Abstract
Ultrasound-mediated targeted delivery (UMTD), a novel delivery modality of therapeutic materials based on ultrasound, shows great potential in biomedical applications. By coupling ultrasound contrast agents with therapeutic materials, UMTD combines the advantages of ultrasound imaging and carrier, which benefit deep tissue penetration and high concentration aggregation. In this paper we introduced recent advances in ultrasound contrast agents and applications in tumor therapy. Ultrasound contrast agents were categorized by their functions, mainly including thermosensitive, pH-sensitive and photosensitive ultrasound contrast agents. The various applications of UMTD in tumor treatment were summarized as follows: drug therapy, transfection of anti-oncogene, RNA interference, vaccine immunotherapy, monoclonal antibody immunotherapy, adoptive cellular immunotherapy, cytokine immunotherapy, and so on. In the end, we elaborated on the current challenges and provided perspectives of UMTD for clinical applications.
Introduction
Cancer has traditionally been one of the world’s most lethal diseases. Surgery, chemotherapy, and radiotherapy are commonly used treatments. In spite of significant progress, these treatments are limited in efficacy. Although chemotherapy is still one of the main treatments, traditional chemotherapy can also damage normal tissue while treating the tumor, and exhibit systemic toxicity. Therefore more effective delivery strategies are urgently being sought. Therapeutic materials carriers that have recently emerged include microemulsions, liposomes, lipoplexes, and nanoparticles.Citation1,Citation2 Some of them have been reported in clinical trials.Citation3 Among efforts to seek ideal carriers, microbubbles have been considered as a promising approach for therapeutic materials delivery. They cannot only deliver therapeutic materials but also serve as ultrasound contrast agents. Compared to other carriers, the advantage of microbubbles in combination with ultrasound is the ability to release a given therapeutic material precisely at the tumor site. After ultrasound irradiation, microbubbles rupture and precisely release large amounts of loaded drugs at tumor sites, maximizing the drug efficacy and simultaneously minimizing the drug toxicity. Ultrasound-mediated microbubble destruction leads to pore formation in the cell membranes, thus promoting the therapeutic materials deposition.Citation4 Compared to drugs alone, this greatly reduces the amount of drugs used, thus decreasing the drug toxicity.
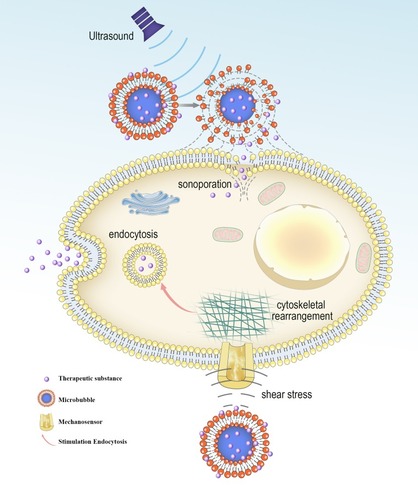
A recent study proved that there are three principal effects of ultrasound that can be useful in the process of targeted delivery: cavitational, thermal, and acoustic radiation force effects.Citation5 The cavitation effect is emerged by the response of the microbubbles to the acoustic excitation.Citation6–Citation9 In cavitation, the cell membrane is penetrated to generate a reversible pore with a diameter of hundreds of nanometers.Citation10–Citation12 Also, the cavitation effect can produce shear pressure to change the morphology of the cell membrane, promoting substance to enter cells via endocytosis ().Citation9,Citation13–Citation17 Cavitation effect has been extensively utilized to facilitate the targeted delivery. For example, it was reported that microbubbles combining with ultrasound induce acoustic cavitation. The results manifest that cavitation improves the permeability of curcumin and its anti-tumor effect.Citation18
Figure 1 Biological effects of cavitating microbubbles. After ultrasound exposure, microbubbles rupture near the cell membrane, leading to the formation of sonoporation. This allows the therapeutic substance to diffuse passively into cells. The shear stress generated by microbubble cavitation results in cytoskeletal rearrangements, altering the membrane tension. The change of cell membrane tension can be sensed by mechanosensors, thus modulating the process of endocytosis.
In addition to the cavitation effect, another important mechanism for ultrasound-mediated targeted delivery is the thermal effect. The partial ultrasonic energy is absorbed by the tissue. Thus converted into heat and caused thermal effects; therefore tumor cells can be killed through high intensity focused ultrasound irradiation.Citation19 Xia et alCitation20 found that owing to the thermal effects of ultrasound, tumor-specific cytotoxic T lymphocytes were activated, thus inducing the immune response of anti-tumor cells. Also, Deng et alCitation21 have suggested that drug delivery from temperature-sensitive liposomes under high intensity focused ultrasound can significantly increase the anti-tumor efficacy. Under ultrasonic irradiation, Au-nanoparticle coated mesoporous silica nanocapsules can enhance the temperature of tissues, thus increasing the delivery of drug.Citation22
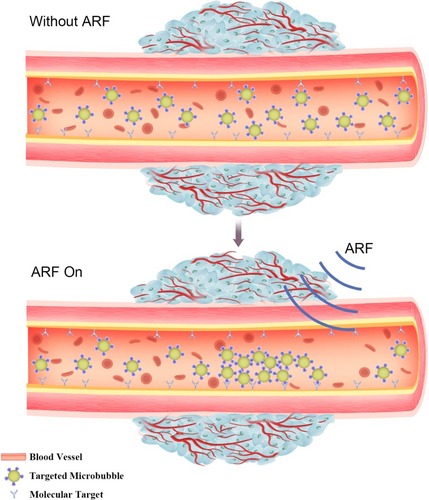
Acoustic radiation force (ARF) also plays an important role in ultrasound-mediated targeted delivery, as ARF can promote the movement of microbubbles to the wall of blood vessels,Citation23 thus effectively increasing the concentration of microbubbles at localized lesion sites ().Citation24–Citation27 Furthermore, the shearing force generated by acoustic radiation can increase the permeability of capillaries. ARF and ultrasound targeted microbubble destruction (UTMD) have a synergistic effect of targeted delivery while reducing the damage to normal tissue.Citation28,Citation29 In brief, ultrasound-mediated targeted delivery (UMTD) system is a promising method for therapeutic materials delivery in the treatment of cancer.
Figure 2 Evaluation of molecular targeted attachment of microbubbles using ARF. Without ARF, targeted microbubbles moving towards the direction of blood flow, thereby low adherence to the target. When ARF pulse exposure, the pulse pushes targeted microbubbles to the contralateral vascular wall, thus increasing targeted attachment of microbubbles.
Ultrasound Contrast Agent
Ultrasound Contrast Agent in Clinical Application
Currently, most commonly used clinical ultrasound contrast agents are microbubbles, including Optison, Sonazoid, SonoVue, and Luminity ().
Table 1 Clinical Microbubbles
Functional Targeted Ultrasound Contrast Agent
In the past few decades, a great quantity of innovations has been applied to the ultrasound contrast agent, the various purpose of ultrasound contrast agents has been achieved. Thus, we summarize some of these in the next part.
Thermosensitive Ultrasound Contrast Agent
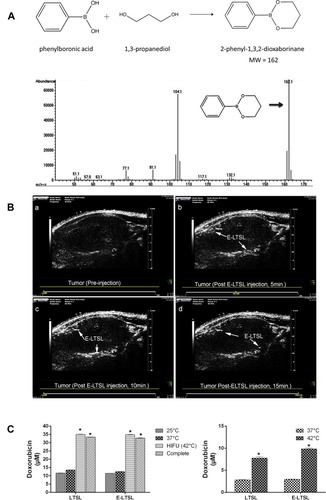
Low temperature-sensitive liposomes (LTSL) have been developed as a novel carrier for temperature-triggered drug release at the lesion site by local hyperthermia.Citation30–Citation32 Through the use of LTSL, the drug is internal loaded and remains in the liquid phase of the LTSL at body temperature, but released at the melting phase transition temperature of the bimolecular lipid layer at the range of 40–45°C.Citation30 In a recent study, Maples et alCitation33 developed echogenic low temperature-sensitive liposomes (E-LTSL) as highly efficient drug delivery carrier for in vivo doxorubicin (Dox) uptake by a 3D tumor spheroid model. As showed in , 1,3-PD was validated to be encapsulated into E-LTSL composed of amphiphilic phospholipids and perfluoropentane (PFP). The resulting 1,3-PD-based E-LTSL were used for imaging of the xenograft model in nude mice (). Furthermore, in combination with high intensity focused ultrasound (HIFU). The Dox release of E-LTSL group was clearly mapped (). Also, Zhang et alCitation34 designed a thermosensitive liposome drug delivery system consisted of ammonium bicarbonate to allow both ultrasound imaging and the release of Dox with local hyperthermia. The key point, ammonium bicarbonate, provides a rapid, controlled release of Dox to come to an effective drug concentration at the tumor site.
Figure 3 (A) Schematic representation showing the structure of derivatized 1,3-PD (MW: 162) and determination of derivatized 1,3-PD encapsulation in E-LTSLs using gas chromatography-mass spectrometry (GC-MS). (B) Continuous high-resolution tumor blood vessels US images following intravenous injection of E-LTSL in a mouse model. A gradual increase in contrast after injection was shown at (a) 0 min, (b) 5 min, (c) 10 min, (d) 15 min. (C) Significantly greater drug release in heated sample (LTSL & ELTSL, HIFU) relative to unheated control at 37°C were respectively noted in cell supernatant (left) and 3D tumour spheroid (right) (*p<0.05). Adapted with permission from Maples D, McLean K, Sahoo K, et al. Synthesis and characterisation of ultrasound imageable heat-sensitive liposomes for HIFU therapy. International Journal of Hyperthermia. 2015;31(6):674–685. Copyright 2015 Taylor & Francis Ltd; http://www.tandfonline.com; reprinted by permission of the publisher.Citation33
PH-Sensitive Ultrasound Contrast Agent
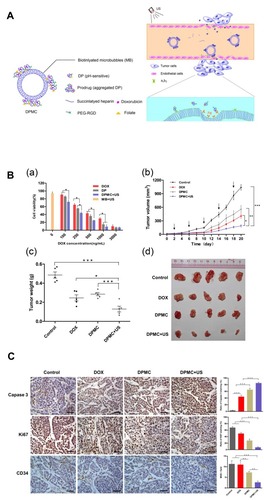
PH-sensitive nanoparticles have been widely used in cancer therapy.Citation35–Citation37 The tumor tissue in low perfusion regions is highly acidic in contrast to the surrounding normal tissues as a result of high lactate metabolism and insufficient oxygen supply of tumor cells.Citation38–Citation40 Depending on its characteristics activated by low pH, pH-sensitive nanoparticles can protect encapsulated drugs from loss during blood circulation until the loaded drugs were released into the acidic extracellular space of tumors.Citation41 Lv et alCitation42 fabricated pH-sensitive nanoparticles carrying resveratrol and loaded into microbubbles, thus combining advantages of targeted therapy, ultrasound imaging, and pH responsiveness. The results showed that the anti-tumor efficacy of resveratrol on tumor-bearing mice was significantly enhanced. With ultrasound coordination, Luo et alCitation43 devised pH-sensitive-microbubble complex, which consists of a succinylated-heparin carrier combined with Dox through hydrazone linkage and conjugated with dual targeting ligands through biotin-avidin binding (). In particular, the pH-sensitive nanoparticles attained high tumor inhibition rates in the experiment of inhibiting cell proliferation, inducing apoptosis and anti-tumor angiogenesis, providing an effective strategy for targeting drug delivery and ultrasound imaging ().
Figure 4 (A) Illustration showing the mechanism of the US combined with DPMC to deliver DOX into nuclei. (B) In vitro and In vivo antitumor efficacy. (a) In vitro cytotoxicity of MCF-7 cells respectively incubated with DOX, DP, DPMC with US, and MB with US. (b) In vivo tumor growth inhibition of DPMC with or without US, DOX and saline in a breast tumor model. DPMC with US obtained significant tumor inhibition. At the end of the experiment, tumor tissues were collected from sacrificed animals, photographed (d) and weighed (c) (***p<0.001; ** p<0.01; * p<0.05). (C) Histological analysis of tumors from mice with different treated groups (left) and corresponding quantification of Caspase-3, Ki67 and CD34 staining (right) (***p<0.001; ** p<0.01). Adapted from Luo W, Wen G, Yang L, et al. Dual-targeted and pH-sensitive Doxorubicin Prodrug-Microbubble Complex with Ultrasound for Tumor Treatment. Theranostics. 2017;7(2):452–465. Copyright 2017 Ivyspring International Publisher (https://creativecommons.org/licenses/by-nc/4.0/legalcode).Citation43
Photosensitive Ultrasound Contrast Agent
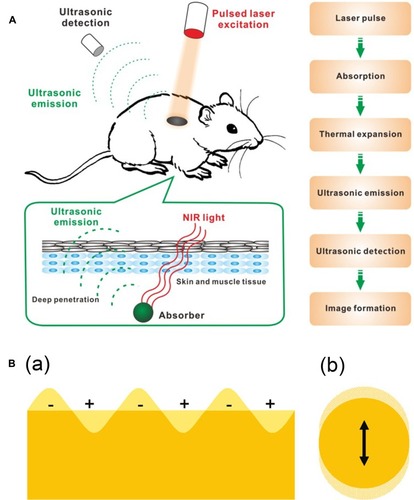
Photosensitive ultrasound contrast agents refer to carry photosensitive materials.Citation44–Citation47 When laser pulses are used, the optical energy can be absorbed by photosensitive materials and generated into heat, then the transient thermal expansion results in the generation of a broadband ultrasonic emission, which can be detected by ultrasonic transducers and analyzed to form images ().Citation48 To date, a variety of photosensitive materials have been widely used in photoacoustic imaging, and therein gold nanomaterialsCitation49 can reach high efficient photothermal transformation by means of plasmon resonance, which occurs when the frequency of surface electron and that of incident photons match mutually ().Citation50 Furthermore, gold nanomaterials possess good biocompatibility and excellent plasmonic characteristics.Citation51 Also, carbon nanomaterials, especially graphenes,Citation52–Citation55 has been explored in the field of biomedicine. Graphene oxide, a derivative of graphene, shows broad absorbance in the near infrared region. In addition, compared to other carbon nanomaterials, graphene oxide has many merits, such as outstanding water solubility and physicochemical stability owing to its oxygen functional groups, and easy to obtain because of an abundant and low manufacture cost material.Citation56–Citation59
Figure 5 (A) Schematic illustration showing the process of photoacoustic imaging (PAI). Adapted from Wang S, Lin J, Wang T, Chen X, Huang P. Recent Advances in Photoacoustic Imaging for Deep-Tissue Biomedical Applications. Theranostics. 2016;6(13):2394–2413. © 2016 Ivyspring International Publisher (https://creativecommons.org/licenses/by-nc/4.0/legalcode).Citation48 (B) Schematic illustration of (a) surface plasmons and (b) a localized surface plasmon. Adapted with permission from Mayer KM, Hafner JH. Localized surface plasmon resonance sensors. Chemical Reviews. 2011;111(6):3828–3857. Copyright © 2011 American Chemical Society.Citation50
Application of UMTD in Tumor Treatment
Tumor
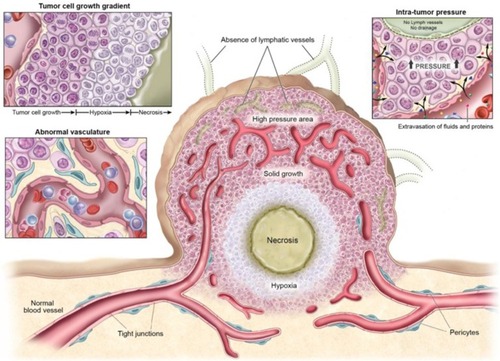
The incidence of cancer and the number of deaths is rising year by year. Although some anti-tumor drugs can induce tumor cell death in vitro, the curative effect of clinical application is not ideal, which may be related to the special microenvironment of the tumor. Previous studies have demonstrated that tumor cells can secrete various growth factors and proteases to alter the characteristics of tumor tissue microenvironment, such as hypoxia, angiogenesis, and high interstitial pressure, thus reducing the sensitivity of the tumor to radiotherapy and chemotherapy ().Citation60–Citation62 Therefore, different combination therapies, targeting tumor cells and tumor microenvironment, have become the new trend in cancer treatment. Presently, numerous researches have been done on tumor therapy through UMTD technique. Next, we will respectively discuss the application development of UMTD in tumor drug therapy, gene therapy and immunotherapy.
Figure 6 Physiological characteristics of tumor tissues and vasculatures that can restrain drug delivery. Adapted from Kobayashi H, Watanabe R, Choyke PL. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics. 2013;4(1):81–89. Copyright 2013 Ivyspring International Publisher (https://creativecommons.org/licenses/by-nc/4.0/legalcode).Citation62
Tumor Drug Therapy
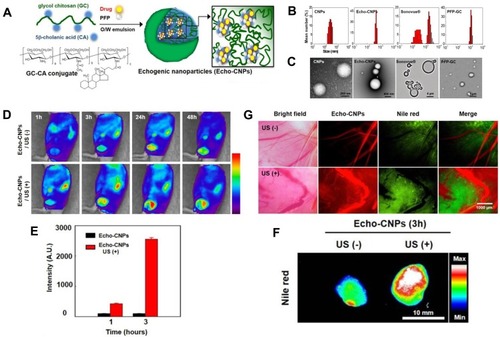
The effective concentration of chemotherapeutic drugs in tumor tissue directly affects the effect of chemotherapy. Despite the fact that traditional chemotherapy can effectively inhibit tumor cell growth in vitro, it excreted rapidly in vivo due to blood circulation, thus, the amount of intravenous medication is usually larger, increasing the systemic toxic side effects. UMTD technique has become a hot spot in the field of drug delivery, because it can achieve directional drug delivery, improve local drug concentration and reduce side effects. Rapport et alCitation63 prepared dox-loaded and acoustic-sensitive nanoparticles by encapsulated perfluoropentane with polymeric micelles. At physiologic temperatures, liquid nanodroplets converted into microbubbles. Dox was steadily retained in the microbubbles but released under ultrasound exposure. Meanwhile, the cavitation effect of microbubbles occurred, which increased intracellular drug uptake by tumor cells and resulted in tumor regression in the mouse model. Also, Min et alCitation64 used the oil in water emulsion method to construct tumor-targeted and glycol chitosan-based nanoparticles, which enwrapped an anti-tumor drug and perfluoropentane (). Compared to the conventional microbubbles, the nanoparticles had a smaller size of 432nm () and presented significantly increasing tumor-targeted ability with lower non-specific uptake by other tissues in tumor-bearing mice ().
Figure 7 (A) Schematic illustration of drug-loaded and echogenic chitosan-based nanoparticles (Echo-CNPs). Size distribution (B) and TEM images (C) of Echo-CNPs compared with CNPs, Sonovue®, and PFP-GC. (D) In vivo biodistribution of fluorescent FlammaTM labeled Echo-CNPs after 1 h, 3 h, 24 h, and 48 h post-tail vein injection with or without US irradiation. (E) Fluorescent intensities on the target tumor tissue after 1 h and 3 h post-injection with or without US treatment. (F) In vitro fluorescence imaging of the excised tumor tissues with or without US treatment. (G) Real-time dynamic drug release process visualized by OV-100 micro-vessel imaging system in tumor tissue after 10 min tail vein injection, and subsequently exposed to US destruction mode for 5 min. Adapted from Min HS, You DG, Son S, et al. Echogenic Glycol Chitosan Nanoparticles for Ultrasound-Triggered Cancer Theranostics. Theranostics. 2015;5(12):1402–1418. Copyright 2015 Ivyspring International Publisher (https://creativecommons.org/licenses/by-nc/4.0/legalcode).Citation64
Tumor hypoxia and angiogenesis present further obstacles for effective therapy in various solid tumors. Tumor hypoxia triggers various cellular defense mechanisms that enhance the drug-resistance of tumor cells.Citation65–Citation69 Therefore, some studies showed that oxygen treatment prescribed before radiotherapy or chemotherapy can boost tumor oxygenation, and improve drug uptake.Citation65,Citation70,Citation71 As a novel drug carrier, microbubbles, assisted by ultrasound, have the potential to simultaneously deliver oxygen and anti-tumor drugs for chemotherapy.Citation72,Citation73 Tumor angiogenesis, the proliferation of abnormal blood vessels, results in high interstitial pressure and poor perfusion that contribute to the low effective uptake of anti-tumor drugs.Citation74–Citation76 Recently, research has shown that microbubbles combined with low-frequency ultrasound for delivery of anti-angiogenic drugs can effectively improve the anti-tumor efficacy of drugs.Citation77
Tumor Gene Therapy
With the rapid development of genetic engineering and gradual explanation of molecular pathogenesis of tumor, gene therapy has emerged as a promising and efficient therapeutic strategy for treating tumor.Citation78,Citation79 Gene therapy is widely known as the transfection of a therapeutic gene into tumor cells or selective silencing of the oncogene to alter gene expression as a means to treat the tumor. Current gene transfection methods, including viral and non-viral vector systems, have various limitations.Citation80–Citation82 For example, viral vectors are highly efficient in gene transfection but cause insertional mutagenesis and immune responses.Citation83–Citation86 Most non-viral vectors are limited by low transfection efficiency and lack of targeting.Citation87,Citation88 UMTD technology represents an appealing, efficient and non-virus transfer method, which could deliver therapeutic genetic material, such as oligonucleotides and plasmid DNA, to the tumor site in a simple and noninvasive way. This is based on the fact that microbubbles after ultrasound irradiation occur cavitation, which generate microflow around the cell membranes to cause sonoporation, allowing for gene directly transfected into the cells.Citation89
Transfection of Anti-Oncogene
P53, a commonly used tumor suppressor gene, plays a vital role in the onset of tumor development, proliferation, and metastasis. Transfection of p53 into tumor cells can effectively inhibit tumor cell growth and promote cell apoptosis. However, there is a risk of mutagenesis using viral vectors to transfect genes into the tumor. UMTD technology can avoid this. For example, Chang et alCitation90 described that the application of ultrasound on ovarian cancer cells following incubation with tumor-targeted microbubbles resulted in a higher cell apoptosis rate, compared to those of the other groups. Similarly, transfection of wild-type p53 using microbubble-assisted ultrasound led to significantly higher transfection efficiency as compared to treatment with microbubbles alone. MicroRNAs (MiRs) are short noncoding RNAs and involved in several pathways related to the pathogenesis of tumor.Citation91–Citation95 It has been observed that multiple tumors possess aberrant miRNA expression. Restoration of the MiR expression levels can inhibit tumor development. Besides, some MiRs can be downregulated in several tumor types,Citation96 therefore some tumor patients are benefiting from successful transfection of specific MiRs. Previous studies have shown that over-expression of miR-122 cannot only inhibit tumor cell proliferation, but also induce cell apoptosis and cell cycle arrest, and recovery of tumor sensitivity to chemotherapy.Citation97–Citation100 Successful delivery of miR-122 into the tumor, playing the part of a novel anti-tumor agent, is of crucial importance. However, MiRs are easy to be degraded by nucleases present in blood circulation. Thus, Wang et alCitation79 proposed encapsulating the miR-122 into nanoparticles to guard against nuclease degradation. Delivery of miR-122 loaded nanoparticles at the tumor site was then enhanced by ultrasound. Results showed that local miR-122 expression in the tumor after treatment with ultrasound was 7.9-fold higher compared to treatment without ultrasound.
RNA Interference
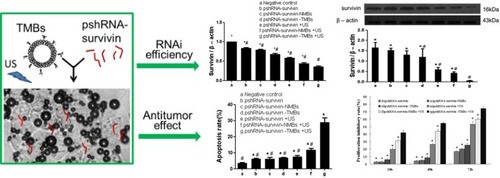
Gene therapy, based on RNA interference, is a highly efficient gene disruption approach, permitting selective silencing of a specific gene. Small interfering RNA (siRNA) can be implemented to target a specific messenger RNA (mRNA), which results in down-regulation of the encoded protein. This process has been reported for the treatment of various tumors, in which some proteins are found to be upregulated.Citation101 For instance, vascular endothelial growth factor (VEGF) is one of that overexpressed in some malignancies, and is responsible for accelerating tumor angiogenesis, that results in rich blood flow in the tumor, thus enhancing tumor growth.Citation102–Citation105 Delivering siRNA, that can target VEGF mRNA and down-regulate protein, has been reported to be a novel strategy to treat tumors revealing enhanced angiogenesis.Citation106–Citation108 For attaining the desired effect, the siRNA, delivered to the tumor site, must reach a therapeutically sufficient concentration.Citation109 However, naked siRNA shows poor cellular uptake and easily degraded by ribonucleases in serum.Citation101 To overcome the aforementioned shortcomings, Florinas et alCitation110 applied cationic polymer as an encapsulated shell to protect siRNA from being degraded by ribonucleases, meanwhile in combination with ultrasound microbubbles to synergistically transfect VEGF-siRNA. Results showed significantly higher siRNA uptake in vitro and stronger tumor growth inhibition in vivo. Survivin, a member of the inhibitor of apoptosis proteins family, is generally over-expressed in a variety of tumors but low expressed or not found in normal tissues.Citation111–Citation114 Also, survivin has been reported as a preferential target for selective cancer treatment because of its functions, contributing to the formation and progression of the tumor.Citation115,Citation116 Zhang et alCitation117 constructed LHRHa targeted microbubble agent for transfecting short hairpin RNA to inhibit survivin gene expression followed by ultrasound exposure. Results showed that UMTD method yielded higher RNAi efficiency, cell apoptosis rate, and cell proliferation inhibitory rate (). Considering that the X-linked inhibitor of apoptosis protein (XIAP), also a member of the inhibitor of apoptosis proteins family, protecting tumor from apoptosis stimulation damage,Citation118,Citation119 is typically over-expressed in malignant tumors, therefore it can be used as an RNA interfering target in tumor therapy. For instance, XIAP-siRNA encapsulated ultrasound-responsive microbubble was developed from polymeric siRNA micelles and liposomal microbubbles through hetero-assembling method.Citation120 Intratumoral injection of microbubbles, carrying XIAP-siRNA followed by low-frequency ultrasound irradiation at the tumor site, resulted in increased permeability of tumor regions for much more siRNA delivery into deep tumor tissues. Significant enhancement of XIAP gene silencing led to a satisfactory therapeutic effect on human cervical cancer subcutaneous xenograft model in nude mice. Moreover, microbubbles carrying XIAP-siRNA were also used as ultrasound contrast agents to monitor real-time tumor during the therapeutic process.
Figure 8 Targeted microbubble for ultrasound-mediated short hairpin RNA plasmid transfection to silence survivin gene and exert the antitumor effect (#p<0.05; *p<0.05). Adapted with permission from Zhang Y, Chang S, Sun J, et al. Targeted Microbubbles for Ultrasound Mediated Short Hairpin RNA Plasmid Transfection to Inhibit Survivin Gene Expression and Induce Apoptosis of Ovarian Cancer A2780/DDP Cells. Mol Pharm. 2015;12(9):3137–3145. Copyright © 2015 American Chemical Society.Citation117
Tumor Immunotherapy
Tumor immunotherapy is a promising means of therapy, that has become a crucial part of many treatment plans, and offers the possibility for the better cure by targeting tumor cells more specifically than other conventional treatments. Broadly speaking, immunotherapy is a treatment strategy that refers to the stimulation of the body’s immune system against tumor cells through the introduction of tumor vaccines, monoclonal antibodies, cytokines or immune cells.Citation121 Immunotherapies can be classified as active immunotherapy and passive immunotherapy. Therein, active immunotherapies rely on stimulating one’s own immune system to eliminate malignant cells, and passive immunotherapies contains cytokines, monoclonal antibodies and immune cells acting directly on the tumor cells. However, no matter it is a tumor vaccine or an antibody, intravenous injection is applied for them to enter the body, thus leading to poor delivery efficiency. UMTD technology has made some progress in tumor immunotherapy. Next, we give a brief introduction to them in the following part.
Tumor Vaccine Immunotherapy
Tumor vaccines, where the patient’s own immune system is triggered to target and eliminate tumor tissue, have emerged as a novel and promising therapeutic strategy. Dendritic cells (DCs) vaccines have made some progress in the clinical and subclinical studies of anti-tumor immunotherapy.Citation122–Citation125 DCs are well known as antigen-presenting cells that activate antigen-specific T cell responses. DCs can be modified to present tumor-derived antigens to T cells. Thus harnessing the patient’s own immune system to battle against tumor.Citation126 For example, Dewitten et alCitation127 evaluated the potential of DC using microbubbles loaded with antigen and TriMix mRNA in combination with ultrasound for tumor immunotherapy. In vivo therapeutic setting, the application of mRNA sonoporated DCs led to a significant inhibition of tumor growth and prolongation of overall survival. Furthermore, complete tumor regression and long-term immunological memory occurred in about 30% of antigen + TriMix DC vaccinated animals. DNA vaccination has emerged as a potential immunotherapeutic approach against tumor due to its stability and safety. It has been noted that mammalian cells are able to express genes encoded on plasmid DNA after transfection.Citation128 What is more, it was also proved that intramuscular injection of plasmid DNA can result in the induction of humoral and cellular immune responses against the encoded antigen.Citation129 In addition, numerous clinical trials on various DNA vaccines against different tumors have been reported.Citation130–Citation133 Un et alCitation134 developed a DNA vaccination for inhibition of melanoma growth and metastasis using an ultrasound-responsive and mannose-modified bubble lipoplexes. Following US exposure, the cytotoxic T lymphocytes were specifically activated in the presence of melanoma-specific antigens, thus obtaining potent DNA vaccine effects against melanoma. However, therapeutic effects of DNA vaccine, regarding a melanoma solid tumor, were insufficient due to the high growth rate of the tumor. On the basis of research of Un et al, Yoshida et alCitation135 further investigated a method, involving the use of Dox-encapsulated liposomes and transfection using mannose-modified bubble lipoplexes in combination with US irradiation for anti-tumor effect. It cannot only inhibit tumor growth but also improve transfection efficacy in antigen-presenting cells, thus improving the therapeutic effects of a DNA vaccine against the tumor.
Tumor Monoclonal Antibody Immunotherapy
The monoclonal antibody can be used to treat the tumor by blocking specific signaling pathways. One approved therapeutic monoclonal antibody, which is effective for HER2-positive breast cancer and significantly improves overall survival of patients with advanced breast cancer, is trastuzumab.Citation136 However, the response of brain metastases to trastuzumab is still poor, due to the restriction of the blood-brain barrier. Previously, it has been reported that the site-specific local delivery of trastuzumab to the mouse brain can be heightened by blood-brain barrier disruption using focused ultrasound in combination with microbubbles ().Citation137 Furthermore, in research using a breast cancer brain metastasis model in nude rats, it was proved that mean tumor volume decreased evidently in rats that injected with trastuzumab in combination with ultrasound disruption of the blood-brain barrier compared to other treatment groups.Citation138 In addition, Kobus et alCitation139 evaluated the anti-tumor effect of trastuzumab and pertuzumab through ultrasound-mediated blood-brain barrier disruption using HER2-positive cell lines that were derived from brain metastases in patients with breast cancer. It was shown that the growth of brain metastases from breast cancer was significantly inhibited.
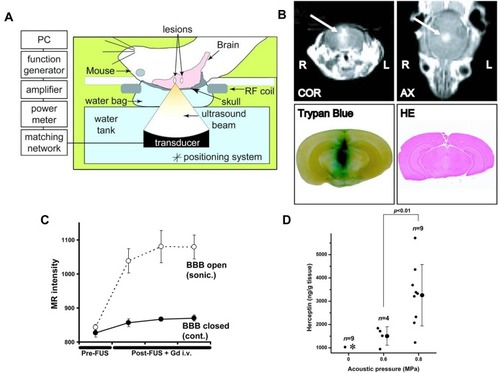
Figure 9 (A) Pattern diagram for the blood–brain barrier (BBB) opening in mice induced by MRI-guided focused ultrasound. (B) The mice BBB opening monitored by coronal (COR) and axial (AX) MR images (arrows). (Lower Left) Trypan blue staining the location of the BBB opening. (Lower Right) HE staining shows no apparent macroscopic damage related to BBB disruption. (C) MR-intensity change between the sonicated target (○) and the contralateral side (control; ●). (D) Graphs show Herceptin concentrations in the sonicated or control groups as a function of the applied acoustic pressure. *In the control (0 MPa), herceptin was below the lower limit of the detection range (780 ng/g of tissue) in eight of nine cases. Adapted from Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci USA. 2006;103(31):11, 719–11, 723. © 2006 by The National Academy of Sciences of the USA.Citation137
Tumor Adoptive Cellular Immunotherapy
Adoptive cellular immunotherapy is the reinfusion of natural or genetically modified autologous lymphocytes that have been expanded ex vivo into patients to treat tumors. The critical role of transferring immune cells into tumor patients has been reported by various articles.Citation140–Citation142 NK cell, nowadays one of the most commonly used adoptive cells, can exert innate immune response to tumor cells ().Citation143 Compared with other systemic therapies, targeted NK cells can lead to more specific cytotoxicity to tumor cells, which are enhanced when recognizing those tumor cells expressing the target antigen.Citation144 Studies have reported that the potential for focused ultrasound to deliver targeted NK cells to the brain using a xenograft model of human metastatic breast cancer in nude rats. Following the disruption of the blood-brain barrier using focused ultrasound in the presence of microbubbles, the average ratio of NK cells to tumor cells was greatly improved.Citation145 In order to further explore the salutary effect of targeted NK cells, Alkins et alCitation146 built an orthotopic HER2-amplified rodent brain tumor model using human breast cancer. Results showed that ultrasound-mediated targeted delivery of NK cells to the tumor site could slow tumor growth and improve survival of tumor-bearing rats.
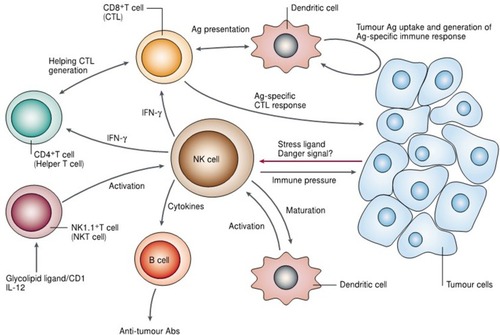
Figure 10 Illustration indicating the potential role of natural killer (NK) cells in tumor immune surveillance and tumor immune response. Adapted with permission from Smyth MJ, Yoshihiro H, Kazuyoshi T, et al. New aspects of natural-killer-cell surveillance and therapy of cancer. Nature Reviews Cancer. 2002;2(11):850–861. Copyright © 2002, Springer Nature. https://www.nature.com/articles/nrc928.Citation143
Tumor Cytokine Immunotherapy
Cytokines, mainly regulation of immunity and inflammation, are biologic immune modulators that are naturally generated by various cell types. Cytokines can regulate the immune response, thus it is crucial to balance the properties of their immune stimulatory and inhibitory for host immunity against tumor cells.Citation147 Interleukin-12 (IL-12) has shown promise in triggering an anti-tumor immune response and establishing a long-term immune memory against tumor recurrence in the host, but the dosage was limited by obvious systemic immunotoxicity. Suzuki et alCitation148 assessed the utility of UMTD method to deliver IL-12 corded plasmid DNA and successfully achieved high concentration aggregation of IL-12 in local tumor tissue, thus minimizing the systemic toxicity. The blood-brain barrier has long been a hindrance of chemotherapeutic drugs for brain tumors, limiting the delivery of the therapeutic materials and ability to reach an effective dose at the tumor site. Chen et alCitation149 reported the use of focused ultrasound-induced blood-brain barrier opening in enhance IL-12 transfer for treatment of a rat glioma model and demonstrated that ultrasound-mediated delivery of gene-transfer IL-12 suppressed tumor progression and prolonged survival in a C-6 glioma model.
Conclusion
In summary, the method of UMTD has shown remarkable promise in clinical therapeutic materials delivery and significantly improved therapeutic effects. In this review, we have described functional ultrasound contrast agents and various strategies for their applications in tumor therapy.
Regardless of the fact that a lot of progress has been made in the application of UMTD to deliver therapeutic materials to various tumors, there are still many difficulties to be solved in clinical practice. Taking microbubbles as an example. First of all, microbubbles, as foreign substances, may cause unnecessary immune system in vivo. Secondly, it is not enough only to modify microbubbles with targeting ligands. Ideally, it is essential to clarify the mechanisms whereby ultrasound controls therapeutic materials fixed-point release. What is more, the size of the microbubbles should also be considered, larger microbubbles have poor vascular permeability. Finally, how to excrete microbubbles from the body is also worth thinking. We hope that these problems will be solved with further research of UMTD in future.
Abbreviations
UMTD, ultrasound-mediated targeted delivery; ARF, acoustic radiation force; UTMD, ultrasound targeted microbubble destruction; LTSL, Low temperature-sensitive liposomes; E-LTSL, echogenic low temperature-sensitive liposomes; Dox, doxorubicin; PFP, perfluoropentane; HIFU, high intensity focused ultrasound; MiRs, MicroRNAs; siRNA, Small interfering RNA; mRNA, messenger RNA; VEGF, vascular endothelial growth factor; XIAP, X-linked inhibitor of apoptosis protein; DCs, Dendritic cells; IL-12, Interleukin-12.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers 81571682, 2016; and 81873900, 2019), and the Hai Yan Science Foundation of Harbin Medical University Cancer Hospital (grant number JJQN2018-18).
Disclosure
The authors have declared no conflicts of interest in this work.
References
- Jeong K, Kang CS, Kim Y, et al. Development of highly efficient nanocarrier-mediated delivery approaches for cancer therapy. Cancer Lett. 2016;374(1):31–43. doi:10.1016/j.canlet.2016.01.05026854717
- Min HS, Dong GY, Son S, et al. Echogenic glycol chitosan nanoparticles for ultrasound-triggered cancer theranostics. Theranostics. 2015;5(12):1402–1418. doi:10.7150/thno.1309926681985
- Wicki A, Witzigmann D, Balasubramanian V, et al. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Controlled Release Off. 2015;200:138–157. doi:10.1016/j.jconrel.2014.12.030
- Lakshmanan S, Gupta GK, Avci P, et al. Physical energy for drug delivery; poration, concentration and activation ☆. Adv Drug Deliv Rev. 2014;71(2):98–114. doi:10.1016/j.addr.2013.05.01023751778
- Steven M, Constantin C, Len S, et al. Ultrasound-enhanced drug delivery for cancer. Expert Opin Drug Deliv. 2012;9(12):1525–1538. doi:10.1517/17425247.2012.73960323121385
- Annemieke VW, Ayache B, Michel V, et al. Micromanipulation of endothelial cells: ultrasound-microbubble-cell interaction. Ultrasound Med Biol. 2004;30(9):1255–1258. doi:10.1016/j.ultrasmedbio.2004.07.01515550330
- Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng. 2007;9(9):415–447. doi:10.1146/annurev.bioeng.8.061505.09585217651012
- Sboros V. Response of contrast agents to ultrasound ☆. Adv Drug Deliv Rev. 2008;60(10):1117–1136. doi:10.1016/j.addr.2008.03.01118486270
- Wu J, Nyborg W. Ultrasound, cavitation bubbles and their interaction with cells. Adv Drug Deliv Rev. 2008;60(10):1103–1116. doi:10.1016/j.addr.2008.03.00918468716
- Vanbavel E. Effects of shear stress on endothelial cells: possible relevance for ultrasound applications. Prog Biophys Mol Biol. 2007;93(1):374–383. doi:10.1016/j.pbiomolbio.2006.07.01716970981
- Delalande A, Kotopoulis S, Postema M, et al. Sonoporation: mechanistic insights and ongoing challenges for gene transfer. Gene. 2013;525(2):191–199. doi:10.1016/j.gene.2013.03.09523566843
- Lentacker I, Cock ID, Deckers R, et al. Understanding ultrasound induced sonoporation: definitions and underlying mechanisms ☆. Adv Drug Deliv Rev. 2014;72:49–64. doi:10.1016/j.addr.2013.11.00824270006
- Ingber DE. TENSEGRITY: THE ARCHITECTURAL BASIS OF CELLULAR MECHANOTRANSDUCTION. Annu Rev Physiol. 2003;59(1):575–599. doi:10.1146/annurev.physiol.59.1.575
- Apodaca G. Modulation of membrane traffic by mechanical stimuli. Am J Physiol Renal Physiol. 2002;282(2):F179. doi:10.1152/ajprenal.2002.282.2.F17911788431
- Lionetti V, Fittipaldi A, Agostini S, et al. Enhanced caveolae-mediated endocytosis by diagnostic ultrasound in vitro. Ultrasound Med Biol. 2009;35(1):136–143. doi:10.1016/j.ultrasmedbio.2008.07.01118950933
- Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75(3):519–560. doi:10.1152/physrev.1995.75.3.5197624393
- Junru W. Theoretical study on shear stress generated by microstreaming surrounding contrast agents attached to living cells. Ultrasound Med Biol. 2002;28(1):125–129. doi:10.1016/S0301-5629(01)00497-511879959
- Li Y, Wang P, Chen X, et al. Activation of microbubbles by low-intensity pulsed ultrasound enhances the cytotoxicity of curcumin involving apoptosis induction and cell motility inhibition in human breast cancer MDA-MB-231 cells. Ultrason Sonochem. 2016;33:26–36. doi:10.1016/j.ultsonch.2016.04.01227245953
- Dubinsky TJ, Carlos C, Dighe MK, et al. High-intensity focused ultrasound: current potential and oncologic applications. AJR Am J Roentgenol. 2008;190(1):191–199. doi:10.2214/AJR.07.267118094311
- Ji-Zhu X, Fang-Lin X, Li-Feng R, et al. High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes. Ultrasound Med Biol. 2012;38(8):1363–1371. doi:10.1016/j.ultrasmedbio.2012.03.00922633269
- Deng Z, Xiao Y, Pan M, et al. Hyperthermia-triggered drug delivery from iRGD-modified temperature-sensitive liposomes enhances the anti-tumor efficacy using high intensity focused ultrasound. J Controlled Release. 2016;243:333–341. doi:10.1016/j.jconrel.2016.10.030
- Wang X, Chen H, Zheng Y, et al. Au-nanoparticle coated mesoporous silica nanocapsule-based multifunctional platform for ultrasound mediated imaging, cytoclasis and tumor ablation. Biomaterials. 2013;34(8):2057–2068. doi:10.1016/j.biomaterials.2012.11.04423246067
- Kilroy JP, Klibanov AL, Wamhoff BR, et al. Intravascular ultrasound catheter to enhance microbubble-based drug delivery via acoustic radiation force. Ultrason Ferroelectr Freq Control IEEE Trans. 2012;59(10):2156–2166. doi:10.1109/TUFFC.2012.2442
- Kaya M, Toma C, Wang J, et al. Acoustic radiation force for vascular cell therapy: in vitro validation. Ultrasound Med Biol. 2012;38(11):1989–1997. doi:10.1016/j.ultrasmedbio.2012.07.01922975034
- Toy R, Bauer L, Hoimes C, et al. Targeted nanotechnology for cancer imaging ☆. Adv Drug Deliv Rev. 2014;76(1):79–97. doi:10.1016/j.addr.2014.08.00225116445
- Theek B, Gremse F, Kunjachan S, et al. Characterizing EPR-mediated passive drug targeting using contrast-enhanced functional ultrasound imaging. J Controlled Release. 2014;182(1):83–89. doi:10.1016/j.jconrel.2014.03.007
- Shortencarier MJ, Dayton PA, Bloch SH, et al. A method for radiation-force localized drug delivery using gas-filled lipospheres. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51(7):822–831. doi:10.1109/TUFFC.2004.132074115301001
- Fabian K, Stanley F, Jessica B, et al. Recent advances in molecular, multimodal and theranostic ultrasound imaging. Adv Drug Deliv Rev. 2014;72:15–27. doi:10.1016/j.addr.2013.11.01324316070
- Chuang YH, Cheng PW, Li PC. Combining radiation force with cavitation for enhanced sonothrombolysis. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60(1):97–104. doi:10.1109/TUFFC.2013.254123287916
- Yatvin MB, Weinstein JN, Dennis WH, et al. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202(4374):1290–1293. doi:10.1126/science.364652364652
- Dou YN, Jinzi Z, Foltz WD, et al. Heat-activated thermosensitive liposomal cisplatin (HTLC) results in effective growth delay of cervical carcinoma in mice. J Controlled Release Off. 2014;178(1):69–78. doi:10.1016/j.jconrel.2014.01.009
- Sun MP, Min SK, Park SJ, et al. Novel temperature-triggered liposome with high stability: formulation, in vitro evaluation, and in vivo study combined with high-intensity focused ultrasound (HIFU). J Controlled Release. 2013;170(3):373–379. doi:10.1016/j.jconrel.2013.06.003
- Maples D, McLean K, Sahoo K, et al. Synthesis and characterisation of ultrasound imageable heat-sensitive liposomes for HIFU therapy. Int J Hyperthermia. 2015;31(6):674–685. doi:10.3109/02656736.2015.105762226185910
- Zhang N, Li J, Hou R, et al. Bubble-generating nano-lipid carriers for ultrasound/CT imaging-guided efficient tumor therapy. Int J Pharm. 2017;534(1):S0378517317306907. doi:10.1016/j.ijpharm.2017.07.081
- Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nature Rev Drug Discovery. 2014;13(11):813–827. doi:10.1038/nrd433325287120
- Neri D, Supuran CT. Interfering with pH Regulation in Tumours as a Therapeutic Strategy. Nature Rev Drug Discov. 2011.
- Hongjun LI, Wang J. Tumor extracellular acidity-activated nanoparticles as drug delivery systems. Biotechnol Adv. 2014;32(4):789–803. doi:10.1016/j.biotechadv.2013.08.00223933109
- Tan S, Wu T, Zhang D, et al. Cell or cell membrane-based drug delivery systems. Theranostics. 2015;5(8):863–881. doi:10.7150/thno.1185226000058
- Jain RK; The Eugene M. Landis Award Lecture 1996. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2015;4(1):1–23.
- Mani P, Grailer JJ, Srikanth P, et al. Gold nanoparticles with a monolayer of doxorubicin-conjugated amphiphilic block copolymer for tumor-targeted drug delivery. Biomaterials. 2009;30(30):6065–6075. doi:10.1016/j.biomaterials.2009.07.04819674777
- Juan L, Yuran H, Anil K, et al. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol Adv. 2014;32(4):693–710. doi:10.1016/j.biotechadv.2013.11.00924309541
- Lv Y, Hao L, Hu W, et al. Novel multifunctional pH-sensitive nanoparticles loaded into microbubbles as drug delivery vehicles for enhanced tumor targeting. Sci Rep. 2016;6:29321. doi:10.1038/srep2932127378018
- Luo W, Wen G, Yang L, et al. Dual-targeted and pH-sensitive doxorubicin prodrug-microbubble complex with ultrasound for tumor treatment. Theranostics. 2017;7(2):452–465. doi:10.7150/thno.1667728255342
- Weitao Y, Weisheng G, Xiaoqun G, et al. Facile synthesis of Gd-Cu-In-S/ZnS bimodal quantum dots with optimized properties for tumor targeted fluorescence/MR in vivo imaging. ACS Appl Mater Interfaces. 2015;7(33):18759–18768. doi:10.1021/acsami.5b0537226257133
- Weisheng G, Xiaolian S, Orit J, et al. Intrinsically radioactive [64Cu]CuInS/ZnS quantum dots for PET and optical imaging: improved radiochemical stability and controllable Cerenkov luminescence. ACS Nano. 2015;9(1):488–495. doi:10.1021/nn505660r25549258
- Li C, Feng L, Zhang Y, et al. Real-time monitoring surface chemistry-dependent in vivo behaviors of protein nanocages via encapsulating an NIR-II Ag2S quantum dot. ACS Nano. 2015;9(12):12255. doi:10.1021/acsnano.5b0550326496067
- Liming N, Xiaoyuan C. Structural and functional photoacoustic molecular tomography aided by emerging contrast agents. Chem Soc Rev. 2014;43(20):7132–7170. doi:10.1039/C4CS00086B24967718
- Wang S, Lin J, Wang T, et al. Recent advances in photoacoustic imaging for deep-tissue biomedical applications. Theranostics. 2016;6(13):2394–2413. doi:10.7150/thno.1671527877243
- Wanwan L, Xiaoyuan C. Gold nanoparticles for photoacoustic imaging. Nanomedicine. 2015;10(2):299–320. doi:10.2217/nnm.14.16925600972
- Mayer KM, Hafner JH. Localized surface plasmon resonance sensors. Chem Rev. 2011;111(6):3828–3857. doi:10.1021/cr100313v21648956
- Zhong J, Wen L, Yang S, et al. Imaging-guided high-efficient photoacoustic tumor therapy with targeting gold nanorods. Nanomed Nanotechnol Biol Med. 2015;11(6):1499–1509. doi:10.1016/j.nano.2015.04.002
- Lin J, Chen X, Huang P. Graphene-based nanomaterials for bioimaging ☆. Adv Drug Deliv Rev. 2016;105(Pt B):242–254. doi:10.1016/j.addr.2016.05.01327233213
- Xuefeng Y, Gang N, Jing L, et al. Enhanced fluorescence imaging guided photodynamic therapy of sinoporphyrin sodium loaded graphene oxide. Biomaterials. 2015;42(42):94–102. doi:10.1016/j.biomaterials.2014.11.04025542797
- Huang P, Wang S, Wang X, et al. Surface functionalization of chemically reduced graphene oxide for targeted photodynamic therapy. J Biomed Nanotechnol. 2015;11(1):117. doi:10.1166/jbn.2015.205526301305
- Lalwani G, Xin C, Nie L, et al. Graphene-based contrast agents for photoacoustic and thermoacoustic tomography ☆. Photoacoustics. 2013;1(3–4):62–67. doi:10.1016/j.pacs.2013.10.00124490141
- Kim H, Kim J, Lee M, et al. Photothermal gene delivery: stimuli-regulated enzymatically degradable smart graphene-oxide-polymer nanocarrier facilitating photothermal gene delivery. Adv Healthcare Mat. 2016;5(15):1917. doi:10.1002/adhm.201670080
- Kalluru P, Vankayala R, Chiang C-S, et al. Nano-graphene oxide-mediated in vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors. Biomaterials. 2016;95:1–10. doi:10.1016/j.biomaterials.2016.04.00627108401
- Li Z, Ke H, Wang J, et al. Graphene oxide and gadolinium-chelate functionalized Poly(lactic acid) nanocapsules encapsulating perfluorooctylbromide for ultrasound/magnetic resonance bimodal imaging guided photothermal ablation of cancer. J Nanosci Nanotechnol. 2016;16(3):2201. doi:10.1166/jnn.2016.1095027455619
- Yang Y, Shi H, Wang Y, et al. Graphene oxide/manganese ferrite nanohybrids for magnetic resonance imaging, photothermal therapy and drug delivery. J Biomater Appl. 2016;30(6):810–822. doi:10.1177/088532821560192626296777
- Corbet C, Feron O. Tumour acidosis: from the passenger to the driver’s seat. Nat Rev Cancer. 2017;17(10):577–593. doi:10.1038/nrc.2017.7728912578
- Nishida N, Kudo M. Oncogenic signal and tumor microenvironment in hepatocellular carcinoma. Oncology. 2017;93(Suppl 1):160–164. doi:10.1159/00048124629258072
- Kobayashi H, Watanabe R, Choyke PL. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics. 2013;4(1):81–89. doi:10.7150/thno.719324396516
- Rapoport N, Gao Z, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J Natl Cancer Inst. 2007;99(14):1095–1106. doi:10.1093/jnci/djm04317623798
- Min HS, You DG, Son S, et al. Echogenic glycol chitosan nanoparticles for ultrasound-triggered cancer theranostics. Theranostics. 2015;5(12):1402–1418. doi:10.7150/thno.1309926681985
- Milane L, Ganesh S, Shah S, et al. Multi-modal strategies for overcoming tumor drug resistance: hypoxia, the Warburg effect, stem cells, and multifunctional nanotechnology. J Controlled Release. 2011;155(2):237–247. doi:10.1016/j.jconrel.2011.03.032
- Jennifer A, Andrew P, Caroline D, et al. Hypoxia-induced cytotoxic drug resistance in osteosarcoma is independent of HIF-1Alpha. PLoS One. 2013;8(6):e65304. doi:10.1371/journal.pone.006530423785417
- Rohwer N, Cramer T. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways ☆. Drug Resist Updat. 2011;14(3):191–201. doi:10.1016/j.drup.2011.03.00121466972
- Mayer A, Vaupel P. Hypoxia, lactate accumulation, and acidosis: siblings or accomplices driving tumor progression and resistance to therapy? Adv Exp Med Biol. 2013;789:203–209.23852496
- Milane L, Duan Z, Amiji M. Role of hypoxia and glycolysis in the development of multi-drug resistance in human tumor cells and the establishment of an orthotopic multi-drug resistant tumor model in nude mice using hypoxic pre-conditioning. Cancer Cell Int. 2011;11(1):3. doi:10.1186/1475-2867-11-321320311
- Moen I, Stuhr LEB. Hyperbaric oxygen therapy and cancer—a review. Target Oncol. 2012;7(4):233–242. doi:10.1007/s11523-012-0233-x23054400
- Moen I, Tronstad KJ, Kolmannskog O, et al. Hyperoxia increases the uptake of 5-fluorouracil in mammary tumors independently of changes in interstitial fluid pressure and tumor stroma. BMC Cancer. 2009;9(1):1–9. doi:10.1186/1471-2407-9-44619118499
- Liu L, Chang S, Sun J, et al. Ultrasound-mediated destruction of paclitaxel and oxygen loaded lipid microbubbles for combination therapy in ovarian cancer xenografts. Cancer Lett. 2015;361(1):147–154. doi:10.1016/j.canlet.2015.02.05225754815
- Sun J, Yin M, Zhu S, et al. Ultrasound-mediated destruction of oxygen and paclitaxel loaded lipid microbubbles for combination therapy in hypoxic ovarian cancer cells. Ultrason Sonochem. 2016;28:319–326. doi:10.1016/j.ultsonch.2015.08.00926384914
- Dai F, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74(2):72–84. doi:10.1016/j.mvr.2007.05.00317560615
- Boucher Y, Leunig M, Jain RK. Tumor angiogenesis and interstitial hypertension. Cancer Res. 1996;56(18):4264.8797602
- Mcdonald DM, Baluk P. Significance of blood vessel leakiness in cancer. Cancer Res. 2002;62(18):5381.12235011
- Chao Z, Pintong H, Ying Z, et al. Anti-tumor efficacy of ultrasonic cavitation is potentiated by concurrent delivery of anti-angiogenic drug in colon cancer. Cancer Lett. 2014;347(1):105–113. doi:10.1016/j.canlet.2014.01.02224530229
- Chowdhury SM, Wang TY, Bachawal S, et al. Ultrasound-guided therapeutic modulation of hepatocellular carcinoma using complementary microRNAs. J Controlled Release. 2016;238:272–280. doi:10.1016/j.jconrel.2016.08.005
- Pu K. Ultrasound-guided delivery of microRNA loaded nanoparticles into cancer. J Controlled Release Off. 2015;203:99–108. doi:10.1016/j.jconrel.2015.02.018
- Husain SR, Han J, Au P, et al. Gene therapy for cancer: regulatory considerations for approval. Cancer Gene Ther. 2015;22(12):554. doi:10.1038/cgt.2015.5826584531
- Shillitoe EJ. Gene therapy: the end of the rainbow? Head Neck Oncol. 2009;1(1):7. doi:10.1186/1758-3284-1-719331651
- Tong AW, Jay CM, Neil S, et al. Systemic therapeutic gene delivery for cancer: crafting Paris’ arrow. Curr Gene Ther. 2009;9(1). doi:10.2174/156652309787354630
- Michael R, Axel S, Luca B. Safety of gene therapy: new insights to a puzzling case. Curr Gene Ther. 2014;14(6):429–436.25245088
- Doi K, Takeuchi Y. Gene therapy using retrovirus vectors: vector development and biosafety at clinical trials. Uirusu. 2015;65(1):27–36. doi:10.2222/jsv.65.2726923955
- Tse LV, Sven MT, Aravind A. Strategies to circumvent humoral immunity to adeno-associated viral vectors. Expert Opin Biol Ther. 2015;15(6):845–855. doi:10.1517/14712598.2015.103564525985812
- Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17(3):295–304. doi:10.1038/gt.2009.14819907498
- Chi H, Sum SW, Slavcev RA. Impact of DNA vector topology on non-viral gene therapeutic safety and efficacy. Curr Gene Ther. 2014;14(4):309–329.25039613
- Morille M, Passirani C, Vonarbourg A, et al. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials. 2008;29(24):3477–3496. doi:10.1016/j.biomaterials.2008.04.03618499247
- Huang C, Zhang H, Bai R. Advances in ultrasound-targeted microbubble–mediated gene therapy for liver fibrosis. Acta Pharmaceutica Sinica B. 2017;7(4):447–452. doi:10.1016/j.apsb.2017.02.00428752029
- Shufang C, Juan G, Jiangchuan S, et al. Targeted microbubbles for ultrasound mediated gene transfection and apoptosis induction in ovarian cancer cells. Ultrason Sonochem. 2013;20(1):171–179. doi:10.1016/j.ultsonch.2012.06.01522841613
- Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol. 2012;6(6):590–610. doi:10.1016/j.molonc.2012.09.00623102669
- Croce CM. Causes and consequences of microRNA dysregulation in cancer. Cancer J. 2012;48(3):S8–S9.
- Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006;94(6):776–780. doi:10.1038/sj.bjc.660302316495913
- Jovanovic M, Hengartner MO. miRNAs and apoptosis: rNAs to die for. Oncogene. 2006;25(46):6176–6187. doi:10.1038/sj.onc.120991217028597
- Somesh B, Alahari SK. miRNA control of tumor cell invasion and metastasis. Int J Cancer. 2010;126(6):1283–1290. doi:10.1002/ijc.2501419877123
- Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu Rev Med. 2009;60(1):167–179. doi:10.1146/annurev.med.59.053006.10470719630570
- Ma L, Liu J, Shen J, et al. Expression of miR-122 mediated by adenoviral vector induces apoptosis and cell cycle arrest of cancer cells. Cancer Biol Ther. 2010;9(7):554–561. doi:10.4161/cbt.9.7.1126720150764
- Wei-Chih T, Paul Wei-Che H, Tsung-Ching L, et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2010;49(5):1571–1582.
- He J, Xie G, Tong J, et al. Overexpression of microRNA-122 re-sensitizes 5-FU-resistant colon cancer cells to 5-FU through the inhibition of PKM2 in vitro and in vivo. Cell Biochem Biophys. 2014;70(2):1343–1350. doi:10.1007/s12013-014-0062-x24898807
- Shoumei B, Nasser MW, Bo W, et al. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Bio Chem. 2009;284(46):32015. doi:10.1074/jbc.M109.01677419726678
- Wang T, Shigdar S, Shamaileh HA, et al. Challenges and opportunities for siRNA-based cancer treatment. Cancer Lett. 2017;387:77–83. doi:10.1016/j.canlet.2016.03.04527045474
- Napoleone F. VEGF-A: a critical regulator of blood vessel growth. Eur Cytokine Netw. 2009;20(4):158–163. doi:10.1684/ecn.2009.017020167554
- Costache MI, Ioana M, Iordache S, et al. VEGF expression in pancreatic cancer and other malignancies: a review of the literature. Rom J Intern Med. 2015;53(3):199–208. doi:10.1515/rjim-2015-002726710495
- Carter JG, Gammons MVR, Damodaran G, et al. The carboxyl terminus of VEGF-A is a potential target for anti-angiogenic therapy. Angiogenesis. 2015;18(1):23–30. doi:10.1007/s10456-014-9444-325274272
- Prakash V, Popel AS, Feilim MG. Extracellular regulation of VEGF: isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 2014;25(1):1–19. doi:10.1016/j.cytogfr.2013.11.00224332926
- Huang HY, Kuo WT, Chou MJ, et al. Co-delivery of anti-vascular endothelial growth factor siRNA and doxorubicin by multifunctional polymeric micelle for tumor growth suppression. J Biomed Mat Res Part A. 2011;97A(3):330–338. doi:10.1002/jbm.a.33055
- Won Jong K, Christensen LV, Seongbong J, et al. Cholesteryl oligoarginine delivering vascular endothelial growth factor siRNA effectively inhibits tumor growth in colon adenocarcinoma. Mol Ther J Am Soc Gene Ther. 2006;14(3):343–350. doi:10.1016/j.ymthe.2006.03.022
- Kim J, Kim SW, Kim WJ. PEI-g-PEG-RGD/small interference RNA polyplex-mediated silencing of vascular endothelial growth factor receptor and its potential as an anti-angiogenic tumor therapeutic strategy. Oligonucleotides. 2015;21(2):101–107. doi:10.1089/oli.2011.0278
- Hammond SM, Caudy AA, Hannon GJ. Post-transcriptional gene silencing by double-stranded RNA. Nature Revi Genet. 2001;2(2):110–119. doi:10.1038/35052556
- Florinas S, Kim J, Nam K, et al. Ultrasound-assisted siRNA delivery via arginine-grafted bioreducible polymer and microbubbles targeting VEGF for ovarian cancer treatment. J Control Release. 2014;183:1–8. doi:10.1016/j.jconrel.2014.03.02524657947
- Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nature Rev Cancer. 2008;8(1):61–70. doi:10.1038/nrc229318075512
- Waligã Rska-Stachura J, Jankowska A, Waå›Ko R, et al. Survivin–prognostic tumor biomarker in human neoplasms–review. Ginekol Pol. 2012;83(7):537–540.22880480
- Tanaka K. Iwamoto S. Gon G, et al. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000;6(1):127–134.10656440
- Grossman D, Mcniff JM, Li F, et al. Expression and targeting of the apoptosis inhibitor, survivin, in human melanoma. J Invest Dermatol. 1999;113(6):1076–1081. doi:10.1046/j.1523-1747.1999.00776.x10594755
- Chang JY, Chang YC, Tsai FY, et al. Survivin – biology and potential as a therapeutic target in oncology. Oncotargets Ther. 2013;6(default):1453–1462. doi:10.2147/OTT.S33374
- Mobahat M, Narendran A, Riabowol K. Survivin as a preferential target for cancer therapy. Int J Mol Sci. 2014;15(2):2494–2516. doi:10.3390/ijms1502249424531137
- Zhang Y, Chang S, Sun J, et al. Targeted microbubbles for ultrasound mediated short hairpin RNA plasmid transfection to inhibit survivin gene expression and induce apoptosis of ovarian cancer A2780/DDP cells. Mol Pharm. 2015;12(9):3137–3145. doi:10.1021/mp500835z26212628
- Orzáez M, Gortat A, Sancho M, et al. Characterization of dequalinium as a XIAP antagonist that targets the BIR2 domain. Apoptosis. 2011;16(5):460–467. doi:10.1007/s10495-011-0582-421340509
- Jingguo L, Du C, Tinghui Y, et al. Copolymer of poly(ethylene glycol) and poly(L-lysine) grafting polyethylenimine through a reducible disulfide linkage for siRNA delivery. Nanoscale. 2014;6(3):1732–1740. doi:10.1039/C3NR05024F24346086
- Wang P, Yin T, Li J, et al. Ultrasound-responsive microbubbles for sonography-guided siRNA delivery. Nanomedicine. 2016;12(4):1139–1149. doi:10.1016/j.nano.2015.12.36126733262
- Gheath A, Haroon J, Stafford PD, et al. Cancer immunotherapies, their safety and toxicity. Expert Opin Drug Saf. 2013;12(5):631–645. doi:10.1517/14740338.2013.79594423668362
- Mohammed S, Bakshi N, Chaudri N, et al. Cancer vaccines: past, present, and future. Adv Anat Pathol. 2016;23(3):180–191. doi:10.1097/PAP.000000000000011627058246
- Yaddanapudi K, Mitchell RA, Eaton JW. Cancer vaccines: looking to the future. Expert Rev Vaccines. 2013;12(10):1125–1126. doi:10.1586/14760584.2013.83776024093953
- Alex K. Overview of cancer vaccines: considerations for development. Hum Vaccin Immunother. 2012;8(9):1335–1353. doi:10.4161/hv.2051822894970
- Harris TJ, Drake CG. Primer on tumor immunology and cancer immunotherapy. J Immunother Cancer. 2013;1(1):12. doi:10.1186/2051-1426-1-1224829749
- Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39(1):38–48. doi:10.1016/j.immuni.2013.07.00423890062
- Dewitte H, Van Lint S, Heirman C, et al. The potential of antigen and TriMix sonoporation using mRNA-loaded microbubbles for ultrasound-triggered cancer immunotherapy. J Control Release. 2014;194:28–36. doi:10.1016/j.jconrel.2014.08.01125151979
- Wolff JA, Malone RW, Williams P. et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949):1465–1468.1690918
- Raz E, Carson DA, Parker SE, et al. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci USA. 1994;91(20):9519–9523. doi:10.1073/pnas.91.20.95197937799
- Chudley L, Mccann K, Mander A, et al. DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency CD8 T-cell responses and increases PSA doubling time. Cancer Immunol Immunother. 2012;61(11):2161–2170. doi:10.1007/s00262-012-1270-022729556
- Diaz CM, Chiappori A, Aurisicchio L, et al. Phase 1 studies of the safety and immunogenicity of electroporated HER2/CEA DNA vaccine followed by adenoviral boost immunization in patients with solid tumors. J Transl Med. 2013;11(1):62. doi:10.1186/1479-5876-11-6223497415
- Butterfield LH, Economou JS, Gamblin TC, et al. Alpha fetoprotein DNA prime and adenovirus boost immunization of two hepatocellular cancer patients. J Transl Med. 2014;12(1):1–9. doi:10.1186/1479-5876-12-8624387290
- Lee SH, Danishmalik SN, Sin JI. DNA vaccines, electroporation and their applications in cancer treatment. Hum Vaccin Immunother. 2015;11(8):1889–1900. doi:10.1080/21645515.2015.103550225984993
- Un K, Kawakami S, Suzuki R, et al. Suppression of melanoma growth and metastasis by DNA vaccination using an ultrasound-responsive and mannose-modified gene carrier. Mol Pharm. 2011;8(2):543–554. doi:10.1021/mp100369n21250746
- Yoshida M, Kawakami S, Kono Y, et al. Enhancement of the anti-tumor effect of DNA vaccination using an ultrasound-responsive mannose-modified gene carrier in combination with doxorubicin-encapsulated PEGylated liposomes. Int J Pharm. 2014;475(1–2):401–407. doi:10.1016/j.ijpharm.2014.09.00525218184
- Brollo J, Curigliano G, Disalvatore D, et al. Adjuvant trastuzumab in elderly with HER-2 positive breast cancer: a systematic review of randomized controlled trials. Cancer Treat Rev. 2013;39(1):44–50. doi:10.1016/j.ctrv.2012.03.00922541668
- Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci USA. 2006;103(31):11719–11723. doi:10.1073/pnas.060431810316868082
- Park EJ, Zhang YZ, Vykhodtseva N, et al. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Control Release. 2012;163(3):277–284. doi:10.1016/j.jconrel.2012.09.00723000189
- Kobus T, Zervantonakis IK, Zhang Y, et al. Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated blood-brain barrier disruption. J Control Release. 2016;238:281–288. doi:10.1016/j.jconrel.2016.08.00127496633
- Dudley ME, Yang JC, Richard S, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233. doi:10.1200/JCO.2008.16.544918809613
- Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–4557. doi:10.1158/1078-0432.CCR-11-011621498393
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nature Rev Immunol. 2012;12(4):269–281. doi:10.1038/nri319122437939
- Smyth MJ, Yoshihiro H, Kazuyoshi T, et al. New aspects of natural-killer-cell surveillance and therapy of cancer. Nature Rev Cancer. 2002;2(11):850–861. doi:10.1038/nrc92812415255
- Christoph U, Torsten T, Barbara U, et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood. 2002;100(4):1265–1273. doi:10.1182/blood.V100.4.1265.h81602001265_1265_127312149207
- Alkins R, Burgess A, Ganguly M, et al. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 2013;73(6):1892–1899. doi:10.1158/0008-5472.CAN-12-260923302230
- Alkins R, Burgess A, Kerbel R, et al. Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro Oncol. 2016;18(7):974–981. doi:10.1093/neuonc/nov31826819443
- Banchereau J, Pascual V, O’Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol. 2012;13(10):925–931. doi:10.1038/ni.240622990890
- Suzuki R, Namai E, Oda Y, et al. Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J Control Release. 2010;142(2):245–250. doi:10.1016/j.jconrel.2009.10.02719883708
- Chen PY, Hsieh HY, Huang CY, et al. Focused ultrasound-induced blood-brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study. J Transl Med. 2015;13:93. doi:10.1186/s12967-015-0451-y25784614

