superparamagnetic colloid suspensions: Water magnetic relaxation and clusteringJ Magn Magn Mater2935329
- RogerJPonsJNMassartR1999Some biomedical applications of ferrofluidsEur Phys J AP53215
- Romero-CanoMSMartin-RodriguezAde las NievesFJ2001Electrosteric stabilization of polymer colloids with different functionalityLangmuir17350511
- RusselASScalesPJMangelsdorfCS1995High-frequency dielectric response of highly charged sulfonate laticesLangmuir11155358
- SadunCBucciRMagriAL2002Structural analysis of the solid amorphous binuclear complexes of Iron(III) and Aluminum(III) with Chromium(III)-DTPA chelator using energy dispersive X-ray DiffractionJ Am Chem Soc12430364111902895
- SahooYPizemHFriedT2001Alkyl phosphonate/phosphate coating on magnetite nanoparticles: a comparison with fatty acidsLangmuir17790711
- SchudelMBehrensSHHolthoffH1997Absolute aggregation rate constants of hematite particles in aqueous suspensions: A comparison of two different surface morphologiesJ Colloid Interf Sci19624153
- SeeberghJEBergJC1995Evidence of a hairy layer at the surface of polystyrene latex particlesColloids Surf A10013953
- SernaCJBodkerFMorupS2001Spin frustration in maghemite nanoparticlesSolid State Commun11843740
- ShenLStachowiakAFateenSEK2001Structure of alkanoic acid stabilized magnetic fluids. A small-angle neutron and light scattering analysisLangmuir1728899
- SonvicoFDubernetCColomboP2005Metallic colloid nano-technology, applications in diagnosis and therapeuticsCurr Pharm Design112095105
- SonvicoFMornetSVasseurS2005Folate-conjugated Iron Oxide nanoparticles for solid tumor targeting as potential “specific magnetic hyperthermia mediators: synthesis, physicochemical characterization, and in vitro experimentsBioconjug Chem161181816173796
- StummW1992Chemistry of the solid-water interfaceNew YorkWiley & Sons
- TartajPMoralesMPVeintemillas-VerdaguerS2003The preparation of magnetic nanoparticles for applications in biomedicineJ Phys D: Appl Phys36R18297
- TartajPMoralesMPVeintemillas-VerdaguerS2005Advances in magnetic nanoparticles for biotechnology applicationsJ Magn Magn Mater2902834
- TartajPMoralesMPVeintemillas-VerdaguerS2006Synthesis, properties and biomedical applications of magnetic nanoparticlesBuschowKHJHandbook of magnetic materialsVol 16Elsevier40382
- TaupitzMWagnerSSchnorrJ2004Phase I clinical evaluation of citrate-coated monocrystalline very small superparamagnetic iron oxide particles as a new contrast medium for magnetic resonance imagingInvest Radiol3939440515194910
- Tejedor-TejedorMIAndersonMA1990Protonation of phosphate on the surface of goethite as studied by CIR-FTIR and electrophoretic mobilityLangmuir660211
- TextorMRuizLHoferR2000Structural chemistry of self-assembled monolayers of octadecylphosphoric acid on tantalum oxide surfacesLangmuir16325771
- ThodeKMullerRHKresseM2000Two-time window and multiangle photon correlation spectroscopy size and zeta potential analysis – highly sensitive rapid assay for dispersion stabilityJ Pharm Sci8913172410980506
- TirrellMKokkoliEBiesalskiM2002The role of surface science in bioengineered materialsSurface Science5006183
- UlmanA1996Formation and Structure of Self-Assembled MonolayersChem Rev9615335411848802
- Valle-DelgadoJJMolina-BolivarJAGalisteo-GonzalezF2003Study of the colloidal stability of an amphoteric latexColloid Polym Sci8170815
- VerveyEJWOverbeekJTG1948Theory of stability of lyophobic colloidsAmsterdamElsevier
- VestalCRZhangZJ2003Effects of surface coordination chemistry on the magnetic properties of MnFe2O4 spinel ferrite nanoparticlesJ Am Chem Soc12598283312904049
- ViotaJLde VicenteJDuránJDG2005Stabilization of magnetorheological suspensions by polyacrylic acid polymersJ coll interf sci28452741
- WapnerKGrundmeierG2005Spectroscopic analysis of the interface chemistry of ultra-thin plasma polymer films on ironSurface & Coatings Technology2001003
- WeisslederRElizondoGWittenbergJ1990Ultrasmall superpara-magnetic iron oxide: characterization of a new class of contrast agents for MR imagingRadiology175489932326474
- WestallJHohlH1980A comparison of electrostatic models for the oxide/solution interfaceAdv Colloid Interface Sci1226594
- WhiteMAJohnsonJAKobersteinJT2006Toward the syntheses of universal ligands for metal oxide surfaces: controlling surface functionality through click chemistryJ Am Chem Soc12811356716939250
- XuR1998Shear plane and hydrodynamic diameter of microspheres in suspensionLangmuir1425937
- YeeCKatabyGUlmanA1999Self-assembled monolayers of alkanesulfonic and -phosphonic acids on amorphous iron oxide nanoparticlesLangmuir1571115
- ZengLLiXLiuJ2004Adsorptive removal of phosphate from aqueous solutions using iron oxide tailingsWater Research3813182614975665
- ZhangYKohlerNZhangM2002Functionalisation of magnetic nanoparticles for applications in biomedicineBiomaterials2315536111922461
