Abstract
The importance of timely diagnosis and the complete treatment of lung cancer for many people with this deadly disease daily increases due to its high mortality. Diagnosis and treatment with helping the nanoparticles are useful, although they have reasonable harms. This article points out that the side effects of using carbon nanotube (CNT) in this disease treatment process such as inflammation, fibrosis, and carcinogenesis are very problematic. Toxicity can reduce to some extent using the techniques such as functionalizing to proper dimensions as a longer length, more width, and greater curvature. The targeted CNT sensors can be connected to various modified vapors. In this regard, with helping this method, screening makes non-invasive diagnosis possible. Researchers have also found that nanoparticles such as CNTs could be used as carriers to direct drug delivery, especially with chemotherapy drugs. Most of these carriers were multi-wall carbon nanotubes (MWCNT) used for cancerous cell targeting. The results of laboratory and animal researches in the field of diagnosis and treatment became very desirable and hopeful. The collection of researches summarized has highlighted the requirement for a detailed assessment which includes CNT dose, duration, method of induction, etc., to achieve the most controlled conditions for animal and human studies. In the discussion section, 4 contradictory issues are discussed which are invited researchers to do more research to get clearer results.
Introduction
Lung cancer was the most significant reason for the mortality in consequence of cancers in which the women were more suffered.Citation1 Moreover, a recent review article noted that lung cancer is the second most common cancer in humans. Due to the growth in silence, this cancer is often associated to the death. Lung cancer is in two basic forms with the names of small cell and non-small cell lung cancer.Citation2 Lung cancer attempted to treat with methods as chemotherapy, radiation therapy, and various medications.Citation3 These methods were not highly effective due to the non-targeted and damaging healthy tissues such as hair follicles. Based on these approaches, damage in the cell cycle, break in the double strands of DNA, inflammatory responses, tissue fibrosis, etc., will have occurred. On the other hand, there were a series of treatment obstacles; low stability, solid solubility in water, and cell resistance to treatment in chemotherapy method.Citation4,Citation5 Finally, in 2017, a review article collected the new research achievements which is called nano-drug delivery as a new cancer treatment strategy.Citation6 By advent of the nanoparticles, a new approach to treatment emerged to discuss purposefulness and effectiveness in therapy.Citation5 However, tumor-targeting has many difficulties even with using the specific antibodies to bind to cancer cells; therefore, the activity of specialists is required in this field. Nevertheless, pulmonary tumors are among the invincible types of cancer, on which the researchers work to solve this issue.Citation7
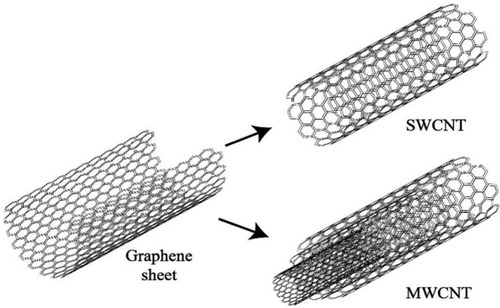
Several nanocarriers were used for drug delivery and improved treatment like Liposome, dendrimer, polymeric micelle, carbon nanotube (CNT), gold nanoparticle, magnetic nanoparticle, solid lipid nanocapsules, and inhalable nanocomposites. Besides, quantum dots, gold nanoparticles, CNTs, and magnetic nanoparticles were a series of nanomaterials that could be utilized to detect lung cancer.Citation1,Citation2,Citation8 Researchers have recently applied CNTs to diagnose and treat cancers such as lung, breast, prostate, liver, colon, etc.Citation9 Indeed, CNT structure explained in the carbon atoms were placed next to each other in a honeycomb structure, creating a tube with high physicochemical strength.Citation10 In other words, CNTs are known as tubes made of carbon which has nanometers dimensions. The dimensions of CNTs expressed in various ratios with 10–15 nm diameters. Moreover, two long length ranges of CNTs in 545 ± 230, and 10,451 ± 8422 nm and short length with lower toxicity with 192 nm lengths are synthesized. Also, CNTs are divided into two groups of single-walled (SW) and multi-walled (MW) nanotubes, and they have different dimensions ().Citation11 CNTs are widely used in many fields due to their thermal conductivity, electrical properties, strength, and excellent hardness.Citation12 Nowadays, the CNTs are utilized in drug delivery carriers, biomedical purposes, genetic engineering, artificial implants, imaging, cancer treatment, antioxidant activity, bio-sensing, etc.Citation13,Citation14 In fact, CNTs are made in five ways, which are described as arc discharge, laser ablation, chemical vapor deposition, flame synthesis, and silane solution methods. Also, CNTs are purified in three ways as air oxidation, sonication, and acid refluxing.Citation13 On the other hand, the CNTs are a fascinating substance that can be employed to bind proteins, peptides, nucleic acids, and various drugs.Citation15 Furthermore, CNTs have a high potential for drug delivery due to their tubular and fiber-like structure.Citation16 The usable techniques to evaluate the CNTs and drugs with each other were collected as transmission electron microscopy (TEM), scanning electron microscopy (SEM), Raman spectroscopy, Fourier transforms infrared spectroscopy (FTIR), and X-ray diffraction (XRD), etc.Citation17 However, the side effects of pulmonary fibrosis and exacerbation have been seen in the use of this approach in rodents with a history of the previous pulmonary disease.Citation16 The toxicity of these carriers is fully evaluated in the “toxicity assessment of CNTs” section. On the other hand, a review paper presented that the CNTs are easily recognizable and transparent due to their intense light absorption in biological imaging.Citation18 As well as, other features of functional MWCNTs were their detectability by multiphoton near-infrared imaging in induced region.Citation19,Citation20
Figure 1 Scheme of SWCNT originating from a graphene sheet, while MWCNT has several graphene sheets according to the size. Carbon atoms were placed next to each other in a honeycomb structure. Reprinted from International Communications in Heat and Mass Transfer, Vol 78, Yazid MNAWM, Sidik NAC, Mamat R, et al, A review of the impact of preparation on stability of carbon nanotube nanofluids, Pages No. 253–263, Copyright (2016), with permission from Elsevier.Citation20
There is no study conducted on the preference of use or not to use CNTs. In this study, while mentioning the disadvantages, the effectiveness of these nanotubes is discussed. Moreover, other traditional treatments have had too destructive impacts than this method. However, it is recommended that the tissue compatibility of nanotubes should be increased to increase efficiency. Despite the collection and categorization of new methods of diagnosis and treatment of lung cancer with CNT, the feasibility of these methods in humans has been discussed. Besides, the ways to reduce the toxic effects of these CNTs are some of the achievements of this article which could be useful for developing Nanomedicine. Also, the treatment of this cancer, all drugs and substances used by experts due to their type of work, are summarized.
The Pulmonary Toxicity Assessment of Carbon Nanotubes
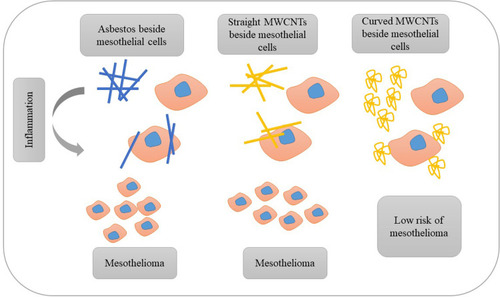
CNTs could quickly enter the lungs through the respiratory tract and then rapidly enter and affect the nervous, lymphatic, and circulatory systems, leading to toxic effects.Citation21 The main reasons of these toxic effects can be durability, the amount of residual oxygen reactive metal, and size. By removing the residual metals and selecting appropriate dimensions, the CNTs can be safe in drug transmission.Citation16 The smaller sizes were with less toxicity; furthermore, the concentration of metal impurities such as iron did not contribute to toxicity.Citation11 On the other hand, the curvature was also among the considerable parameters in MWCNTs toxicity. Indeed, the greater the curvature, the less damage occurred to the cells.Citation22 This issue is illustrated in . In animal research, CNT-long exposure could induce persistent inflammation, lung cancer, fibrosis, and gene destruction in the lung.Citation23,Citation24 In general, the mechanisms of creating toxicity are divided into five sections as apoptosis, reactive oxygen species, free radical formation, the formation of granuloma, and increased inflammatory response. Due to the above mechanisms, types of toxicities in cells, skin, gene, liver, cardiovascular, pulmonary, and carcinogens substance, are created.Citation25
Figure 2 Evaluation of the similarity of straight MWCNTs and asbestos, and the effect of straight, curved MWCNTs and asbestos on mesothelial cells. Indeed, curved nanotubes are less dangerous for the cell than a straight CNTs or asbestos, and could better play their carrier role.
Carbon Nanotubes and Asbestos
Due to the high structural similarity of CNTs with asbestos, DNA microarray analysis showed similar physiological effects on the human bronchial epithelial cells.Citation12 In humans exposed to asbestos, the malignant plaques and malignant mesothelioma are created and developed. Also, animal studies showed that the CNTs could induce lung and pleural lesions, inflammation, pleural fibrosis, lung tumors, and malignant mesothelioma when animals were inhaling.Citation26 A study in 2018 confirmed that stiffness, hardness, length, width, and CNT longevity were five factors that could induce harmful effects as asbestos.Citation27 In addition to the mentioned factors, the review report also introduced two other effective factors in causing toxicity as exposure time and the amount of accumulation in the target tissue.Citation28 Forty microgram was the lowest dose of MWCNTs which could induce pulmonary fibrosis, whereas 120 µg of asbestos should be inhaled to produce the same fibrosis. Also, MWCNTs, like asbestos, could alter the expression of several genes and cell survival and proliferation.Citation29 Another article in 2010 stated that the SWCNT produced the least toxicity, and asbestos brought the most toxicity in mesothelial cells, in the identical dose of MWCNT, SWCNT, and asbestos.Citation30 Moreover, an article compared MWCNT and SWCNT in this sentence which the exposure to MWCNTs mainly causes inflammation whereas SWCNTs induce apoptosis and mitochondrial dysfunction.Citation31 On the other side, functional CNT showed less toxicity with higher activity toward CNT without functionalization. The safe applied dose of CNTs was not determined.Citation32,Citation33 Although two agents for obtaining the non-toxic dose of MWCNTs are tetrazolium bromide salt, and lactate dehydrogenase. This dose could induce the apoptosis and create the oxidative stress in A549 cancer cells.Citation34 An article stated that the consequences of utilizing unsafe MWCNTs dose in the lungs include oxidative stress in healthy tissue, non-malignant lung disease, and cardiovascular disorders.Citation35
Toxicity of Multi-Wall Carbon Nanotubes
The presence of MWCNTs in vivo resulted in cytokines production such as TNF‐α and IL‐1β from immune cells which involved in creating the toxicity.Citation36 In a 90-day study by the intravenous injection of MWCNT, no changes in mice weight were observed, while the toxic effects were observed, and also the mice survive, despite the toxicity.Citation37 In another study on Sprague–Dawley rats with injected intratracheally of 0.5, 2, and 5 mg MWCNTs, TNF-α discharge was increased from macrophages. After 60 days, the inflammatory and fibrotic reactions were observed. After 2 months, pulmonary lesions were formatted by the aggregation of collagen in the bronchial lumen of lungs.Citation38 The researches in 2012, and 2019 have shown that the amount of inflammation is directly related to MWCNTs dose volume.Citation39,Citation40 Nevertheless, the MWCNT can decrease tumor metastasis.Citation41 In another study, due to the microarray survey results in different doses and various days, the MWCNTs led to the induction of pulmonary inflammation and fibrotic damage from miRNA and mRNA regulatory networks.Citation42,Citation43 Another article has inferred that the development of inflammation, pulmonary fibrosis, and the induction of lung cancer by MWCNT were due to the elevated and decreased levels of blood mRNAs and miRNAs.Citation44
On the other side, MWCNT-COOH activating TLR4/NF-κB signaling led to inhibiting lung tumor metastasis which happened due to the changes in polarized macrophages.Citation41 The MWCNT-induced carcinogenesis may involve low levels of DNA damage, and parallel increases or decreases in the expression of genes involved in several pro-carcinogenic pathways.Citation45 An adverse event which seen for lungs was adenocarcinoma, created by exposing with MWCNTs via inhalation.Citation46 The MWCNTs provoked hypomethylation in the promoters of genes area and CpG sites. Those were the other genetic alterations due to the MWCNT exposures to cells.Citation47 Suzui et alCitation48 in 2016 reported that the MWCNTs induce developing the pleural malignant mesothelioma and lung tumors. Nevertheless, the other examination on 344 rats exposed to inhale with MWCNTs showed no pleural mesothelioma during 104 weeks. Although there was the evidence of toxicity for the lungs, such as localized fibrosis, granulomatous change, and epithelial hyperplasia existed clearly.Citation49 Pacurari et alCitation50 presented that the MWCNTs can induce the lung tumor and mesothelioma during long-term usage due to creating changes in the expression of several cancer-causing genes. Another research work has mentioned a significant relationship between MWCNT and altered expression of cancer inducer genes, especially lung cancer.Citation51 In an animal model study, it was reported that the intact MWCNTs were more toxic to healthy cells than MWCNTs with an acidic agent.Citation52 A fascinating statement in 2020 expressed that no documented cell carcinoma effects were observed in the controlled injection of MWCNTs in their mice.Citation53
Toxicity of Single-Wall Carbon Nanotubes
The following results were obtained from a series of researches performed on mice exposed to SWCNTs. SWCNTs provoke acute effects in lungs, such as inflammation, granuloma synthesis, the deposition of collagen, fibrosis, and genotoxic. Perfused SWCNTs are distributed in most organs which is mainly stored in liver, lungs, and spleen. SWCNTs are destroyed by kidney and bile ducts.Citation54 Sanpui et alCitation55 in 2014 demonstrated that using SWCNTs resulted in the suppression of some anti-inflammatory and anti-virus genes. Therefore, this type of nanotube could increase airway epithelial cells’ sensitivity to viral infections such as influenza A H1N1, as a side effect. In the other research which conducted in 2017, SWCNTs association utilization and SOX9 gene expression increase has been observed. This expression further induces tumorigenesis and metastasis through the body. Also, barricaded this gene expression led to repress tumor cells and suppresses the growth of tumor cells.Citation56 The long-term presence of these nanotubes can lead to the overgrowth of cells, the creation of colonies, the enhancement of cell migration, and angio-genicity.Citation57 Research has recorded that solo SWCNTs in adjacent epithelial cells were one of the fundamental forces of creating tumors.Citation58 Chen et alCitation59 declared that SWCNT’s presence for six months led to apoptotic resistant phenotype, which could cause a tumor. Also, the changes in expressing a set of genes like increased activation of pAkt/p53/Bcl-2 signaling axis, Ras family, and Dsh-mediated Notch 1 were seen as agents of this apoptotic resistant. Besides, the decreased expression of genes regulating apoptosis such as BAX and Noxa genes occurred. On the other hand, protein caveolin-1 was known to produce cancer cells such as stem cells and the inactivation of protein P53 in epithelial cells. Research in 2014 represented that this protein could induce tumors, in the vicinity of SWCNTs for six months.Citation60 SWCNTs could lead to the induction of transcription factor slug enhances cell migration, invasion, and independent cell development.Citation61 Besides, the discussion of the toxicity of pure SWCNT compared to raw SWCNT was observed the higher cell death, reduced antioxidant amount, and the activation of caspase cascade.Citation62 Furthermore, the SWCNTs can induce the resistance to apoptosis through the natural (antimycin A and CDDP) or external (FasL and TNF-a) pathways.Citation63 Researchers have found that the SWCNTs led to myeloid-derived suppressor cell accumulation which increased the cancer cells’ induction of cancer.Citation64 A study in 2016 showed that the mesothelin gene expression reduced the bronchial part of epithelial cells, which were exposed to SWCNT.Citation65
To conclude, the toxicity of CNTs for healthy tissue and a series of side effects for lung inflammation and fibrosis were reported. The set of effects generated is shown in . This issue could be controlled using the appropriate dose. Also, researchers can reduce the toxic effects by choosing the appropriate dimensions (shorter length and higher width), more curved nanotubes, and using the functionalized form. Moreover, the MWCNT was used more efficiently due to less toxicity toward SWCNT. On the other side, there were significant contrasts in using the nanotubes which induced tumor growth and tumor suppressor protein.
Table 1 Summary of Toxic Effects Obtained from Studies by Year Order
Using Carbon Nanotubes in Lung Cancer Detection
Generally, lung cancer’s clinical manifestations include fatigue, coughing, wheezing, the pain in the chest, the brevity of breath, swallowing hardness, anxiety, and yellow fingers.Citation66 Also, a lung cancer diagnosis is possible with helping 4 methods with the names of radiological, non-radiological imaging such as MRI, endoscopic, and biochemical methods.Citation67 In usual, diagnosis, type, and degree of lung cancer are performed by CT scan imaging.Citation68,Citation69 Unfortunately, the low-dose CT scans may bring false-positive answers about having lung cancer.Citation70 Today, the biomarkers of both protein and genetic modifications are known for lung cancer. Numerous biosensors were studied to bind to these biomarkers for non-invasive detection.Citation71 The detection of VOCs and tumor markers by breath analysis with helping CNT is a modern and studied method.Citation67
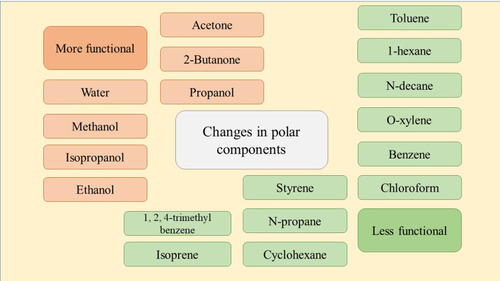
The sensor array of CNTs has demonstrated a discrepancy between the healthy and the patient respiratory sample in the volatile organic components (VOC). Due to mass-spectroscopy and gas-chromatography, the rate of these components increased in the patients affected by lung cancer.Citation72 The sensors identify different biomarkers due to the solubility, polarity, and chemical associations, especially for tuberculosis disease.Citation73 The design of an electronic nose with CNTs to delete the VOC of lung cancer patients was an inexpensive and rapid method. Water, methanol, isopropanol, ethanol, acetone, 2-butanone, and propanol were found as polar vapors lung cancer biomarkers. Chloroform, benzene, o-xylene, n-decane, 1-hexene, toluene, styrene, n-propane, cyclohexane, 1, 2, 4-trimethyl benzene, and isoprene were discovered as nonpolar vapors.Citation74 SWCNTs, coated with nonpolymeric organic substances, can detect VOC changes as the cancer biomarkers.Citation72 This method was a convenient, non-invasive, inexpensive, and rapid screening for diagnosing the biomarkers of lung cancer. In this study, the SWCNT was functionalized with tricosane, and pentadecane to have a high sensitivity for diagnosis nonpolar and VOC molecules.Citation75 The CNTs, doped with platinum, can detect styrene and benzene vapors which existed in the exhale of lung cancer patients. This sensitivity was very low in natural nanotubes.Citation76 SWCNTs decorated with Pd, Pt, Ru, or Rh elements could also be used to detect toluene gas as an indicator of lung cancer.Citation77 Although the sensitivity of CNT sensors toward nonpolar volatile organic compounds was low and led to the limitation of the diagnosis. The existence of derivatives Hexa-peri-hexabenzocoronene can increase this sensitivity for developing detection.Citation78 A research, conducted in 2018, stated that a biosensor was created by CNT which drugged with Rh, can distinguish and absorb C6H7N, and C6H6 in the exhaled air of lung cancer patients.Citation79 In another study, it was reported that infrared CNT could detect o-toluidine and aniline as two common gases in lung cancer.Citation80
Moreover, a selective chemiresistive sensor for cancer-related VOC hexanal using molecularly imprinted polymers and multiwalled carbon nanotubes has been designed by Janfaza et al.Citation81 Another scheme used to enhance lung cancer detection was preparing a combination of SWCNT and chitosan.Citation82 Due to the differences in nicotinic acetylcholine receptors in normal and small cells of lung cancer, the nanotube-based electrode sensor for the quantitative electrophysiological monitoring of a nonadherent cell has been demonstrated.Citation83 Furthermore, nitrogen-filled CNT, attached to iron, could detect specific microRNAs of cancer cells which was known as a sensitive electrocatalysis biosensor.Citation84 Also, the changes in mRNAs and miRNAs predicted to indicate pulmonary fibrosis in individuals exposed to MWCNTs.Citation85
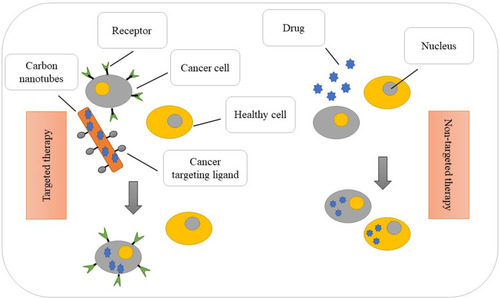
As a result, by the advent of CNTs in the field of lung cancer detection, another way was opened in the aspect of the efficiency of these nanotubes in medicine (). Besides, the alteration of micro RNAs and RNAs of cancerous cells was acquirable with CNTs. CNTs could assemble several biomarkers of lung cancer by the electrode and electrical current.
Using Carbon Nanotubes in Lung Cancer Treatment
Despite many efforts by the lung cancer treatment teams, many people still die for this reason annually. It is known to be among the most common cancers in the world; the nano therapy is a new method that researchers work on. CNTs themselves are effective in treating the disease such as the activation of apoptosis pathway by targeting mitochondrial organelle in cancer cells. In this regard, CNTs attached to polyethylene glycol could focus better on cancer cell repertoires which had the potential to improve the performance in Nano-drug delivery.Citation86 In this section, studies are divided due to the type of CNTs, drugs, and protein.
Single-Wall Carbon Nanotubes in the Presence of Effective Lung Cancer Drugs
Due to the low toxicity of SWCNT as a carrier, it was widely used in cancer therapy and drug delivery. However, the biochemical changes occurred in serum and pulmonary inflammation.Citation87
Paclitaxel
Paclitaxel utilized as an anti-cancer drug which bonded with SWCNTs beside graphene oxide. This nanostructure led to enhance the effectiveness and induce death on A549 and NCI-H460 cancer cells.Citation88 Another study to deliver this drug was about SWCNT modified with chitosan, which led to increase the compatibility in vivo. Also, the layer of chitosan has been combined with hyaluronic acid to target A549 cells.Citation89
TRAIL
Apo2L or TRAIL is a protein that can bind to cancer cell receptors and induce apoptosis without a toxic effect on healthy cells. In contrast, the presence of SWCNTs, due to TRAIL due to and its high solubility in the blood led to the proper distribution of this protein and rapid pulmonary tumor eradication.Citation90
Doxorubicin
Delivering doxorubicin with SWCNTs due to magnetic localization and better distribution can enhance the targeting and increased therapeutic efficiency. Indeed, this project which was performed on mice, confirmed the results of increasing the efficiency of therapy by MRI technique.Citation91
Curcumin
In a study in 2018, Curcumin, a chemical produced which has therapeutic potential for A549 cancer cells, was investigated in a nano-state with an SWCNT carrier. The carrier, which was functional with chitosan and alginate polysaccharides, increased the efficacy of the drug.Citation92
Survivin siRNA and Doxorubicin
A study in 2019 showed using survivin siRNA, and doxorubicin with SWCNT carrier, as two factors for increasing apoptosis and expressing less survivin as an inhibitor gene apoptosis. The carrier was functionalized with polyethyleneimine (PEI) besides betaine.Citation93
Gemcitabine
Also, Gemcitabine is among the anti-cancer drugs for non-small cell lung cancer. In a clinical trial on B6-mice, the drug was tested with an SWCNT carrier. In this study, the cell line of A549 was examined which shows interesting results repression. In fact, this research has identified SWCNTs as encouraging carriers for drug delivery due to the high loading inclination of the drug, prolonged distribution time, and notable cell membrane permeability.Citation94
Multi-Wall Carbon Nanotubes in the Presence of Effective Lung Cancer Drugs
The presence of plasma-functioned MWCNT with graphene oxide decreased the expression of telomerase reverse transcriptase.Citation95 In another study on targeting drug-resistant cancer cells, researchers modified MWCNT with a coating ethylene glycol and antibodies against p-glycoprotein as a multidrug resistance protein. The results showed that in these cancer cells, the toxicity was significantly increased after light irradiation whereas it did not have any toxic effect on healthy cells.Citation96 CNTs acted as drug carriers which are categorized in this article due to the drugs used in research works.
SiRNA
CNTs are used as a highly effective vector in clinical treatment due to their high antitumor and durability properties. MWCNT NH+3 with Si RNA can increase the viability and prevent tumor growth in the animal model.Citation97 Furthermore, tumor eradication via targeting the Polo-like Kinase gene for delivery siRNA by MWCNT-NH3+ was suggested to treat lung cancer.Citation98
Doxorubicin
Doxorubicin was known as an anti-cancer growth inhibitor. Conjugated MWCNT with this drug and hyaluronic acid had more toxicity and apoptosis effect on A549 cells. This complex showed free of toxicity for kidneys, heart, and liver. Optical imaging and scintigraphic techniques could also be used to identify the location and operation of the complex.Citation99 The study of Lodhi et alCitation100 presented that Doxorubicin HCL besides MWCNTs and MWCNTs conjugated with Dexamethasone can affect the epithelial cancer cells. The complex of MWCNTs, Dexamethasone, and Doxorubicin had more cytotoxicity on these cancer cells, less hemolytic effect, and superior and quicker diffusion and dispersion.
Methotrexate
Methotrexate could conjugate with MWCNTs for lung cancer therapy to decrease drug waste and use it in the target way. Moreover, the animal tests indicated that this complex was free of toxin effects on cardiac, hepatic, and nephrotic systems.Citation101 In another study, chitosan-coated CNTs were used as carriers of methotrexate. In this in vitro research, less toxicity of this drug on healthy lung cells (MRC-5) and improved their anti-cancer activity in the cancerous lung cells (H1299) were admitted.Citation102
Cisplatin
Li et alCitation103 presented that MWCNTs conjugated with cisplatin as a chemotherapeutic agent led to increase treatment and decrease the toxicity for liver and kidney as typical side effects. This efficient carrier can improve the platinum levels of accumulation in target organs such as the lungs. The study was conducted on mice showed that CNTs do not have abnormal immune and inflammatory responses.
Betulinic Acid
The acid-functionalized MWCNTs loaded with betulinic acid had an anti-cancer activity which can be analyzed by ultraviolet light and thermogravimetric. In fact, lung cancer cells were more sensitive to certain concentrations of this drug.Citation104
Docetaxel
In a study, conducted by Singh et al,Citation105 it is mentioned that docetaxel in the presence of an MWCNT carrier conjugated with the transferrin protein, 136 times more effective than the drug used alone. The drug uptake was reported to be much higher in A549 cancer cells. In another research in 2016, it was declared that this drug and MWCNT coated by d-alpha-tocopheryl polyethylene glycol 1000 succinate is more effective than non-targeted therapy.Citation106 Another research in 2017 demonstrated that docetaxel, loaded on MWCNTs conjugated with chitosan-folate, was 89-fold more effective in targeting A549 cells.Citation107
Etoposide
Transferring this chemotherapy drug with functionalized MWCNT used in other research for the treatment of cancer. In this research, simultaneously delivery of Bcl-2 and VP-16 targeted antisense by MWCNTs for anti-cancer effectiveness in both kinds of lung cancer cells was done.Citation108
IRGD Peptide and Candesartan
By placing polyethyleneimine and cystamine ligands on MWCNT, researchers modified the attachment to iRGD peptide and candesartan. These two substances boosted the efficiency of MWCNTs transfer and cell absorption. The positioning of these ligands led to the targeting of parenchymal and endothelial cancer cells, and treatment of lung cancer occurred.Citation109
To conclude, in the treatment of this cancer, nanotubes can lead to recovery or serve as a carrier for several functional substances (). In the discussion of drug delivery, nanotubes were functionalized with various substances which led to targeted binding to the tumor tissue. In general, the studies with MWCNTs were more frequent. Indeed, due to researches, it could be stated that CNTs are also useful carriers for nano-drug delivery. CNTs could induce the apoptotic pathway in targeted cells. Besides, lower efficiency may sometimes occur due to the drug resistance. classifies the set of researches conducted under the relevant conditions and the results obtained.
Table 2 Review of Studies and Results of Nano-Drug Delivery by CNT in the Treatment of Lung Cancer (by Year Order)
Discussion
In this section, 4 challenging and contradictory issues are discussed. Also, it invites researchers to do more research to get clearer results.
The efficacy and toxicity of CNTs are always an excellent opportunity for discussion.Citation11,Citation16 One point of contention is that an article stated that a low mount of CNT has more potential to produce toxic effects than asbestos.Citation29 In contrast, Pacurari et alCitation30 stated that asbestos is much more toxic. As well as, the other article confirmed and mentioned that SWCNT had less toxicity in the organs or changes in immune indicators.Citation87
On the other side, two articles stated that the amount of pulmonary inflammation was directly related to CNT dose consumption.Citation39,Citation40 However, another article declared that different doses of CNTs at the same time can cause inflammation.Citation38 Also, increasing the length of CNTs led to heightened toxicity for healthy cells and expanded CNT, despite increased utilization resulting in tumor tissue destruction.Citation7,Citation11
An article stated that SWCNT led to the expression of SOX9 created tumorigenesis and metastasis.Citation56 In contrast, the activation of P53 as tumor suppressor protein was seen in the presence of MWCNTs without mutagenic effectiveness.Citation45 Also, the insertion of nanotubes into the body resulted in increased cytokines such as TNFα and IL1β, which results in toxicity. On the other hand, enhanced cytokines IL-10, and TGF-β, restricted tumor metastasis.Citation36,Citation41 Indeed, the role of tumor destruction of CNTs did not rule out. Another study on breast cancer was performed with CNTs, which confirmed that CNTs could reduce tumor size.Citation110 Furthermore, an in vitro study on A569 cells also approved an increase in the anti-cancer properties of CNT decorated with naringenin.Citation111 A very interesting report in 2020 stated that no documented carcinogenesis had been observed in the locally controlled injection of MWCNS in their mice analyzed. In this study, the use of CNT in a controlled manner is considered safe.Citation53 Another study presented the other advantage of using CNT in nano-drug delivery of hepatocellular as target cells. In this article, the drug was ruthenium polypyridyl, in which cancer cells had resistance to drug and radio waves. CNT can cause to induce more apoptosis with the increased uptake and reduced toxicity.Citation112
An investigation stated which CNTs have antioxidant activity in two groups as fight free radicals, and anti-aging activity.Citation14 Two other studies reported a decrease in antioxidant activity due to using CNTs.Citation25,Citation62 This controversial issue needs further study in this area.
Conclusion and Future Perspective
Due to the researches, it can be mentioned using nanotubes in the diagnosis of disease, has been known to be an effective method. Additionally, studies have suggested that the immune response is not unusual in the in vivo. However, researchers should seek to increase the compatibility of these carriers. The findings also showed that the treatment with CNT was much more effective and more successful than the traditional treatments for this cancer. Fortunately, this type of cancer treatment is rapidly investigated by researchers. Also, using the new methods such as functionalization, nanotubes with a longer length, more width, and greater curvature partially can be done with lower toxicity. The collection of studies summarized has highlighted the need for a detailed assessment which includes the dose of CNT, duration, method of induction, etc., to achieve the most controlled conditions for animal and human studies. Finally, other drugs have also been used to target cancer cells using other Nano methods that could be evaluated for their efficacy using CNTs.Citation113–Citation115
Disclosure
The authors declare that there are no conflicts of interest.
References
- Badrzadeh F, Rahmati-Yamchi M, Badrzadeh K, et al. Drug delivery and nanodetection in lung cancer. Artif Cells Nanomed Biotechnol. 2016;44(2):618–634. doi:10.3109/21691401.2014.97523725386728
- Woodman C, Vundu G, George A, et al. Applications and strategies in nanodiagnosis and nanotherapy in lung cancer. Semin Cancer Biol. 2020. doi:10.1016/j.semcancer.2020.02.009
- Blom EF, Ten Haaf K, Arenberg DA, et al. Disparities in receiving Guideline-Concordant treatment for lung cancer in the United States. Ann Am Thorac Soc. 2020;17(2):186–194. doi:10.1513/AnnalsATS.201901-094OC31672025
- Gao Q, Zhou G, Lin S-J, et al. How chemotherapy and radiotherapy damage the tissue: comparative biology lessons from feather and hair models. Exp Dermatol. 2019;28(4):413–418. doi:10.1111/exd.1384630457678
- England G, Ng C, van Berkel V, Frieboes H. A review of pharmacological treatment options for lung cancer: emphasis on novel nanotherapeutics and associated toxicity. Curr Drug Targets. 2015;16(10):1057–1087.25944016
- Sheikhpour M, Barani L, Kasaeian A. Biomimetics in drug delivery systems: a critical review. J Controlled Release. 2017;253:97–109. doi:10.1016/j.jconrel.2017.03.026
- Kim SW, Park JY, Lee S, Kim SH, Khang D. Destroying deep lung tumor tissue through lung-selective accumulation and by activation of caveolin uptake channels using a specific width of carbon nanodrug. ACS Appl Mater Interfaces. 2018;10(5):4419–4428.29309112
- Sharma P, Mehta M, Dhanjal DS, et al. Emerging trends in the novel drug delivery approaches for the treatment of lung cancer. Chem Biol Interact. 2019;309:108720. doi:10.1016/j.cbi.2019.06.03331226287
- Sheikhpour M, Golbabaie A, Kasaeian A. Carbon nanotubes: a review of novel strategies for cancer diagnosis and treatment. Mater Sci Eng C. 2017;76:1289–1304. doi:10.1016/j.msec.2017.02.132
- van Zandwijk N, Frank AL. Awareness: potential toxicities of carbon nanotubes. Transl Lung Cancer Res. 2019;8(Suppl 4):S471. doi:10.21037/tlcr.2019.12.0532038941
- Kim JS, Song KS, Joo HJ, et al. Determination of cytotoxicity attributed to multiwall carbon nanotubes (MWCNT) in normal human embryonic lung cell (WI-38) line. J Toxicol Environ Health A. 2010;73(21–22):1521–1529. doi:10.1080/15287394.2010.51157720954078
- Kim JS,Song KS, Lee JK, et al. Toxicogenomic comparison of multi-wall carbon nanotubes (MWCNTs) and asbestos. Arch Toxicol. 2012;86(4):553–562.22076105
- Jha R, Singh A, Sharma PK, et al. Smart carbon nanotubes for drug delivery system: A comprehensive study. J Drug Deliv Sci Technol. 2020;58:101811. doi:10.1016/j.jddst.2020.101811
- Saliev T. The advances in biomedical applications of carbon nanotubes. C J Carbon Res. 2019;5(2):29. doi:10.3390/c5020029
- Porter SL, Coulter SM, Pentlavalli S, et al. Pharmaceutical formulation and characterization of dipeptide nanotubes for drug delivery applications. Macromol Biosci. 2020;20(7):2000115. doi:10.1002/mabi.202000115
- Bonner JC. Carbon nanotubes as delivery systems for respiratory disease: do the dangers outweigh the potential benefits? Expert Rev Respir Med. 2011;5(6):779–787. doi:10.1586/ers.11.7222082164
- Alqaheem Y, Alomair AA. Microscopy and spectroscopy techniques for characterization of polymeric membranes. Membranes. 2020;10(2):33. doi:10.3390/membranes10020033
- Heister E, Brunner EW, Dieckmann GR, et al. Are carbon nanotubes a natural solution? Applications in biology and medicine. ACS Appl Mater Interfaces. 2013;5(6):1870–1891. doi:10.1021/am302902d23427832
- Rubio N, Hirvonen LM, Chong EZ, et al. Multiphoton luminescence imaging of chemically functionalized multi-walled carbon nanotubes in cells and solid tumors. Chem Commun (Camb). 2015;51(45):9366–9369. doi:10.1039/C5CC02675J25959229
- Yazid MNAWM, Sidik NAC, Mamat R, et al. A review of the impact of preparation on stability of carbon nanotube nanofluids. Int Commun Heat Mass Transfer. 2016;78:253–263. doi:10.1016/j.icheatmasstransfer.2016.09.021
- Kayat J, Gajbhiye V, Tekade RK, et al. Pulmonary toxicity of carbon nanotubes: a systematic report. Nanomedicine. 2011;7(1):40–49. doi:10.1016/j.nano.2010.06.00820620235
- Rittinghausen S, Hackbarth A, Creutzenberg O, et al. The carcinogenic effect of various multi-walled carbon nanotubes (MWCNTs) after intraperitoneal injection in rats. Part Fibre Toxicol. 2014;11(1):59. doi:10.1186/s12989-014-0059-z25410479
- Dong J, Ma Q. Integration of inflammation, fibrosis, and cancer induced by carbon nanotubes. Nanotoxicology. 2019;13(9):1244–1274. doi:10.1080/17435390.2019.165192031537143
- Kobayashi N, Izumi H, Morimoto Y. Review of toxicity studies of carbon nanotubes. J Occup Health. 2017;17–0089–RA.
- Prajapati SK, Malaiya A, Kesharwani P. et al. Biomedical applications and toxicities of carbon nanotubes. Drug Chem Toxicol;2020 1–16. doi:10.1080/01480545.2019.1709492
- Wang Q, et al. Pleural translocation and lesions by pulmonary exposed multi-walled carbon nanotubes. J Toxicol Pathol. 2020;2019–2075.
- Kane AB, Hurt RH, Gao H. The asbestos-carbon nanotube analogy: an update. Toxicol Appl Pharmacol. 2018;361:68–80. doi:10.1016/j.taap.2018.06.02729960000
- Francis A, Devasena T. Toxicity of carbon nanotubes: a review. Toxicol Ind Health. 2018;34(3):200–210. doi:10.1177/074823371774747229506458
- Dymacek JM, Snyder-Talkington BN, Raese R, et al. Similar and differential canonical pathways and biological processes associated with multiwalled carbon nanotube and asbestos-induced pulmonary fibrosis: a 1-year postexposure study. Int J Toxicol. 2018;37(4):276–284. doi:10.1177/109158181877903829916280
- Pacurari M, Castranova V, Vallyathan V. Single-and multi-wall carbon nanotubes versus asbestos: are the carbon nanotubes a new health risk to humans? J Toxicol Environ Health A. 2010;73(5–6):378–395.20155580
- Nahle S, Safar R, Grandemange S, et al. Single wall and multiwall carbon nanotubes induce different toxicological responses in rat alveolar macrophages. J Appl Toxicol. 2019;39(5):764–772. doi:10.1002/jat.376530605223
- Stueckle TA, Davidson DC, Derk R, et al. Effect of surface functionalizations of multi-walled carbon nanotubes on neoplastic transformation potential in primary human lung epithelial cells. Nanotoxicology. 2017;11(5):613–624. doi:10.1080/17435390.2017.133225328513319
- Zhou L, Forman HJ, Ge Y, et al. Multi-walled carbon nanotubes: a cytotoxicity study in relation to functionalization, dose and dispersion. Toxicol in Vitro. 2017;42:292–298. doi:10.1016/j.tiv.2017.04.02728483489
- Srivastava RK, Pant AB, Kashyap MP, et al. Multi-walled carbon nanotubes induce oxidative stress and apoptosis in human lung cancer cell line-A549. Nanotoxicology. 2011;5(2):195–207. doi:10.3109/17435390.2010.50394420804439
- Vlaanderen J, Pronk A, Rothman N, et al. A cross-sectional study of changes in markers of immunological effects and lung health due to exposure to multi-walled carbon >nanotubes. Nanotoxicology. 2017;11(3):395–404. doi:10.1080/17435390.2017.130803128301273
- Fukai E, Sato H, Watanabe M, et al. Establishment of an in vivo simulating co-culture assay platform for genotoxicity of multi-walled carbon nanotubes. Cancer Science. 2018;109(4):1024–1031. doi:10.1111/cas.1353429444368
- Liu AH. In Vivo studies of the toxicity of multi-wall carbon nanotubes. Advan Mater Res. 2012.
- Muller J, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207(3):221–231. doi:10.1016/j.taap.2005.01.00816129115
- Gaté L, Knudsen KB, Seidel C, et al. Pulmonary toxicity of two different multi-walled carbon nanotubes in rat: comparison between intratracheal instillation and inhalation exposure. Toxicol Appl Pharmacol. 2019;375:17–31.31075343
- Morimoto Y, Hirohashi M, Ogami A, et al. Pulmonary toxicity of well-dispersed multi-wall carbon nanotubes following inhalation and intratracheal instillation. Nanotoxicology. 2012;6(6):587–599. doi:10.3109/17435390.2011.59491221714591
- Wu L, Tang H, Zheng H, et al. Multiwalled carbon nanotubes prevent tumor metastasis through switching M2-polarized macrophages to M1 via TLR4 activation. J Biomed Nanotechnol. 2019;15(1):138–150. doi:10.1166/jbn.2019.266130480521
- Dymacek J, Snyder-Talkington BN, Porter DW, et al. mRNA and miRNA regulatory networks reflective of multi-walled carbon nanotube-induced lung inflammatory and fibrotic pathologies in mice. Toxicol Sci. 2015;144(1):51–64. doi:10.1093/toxsci/kfu26225527334
- Snyder-Talkington BN, Dong C, Porter DW, et al. Multiwalled carbon nanotube-induced pulmonary inflammatory and fibrotic responses and genomic changes following aspiration exposure in mice: a 1-year postexposure study. J Toxicol Environ Health A. 2016;79(8):352–366.27092743
- Snyder-Talkington BN, Dong C, Porter DW, et al. mRNAs and miRNAs in whole blood associated with lung hyperplasia, fibrosis, and bronchiolo-alveolar adenoma and adenocarcinoma after multi-walled carbon nanotube inhalation exposure in mice. J Appl Toxicol. 2016;36(1):161–174.25926378
- Rahman L, et al. Multi-walled carbon nanotube-induced genotoxic, inflammatory and pro-fibrotic responses in mice: investigating the mechanisms of pulmonary carcinogenesis. Mutat Res. 2017;823:28–44.28985945
- Sargent LM, Porter DW, Staska LM, et al. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol. 2014;11:3.24405760
- Öner D, Ghosh M, Coorens R, et al. Induction and recovery of CpG site specific methylation changes in human bronchial cells after long-term exposure to carbon nanotubes and asbestos. Environ Int. 2020;137:105530.32062310
- Suzui M, Futakuchi M, Fukamachi K, et al. Multiwalled carbon nanotubes intratracheally instilled into the rat lung induce development of pleural malignant mesothelioma and lung tumors. Cancer Sci. 2016;107(7):924–935.27098557
- Kasai T, Umeda Y, Ohnishi M, et al. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part Fibre Toxicol. 2016;13(1):53.27737701
- Pacurari M, et al. Multi-walled carbon nanotube-induced gene expression in the mouse lung: association with lung pathology. Toxicol Appl Pharmacol. 2011;255(1):18–31.21624382
- Guo NL, Wan Y-W, Denvir J, et al. Multiwalled carbon nanotube-induced gene signatures in the mouse lung: potential predictive value for human lung cancer risk and prognosis. Journal of Toxicology and Environmental Health, Part A. 2012;75(18):1129–1153. doi:10.1080/15287394.2012.69985222891886
- Yu KN, Kim JE, Seo HW, Chae C, Cho MH. Differential toxic responses between pristine and functionalized multiwall nanotubes involve induction of autophagy accumulation in murine lung. J Toxicol Environ Health A. 2013;76(23):1282–1292.24283420
- Aoki K, Saito N. Biocompatibility and carcinogenicity of carbon nanotubes as biomaterials. Nanomaterials. 2020;10(2):264. doi:10.3390/nano10020264
- Ema M, Gamo M, Honda K. A review of toxicity studies of single-walled carbon nanotubes in laboratory animals. Regul Toxicol Pharmacol. 2016;74:42–63. doi:10.1016/j.yrtph.2015.11.01526619783
- Sanpui P, Zheng X, Loeb JC, et al. Single-walled carbon nanotubes increase pandemic influenza A H1N1 virus infectivity of lung epithelial cells. Part Fibre Toxicol. 2014;11(1):66. doi:10.1186/s12989-014-0066-025497303
- Voronkova MA, Luanpitpong S, Rojanasakul LW, et al. SOX9 regulates cancer stem-like properties and metastatic potential of single-walled carbon nanotube-exposed cells. Sci Rep. 2017;7(1):11653. doi:10.1038/s41598-017-12037-828912540
- Wang L, Luanpitpong S, Castranova V, et al. Carbon nanotubes induce malignant transformation and tumorigenesis of human lung epithelial cells. Nano Lett. 2011;11(7):2796–2803. doi:10.1021/nl201121421657258
- Luanpitpong S, Wang L, Castranova V, et al. Induction of stem-like cells with malignant properties by chronic exposure of human lung epithelial cells to single-walled carbon nanotubes. Part Fibre Toxicol. 2014;11(1):22. doi:10.1186/1743-8977-11-2224885671
- Yang J-S, Li B-J, Lu H-W, et al. Serum miR-152, miR-148a, miR-148b, and miR-21 as novel biomarkers in non-small cell lung cancer screening. Tumour Biol. 2015;36(4):3035–3042. doi:10.1007/s13277-014-2938-125501703
- Luanpitpong S, Wang L, Stueckle TA, et al. Caveolin-1 regulates lung cancer stem-like cell induction and p53 inactivation in carbon nanotube-driven tumorigenesis. Oncotarget. 2014;5(11):3541–3554. doi:10.18632/oncotarget.195624939878
- Wang P, Voronkova M, Luanpitpong S, et al. Induction of slug by chronic exposure to single-walled carbon nanotubes promotes tumor formation and metastasis. Chem Res Toxicol. 2017;30(7):1396–1405. doi:10.1021/acs.chemrestox.7b0004928598615
- Goornavar V, Biradar S, Ezeagwu C, et al. Toxicity of raw and purified single-walled carbon nanotubes in rat’s lung epithelial and cervical cancer cells. J Nanosci Nanotechnol. 2015;15(3):2105–2114. doi:10.1166/jnn.2015.952426413627
- Pongrakhananon V, Luanpitpong S, Stueckle TA, et al. Carbon nanotubes induce apoptosis resistance of human lung epithelial cells through FLICE-inhibitory protein. Toxicol Sci. 2015;143(2):499–511. doi:10.1093/toxsci/kfu25125412619
- Shvedova AA, Kisin ER, Yanamala N, et al. MDSC and TGF are required for facilitation of tumor growth in the lungs of mice exposed to carbon nanotubes. Cancer Res. 2015;75(8):1615–1623. doi:10.1158/0008-5472.CAN-14-237625744719
- He X, Despeaux E, Stueckle TA, et al. Role of mesothelin in carbon nanotube-induced carcinogenic transformation of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2016;311(3):L538–49. doi:10.1152/ajplung.00139.201627422997
- Nasser IM, Abu-Naser SS. Lung cancer detection using artificial neural network. Int J Eng Inf Syst. 2019;3(3):17–23.
- Zhou J, Huang ZA, Kumar U, Chen DD. Review of recent developments in determining volatile organic compounds in exhaled breath as biomarkers for lung cancer diagnosis. Anal Chim Acta. 2017;996:1–9.29137702
- Shakeel PM, Burhanuddin MA, Desa MI. Lung cancer detection from CT image using improved profuse clustering and deep learning instantaneously trained neural networks. Measurement. 2019;145:702–712.
- Bhatia S, Sinha Y, Goel L. Lung Cancer Detection: A Deep Learning Approach, in Soft Computing for Problem Solving. Springer; 2019:699–705.
- Crucitti P, et al. e-nose technology: the state of the art on lung cancer diagnosis. Breath Anal. 2019:121–129.
- Khanmohammadi A, Aghaie A, Vahedi E, et al. Electrochemical biosensors for the detection of lung cancer biomarkers: a review. Talanta. 2020;206:120251. doi:10.1016/j.talanta.2019.12025131514848
- Peng G, Track E, Haick H. Detecting simulated patterns of lung cancer biomarkers by random network of single-walled carbon nanotubes coated with nonpolymeric organic materials. Nano Lett. 2008;8(11):3631–3635. doi:10.1021/nl801577u18839997
- Park CH, Schroeder V, Kim BJ, et al. Ionic liquid-carbon nanotube sensor arrays for human breath related volatile organic compounds. ACS Sensors. 2018;3(11):2432–2437. doi:10.1021/acssensors.8b0098730379539
- Chatterjee S, Castro M, Feller JF. An e-nose made of carbon nanotube based quantum resistive sensors for the detection of eighteen polar/nonpolar VOC biomarkers of lung cancer. J Mater Chem B. 2013;1(36):4563–4575. doi:10.1039/c3tb20819b32261199
- Liu FL, Xiao P, Fang HL, et al. Single-walled carbon nanotube-based biosensors for the detection of volatile organic compounds of lung cancer. Physica E Low Dimens Syst Nanostruct. 2011;44(2):367–372. doi:10.1016/j.physe.2011.08.033
- Wang F, Zhou J, Zhang Y, et al. The value of MicroRNA-155 as a prognostic factor for survival in non-small cell lung cancer: a meta-analysis. PLoS One. 2015;10(8):e0136889. doi:10.1371/journal.pone.013688926322518
- Aasi A, Aghaei SM, Panchapakesan B. A Density Functional Theory (DFT) study on the interaction of toluene with transition metal decorated carbon nanotubes: a promising platform for early detection of lung cancer from human breath. Nanotechnology. 2020.
- Zilberman Y, Tisch U, Pisula W, et al. Spongelike structures of hexa-peri -hexabenzocoronene derivatives enhance the sensitivity of chemiresistive carbon nanotubes to nonpolar volatile organic compounds of cancer. Langmuir. 2009;25(9):5411–5416. doi:10.1021/la804292819344156
- Wan Q, Xu Y, Chen X, et al. Exhaled gas detection by a novel Rh-doped CNT biosensor for prediagnosis of lung cancer: a DFT study. Mol Phys. 2018;116(17):2205–2212. doi:10.1080/00268976.2018.1467057
- Wan Q, Xu Y, Xiao H. Exhaled gas detection by Ir-doped CNT for primary diagnosis of lung cancer. AIP Adv. 2018;8:10.
- Janfaza S, et al. A selective chemiresistive sensor for the cancer-related volatile organic compound hexanal by using molecularly imprinted polymers and multiwalled carbon nanotubes. Microchimica Acta. 2019;186(3):137.30707323
- Choudhary M, Singh A, Kaur S, et al. Enhancing lung cancer diagnosis: electrochemical simultaneous bianalyte immunosensing using carbon nanotubes–chitosan nanocomposite. Appl Biochem Biotechnol. 2014;174(3):1188–1200. doi:10.1007/s12010-014-1020-125024132
- Ta V-T, Park J, Park EJ, et al. Reusable floating-electrode sensor for the quantitative electrophysiological monitoring of a nonadherent cell. ACS Nano. 2014;8(3):2206–2213. doi:10.1021/nn405315524490836
- Cui L, Wang M, Sun B, Ai S, Wang S, Zhang CY. Substrate-free and label-free electrocatalysis-assisted biosensor for sensitive detection of microRNA in lung cancer cells. Chem Commun (Camb). 2019;55(8):1172–1175.30632563
- Snyder-Talkington BN, et al. Multi-walled carbon nanotube-induced gene expression biomarkers for medical and occupational surveillance. Int J Mol Sci. 2019;20(11).
- Kim SW, Lee YK, Lee JY, Hong JH, Khang D PEGylated anticancer-carbon nanotubes complex targeting mitochondria of lung cancer cells. Nanotechnology. 2017.
- Yang S-T, Wang X, Jia G, et al. Long-term accumulation and low toxicity of single-walled carbon nanotubes in intravenously exposed mice. Toxicol Lett. 2008;181(3):182–189. doi:10.1016/j.toxlet.2008.07.02018760340
- Arya N, Arora A, Vasu KS, et al. Combination of single walled carbon nanotubes/graphene oxide with paclitaxel: a reactive oxygen species mediated synergism for treatment of lung cancer. Nanoscale. 2013;5(7):2818–2829. doi:10.1039/c3nr33190c23443459
- Yu B, Tan L, Zheng R, et al. Targeted delivery and controlled release of Paclitaxel for the treatment of lung cancer using single-walled carbon nanotubes. Materials Science and Engineering: C. 2016;68:579–584. doi:10.1016/j.msec.2016.06.02527524057
- Al Zakaria AB, Picaud F, Rattier T, et al. Nanovectorization of TRAIL with single wall carbon nanotubes enhances tumor cell killing. Nano Lett. 2015;15(2):891–895. doi:10.1021/nl503565t25584433
- Al Faraj A, Shaik AS, Halwani R, et al. Magnetic targeting and delivery of drug-loaded SWCNTs theranostic nanoprobes to lung metastasis in breast cancer animal model: noninvasive monitoring using magnetic resonance imaging. Mol Imaging Biol. 2016;18(3):315–324. doi:10.1007/s11307-015-0902-026486793
- Singh N, Sachdev A, Gopinath P. Polysaccharide functionalized single walled carbon nanotubes as nanocarriers for delivery of curcumin in lung cancer cells. J Nanosci Nanotechnol. 2018;18(3):1534–1541. doi:10.1166/jnn.2018.1422229448627
- Cao Y, Huang H-Y, Chen L-Q, et al. Enhanced lysosomal escape of pH-responsive polyethylenimine-betaine functionalized carbon nanotube for the codelivery of survivin small interfering RNA and doxorubicin. ACS Appl Mater Interfaces. 2019;11(10):9763–9776. doi:10.1021/acsami.8b2081030776886
- Razzazan A, Atyabi F, Kazemi B, et al. In vivo drug delivery of gemcitabine with PEGylated single-walled carbon nanotubes. Mater Sci Eng C. 2016;62:614–625. doi:10.1016/j.msec.2016.01.076
- Attri P, Gaur J, Choi S, et al. Interaction studies of carbon nanomaterials and plasma activated carbon nanomaterials solution with telomere binding protein. Sci Rep. 2017;7(1):2636. doi:10.1038/s41598-017-02690-428572671
- Suo X, Eldridge BN, Zhang H, et al. P-glycoprotein-targeted photothermal therapy of drug-resistant cancer cells using antibody-conjugated carbon nanotubes. ACS Appl Mater Interfaces. 2018;10(39):33464–33473. doi:10.1021/acsami.8b1197430188117
- Podesta JE, et al. Antitumor activity and prolonged survival by carbon-nanotube-mediated therapeutic siRNA silencing in a human lung xenograft model. Small. 2009;5(10):1176–1185.19306454
- Guo C, Al-Jamal WT, Toma FM, et al. Design of cationic multiwalled carbon nanotubes as efficient siRNA vectors for lung cancer xenograft eradication. Bioconjug Chem. 2015;26(7):1370–1379. doi:10.1021/acs.bioconjchem.5b0024926036843
- Datir SR, Das M, Singh RP, et al. Hyaluronate tethered, “smart” multiwalled carbon nanotubes for tumor-targeted delivery of doxorubicin. Bioconjug Chem. 2012;23(11):2201–2213. doi:10.1021/bc300248t23039830
- Lodhi N, Mehra NK, Jain NK. Development and characterization of dexamethasone mesylate anchored on multi walled carbon nanotubes. J Drug Target. 2013;21(1):67–76. doi:10.3109/1061186X.2012.72921323039174
- Das M, Datir SR, Singh RP, et al. Augmented anticancer activity of a targeted, intracellularly activatable, theranostic nanomedicine based on fluorescent and radiolabeled, methotrexate-folic Acid-multiwalled carbon nanotube conjugate. Mol Pharm. 2013;10(7):2543–2557. doi:10.1021/mp300701e23683251
- Cirillo G, Vittorio O, Kunhardt D, et al. Combining carbon nanotubes and chitosan for the vectorization of methotrexate to lung cancer cells. Materials. 2019;12(18):2889. doi:10.3390/ma12182889
- Li J, Pant A, Chin CF, et al. In vivo biodistribution of platinum-based drugs encapsulated into multi-walled carbon nanotubes. Nanomedicine. 2014;10(7):1465–1475. doi:10.1016/j.nano.2014.01.00424486857
- Tan JM, Karthivashan G, Arulselvan P, et al. Characterization and in vitro studies of the anticancer effect of oxidized carbon nanotubes functionalized with betulinic acid. Drug Des Devel Ther. 2014;8:2333–2343. doi:10.2147/DDDT.S70650
- Singh RP, Sharma G, Singh S, et al. Effects of transferrin conjugated multi-walled carbon nanotubes in lung cancer delivery. Mater Sci Eng C. 2016;67:313–325. doi:10.1016/j.msec.2016.05.013
- Singh RP, Sharma G, Singh S, et al. Vitamin E TPGS conjugated carbon nanotubes improved efficacy of docetaxel with safety for lung cancer treatment. Colloids Surf B Biointerfaces. 2016;141:429–442. doi:10.1016/j.colsurfb.2016.02.01126895505
- Singh RP, Sharma G, Singh S, et al. Chitosan-folate decorated carbon nanotubes for site specific lung cancer delivery. Mater Sci Eng C. 2017;77:446–458. doi:10.1016/j.msec.2017.03.225
- Heger Z, Polanska H, Krizkova S, et al. Co-delivery of VP-16 and Bcl-2-targeted antisense on PEG-grafted oMWCNTs for synergistic in vitro anti-cancer effects in non-small and small cell lung cancer. Colloids Surf B Biointerfaces. 2017;150:131–140. doi:10.1016/j.colsurfb.2016.11.02327907860
- Su Y, Hu Y, Wang Y, et al. A precision-guided MWNT mediated reawakening the sunk synergy in RAS for anti-angiogenesis lung cancer therapy. Biomaterials. 2017;139:75–90. doi:10.1016/j.biomaterials.2017.05.04628595131
- Kavosi A, Hosseini Ghale Noei S, Madani S, et al. The toxicity and therapeutic effects of single-and multi-wall carbon nanotubes on mice breast cancer. Sci Rep. 2018;8(1):1–12. doi:10.1038/s41598-018-26790-x29311619
- Morais RP, Novais GB, Sangenito LS, et al. Naringenin-functionalized multi-walled carbon nanotubes: a potential approach for site-specific remote-controlled anticancer delivery for the treatment of lung cancer cells. Int J Mol Sci. 2020;21(12):4557. doi:10.3390/ijms21124557
- Wang N, Feng Y, Zeng L, et al. Functionalized multiwalled carbon nanotubes as carriers of ruthenium complexes to antagonize cancer multidrug resistance and radioresistance. ACS Appl Mater Interfaces. 2015;7(27):14933–14945. doi:10.1021/acsami.5b0373926107995
- Shaikhpoor M, et al. Significant changes in D2-like dopamine gene receptors expression associated with non-small-cell lung cancer: could it be of potential use in the design of future therapeutic strategies? Curr Cancer Ther Rev. 2012;8(4):304–310.
- Sheikhpour M, Ahangari G, Sadeghizadeh M, Deezagi A. A novel report of apoptosis in human lung carcinoma cells using selective agonist of D2-like dopamine receptors: a new approach for the treatment of human non-small cell lung cancer. Int J Immunopathol Pharmacol. 2019;8(S4):393–402. doi:10.1177/039463201302600212
- Sheikhpour M, Sadeghizadeh M, Yazdian F, et al. Co-administration of curcumin and bromocriptine nano-liposomes for induction of apoptosis in lung cancer cells. Iran Biomed J. 2020;24(1):24. doi:10.29252/ibj.24.1.2431454860